Abstract
Background
Staphylococcus aureus (S. aureus) is an organism of great public health importance, causing 20,000 deaths annually. Decolonization of patients with S. aureus may prevent infections, yet current options are limited to antimicrobials that promote antibiotic resistance and can cause adverse side effects. Probiotics have potential to reduce colonization of pathogenic bacteria, representing a promising alternative for S. aureus decolonization, but thus far lack rigorous evaluation.
Methods
Potential subjects were recruited from inpatient and outpatient settings within a VA medical center and screened for S. aureus gastrointestinal (GI) or extra-GI colonization using swabs at multiple body sites. Positive, eligible, consenting participants were stratified by colonization site and randomized in a 1:1 ratio to 4-weeks of daily placebo or Lactobacillus rhamnosus (L. rhamnosus) HN001 probiotic treatment. Blood and stool samples, and treatment adherence reports were collected from each subject throughout the study, along with a final set of swabs at study completion to detect S. aureus carriage. The outcomes of this study are GI or extra-GI carriage by S. aureus at the end of 4 weeks of therapy, change in phagocytic activity of polymorphonuclear cells from pre-intervention to post-intervention, and symptomatic S. aureus infection at any site during the study period.
Conclusion
114 participants have been recruited for this study. Analysis of outcomes is underway. This is the first clinical trial to examine the efficacy of L. rhamnosus HN001 for decolonization of S. aureus, and investigates the mechanism by which L. rhamnosus HN001 mediates its effect on S. aureus colonization.
Keywords: Clinical trial, Staphylococcus aureus, MRSA, Lactobacilli, Probiotics, Veterans
1. Introduction
Staphylococcus aureus (S. aureus) is a pathogen of tremendous public health importance. Nearly 100,000 serious methicillin-resistant S. aureus (MRSA) infections occur in the US annually, resulting in close to 20,000 deaths [1]. Between 45 and 75% of these infections are the consequence of a healthcare-associated transmission [2,3]. Despite the reduction in incidence of hospital-acquired MRSA infections since 2005, invasive S. aureus infections continue to increase [4].
Methicillin-susceptible S. aureus (MSSA) is much more common than MRSA. Nearly 30% of the general population has nasal colonization of S. aureus and 1% of that is MRSA [5–8]. Although the anterior nares are the primary ecological niche for strains of S. aureus, including MRSA, several recent studies have shown that individuals may be colonized in the axillae, gastrointestinal (GI) tract, and oropharynx [9–11]. Studies assessing MRSA colonization among hospitalized patients have found their risk of acquiring S. aureus to be particularly high, with between 2 and 7% of patients colonized with MRSA on admission, and an additional 2–10% acquiring it during their hospital stay. Studies have further shown that colonization tends to be persistent and prolonged [12–27].
Asymptomatic colonization with S. aureus is a strong risk factor for subsequent invasive infection [12–28]. MRSA carriage poses a four-fold to thirteen-fold higher risk of infection compared with MSSA colonization [28], and MRSA colonization at hospital admission increases the risk of subsequent MRSA infection, compared with MSSA colonization [29]. Factors that trigger infection in colonized patients include invasive devices, surgical procedures, comorbid illnesses, and immune status. Many of these are not modifiable, thus it seems most logical to intervene at the colonization stage of the illness [29]. Yet, strategies to reduce colonization are currently limited to antimicrobials, which carry their own side effects and have been shown to promote antibiotic resistance [30–32].
Probiotics are live microorganisms that are available over the counter and are widely used as dietary supplements or in nutritional foods. They represent a low-cost, well tolerated, non-antibiotic potential decolonization strategy with no risks of promoting antimicrobial resistance. A limited number of studies have shown that probiotics may reduce colonization of pathogenic bacteria, however, little is known about the efficacy of probiotics for reducing S. aureus colonization specifically [33–35]. The probiotic Lactobacillus rhamnosus (L. rhamnosus), stimulates systemic immune functions, likely enhancing the body's ability to eradicate S. aureus at any colonization site [34,35].
We propose that the probiotic L. rhamnosus HN001,when compared to placebo, will reduce S. aureus nasal colonization when taken for four weeks, by increasing phagocytic activity of white cells, and that it will reduce S. aureus GI colonization over this same 4-week period. To investigate these hypotheses, we are conducting a randomized, double-blinded, placebo-controlled, phase II clinical trial.
2. Methods
2.1. Study design
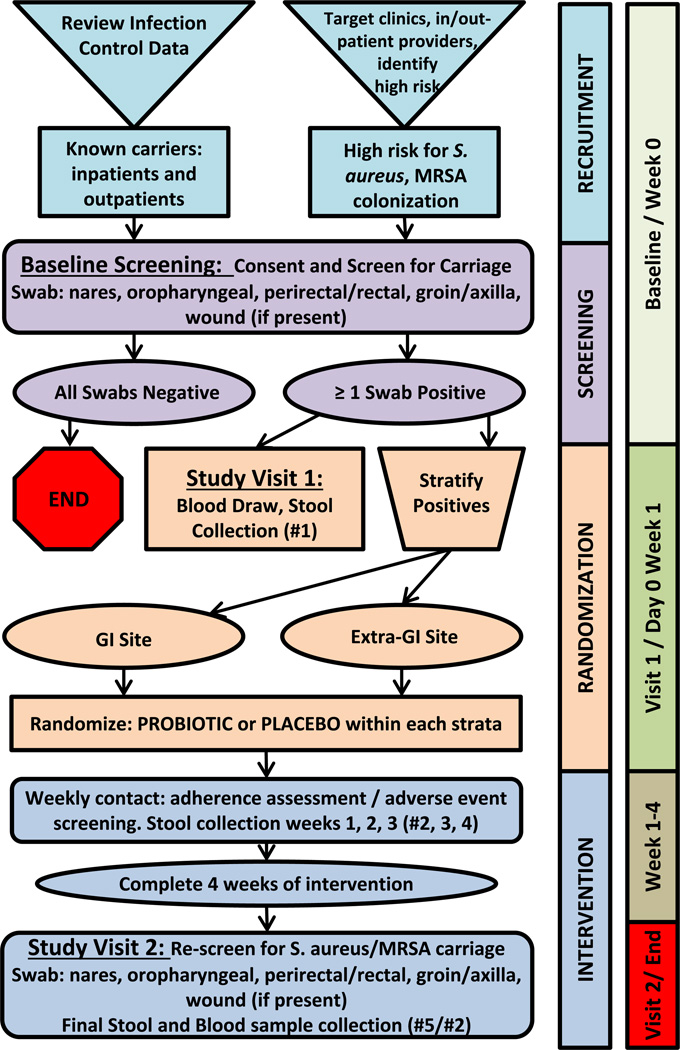
This is a randomized, placebo-controlled, double blind, phase II clinical trial in veterans colonized by S. aureus. Study enrollment took place from February 2012 to December 2015. Fig. 1 displays each step of the study protocol. All study procedures and informed consent documents were approved by the University of Wisconsin-Madison institutional review board and the Veterans Affairs (VA) Research and development committee.
Fig. 1.
Schematic representation of the IMPROVE protocol.
2.2. Participant selection
The inclusion and exclusion criteria for subject enrollment are summarized in Table 1.
Table 1.
Study inclusion and exclusion criteria.
| Inclusion criteria |
|
S. aureus colonization at GI or extra-GI (nares, axillae, wound) source, screened at VAMC |
| Age 18 years or older |
| Able to take oral medications |
| Able to provide informed consent |
| Exclusion criteria |
| Uncontrolled psychiatric illness |
| Already on MRSA decolonization protocol (mupirocin, tea tree oil, etc.) |
| Currently involved in another investigational trial |
| Pregnancy |
| Persistent diarrhea, defined as >3 loose stools per day for at least 2 consecutive days |
| Critical illness (admitted in the ICU at time of enrollment) |
| Cognitive decline (clinical diagnosis of dementia for outpatients, and mention of delirium or dementia in the medical record for inpatients) |
| Active S. aureus infection, including MRSA, currently being treated by therapeutic doses of vancomycin, daptomycin, clindamycin, minocycline, or linezolid |
2.2.1. Inclusion criteria
Adult patients, age 18 years or older of either gender, who screened positive for S. aureus colonization at GI or extra-GI sites at the William S. Middleton Veterans Affairs Hospital (VAMC), were able to take oral medication, and provide informed consent were eligible for this study. S. aureus extra-GI colonization is defined as the recovery of S. aureus, including MRSA, from the nose, axillae, and wound sites. GI carriage is defined as the recovery of S. aureus from an oropharyngeal and/or a perirectal swab or stool specimen. Only 1 swab needed to be positive for the patient to be considered positive for S. aureus colonization.
2.2.2. Exclusion criteria
Patients were excluded from the study if they had uncontrollable psychiatric illness (defined as being admitted to the psychiatric ward of the hospital for inpatients, and mental health clinic visit notes documenting uncontrolled illness), persistent diarrhea (defined as >3 loose stools per day for at least 2 consecutive days), or an active infection with S. aureus (defined as being on vancomycin, daptomycin, or therapeutic doses of clindamycin, minocycline, or linezolid). Any suspected S. aureus infection was confirmed by reviewing microbiology records going back 30 days before enrollment for positive cultures from a sterile site. Other exclusion criteria include being pregnant, admission in the intensive care unit (ICU) at time of enrollment, on a decolonization protocol for MRSA, including mupirocin and tea tree oil, or currently involved in another investigational trial. Women who become pregnant during the study period discontinued the study medication but continued the procedures as listed in the protocol as well as continuing to self-monitor for adverse events. Patients with cognitive decline, as defined by a clinical diagnosis of dementia for outpatients, and mention of delirium or dementia in the medical record for inpatients, were also excluded from the trial.
2.3. Recruitment
This study recruited both inpatients and outpatients seeking care at the VAMC, as well as members of the VA community not currently seeking care. In order to increase rate of positive screening of patients with S. aureus colonization, we used infection control data to determine which patients and clinics were at highest risk for MSSA or MRSA colonization. Several high-risk factors were identified in the literature, from which seven were selected based on easy identifiability in the outpatient setting (Table 2). All potential study subjects were identified through the VAMC, local Veterans of Foreign Wars (VFW) posts, The Dry Hootch, and local papers and VA newsletters. A total of 114 participants were recruited for this study.
Table 2.
Risk factors for colonization with S. aureus.
| Age ≥ 60 years |
| Wound present for 4 weeks or more |
| Diabetes |
| Hospitalization in the last 6 months |
| Resident in a skilled nursing facility |
| Hemodialysis |
| Solid organ or bone marrow transplant patient |
2.3.1. Outpatients
Ten outpatient clinics were targeted for baseline screening, including the primary care, infectious diseases, women's health, geriatrics, dermatology, pulmonary, endocrine, nephrology, cardiology, and orthopedic surgery clinics. Providers at these clinics were asked to call the research team when a patient at high risk for S. aureus colonization was being seen, based on an informational flyer that was distributed to all target clinics that defined high-risk patients. For patients known to be colonized by S. aureus, a weekly list of patients coming to the outpatient clinics was generated by infection control and the research team made contact with them during their scheduled clinic visit.
The consent form and informational material was mailed to all potential subjects who met eligibility criteria and expressed interest in the study. One-week later a follow-up phone call was made to assess the subject's interest, answer questions, and schedule a baseline study visit.
2.3.2. Community sites
Several presentations regarding the study were given at The Dry Hootch and VFWs throughout the study to increase veteran recruitment. If individuals were interested, a study team member contacted the potential subject's primary care physician and evaluated their medical records to assess the appropriateness of study enrollment.
2.3.3. Inpatients
To recruit inpatients, a member of the potential subject's clinical treatment team sought permission from the subject to talk to the study team and introduced the study. A member of the research team gave the participant study details and obtained informed consent.
2.4. Compensation
A total of $100 was provided as compensation to each participant who enrolled in and completed the study, in two $50 payments. One payment was given for completion of Study Visit 1 (Day 0/Week 1) and the second for Study Visit 2 (Week 4), at the end of study. Compensation was only provided for subjects who were found to be colonized with S. aureus, and enrolled in the study. No compensation was given for subjects who were negative for S. aureus on screening, or who underwent screening but did not enroll in the study.
2.5. Randomization
All subjects with a positive S. aureus screen who enrolled in the study were randomized at the first study visit using a blocked randomization scheme in a 1:1 ratio. Randomization was conducted by the VA pharmacy and stratified based on site of S. aureus colonization: GI, or extra-GI sites, to account for differences inherent to various colonization sites. Within each stratum, a permuted blocked randomization was used with varying block sizes. Sequence generation was completed using a computer random number generator. Both the investigators and subjects were blinded to the treatment allocation assignments.
2.6. Intervention
The intervention is daily oral administration of L. rhamnosus HN001 or matched placebo for four weeks.
2.6.1. Probiotic
The probiotic, L. rhamnosus HN001, is administered in capsule form containing 1 × 1010 organisms. The study drug is provided by the manufacturer, DuPont Nutrition and Health (Madison, WI). Random samples of study drug are cultured once monthly to ensure that colony counts remain stable. Subjects take 1 capsule daily for 28 days.
2.6.2. Placebo
The matched placebo contains the same inert filler as the active product and is identical to study treatment in appearance and taste. The manufacturer, DuPont Nutrition and Health, also supplies the placebo.
2.7. GI tract survival and intestinal adhesion of L. rhamnosus HN001
L. rhamnosus HN001 has good viability and survival in the human GI tract as demonstrated by fecal analysis for L. rhamnosus HN001 of 10 subjects who consumed 1.6 × 109 colony-forming units (cfu) L. rhamnosus HN001 per day for 6 months [36]. L. rhamnosus HN001 has also demonstrated strong adhesion to human intestinal epithelial cells [37]. The dose of 1 × 1010 organisms once daily for 28 days was chosen due to its similarity to what has been used in other clinical trials of L. rhamnosus HN001 [35,38–40].
2.8. Intervention timeline
The study timeline is shown in Fig. 1, and intervention details are outlined below.
2.8.1. Baseline screening visit
Once the consent form was signed, the patient was screened for MRSA carriage and the first set of swabs (set #1) was obtained to culture for S. aureus at nose, skin, oropharyngeal and perirectal sites. If an open wound was present, a wound swab was obtained as well. The participant is given 4 stool-collection kits and trained in stool collection.
2.8.2. Study Visit 1
If the participant was found to be colonized with S. aureus, outpatients were told to collect their next stool (#1) and either mail it in, or bring it to clinic at Study Visit 1 (Week 0), where a blood draw was also performed. A blood draw and stool sample were collected for inpatients at this visit as well. All study participants were randomized to receive either placebo or probiotic for 4 weeks. Outpatients and inpatients that had been discharged were asked to mail-in their stool at 1 week, 2 weeks, and 3 weeks from this time point (#2, 3, 4). Study team members collected stool samples from inpatients that had not been discharged.
2.8.3. Study Visit 2
At the end of Week 4 outpatient and discharged inpatient subjects were asked to return to clinic with a stool sample (#5) and were resampled for S. aureus colonization by swabbing nose, skin, oropharyngeal and perirectal sites (set #2). Inpatient subjects that had not been discharged also submitted a final stool sample, and were re-swabbed. At this point, the second blood draw (#2) was performed to detect changes in phagocytic polymorphonuclear (PMN) cell activity.
2.9. Adherence
During the visit at week 4,we collected self-report data using simple validated questions that assess adherence over the past 7 days, for example “Many people don't take their medication perfectly all the time. Over the past 7 days, how many times did you miss a dose of study medication? When was the last time that you missed any of the study medication?”
2.10. Subject withdrawal
All subjects were informed during enrollment that they may discontinue participation in the study at any time. We asked that they contact the study coordinator if they decided to drop-out of the study. The second half of the compensation, $50, was given to all participants who completed at least 75% of the study period. If the subject was willing, the research coordinator arranged a research clinic visit which was used to collect a final set of samples.
2.11. Outcomes
The primary outcome of this study is GI (oropharyngeal, perirectal swab, or stool sample positive) or extra-GI (nares, axillae, or wound swab positive) carriage by S. aureus at the end of 4 weeks of therapy. The secondary outcome is the change in phagocytic activity of PMN cells from pre-intervention (Study Visit 1/week 0) to post-intervention (Study Visit 2/week 4). The exploratory outcome is symptomatic S. aureus infections at any site at any time during the trial period, which will be used for descriptive purposes only.
We hypothesize that the ingestion of L. rhamnosus HN001 will decrease S. aureus colonization by 72%, significantly improve phagocytic functioning of PMN and monocyte cells, and decrease the rate of S. aureus clinically symptomatic infection.
2.12. Sample collection and microbiologic analysis
2.12.1. Blood specimens
We anticipate that there will be a mean increase in the percentages of PMN and monocyte cells with phagocytic activity among subjects in the treatment group compared with the placebo group by at least 15%. This is in line with previous studies that found significant increases ranging from 14 to 35% in PMN phagocytic activity and 40% mean increases in the percentage of monocyte phagocytic activity for subjects taking probiotics [35,40,41].
Once collected, blood samples were processed within 24 h of collection. The phagocytic activity of Monocytes and Granulocytes in whole blood is determined using PHAGOTEST; clinical Diagnostic for the Qualitative Analysis of Leukocyte Phagocytosis in Human Whole blood (Glycotope Biotechnology GmbH, Biocarta San Diego CA). Test kits are analyzed by flow cytometry using the 488 nm argon-ion laser of a Becton-Dickinson FACSCalibur (Becton-Dickinson, Franklin Lakes NJ) controlled by Becton-Dickinson CellQuest software. Results are expressed as % Phagocytizing Granulocytes and % Phagocytizing Monocytes.
2.12.2. Assessment of S. aureus Colonization in Nose, Oropharyngeal, skin Stool and Perirectal Swabs
Polymerase Chain Reaction (PCR) is undertaken on extra-GI and GI swabs to identify colonization by S. aureus using GeneXpert's Xpert SA Nasal Complete kit (Cepheid, Sunnyvale CA). We also utilize conventional cultures to identify and stock S. aureus isolates for future strain typing. Cultures of these sites and wounds are done using a broth enrichment followed by standard microbiologic techniques. A second nasal sample was taken with a flocculated swab and stored for future analysis.
2.12.3. Assessment of S. aureus strain relatedness
We stock all positive S. aureus cultures for future potential strain typing using pulsed-field gel electrophoresis. This could be used to determine whether subjects stay colonized with the same strain or acquire different or multiple strains from the environment.
2.12.4. Assessment of probiotic presence in fecal samples
This analysis is done to confirm that viable probiotic is able to reach the lower intestinal tract, and thus able to exert a potentially beneficial effect on gutmicrobiota, and eradicate GI tract colonization by S. aureus. Probiotic is detected in stool samples (or perirectal swabs) in participants randomized to the intervention group. Identity of the probiotic recovered from each patient is confirmed by strain specific PCR performed on selected colonies of lactobacillus recovered from stool cultures.
2.13. Adverse events
2.13.1. Safety of probiotics
Lactobacilli have a long history of use in food and dairy products and are commercially available to the public without the consent of a physician. They have been safely used in several studies of various patient populations, including premature infants, pregnant women, immunocompromised hosts, and animal models [34,35,40,42–51]. As a live bacterial organism, it carries some degree of risk for clinical infections, including bacteremia, however these infections due to Lactobacillus species arise most often from the patient's endogenous microbiota. L. rhamnosus HN001 is unable to dissolve mucin, and has not been found to translocate to organs beyond the intestine [50,52,53], and is unlikely to be able to cause invasive disease. When they do occur, the majority of clinical infections due to lactobacilli species from probiotics occur in immunocompromised or severely ill patients, thus we have excluded ICU subjects from our study.
Although we did not anticipate any safety concerns associated with the administration of L. rhamnosus HN001 given its long safety record, close monitoring for adverse effects and clinical infection was maintained to ensure protection of subjects.
2.13.2. Safety of placebo
The placebo used was made of the inert and inactive substance microcrystalline cellulose, normally used as a carrier substance in the formulation of tablets and capsules. There was virtually no likelihood of adverse reaction to the substance, therefore there was no foreseeable risk associated with placebo ingestion. However, safety monitoring was still performed.
2.13.3. Safety monitoring
All participants were instructed to contact the research specialist directly if they developed a fever or other signs of infection. In the case of emergency, such as severe illness, they were instructed to proceed to the nearest emergency room or call 911. Subjects were instructed to keep a copy of the study information in case they needed to be evaluated by an outside physician. In the event of any adverse effects, timely, accurate and complete reporting and analysis of safety information was undertaken.
2.14. Data management
A Data and Safety Monitoring Committee (DSMC) set up by the VA funding agency was used to ensure subject safety, research data integrity, compliance with federal regulations, and serious adverse event monitoring. The frequency of protocol oversight by the DSMC was quarterly.
2.15. Statistical analysis
2.15.1. Sample size justification
Assuming the rate of S. aureus carriage at the time of initial screening for the placebo group is 30% [5–8],- a sample size of 114 subjects (approximately 57 per group)would provide 80% power to detect a 72% reduction in carriage rates using a 2-sided 0.05 level significance test. The sample size of 114 includes a 10% drop out rate inflation. Sample size calculations were performed using R version 2.9.1 (R Foundation for Statistical Computing, Vienna, Austria).
2.15.2. Data analysis
All analyses will be conducted in SAS version 9.4 (SAS Institute, Cary, NC). The primary outcome for this trial is carriage of S. aureus at the end of 4-weeks of treatment, comparing probiotic and placebo treatment groups. The primary analysis will consist of a single comparison of the rates of carriage in the L. rhamnosus HN001 and placebo groups using the Cochran-Mantel-Haenszel test for differences in proportions across strata, as defined by baseline carriage site. A nominal two-sided p-value of 0.05 will be regarded as statistically significant.
The main analyses of the primary outcome will be based on the intention- to-treat principle. Additional exploratory analyses will be conducted using the on-protocol population, which will consist of subjects who took at least 50% of assigned doses of study drug. We anticipate relatively high adherence; however, this additional analysis will help to understand the biological efficacy of treatment.
Repeated measures linear regression models will be used to evaluate the effect of probiotic treatment on phagocytic activity of PMN and monocyte cells (in some cases, after log-transformation) at the two time-points (Study Visit 1/week 0, and week 4/end of treatment). Models will include the treatment contrast of interest, as well as relevant covariates.
To assess the exploratory outcome, the composite incidence rate of all-site S. aureus infections in probiotic and placebo groups will be compared using the Cochran-Mantel-Haenszel test for differences in proportions across strata defined by baseline carriage site.
3. Discussion
Ingestion of lactic acid bacteria has been linked to enhanced host immune response in many human and animal studies [34,35,38–41, 54–57]. Lactic acid bacteria have been shown to induce local immune modulation in the GI tract, and certain strains may also induce a systemic immune response [58–60]. The probiotic used in this study, L. rhamnosus HN001, is a lactic acid bacterium shown to affect significant immunemodulation, and is a recognized immunostimulatory agent [34, 35,38,39,56,57]. L. rhamnosus HN001 consumption in mice and humans is associated with enhanced phagocytic activity of leukocytes, macrophages, PMN cells, and natural killer cells [34,35,40,56,57,61]. The increase in phagocytosis seen with L. rhamnosus HN001 therapy could lead to improved killing of gram positive organisms such as S. aureus, including MRSA.
This is the first clinical trial to examine the efficacy of L. rhamnosus HN001 for decolonization of S. aureus. This study will identify the major mechanisms by which L. rhamnosus HN001 is expected to mediate its effect on S. aureus colonization, and shed light on its efficacy in decolonization at sites outside of the GI tract. If the hypotheses are supported, this study would be strong evidence for the use of probiotics, specifically L. rhamnosus HN001, as a low cost, safe, non-antibiotic adjunct treatment option. By including both inpatients and outpatients in our study population, we hope to elucidate the effects of probiotic therapy in multiple settings. If the treatment is successful, and readily adopted, the use of probiotics would greatly reduce carriage of S. aureus, thus reducing the infection rate, the spread of S. aureus, particularly in the hospital setting, would reduce use of antibiotics and subsequent antibiotic resistance, and significantly reduce the costs associated with care of patients colonized and infected by S. aureus.
The innovation in this trial is assessing the use L. rhamnosus to reduce S. aureus colonization. We examine the efficacy of this treatment option in several innovative ways including using inpatient and outpatient participants, assessing several different sites of colonization, and examining PMN activity as the mediating variable. We also use various methods to monitor study quality, including detailed adherence reporting, and assessment of probiotic presence in fecal samples.
Over the course of this trial, we have learned some valuable lessons about successful study execution. For instance, the use of both inpatients and outpatients helped make our study population more diverse and generalizable, but when subjects, albeit infrequently, transitioned from one setting to the other, brief lapses in adherence did occur. Patients transitioning often forgot about their trial medication when going through the pharmacy on check-in or discharge, causing a 1–2 day lag in administration of the appropriate study drug. Given our regular collection of adherence data, and high level of adherence overall, these brief lapses likely did not affect our trial outcome. However, future studies with a need for more rigorous adherence should closely consider this issue when designing a study using inpatient and outpatient participants.
Clinical staff engagement was also a likely key to successful completion of this trial. Our research team had previously established positive relationships with the pharmacy and patient care teams involved in the study, allowing for increased ease of study coordination. We have also conducted prior interventions using probiotics within this hospital, and climate within the clinical setting for this type of practice was positive.
While this study will add important knowledge to the literature, it does have some limitations. The sample was drawn from the Wisconsin veteran population and is therefore predominantly white and male. The inpatients included in this study, while adding valuable information, have a high risk of re-exposure to S. aureus in the hospital setting. If an inpatient participant was colonized by S. aureus at enrollment, was successfully decolonized by the treatment drug, and was subsequently re-exposed to S. aureus and re-colonized during their hospital stay in the course of the clinical trial, we may not be able to detect the successful decolonization. Cases such as this would bias our results toward the null.
Lastly, by excluding patients with symptomatic infections, we are unable to examine the role of L. rhamnosus HN001 in treating S. aureus infection, however, that aim is outside the scope of the current study. If, as expected, S. aureus colonization is reduced, the next step is to undertake a study to examine the efficacy of probiotics for reducing S. aureus clinical infections. Comparative effectiveness research, comparing the impact of probiotics to currently available methods of decolonization such as topical mupirocin is another logical extension of this work. Future studies could also examine the impact of probiotics on colonization and infection by other multidrug-resistant bacteria, such as vancomycin-resistant enterococcus, and Clostridium difficile.
Acknowledgments
This work was supported by the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (Award Number I01BX007080). The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs. AB is supported under National Institute of Health (awards UL1TR000427 and TL1TR000429, administered by the University of Wisconsin-Madison's Institute for Clinical and Translational Research). These funding sources had no role in the design or implementation of this study, and will have no role in data analysis, in of data, or the decision to submit results.
Status of trial
Recruitment for this study has concluded. Nasia Safdar (nasia.safdarva.gov) is the study contact. This study is funded by the Department of Veterans Affairs.
Abbreviations
- CFU
colony-forming units
- DSMC
Data and Safety Monitoring Committee
- GI
gastrointestinal
- ICU
intensive care unit
- IMPROVE
Impact of Probiotics for Reducing Infections in Veterans
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- PCR
polymerase chain reaction
- PMN
polymorphonuclear
- VA
Veterans Affairs
- VAMC
William S. Middleton Veterans Affairs Hospital
- VFW
Veterans of Foreign Wars
Footnotes
The ClinicalTrials.gov identification number is NCT01321606.
Competing interests
The authors have no conflict of interest to disclose.
Author's contributions
SE drafted and edited manuscript.
AB edited manuscript.
SV recruited subjects, performed sample collection, edited the protocol.
TH is the statistician for the study.
MD drafted and edited microbiology methods.
NS drafted and edited protocol, edited manuscript.
References
- 1.Klevens R, Morrison MA, Nadle J, et al. Invasive methicillin-resistant staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. http://dx.doi.org/10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.P R V, J M. A comparative analysis of community acquired and hospital acquired methicillin resistant Staphylococcus aureus. J Clin Diagn Res JCDR. 2013;7(7):1339–1342. doi: 10.7860/JCDR/2013/5302.3139. http://dx.doi.org/10.7860/JCDR/2013/5302.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Flynn NM, King JH, Monchaud C, Morita M, Cohen SH. Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J Clin Microbiol. 2006;44(7):2423–2427. doi: 10.1128/JCM.00254-06. http://dx.doi.org/10.1128/JCM.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malani PN. National burden of invasive methicillin-resistant staphylococcus aureus infection. JAMA. 2014;311(14):1438–1439. doi: 10.1001/jama.2014.1666. http://dx.doi.org/10.1001/jama.2014.1666. [DOI] [PubMed] [Google Scholar]
- 5.Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 2002;346(24):1871–1877. doi: 10.1056/NEJMoa003069. http://dx.doi.org/10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31(1):13–24. doi: 10.1016/0195-6701(95)90079-9. http://dx.doi.org/10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 7.Kluytmans J, Belkum A van, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997;10(3):505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: Treating S. aureus nasal carriage. Ann Pharmacother. 1998;32(1):S7–S16. doi: 10.1177/106002809803200104. http://dx.doi.org/10.1177/106002809803200104. [DOI] [PubMed] [Google Scholar]
- 9.Eveillard M, de Lassence A, Lancien E, Barnaud G, Ricard J, Joly-Guillou M, et al. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect. Control Hosp. Epidemiol. 2006;27(2):181–184. doi: 10.1086/500627. http://dx.doi.org/10.1086/500627. [DOI] [PubMed] [Google Scholar]
- 10.El-Bouri K, El-Bouri W. Screening cultures for detection of methicillin-resistant Staphylococcus aureus in a population at high risk for MRSA colonisation: identification of optimal combinations of anatomical sites. Libyan J Med. 2013;8(0) doi: 10.3402/ljm.v8i0.22755. http://www.libyanjournalofmedicine.net/index.php/ljm/article/view/22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matheson A, Christie P, Stari T, et al. Nasal swab screening for methicillin-resistant Staphylococcus aureus—how well does it perform? A cross-sectional study. Infect. Control Hosp. Epidemiol. 2012;33(8):803–808. doi: 10.1086/666639. http://dx.doi.org/10.1086/666639. [DOI] [PubMed] [Google Scholar]
- 12.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102. http://dx.doi.org/10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 13.Muder RR, Brennen C, Wagener MM, et al. Methicillin-resistant staphylococcal colonization and infection in a long -term care facility. Ann. Intern. Med. 114(2):1991–1107. doi: 10.7326/0003-4819-114-2-1-107. [DOI] [PubMed] [Google Scholar]
- 14.Roghmann M-C, Siddiqui A, Plaisance K, Standiford H. MRSA colonization and the risk of MRSA bacteraemia in hospitalized patients with chronic ulcers. J Hosp Infect. 2001;47(2):98–103. doi: 10.1053/jhin.2000.0903. http://dx.doi.org/10.1053/jhin.2000.0903. [DOI] [PubMed] [Google Scholar]
- 15.Dupeyron C, Campillo B, Mangeney N, Bordes M, Richardet J, Leluan G. Carriage of Staphylococcus aureus and of gram-negative bacilli resistant to third-generation Cephalosporins in cirrhotic patients: a prospective assessment of hospital-acquired infections. Infect. Control Hosp. Epidemiol. 2001;22(7):427–432. doi: 10.1086/501929. http://dx.doi.org/10.1086/501929. [DOI] [PubMed] [Google Scholar]
- 16.Pacio GA, Visintainer P, Maguire G, Wormser GP, Raffalli J, Montecalvo MA. Natural history of colonization with vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and resistant Gram-negative bacilli among long-term-care facility residents. Infect. Control Hosp. Epidemiol. 2003;24(4):246–250. doi: 10.1086/502201. http://dx.doi.org/10.1086/502201. [DOI] [PubMed] [Google Scholar]
- 17.Garrouste-Orgeas M, Timsit J, Kallel H. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect. Control Hosp. Epidemiol. 2001;22(11):687–692. doi: 10.1086/501846. http://dx.doi.org/10.1086/501846. [DOI] [PubMed] [Google Scholar]
- 18.Campillo B, Dupeyron C, Richardet JP. Epidemiology of hospital-acquired infections in cirrhotic patients: effect of carriage of methicillin-resistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol. Infect. 2001;127(3):443–450. doi: 10.1017/s0950268801006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarts M-AWM, Hancock JN, Heyland DM, McLeod RSM, Marshall JCM. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: a systematic review and meta-analysis of randomized trials. Crit. Care Med. 2008;36(1):108–117. doi: 10.1097/01.CCM.0000297956.27474.9D. http://dx.doi.org/10.1097/01.CCM.0000297956.27474.9D. [DOI] [PubMed] [Google Scholar]
- 20.Pujol M, Peña C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am. J. Med. 1996;100(5):509–516. doi: 10.1016/s0002-9343(96)00014-9. http://dx.doi.org/10.1016/S0002-9343(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 21.Longfield JN, Townsend TR, Cruess DF, et al. Methicillin-resistant Staphylococcus aureus (MRSA): risk and outcome of colonized vs infected patients. Infect. Control. 1985;6(11):445–450. doi: 10.1017/s0195941700064791. [DOI] [PubMed] [Google Scholar]
- 22.Coello R, Glynn JR, Gaspar C, Picazo JJ, Fereres J. Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J Hosp Infect. 1997;37(1):39–46. doi: 10.1016/s0195-6701(97)90071-2. http://dx.doi.org/10.1016/S0195-6701(97)90071-2. [DOI] [PubMed] [Google Scholar]
- 23.Hagiwara S, Miwa A, Yoshida M, et al. Methicillin-resistant Staphylococcus aureus: colonization and development of infection in patients with haematological disorders. Eur. J. Haematol. 1995;55(4):267–271. doi: 10.1111/j.1600-0609.1995.tb00272.x. http://dx.doi.org/10.1111/j.1600-0609.1995.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 24.Fierobe L, Decré D, Mùller C, et al. Methicillin-resistant Staphylococcus aureus as a causative agent of postoperative intra-abdominal infection: relation to nasal colonization. Clin. Infect. Dis. 1999;29(5):1231–1238. doi: 10.1086/313454. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan JA, Titus MG, Gröschel DHM, Getchell-White SI, Farr BM. Effectiveness of contact isolation during a hospital outbreak of methicillin resistant Staphylococcus aureus. Am. J. Epidemiol. 1996;143(5):496–504. doi: 10.1093/oxfordjournals.aje.a008770. [DOI] [PubMed] [Google Scholar]
- 26.Girou E, Pujade G, Legrand P, Cizeau F, Brun-Buisson C. Selective screening of carriers for control of methicillin-resistant Staphylococcus aureus (MRSA) in high-risk hospital areas with a high level of endemic MRSA. Clin. Infect. Dis. 1998;27(3):543–550. doi: 10.1086/514695. http://dx.doi.org/10.1086/514695. [DOI] [PubMed] [Google Scholar]
- 27.Mest DR, Wong DHP, Shimoda KJR, Mulligan ME, Wilson SE. Nasal colonization with methicillin-resistant Staphylococcus aureus on admission to the surgical intensive care unit increases the risk of infection. Anesth. Analg. 1994;78(4):644–650. doi: 10.1213/00000539-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am. J. Med. 2008;121(4):310–315. doi: 10.1016/j.amjmed.2007.07.034. http://dx.doi.org/10.1016/j.amjmed.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 2004;39(6):776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 30.Levy SB. The challenge of antibiotic resistance. Sci. Am. 1998;278(3):32–39. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 31.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. http://dx.doi.org/10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 32.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. http://dx.doi.org/10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glück U, Gebbers J-O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and β-hemolytic streptococci) Am. J. Clin. Nutr. 2003;77(2):517–520. doi: 10.1093/ajcn/77.2.517. [DOI] [PubMed] [Google Scholar]
- 34.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J. Clin. Immunol. 2001;21(4):264–271. doi: 10.1023/a:1010979225018. http://dx.doi.org/10.1023/A:1010979225018. [DOI] [PubMed] [Google Scholar]
- 35.Gill HS, Rutherfurd KJ. Probiotic supplementation to enhance natural immunity in the elderly: effects of a newly characterized immunostimulatory strain Lactobacillus rhamnosus HN001 (DR20™) on leucocyte phagocytosis. Nutr. Res. 2001;21(1–2):183–189. http://dx.doi.org/10.1016/S0271-5317(00)00294-3. [Google Scholar]
- 36.Tannock GW, Munro K, Harmsen HJM, Welling GW, Smart J, Gopal PK. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosusDR20. Appl. Environ. Microbiol. 2000;66(6):2578–2588. doi: 10.1128/aem.66.6.2578-2588.2000. http://dx.doi.org/10.1128/AEM.66.6.2578-2588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopal PK, Prasad J, Smart J, Gill HS. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 2001;67(3):207–216. doi: 10.1016/s0168-1605(01)00440-8. http://dx.doi.org/10.1016/S0168-1605(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 38.Cross ML, Mortensen RR, Kudsk J, Gill HS. Dietary intake of Lactobacillus rhamnosus HN001 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med Microbiol Immunol (Berl) 2002;191(1):49–53. doi: 10.1007/s00430-002-0112-7. http://dx.doi.org/10.1007/s00430-002-0112-7. [DOI] [PubMed] [Google Scholar]
- 39.Gill HS, Rutherfurd KJ. Immune enhancement conferred by oral delivery of Lactobacillus rhamnosus HN001 in different milk-based substrates, 2001. J Dairy Res. 68(4):611–616. doi: 10.1017/s0022029901005155. http://dx.doi.org/10.1017/S0022029901005155. [DOI] [PubMed] [Google Scholar]
- 40.Sheih Y-H, Chiang B-L, Wang L-H, Liao C-K, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J. Am. Coll. Nutr. 2001;20(2):149–156. doi: 10.1080/07315724.2001.10719027. http://dx.doi.org/10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- 41.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001;74(6):833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 42.Österlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br. J. Cancer. 2007;97(8):1028–1034. doi: 10.1038/sj.bjc.6603990. http://dx.doi.org/10.1038/sj.bjc.6603990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.M M, E L, D L, et al. Prevention of febrile neutropenia in cancer patients by probiotic strain Enterococcus faecium M-74. Pilot study phase I. Neoplasma. 2004;52(2):159–164. [PubMed] [Google Scholar]
- 44.Mego M, Koncekova R, Mikuskova E, et al. Prevention of febrile neutropenia in cancer patients by probiotic strain Enterococcus faecium M-74. Phase II study, Support Care Cancer. 2005;14(3):285–290. doi: 10.1007/s00520-005-0891-7. http://dx.doi.org/10.1007/s00520-005-0891-7. [DOI] [PubMed] [Google Scholar]
- 45.Heiser CR, Ernst JA, Barrett JT, French N, Schutz M, Dube MP. Probiotics, soluble fiber, and L-glutamine (GLN) reduce nelfinavir (NFV)or lopinavir/ritonavir (LPV/r)-related diarrhea. J Int Assoc Physicians AIDS Care JIAPAC. 2004;3(4):121–129. doi: 10.1177/154510970400300403. http://dx.doi.org/10.1177/154510970400300403. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham-Rundles S, Ahrné S, Bengmark S, et al. Probiotics and immune response. Am. J. Gastroenterol. 2000;95(1, Supplement 1):S22–S25. doi: 10.1016/s0002-9270(99)00813-8. http://dx.doi.org/10.1016/S0002-9270(99)00813-8. [DOI] [PubMed] [Google Scholar]
- 47.Timsit JF, Cheval C, Gachot B, et al. Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med. 2001;27(4):640–647. doi: 10.1007/s001340000840. http://dx.doi.org/10.1007/s001340000840. [DOI] [PubMed] [Google Scholar]
- 48.Salminen MK, Tynkkynen S, Rautelin H, et al. The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on antiretroviral therapy: a randomized, placebo-controlled. Crossover Study. HIV Clin Trials. 2004;5(4):183–191. doi: 10.1310/6F83-N39Q-9PPP-LMVV. http://dx.doi.org/10.1310/6F83-N39Q-9PPP-LMVV. [DOI] [PubMed] [Google Scholar]
- 49.Pawłowska J, Klewicka E, Czubkowski P, et al. Effect of lactobacillus casei DN-114001 application on the activity of fecal enzymes in children after liver transplantation. Transplant. Proc. 2007;39(10):3219–3221. doi: 10.1016/j.transproceed.2007.03.101. http://dx.doi.org/10.1016/j.transproceed.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 50.Zhou JS, Shu Q, Rutherfurd KJ, Prasad J, Gopal PK, Gill HS. Acute oral toxicity and bacterial translocation studies on potentially probiotic strains of lactic acid bacteria. Food Chem. Toxicol. 2000;38(2–3):153–161. doi: 10.1016/s0278-6915(99)00154-4. http://dx.doi.org/10.1016/S0278-6915(99)00154-4. [DOI] [PubMed] [Google Scholar]
- 51.Zhou JS, Shu Q, Rutherfurd KJ, et al. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol. 2000;56(1):87–96. doi: 10.1016/s0168-1605(00)00219-1. http://dx.doi.org/10.1016/S0168-1605(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhou JS, Gopal PK, Gill HS. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int. J. Food Microbiol. 2001;63(1–2):81–90. doi: 10.1016/s0168-1605(00)00398-6. http://dx.doi.org/10.1016/S0168-1605(00)00398-6. [DOI] [PubMed] [Google Scholar]
- 53.Shu Q, Zhou JS, J. Rutherfurd K, et al. Probiotic lactic acid bacteria (Lactobacillus acidophilus HN017, Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019) have no adverse effects on the health of mice. Int. Dairy J. 1999;9(11):831–836. [Google Scholar]
- 54.Manley KJ, Fraenkel MB, Mayall BC, DA P. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. [Accessed May 26, 2016];Med. J. Aust. 2007 186(9) doi: 10.5694/j.1326-5377.2007.tb00995.x. https://www.mja.com.au/journal/2007/186/9/probiotic-treatment-vancomycin-resistant-enterococci-randomised-controlled-trial. [DOI] [PubMed] [Google Scholar]
- 55.Gill HS, Rutherfurd KJ, Gopal PK. Dietary probiotic supplementation to enhance cellular immunity in the elderly. Br. J. Biomed. Sci. 2001;58(2):94–96. [PubMed] [Google Scholar]
- 56.Gill HS, Shu Q, Lin H, Rutherfurd KJ, Cross ML. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med Microbiol Immunol (Berl) 2001;190(3):97–104. doi: 10.1007/s004300100095. http://dx.doi.org/10.1007/s004300100095. [DOI] [PubMed] [Google Scholar]
- 57.Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) Br. J. Nutr. 2000;83(2):167–176. doi: 10.1017/s0007114500000210. http://dx.doi.org/10.1017/S0007114500000210. [DOI] [PubMed] [Google Scholar]
- 58.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am. J. Clin. Nutr. 2001;73(2):444s–450s. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 59.Paineau D, Carcano D, Leyer G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008;53(1):107–113. doi: 10.1111/j.1574-695X.2008.00413.x. http://dx.doi.org/10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 60.de Vrese M, Winkler P, Rautenberg P, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44–46):6670–6674. doi: 10.1016/j.vaccine.2006.05.048. http://dx.doi.org/10.1016/j.vaccine.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 61.Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20™) against Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 2002;34(1):59–64. doi: 10.1111/j.1574-695X.2002.tb00603.x. http://dx.doi.org/10.1111/j.1574-695X.2002.tb00603.X. [DOI] [PubMed] [Google Scholar]