Summary
To date, studies have demonstrated the dynamic influence of exogenous environmental stimuli on multiple regions of the brain. This environmental influence positively and negatively impacts programs governing myelination, and acts on myelinating oligodendrocyte (OL) cells across the entire human lifespan. Developmentally, environmental manipulation of OL progenitor cells (OPCs) has profound effects on the establishment of functional cognitive, sensory, and motor programs. Furthermore, central nervous system (CNS) myelin remains an adaptive entity in adulthood, sensitive to environmentally induced structural changes. Here, we discuss the role of environmental stimuli on mechanisms governing programs of CNS myelination under normal and pathological conditions. Importantly, we highlight how these extrinsic cues can influence the intrinsic power of myelin plasticity to promote functional recovery.
Why study the environmental effects on myelination and myelin generation?
The strength, duration, and timing of environmental experience influences plasticity in brain circuitry [1,2]. This complex circuitry is comprised of a network of communication cables called axons- which connect neurons in one region of the brain to another, and are coated in a fatty substance called myelin. By ensheathing axons in multiple concentric layers of membrane, myelin allows for the rapid and accurate conduction of nerve impulses [3]. Myelin is crucial for proper brain function throughout life and its integrity is continuously refined in response to environmental stimuli [4]. Importantly, loss of or damage to myelin impairs nerve conduction and often leads to cognitive, motor, and behavioral deficits [5], which constitute substrates of a wide variety of neurological diseases. The majority of neurodevelopmental disorders are associated with defects in microstructural organization of the white matter (WM) - likely involving abnormalities in developmental myelination [6]. In the vertebrate CNS, new myelin is made by OLs that derive from a large pool of precursors called oligodendrocyte progenitor cells (OPCs) [7]. Proliferation and differentiation of OPCs into myelin producing cells continues well into adulthood [8]. Myelin growth can also be reinitiated from OPCs in the adult, as mature myelin sheaths are wrapped with additional layers of membrane [9]. Alterations in myelin are modifiable by environmental experience during critical windows of structural remodeling and functional adaptation (Box 1.). This plasticity is regulated by intrinsic and extrinsic signals that coordinate to control myelination programs in both the healthy and pathological brain [10], and are key to optimizing myelin function.
Box 1. Critical Windows of Plasticity.
Environmental experience instructs WM plasticity during limited temporal periods. Within these critical windows, structural remodeling and functional adaptation of OLs and myelin occur with heightened sensitivity. These epochs of plasticity are particularly important during development, recovery from injury, and remodeling in the adult.
In preterm infants, damage to the developing WM commonly induces chronic neurological disability. Pathogenesis of this WM injury coincides with a critical window of gestational immaturity that precedes the onset of myelination [44] and is marked by regional distribution of vulnerable OPCs throughout the brain [45]. Diffuse WM damage evokes a disruption in maturation that renders OPCs unable to advance along their normal developmental trajectory, thus leading to myelination failure [46].
In the postnatal mouse brain, most OPCs differentiate within a specific temporal window of 3–8 days. Targeted ablation of these OPCs during a critical early postnatal period hinders brain remodeling after injury, which corresponds to myelin and motor deficits [39,47]. OPC maturation and survival also increases within this period in response to injury [48]. Chemical demyelination models identify windows of opportunity for remyelination of experimentally induced lesions. Outside of these windows (beyond 6 weeks), demyelinated axons are functionally irreparable, despite the presence of myelinating OLs [49].
New myelin sheaths are constructed throughout adulthood by newly differentiating OLs [25]. In vivo imaging in zebra fish demonstrated that single OLs only generate myelin sheaths within a restricted five-hour period. [50]. This limited response to the cellular environment is an intrinsic feature of individual OLs, and is irrespective of the number of newly elaborated sheaths. Alterations in myelin sheath thickness also occur within established critical periods. Socially isolated juvenile mice exhibit irregularities in myelin and behavior that fail to normalize after social reintegration. However, individually housed adult mice display no persistent deficits, suggesting a critical period of environmental influence that occurs during adolescence [51].
The protracted progression to neuropsychiatric disorders is, to some extent, a product of destabilization that occurs during critical periods of development. Convergent evidence highlights a central role for OLs and myelin in schizophrenia (SZ) [52,53]. WM pathology during critical periods of adolescence heightens vulnerability to SZ [54], and contributes to disease-related abnormalities in synaptic formation and function [51], OL maturation and density [55], cell-cycle arrest genes [56], and axonal conduction velocity [57].
The brain is remarkably responsive to the environment. Future studies will surely continue to unravel the complexity of cellular and molecular mechanisms that underlie critical periods of plasticity in oligodendroglia under normal physiological conditions, and in injury and disease.
This concept of adaptive myelination - the OL as a plastic entity, is in stark contrast to our traditional understanding of OLs as “second fiddle” to other central cell types, and capable only of passive electrical insulation of axons [11]. In fact, we have readily departed from the “limitations” of OLs as a static cell type; rather, we now understand that OLs establish a crucially important partnership with axons to modulate their metabolic function [12], and that myelin participates in the remodeling of brain circuitry according to experience [4]. Changes in myelin thickness also influence the velocity of neuronal communication [13]. This dynamic feedback loop between myelin plasticity and neuronal excitability is crucial for the elaboration and stabilization of neuronal circuitry, helps strengthen motor and cognitive function, and permits the acquisition of new skills and memories in children and adults. However, the influence of the environment on myelination is not yet fully understood. This review aims to underscore the inherent dynamic capabilities of OLs, and to further elucidate programs of environmentally-induced myelin plasticity in brain. Championing the environment to empower the structure and function of WM provides an avenue through which experiences become embedded in measurable patterns of myelination.
Oligodendrocyte development
In humans, OL development occurs between 23 and 37 weeks gestational age, and continues postnatally until adolescence [14]. In the postnatal brain, OPCs undergo a highly regulated yet impressionable developmental process to become mature, myelinating cells that closely interact with axons to facilitate the process of myelination. Many of these OPCs migrate into a region of the brain called white matter (WM), which occupies almost half the volume of the human brain [15]. Region-specific patterns of myelination correspond to developmental behavioral and functional milestones, starting with homeostatic function of the brain and later progressing to cognitive and executive function [16]. Advancement through this developmental series is orchestrated by multiple cues - both cell autonomous and non-autonomous - that facilitate OL lineage progression in a timely fashion. This transcriptional system of checks and balances include, but are not limited to- DNA binding proteins, micro-RNAs, and transcription factors mediated by changes to the chromatin architecture. As reviewed by Mitew et al., OPCs and OLs also receive important developmental signals from other cells in the WM that activate intracellular pathways and OL transcription (Mitew, 2014). These coordinated efforts work to activate genes that promote differentiation, and repress those genes that prevent it [17].
Chromatin remodeling regulates OL development by either covalent modification of histones to activate/silence genes, or ATP-dependent remodeling. The latter is facilitated by enzymes that restructure the nucleosome to alter DNA accessibility during transcription, replication, and DNA repair [18,19]. In the former, histone deacetylation is induced by secreted morphogens like Sonic hedgehog (Shh)- which promotes OL differentiation, and blocked by bone morphogenic proteins (BMPs), such as BMP4, that opposes Shh and inhibits OL specification. [20,21]. This differential regulation of histone acetylation and chromatin structure is important in OL lineage progression during development and remyelination efficiency in disease. Further, these epigenetic changes are dynamic, and strongly influenced by environmental factors like social isolation [22].
OL lineage gene expression is controlled by a host of transcription factors. These work in unison to engineer the specification, differentiation, and maturation of OLs in a stage-specific manner during normal development. Transcriptional regulation of OL development has been recently and comprehensively reviewed [17]; therefore, we will only briefly highlight a transcription factor that plays in essential role in generating OLs during development, and is critical for myelin plasticity. The transcription factor Myelin Gene Regulatory Factor (MYRF) is a master regulator of myelin gene expression that confers transcriptional control on progression through the OL lineage. Its gene expression in postmitotic OLs precedes that of major myelin genes like PLP and MBP, and is required for both the maintenance of myelin and mature OL identity in the adult CNS [23]. In the absence of MYRF, OPCs differentiate into a pre-myelinating state, but they fail to mature and instead undergo apoptosis [24]. Ablation of MYRF in OPCs also leads to a reduction in expression of myelin genes, including PLP, MAG, MBP, and MOG, and has been demonstrated in mice to prevent the production of new OLs in adulthood during an activity dependent task [25].
Neuronal influence on oligodendrocyte development and myelination
Neuronal activity also plays a substantial role in oligodendrogenesis, myelin remodeling, and related behavioral phenotypes. Pioneering work established that OPC proliferation was reduced by blockade of action potential propagation in the optic nerve [26] and demonstrated that OPCs receive functional synapses from neurons both in gray and white matter regions [27,28,29,30]. Hence, myelin is partly regulated by functional neuronal inputs, and can therefore participate in activity-dependent CNS plasticity.
OPCs are electrically responsive cells, and the synaptic input they receive steers epigenetic changes in gene expression patterns and chromatin remodeling in response to neuronal activity [31]. Recently, Hines et al. found that selective myelination of axons is a refinement response to neuronal activity. In vivo time-lapse microscopy demonstrated that activity-evoked secretion by axons promoted additional stabilization of myelin sheath formation. Conversely, removal of this secretory input shortened extension of myelin sheaths and led to a higher rate of retraction [32]. This discovery supports the notion that neuronal activity enhances myelin plasticity, and biases which axons become myelinated during a critical period of environmental influence. Interestingly, pharmacological induction of brain activity promotes the formation of excess myelin sheaths [33]. Sheath formation by individual OLs was increased by neuronal stimulation of the synaptic vesicle. Conversely, blocking vesicle release with a tetanus toxin reduced the myelinating capacity of OLs [33]. This complex interplay within the neural circuit is crucial for normal brain development, and often leads to neurological impairment and disability when compromised.
Neuronal regulation of adaptive myelinogenesis has also been demonstrated in the healthy adult brain [34]. Optogentic stimulation of layer V projection neurons in awake, young-adult mice provided direct evidence of neuronal-excitation-evoked oligodendrogenesis and myelination in the premotor cortex and subcortical WM. This led to an associated improvement in corresponding limb motor function that was suppressible by epigenetic modification of OL differentiation. In summary, neural circuit plasticity is a dynamic sum of its moving parts - each component synchronously adapting to augment circuit function and ultimately behavior. Dysregulation of the oligodendroglial/myelin component has significant clinical ramifications throughout the human lifespan [35,36], but the potential of modulating myelogenic cells to influence behavior, cognition, and neural regeneration is an exciting clinical prospect.
Myelin plasticity throughout life
OLs inherently adapt to the ever-changing dynamics of the CNS, including specific responses to environmental cues. This operative response to exogenous environmental stimuli widely influences myelin plasticity at various stages of development, health, and disease (Figure 1.).
Figure 1. Environmental influence promotes myelin plasticity to ensure brain health.
Throughout the lifetime, environmental experience, myelin plasticity, and brain health function in a continuous response loop, as each event in the cycle reinvigorates the next.
Development/Adolescence
In utero genetic programs determine the initial architecture of WM infrastructure. However, construction of dendritic complexity, synaptic connectivity, and myelination during early development is readily influenced by a multitude of environmental cues [37]. Various tools are used to study these malleable determinants of WM structure, integrity, and function. These include, but are not limited to, genetic modifications in rodents that target OLs [38], WM-dependent animal neurobehavioral tests [25,39], and advanced neuroimaging techniques (Box 2.) combined with cognitive behavioral tests to assess macroscopic changes in human myelination [40,41].
Box 2. Neuroimaging white matter plasticity.
Neuroimaging tools provide a powerful means to study environmental experience in the dynamic context of WM biology. These noninvasive techniques reveal structural and functional differences in response to a wide range of stimuli, and are an important prognostic indicator in WM development, injury, and disease [60].
One type of advanced neuroimaging is diffusion tensor imaging (DTI), a variant of magnetic resonance imaging (MRI). DTI relies on the directional profile of water diffusion throughout the brain. Since water diffusivity is sensitive to subtle differences in the composition of cellular membranes, it allows for three-dimensional characterization of changes in microstructural architecture, i.e. changes in WM integrity [61]. A common metric of DTI used to describe the direction of water diffusion is fractional anisotropy (FA), which increases with myelination, and decreases with hypomyelination or demyelination.
WM integrity is a product of environmentally driven plasticity. This plasticity begins with microstructural changes in anisotropic myelin sheaths that shape the constitution of WM, which in turn alters neurocircuitry throughout the brain and manifests as a behavioral phenotype. Over the lifespan, DTI identifies marked increases in FA and reductions in diffusivity during normal development, and conversely, rising diffusivity and a decrease in FA during typical age-related demyelination [62]. Interestingly, DTI-detected microstructural alterations in WM during infancy are a useful indicator of executive and motor functioning during childhood [63].
Neuroimaging studies provide insight into the correlation between brain structure and function as it relates to experience [64]. For instance, FA demonstrates that learning a complex motor skill, such as juggling, alters WM architecture when practiced regularly [64]. Study of a second language in adults also influences WM plasticity as identified with DTI [65]. These changes reflect learning-related increases in myelination.
A major challenge in the diagnosis and treatment of neurological disorders is identifying imaging markers that accurately characterize disease while offering prognostic value. Multiple sclerosis (MS) is a chronic demyelinating disease, and as such, DTI has important utility in tracking microscopic changes in WM lesions. By assessing patterns of myelination, the distribution and severity of lesions are correlated to clinical signs and symptoms. This data, especially in parallel with histopathological evaluation of changes occurring at the cellular level, offers important insight into both disease progression and responsiveness to promyelinating therapies [66].
Activity-dependent crosstalk between OPCs and axons helps steer the developmental trajectory, in part by preferential myelination of electrically active axons [42]. This discrimination enables environmental modulation of neural circuits that shape our phenotypes in response to experiential input [43]. It is important to consider that these changes occur within a critical period of experience-dependent plasticity (Box 1.). Microstructural alterations in distinct WM regions, including the corpus callosum (CC), occur in response to changes in altitude [58]. This is especially evident in adolescents that have migrated (for educational purposes) from high altitude to sea level during peak periods of brain development [59]. Most children demonstrated an increase in fractional anisotropy (FA) (Box 2.) in ten major WM tracts versus a high-altitude-only control group, indicative of a widespread increase in myelinated fiber numbers and therefore enhancement of myelination.
Social environment also influences early myelin plasticity. Juvenile mouse studies of the prefrontal cortex demonstrated that two weeks of social isolation led to alterations in OL morphology, a reduction in myelin thickness, and deficits in working memory and sociability [51]. In early post-weaning mice, changes in myelination were not reversed by reintroduction into a social environment. Interestingly, isolation in adult mice had little effect on myelin content as these cohorts fully recovered [51], defining an important critical period in which juvenile social experience readily impacts regional brain development. Human studies on institutionally reared children demonstrate that severe neglect in early life compromises WM microstructure throughout the brain [67]. However, early removal from adverse conditions and subsequent placement into high-quality foster care promotes more normative WM development, and therefore may support long-term motor, cognitive, and sensory remediation [67].
Adult
The ubiquitous OPC presence in the adult rodent brain imparts neuronal influence on OL plasticity and myelination beyond the juvenile period. Studies have demonstrated that approximately one third of all myelinating OLs in the adult mouse CC are generated after 7 weeks of age in the murine brain [68], suggesting that this new population of OLs may shape environmental influence on myelin plasticity. Quantification of OPC division and OL production in the mature rodent CNS reveals that OPCs continue to divide with regional heterogeneity into adulthood, although rates of cell division universally decline with age [69]. Newly generated OLs also help facilitate the maintenance and remodeling of existing myelin sheaths [69]. Presumably, this recalibrates axonal myelination to modify brain circuitry according to present experiences, such as learning a new task or compensating for the burden of injury or disease. Adult-born OLs are necessary to learn a new motor skill, and their ablation in mice prevents mastery of an activity-dependent novel task [25]. Further, neuroimaging reveals that complex visuo-motor skill training modifies WM architecture in healthy human adults [64], and working memory conditioning regionally increases myelination [70]. As humans age, cognitive function deteriorates in response to aberrations in WM integrity [71]. Age-related WM decline is especially pronounced in the anterior brain [72], and these anatomical deficits correlate to reduced cognition [73].
Injury and Disease
Adult OPCs are mitotically active and can differentiate in response to injury and disease [74]. Many of the signaling pathways and trophic factors important in the specification, proliferation, and differentiation of OPCs during development also play a crucial role in repair of the adult injured brain [75,76]. In vivo time-lapse imaging in mice revealed that adult OPCs migrate to areas of focal demyelination and participate in tissue repair by generating new OLs to restore myelin [7]. Remyelination, a form of myelin plasticity, is impaired in progressive multiple sclerosis (MS), which consequently leads to neurological disability. Cognitive impairment partly derives from a lack of structural and trophic support from OLs to axons, which fail to adequately remyelinate, thereby leading to axonal degeneration and disease progression [77]. In traumatic brain injury (TBI), myelin plasticity and remodeling influence recovery of WM integrity and resynchronization of cortical circuits [78,79]. Further, microstructural WM damage sustained during TBI can have lasting effects on cognition [80], behavior [81], and increase susceptibility to psychiatric [82] and neurodegenerative disorders like Parkinson’s disease [83].
Environmentally influenced changes in myelin structure and function
Here, we dissect the complexity of environmental cues influencing adaptive myelination in the CNS, and identify avenues to capitalize on glial plasticity. Clinical manipulation of the environment is an intriguingly viable, noninvasive therapeutic approach to improve WM health, either alone or in combinatorial fashion with other cellular or pharmacological modalities (Figure 1.). To date, myelin’s response to environmental influence has largely been measured using advanced neuroimaging techniques (Box 2.) and cellular and molecular methods. But, are we really harnessing myelin’s full potential? To comprehensively understand how the environment directs these plastic changes, we must pair the macroscopic with the microscopic, and utilize these tools in parallel. New molecular tools like gene trapping and single-cell RNA sequencing have helped to clear the picture, but a better understanding of the roles that OLs play in the context of dynamic brain remodeling will allow us to target environmental factors as therapies.
Positive influences- chalk it up to experience
Enriched Environment
One of the oldest and most widely used experimental approaches to study the influence of experience on the brain is exposure to an enriched environment. Environmental enrichment (EE) refers to a complex and stimulating domain that challenges an organism to continuously adapt to its surroundings in a social, physical, and experiential manner. In animal models, EE more closely emulates the natural environment than a standard research vivarium by incorporating novel objects, physical activity, and a larger number of cagemates to increase socialization. It is therefore a useful means to study conditions that simultaneously influence sensory, motor, and cognitive changes in the brain.
Early EE studies demonstrated widespread effects on numerous CNS cell types and under varied physiological conditions in both small and large mammals [84,85,86,87,88]. Differential rearing in infant rhesus monkey shows that animals raised in larger groups demonstrate expansion of the CC and sustained improvement in cognitive performance versus age-matched, individually raised controls [89]. EE is not strictly a developmental phenomenon either. In a model of Parkinson’s disease, young adult mice demonstrated an increase in new OLs in the substantia nigra after a month of enriched housing [90]. Similarly, EE increases OPC proliferation [91], and influences OPC number and cell fate in the amygdala of adult mice [92]. EE also improves spatial learning in aged rats by increasing the volume and length of myelinated fibers, volume of myelin sheaths, and total CC volume [93]. Further, EE promotes progenitors derived from an endogenous pool of neural stem cells to generate OLs in a murine model of multiple sclerosis (MS), with consequent enhanced remyelination of lesions and reduced functional impairment [94]. In summary, EE globally supports brain health, and is a promising modality to treat a number of traumatic and neurodegenerative disorders, especially those of the WM.
Music, practice makes perfect
Music is a multisensory form of EE that imparts cognitive (learning), auditory (listening), and motor (playing) stimulation, whereby simultaneous collaboration between multiple areas of the brain coordinate an organized response [95]. Musical performance alters WM architecture [96,97]. Professional pianists who began playing during adolescence demonstrate improved WM integrity [98] and WM plasticity, as shown by diffusion tensor imaging (DTI) [99]. DTI studies also revealed that these WM changes are relative to the amount of time spent practicing, and that specific WM regions are uniquely sensitive to piano playing during childhood, adolescence, and adulthood [100]. This suggests age-specific regional plasticity in myelinating tracts, and functional adaptation within a critical period of development in WM undergoing maturation. Further, despite less overall training hours than adults, the larger number of involved brain regions correlated with practice during childhood, reinforcing the influence of early experience on WM plasticity. Pragmatically, these WM changes serve as a foundation to build upon with future experience [101]. This adaptive response to music also occurs in the WM tracts of the adult [102], and in disease [103], emphasizing how life experiences - in this case learning -, alter myelination and are influenced by the environment.
Learning
Interacting with and adapting to our environment forms the basis for learning. Dynamic and influential learning involving WM occurs throughout life, but is especially critical during development, as organisms establish the infrastructure and patterns of circuitry in the brain. Spelling impairment is associated with DTI differences in WM integrity [104], and five weeks of remediation improved spelling and reading comprehension versus controls [105]. This increase in WM FA in language relevant networks highlights the importance of WM plasticity in cognitive learning.
Academic pursuits also require the adult brain to change. Neuroimaging studies reveal that WM plasticity is key in adults learning a second language [65,106] and who learn to read late in life [107]. Training a novel, complex visuo-motor skill (i.e. juggling) also modifies WM architecture in the healthy adult brain [64]. In rodent models, simple motor training stimulates functional myelin plasticity in the motor cortex [108], and in turn, myelin plasticity enhances and is required for motor learning [25]. Ablation of key regulatory transcription factors such as MYRF prevents generation of new OLs, and hinders adult mice from mastering an activity-dependent task without affecting either preexisting OLs or myelin [25]. Additionally, this generation of new OLs influences both early and late stage motor skill learning [109], which has important implications in aging and disease. The pursuit of “brain fitness”- cognitive training and stimulation to confer improved mental function, shapes and preserves WM integrity in the aging individual [110], and is implicated as a means to potentially stave off pathological states like Alzheimer’s disease [111].
Meditation
Like music, meditation offers a wealth of benefits for the body and mind [112,113,114]. Many of these effects on physical and mental health involve alterations in WM. DTI studies demonstrate that 4 weeks of integrative body-mind training alters myelination [115], especially in WM tracts involved in self control [116]. These changes in WM efficiency occur quickly, and are heightened in the anterior cingulate cortex- an area of the brain surrounding the frontal CC associated with higher-level functions like self-regulation, impulse control, and emotion. This implicates meditation as a short-term means to alter the long-term trajectory of disorders of the mind [117]. Not surprisingly, experienced meditators also have structural differences in WM [118], emphasizing the utility of meditation on cognitive and behavioral performance throughout life.
Exercise
Physical activity extensively impacts mammalian brain function and cognition [119,120,121], as well as protects the brain from the detriments of aging [122]. Animal models that study the effects of physical activity on brain often rely on a voluntary wheel running paradigm. Although reductionist, these models somewhat mimic the human experience, as conscious decisions are made with regards to speed, duration, distance, and ultimately, whether to run at all. Voluntary exercise in mice enhances differentiation of OPCs into mature OLs [123], and increases oligodendrogenesis in the intact thoracic spinal cord after only a week of activity [124]. Recent studies also confirm an impact of cardiorespiratory fitness on WM in older humans. DTI established a positive relationship between fitness and spatial working memory that is mediated by WM microstructure [125]. On the contrary, sedentary behavior in adults reduces WM integrity, but avoidance of this practice (i.e. exercise) is beneficial for WM health [126], and memory-related brain networks [127].
Negative influences- an albatross around the neck
Neural plasticity pioneer Marian Diamond was the first to demonstrate that the environment strongly influences the brain. Her research established the notion that our brains change throughout the lifetime- they shrink with impoverishment and grow in an enriched environment. She identified five essentials for a healthy brain: newness, challenge, exercise, diet, and love: quite literally changing the landscape in many arenas of health. Modern investigation reveals that without these five essentials, an organism may lose the ability to carry out basic motor and cognitive functions. As such, many of these effects are a direct result of damage to the OL population, particularly during critical neurodevelopmental periods (Box 1.). OLs are a vulnerable cell population, particularly sensitive to environmental signals in the postnatal brain, and compounding factors like poor nutrition, behavioral disorders (Table 1.), disease, and unfavorable social setting can contribute significantly to their myelinating potential, or lack thereof.
Table 1.
Neurobehavioral Abnormalities, Addictions, and Disorders of White Matter
| Cohort | Disorder | Technique | Important Findings |
Refs |
|---|---|---|---|---|
| Human | Internet Addiction | DTI | Widespread ↓ FA in adolescents | [128] |
| Human | Gaming | fMRI | ↓ WM density in decision-making, behavioral inhibition, and emotional regulation brain regions | [129] |
| Human | Impulsivity | DTI | WM connectivity associated with distinct impulsivity subtypes underlying motor and reward control | [130] |
| Human | Gambling | MRI | ↓ FA in CC and SLF | [131] |
| Human | Mobile Phone Dependence | DTI | Abnormal WM integrity | [128] |
| Human | Kleptomania | DTI | ↓ WM integrity in inferior frontal brain | [132] |
| Human | Obesity | DTI | BMI correlates negatively with WM integrity in fornix and CC | [133] |
| Mouse | High Fat Diet | qRT-PCR IHC | High fat consumption plus SB promotes loss of OPCs and OLs. Exercise training attenuates negative effects of high fat diet on myelin. | [134] |
| Human | Chronic Alcoholism | DTI | Widespread ↓ FA (frontal, temporal, parietal, Cerebellar WM tracts) | [135] |
| Human | Adolescent Alcohol Abuse | MRI | Heavy drinking during adolescence negatively impacts WM maturation | [136] |
| Human | Prenatal Alcohol Exposure | DTI | ↓ WM microstructural integrity | [137] |
| Human | Tobacco | DTI | ↓ FA in right CG, FA in left CG is negatively associated with # cigarettes smoked per day | [138] |
| Mouse | Tobacco | qRT-PCR Histology | Second hand smoke exposure ↓ expression of genes for myelin synthesis and maintenance | [139] |
| Human | Cannabis | DTI | ↑ cannabis use correlates to ↓ FA and impaired performance in verbal learning | [140] |
| Human | Prenatal Cocaine Exposure | DTI | Altered WM development, ↓ FA | [141] |
| Human | Methamphetamine Dependence | DTI | Frontal WM changes and increased aggression | [142] |
| Human | Heroin Abuse | DTI | Extensive abnormal WM connectivity | [143] |
DTI, diffusion tensor imaging; FA, fractional anisotropy, qRT-PCR, quantitative real time polymerase chain reaction; MRI, magnetic resonance imaging; fMRI, functional magnetic resonance imaging
IHC, immunohistochemistry; BMI, body mass index
OPCs, oligodendrocyte progenitor cells; OLs, oligodendrocytes
CC, corpus callosum; WM, white matter; Cingulum, CG; SLF, superior longitudinal fasciculus
SB, sedentary behavior; MPD, mobile phone dependence; PCE, prenatal cocaine exposure
Poor Diet/Nutrition
The nutritional environment impacts brain structure and function, and gross deviations in nutritional status are implicated in many neurological disorders [144]. OLs, especially OPCs, have tremendous energy requirements [145]. Synthesis and maintenance of myelin by OLs is a metabolically taxing process, and thus, adequate dietary intake of a number of key elements is crucial to successful myelination.
Iron deficiency is the most common nutritional health problem in the world [146], and has been linked to persistent hypomyelination. This has important clinical implications during development, as the neurological sequelae (behavioral disorders, decreased cognitive ability, poor school performance) are long-lasting [147,148,149]. Developmental disturbances in myelin synthesis and composition are not corrected with iron repletion [150]. In models of MS, MRI and histological iron distribution reveal that iron maintains myelin integrity and plays an important role in remyelination and repair [151]. Interestingly, age-related iron accumulation in the striatum is linked to demyelination and a reduction in declarative memory [152]. This indicates both a pathogenic and reparative role for iron in OL activity and integrity
Essential fatty acids (EFAs) also play a key role in the structural integrity of myelin. Because the body cannot synthesize EFAs, they must be obtained from dietary intake. EFAs help build the myelin sheath, and their deficiency during infancy can delay brain development [153], as well as accelerate the deterioration of cognitive processing in the adult [119].
Additionally, obesity is associated with reduced myelin (Table 1.). BMI is negatively correlated to WM integrity in multiple regions of the brain, including the CC [133], and has been implicated in the reduction of OL and OPC numbers in the murine spinal cord [134]. Encouragingly, 7 weeks of exercise training reversed these reductions in the OL population. Obesity-related loss of WM integrity is prevalent in the limbic system and tracts connecting the frontal and temporal lobes [154], and could compound the effects of age-related cognitive decline. The reciprocal relationship between diet, exercise, and myelinogenesis highlights that adequate nutrition is required for normal brain development, plays a central role in myelin homeostasis, and importantly, should be self-imposed.
Social isolation and neglect
Socialization tremendously impacts neurological development. Environmental resources like healthcare, education, and housing have a cause and effect relationship on the brain throughout life, and individual differences in cognition, emotion, and psychological well-being can be attributed to deviations in these social parameters [155,156].
Early environmental adversity studies by Harry Harlow demonstrated that rhesus monkeys raised in isolation are detached from the environment, hostile, and cannot form adequate social attachments [157]. Similarly, recent studies investigating social influence on WM plasticity found that monkeys exposed to early life stress, particularly a disrupted infant-mother bond, demonstrate reductions in myelin and WM integrity, and elevations in plasma cortisol levels after maltreatment [158]. These WM alterations are especially evident in regions of the brain associated with motor integration and emotional regulation. Murine studies also implicate neglect as a cause of reduced myelination in the prefrontal cortex [22,51]. Two weeks of social impoverishment reduced myelin thickness and simplified OL morphology, and importantly, social reintegration in young adult mice did not lead to recovery. Further, DTI of language and limbic pathways reveals microstructural WM abnormalities in orphanage-reared children [159]. Importantly, these changes correlate to the period of deprivation and time spent in the orphanage, i.e. in a deprived environment, and could misshape the trajectory of cognitive and behavioral neurodevelopment.
Encephalopathy of prematurity
In mammalian development, pre- and early postnatal periods are heavily influenced by environmental experience. These periods are characterized by rapid alterations in CNS organization, including gliogenesis and glial maturation, that shape developmental outcome. Approximately ten percent of all children are born premature-defined as birth occurring at less than 37 weeks gestational age [160]. Most of these infants survive; however, many are burdened with a complex array of sensory, cognitive, and behavioral impairments [35,44,161]. A number of simultaneous comorbidities contribute to diffuse brain injury in the premature infant [162]. Ordinarily, the OPC pool rapidly expands in the critical period surrounding birth, but is also particularly sensitive to excitotoxic, oxidative, and inflammatory insult [45,163]. Significant loss of the progenitor pool and gross reductions in the number of myelinating OLs underlies the maturation blockade seen with injury. Apneic, ischemic, and inflammatory events cause a delay maturation of neuronal and glial cell populations, and diffuse abnormalities in WM that result in a reduced capacity to synthesize myelin [46,164]. Accordingly, the resultant diffuse WM injury is believed to be a major cause of disability in survivors [165].
Vasculature in the preterm brain is anatomically immature. As such, the meager blood supply to the developing cerebrum amounts to a marked susceptibility of neurovascular perfusion, and a reduced capacity to adequately respond to changes in hemodynamics [166]. Inflammation and oxidative stress also play a key role- not only in susceptibility to additional insults, like hypoxic brain injury, but also in disruption of OL developmental programs [167], and related delays in myelination [168]. A ubiquitous OPC presence heightens susceptibility to oxidative damage and free radical injury, and contributes to a marked reduction in total WM volume in the preterm brain [169].
Compounding this array of intrinsic damage is a barrage of extrinsic, environmental factors. Levels of maternal care [170,171], nutritional status [172], housing [173], exposure [174], anesthesia [175], and stress [176] all provide an additional basis for many of the cognitive and behavioral changes seen in WM injury. Recent neuroimaging studies recognize neonatal alterations in WM that are associated with impaired cognitive, motor [63] and emotional development [177], and reduced social skills during childhood. [178]. These neuroanatomical changes highlight the chronic impact of WM injury in preterm birth, as well as the usefulness of early imaging studies as predictive markers of future disability [60].
In spite of these challenges, neurodevelopment is also a period of adaptation. Myelin plasticity regulated by alternative activities or experiences can evoke reorganization of brain circuitry to reduce disability in children born preterm [179]. The strongest indicators of long-term neurological outcome in preterm infants are the level of maternal education and the presence of a two-parent household [180], highlighting the utility of environmentally dependent changes in brain to yield greater developmental progress.
Concluding remarks and future perspectives
Even the simplest single cell organisms respond to environmental cues. In mammals, these signals are more complex, and the environment evokes short- and long-term chemical, electrical, and behavioral responses that shape the trajectory of brain development and beyond. This task is facilitated by the myelination of axons, which occurs throughout life, and continues in response to the demands of development, health, and disease. Preservation and reparation of WM integrity is crucial. Gross disturbances in myelination contribute to aberrations of the developmental process [44], neuropsychiatric illness (see table), and can, unfortunately, add insult to injury in the process of aging [71]. These experiences shape our environment, and our environment then returns the favor. Therefore, we must continue to program the environmental experience- first recognizing the environment as an efficacious means to modulate WM integrity; and second, capitalizing on the environment as a powerful tool to induce myelin plasticity.
Clinical studies and animal models alike have demonstrated a robust and reproducible response to environmentally based alterations in WM. This reorganization occurs in a region-specific manner as our brain adjusts to the timing, intensity and duration of the driving experience, and manifests as a behavioral phenotype in the context of changing environment. While analysis in previous studies has been crucial in identifying many of the cellular, molecular, and functional benefits of environmental influence on brain myelin, important gaps in knowledge still remain (Box 3).
Box 3. Outstanding Questions.
What are physiological limitations to environmentally-induced myelin plasticity?
Do all OLs have the capacity for plasticity in response to environmental cues, or are subsets of OLs unresponsive?
How reversible and lasting are WM changes induced by the environment?
What are the cellular and molecular mechanisms that regulate the duration of the critical period?
What are the developmental consequences of environmental overstimulation?
How can we best implement standard research protocols of enriched and impoverished environments?
How do we account for the lack of environmental stimuli in “standard” research animal facilities used to model the human condition?
The panorama of interventional strategies aimed at the preservation of WM integrity must employ the environment as a means to manipulate plasticity. Myelin plasticity alters conduction velocity in the neural circuit [13], which has a profound impact on the structure and function of brain throughout the lifetime. In fact, not only would stronger consideration of environmental influence on myelin play an important role in health and disease, but it could be the Archimedes’ Lever to appropriating WM development amongst a limited range of only partially efficacious treatment options.
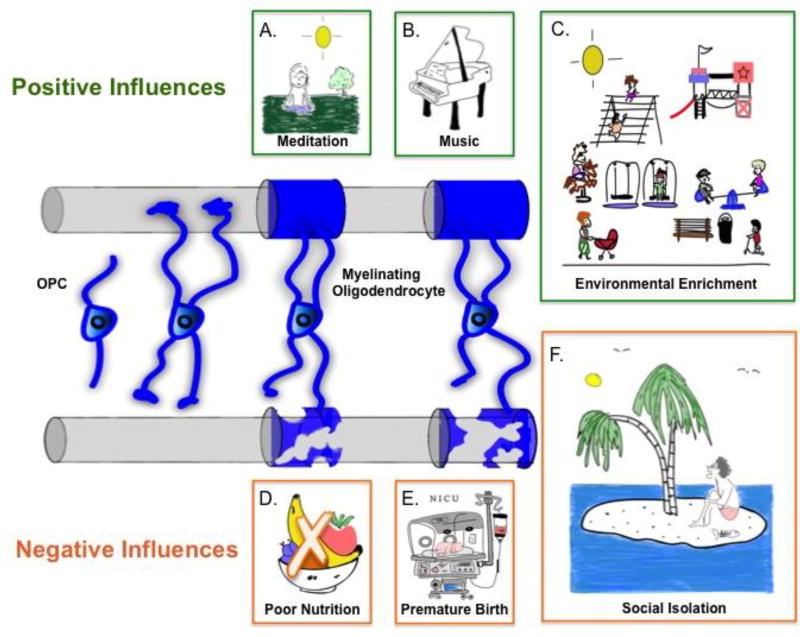
Figure 2. Environmental factors influencing myelination.
Axons are wrapped in myelin sheaths as OPCs differentiate into mature, myelinating OLs. Positive environmental influences like meditation (A), musical practice (B), and environmental enrichment (C) promote myelination (top axon). Conversely, negative environmental influences like poor nutrition (D), premature birth (E), and social isolation (E) can cause abnormalities in myelination (bottom axon).
Trends box.
Environmental influence on myelination plays an important role in development, the aging brain, and in recovery from injury and disease.
Structural and functional changes in white matter architecture occur in response to environmental stimuli, and have significant implications in cognition, behavior, and motor function.
The brain is sensitive to manipulations of the environment during restricted temporal windows, and responds in a region-specific manner.
Recent advances in technology have presented new and exciting opportunities to study developmental myelination and changes associated with neuronal activity, leading to myelin plasticity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Versendaal D, Levelt CN. Inhibitory interneurons in visual cortical plasticity. Cell Mol Life Sci. 2016;73:3677. doi: 10.1007/s00018-016-2264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox S, Levitt P, Nelson C. How the timimg and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornig J, Frob F, Vogl M, Hermans-Borgmeyer I, Tamm E, Wegner M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013;9(10):e1003907. doi: 10.1371/journal.pgen.1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P-W, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barateiro A, Brites D, Fernandes A. Oligodendrocyte development and myelination in neurodevelopment: molecular mechanisms in health and disease. Curr Pharm Des. 2016;22(6):656–679. doi: 10.2174/1381612822666151204000636. [DOI] [PubMed] [Google Scholar]
- 7.Hughes E, Kang S, Fukaya M, Bergles D. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neurosci. 2013;16(6):668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin Lou, Druid H, Frisén J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Snaidero N, Mobius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, Simons M. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD. A new mechanism of nervous system plasticity: activitydependent myelination. Nat Rev Neuro. 2015;16(12):756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 13.Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344(6181):319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Back SA, Rosenberg PA. Pathophysiology of Glia in Perinatal White Matter Injury. Glia. 2014;62(11):1790–1815. doi: 10.1002/glia.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton WT. Basic Neurochemistry. Little, Brown; Boston: 1981. Formation, structure, and biochemistry of myelin; pp. 63–92. [Google Scholar]
- 16.Gerber RJ, Wilks T, Erdie-Lalena C. Developmental milestones: motor development. Pediatr Rev. 2010;31(7):267–277. doi: 10.1542/pir.31-7-267. [DOI] [PubMed] [Google Scholar]
- 17.Emery B, Lu QR. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol. 2015;7(9):a020461. doi: 10.1101/cshperspect.a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu L, Mao M, Chan J, Wu J, Lu R. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocte differentiation. Cell. 2013;152(1–2):248–261. doi: 10.1016/j.cell.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuchero JB, Barres BA. Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol. 2013;23(6):914–920. doi: 10.1016/j.conb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swiss V, Nguyen T, Dugas J, Ibrahim A, Barres B, Androulakis I, Casaccia P. Identification of gene regulatory network necessary for the initiation of oligodendrocyte differentiation. PLoS ONE. 2011;6(4):e18088. doi: 10.1371/journal.pone.0018088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Hernandez M, Shen S, Sabo JK, Kelkar D, Wang J, O’Leary R, Phillips GR, Cate HS, Casaccia P. Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenic protein 4 via opposing effects on histone acetylation. J Neurosci. 2012;32(19):6651–6654. doi: 10.1523/JNEUROSCI.4876-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Dietz K, Dupree J, Casaccia P. Impaired Adult Myelination in the Prefrontal Cortex of Socially Isolated Mice. Nature Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham TJ, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32(36):12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138(1):172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie IA, Ohayon D, Li H, Richardson WD. Motor Skill Learning Requires Active Central Myelination. Science. 2014;17(346):318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361(6409):258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 27.Bergles DED, Roberts JDJ, Somogyi PP, Jahr CEC. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 28.Kukley M, Cepetill0-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nature Neurosci. 2007;10(3):311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 29.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neurosci. 2007;10(3):321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nature Neurosci. 2012;15(9):1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day J, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70(5):813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hines J, Ravanelli A, Schwindt R, Scott E, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nature Neurosci. 2015;18(5):683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18(5):628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of Premature Brain Injury. Nature Neurosci. 2014;17(3):341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesh K, Srikanth L, Vengamma B, Chandrasekhar C, Prasad BC, Sarma PV. In vitro transdifferentiation of human cultured CD34+ stem cells into oligodendrocyte precursors using thyroid hormones. Neurosci Lett. 2015;588:36–41. doi: 10.1016/j.neulet.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 37.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 38.Pfrieger FW, Slezak M. Genetic approaches to study glial cells in the rodent brain. Glia. 2012;60(5):681–701. doi: 10.1002/glia.22283. [DOI] [PubMed] [Google Scholar]
- 39.Scafidi J, Hammond T, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter R, Jr, Hyder F, Horvath T, Gallo V. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506(7487):230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Muircheartaigh J, Dean DC, Ginestet CE, Walker L, Waskiewicz N, Lehman K, Dirks H, Piryatinsky I, Deoni SC. White matter development and early cognition in babies and toddlers. Hum Brain Mapp. 2014;35(9):4475–4487. doi: 10.1002/hbm.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muetzel R, Mous S, van der Ende J, Blanken L, van der Lugt A, Jaddoe V, Verhulst F, Tiemeier H, White T. White matter integrity and cognitive performance in school-age children: a population-based neuroimaging study. NeuroImage. 2015;119:119–128. doi: 10.1016/j.neuroimage.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Wake H, Ortiz F, Woo D, Lee P, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Comm. 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fields RD. Change in the brain’s white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Back SA, Miller SP. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann Neurol. 2014;75:469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XB, Shen Y, Plane J, Deng W. Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neurosci Bull. 2013;29(2):229–238. doi: 10.1007/s12264-013-1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elitt C, Rosenberg P. The challenge of understanding cerebral white matter injury in the premature infant. Neuroscience. 2014;0:216–238. doi: 10.1016/j.neuroscience.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright J, Zhang G, Tzong-Shiue Y, Kernie S. Age-related changes in the oligodendrocyte progenitor pool influence brain remodeling after injury. Dev Neurosci. 2010;32:499–509. doi: 10.1159/000322081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill R, Patel K, Goncalves C, Grutzendler J, Nishiyama A. Modulation of oligodednrocyte generation during a critical temporal window after NG2 cell division. Nature Neurosci. 2014;17(11):1518–1552. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawford DK, Mangiardi M, Xia X, Lopez-Valdes HE, Tiwari-Woodruff SK. Functional recovery of callosal axons following demyelination: a critical window. Neuroscience. 2009;164(4):1407–1421. doi: 10.1016/j.neuroscience.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 50.Czopka T, fFrench-Constant-C. Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makinodan M, Rosen KM, Ito S, Corfas G. A Critical Period for Social Experience-Dependent Oligodendrocyte Maturation and Myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi N, Sakurai T, Davis K, Buxbaum J. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93(1):13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew LJ, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;35:102–129. doi: 10.1159/000346157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;19:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 55.Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011:325789. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, Haroutunian V. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. doi: 10.1038/npp.2008.19. [DOI] [PubMed] [Google Scholar]
- 57.Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophr Bull. 2012;38:486–494. doi: 10.1093/schbul/sbq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Lin J, Sun Y, Huang Y, Ye H, Wang X, Yang T, Jiang X, Zhang J. Compromised white matter microstructural integrity after mountain climbing: evidence from diffusion tensor imaging. High Alt Med Biol. 2012;13:118–125. doi: 10.1089/ham.2011.1073. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Zhang H, Chen J, Fan M, Gong Q. Structural modulation of brain development by oxygen: evidence on adolescents migrating from high altitude to sea level environment. PLoS ONE. 2013;8(7):e67803. doi: 10.1371/journal.pone.0067803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullman H, Spencer-Smith M, Thompson D, Doyle L, Inder TE, Anderson P, Klingberg T. Neonatal MRI is associated with future cognition and academic achievement in preterm children. Brain. 2015;138:3251–3262. doi: 10.1093/brain/awv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang E, Argyelan M, Aggarwal M, Malhotra A. The role of myelination in meausres of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. NeuroImage. 2017;147:253–261. doi: 10.1016/j.neuroimage.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 63.Thompson DK, Lee KJ, Egan GF, Warfield SK, Doyle LW, Anderson PJ, Inder TE. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 2014;52:60–74. doi: 10.1016/j.cortex.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholz J, Klein M, Behrens T, Johansen-Berg H. Training induces changes in white matter architecture. Nature Neurosci. 2009;12(11):1367–1368. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hosoda C, Tanaka K, Nariai T, Honda M, Hanakawa T. Dynamic neural network reorganization associated with second language vocabulary acquisition: a multimodal imaging study. J. Neurosci. 2013;33(34):13663–13672. doi: 10.1523/JNEUROSCI.0410-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stamile C, Kocevar G, Cotton F, Durand-Dubief F, Hannoun S, Frindel C, Guttmann C, Rousseau D, Sappey-Marinier D. A sensitive and auromatic white matter fiber tracts model for longitudinal analysis of diffusion tensor images in multiple sclerosis. PLoS ONE. 2016;11(5):e0156405. doi: 10.1371/journal.pone.0156405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr. 2015;169(3):211–219. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivers L, Young K, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson W. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature Neurosci. 2008;11(12):1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young K, Psachoulia K, Tripathi R, Dunn S-J, Cossell L, Attwell D. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Lange A-M, Brathen A, Gryeland H, Sexton C, Johansen-Berg H, Adnersson J, Rohani D. White matter integrity as a marker for cognitive plasticity in aging. Neurobiol Aging. 2016;47:74–82. doi: 10.1016/j.neurobiolaging.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brickman A, Meier I, Korgaonkar M, Provenzano F, Grieve S, Siedlecki K, Wasserman B, Williams L, Zimmerman M. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol. Aging. 2012;33:1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31(11):1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dietz K, Polanco J, Pol S, Sim F. Targeting human oligodendrocyte progenitors for myelin repair. Exper Neurol. 2016;283(B):489–500. doi: 10.1016/j.expneurol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juarez A, He D, Lu Q. Oligodendrocyte progenitor programming and reprogramming: Toward myelin regeneration. Brain Res. 2016;1638(B):209–220. doi: 10.1016/j.brainres.2015.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ontaneda D, Thompson A, Fox R, Cohen J. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. doi: 10.1016/S0140-6736(16)31320-4. [DOI] [PubMed] [Google Scholar]
- 78.Eirud C, Craddock R, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte S. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez AM, Adler J, Kulkarni N, Strain JF, Womack KB, Diaz-Arrastia R, Marquez de la Plata CD. Longitudinal white matter changes after traumatic axonal injury. J Neurotrauma. 2014;31(17):1478–1485. doi: 10.1089/neu.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laborey M, Masson F, Ribereau-Gayon R, Zongo D, Salmi L, Lagarde E. Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: results from a comparative cohort study. J. Head Trauma Rehabil. 2014;29:E28–E36. doi: 10.1097/HTR.0b013e318280f896. [DOI] [PubMed] [Google Scholar]
- 81.Lagarde E, Salmi L, Holm L, Contrand B, Masson F, Ribereau-Gayon R, Laborey M, Cassidy J. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry. 2014;71:1032–1040. doi: 10.1001/jamapsychiatry.2014.666. [DOI] [PubMed] [Google Scholar]
- 82.Haagsma J, Scholten A, Andriessen T, Vos P, Van Beeck E, Polinder S. Impact of depression and posttraumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J. Neurotrauma. 2014;32(11):853–862. doi: 10.1089/neu.2013.3283. [DOI] [PubMed] [Google Scholar]
- 83.Gardner R, Burke J, Nettiksimmons J, Goldman S, Tanner C, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson’s disease. Ann. Neurol. 2015;77(6):987–995. doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diamond M, Krech D, Rosenzweig M. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neur. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- 85.Kempermann G, Kuhn G, Gage F. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 86.Jha S, Dong B, Xue Y, Delotterie D, Vail M, Sakata K. Antidepressive and BDNF effects of enriched environment treatment across ages in mice lacking BDNF expression through promoter IV. Nat Trans Psych. 2016;6(9):e896. doi: 10.1038/tp.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lambert K, Hyer M, Bardi M, Rzucidlo A, Scott A, Kinsley C. Natural-enriched environments lead to enhanced environmental engagement and altered neurobiological resilience. Neuroscience. 2016;330:386–394. doi: 10.1016/j.neuroscience.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 88.Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, Porzia A, Limatola C. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat. Commun. 2015;6:6623. doi: 10.1038/ncomms7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez MM, Hearn EF, Herndon JG. Differential Rearing Affects Corpus Callosum Size and Cognitive Function of Rhesus Monkeys. Brain Res. 1998:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 90.Klaissle P, Lesemann A, Huehnchen P, Hermann A, Storch A, Steiner B. Physical activity and environmental enrichment regulate the generation of neural precursors in the adult mouse substantia nigra in a dopamine-dependent manner. BMC Neurosci. 2012;13:132. doi: 10.1186/1471-2202-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res. 2009;87(16):3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- 92.Ehninger D, Wang LP, Kettenmann H, Kempermann G. Enriched Environment and Physical Activity Reduce Microglia and Influence the Fate of NG2 Cells in the Amygdala of Adult Mice. Cell Tissue Res. 2011 Jul;345(1):69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y, Shi XY, Qiu X, Tang GH. Enriched Environment Increases the Myelinated Nerve Fibers of Aged Rat Corpus Callosum. Anat Rec. 2012;295(6):999–1005. doi: 10.1002/ar.22446. [DOI] [PubMed] [Google Scholar]
- 94.Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched Environment Promotes Adult Neural Progenitor Cell Mobilization in Mouse Demyelination Models. Eur J Neurosci. 2007 Feb;25(3):761–71. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- 95.Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception. Nat Rev Neurosci. 2007;8(7):547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- 96.Anders D, Garza-Villarreal E, Chakravarty M, Hansen M, Lerch J, Vuust P. Gray- and white- matter anatomy of absolute pitch possessors. Cerebral Cortex. 2015;25:1379–1388. doi: 10.1093/cercor/bht334. [DOI] [PubMed] [Google Scholar]
- 97.Engel A, Hijmans B, Cerliani L, Bangert M, Nanetti L, Keller P, Keysers C. Inter-individual differences in audio-motor learning of piano melodies and white matter fiber tract architecture. Hum Brain Mapp. 2014;35:2483–2497. doi: 10.1002/hbm.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han Y, Yang H, Lv Y, Zhu C, He Y, Tang H, Gong Q, Luo Y, Zang Y, Dong Q. Gray Matter Density and White Matter Integrity in Pianists’ Brains: A Combined Structural and Diffusion Tensor MRI Study. Neurosci Lett. 2009;459:3–6. doi: 10.1016/j.neulet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 99.Imfield A, Oechslin M, Meyer M, Loenneker T, Jancke L. White matter Plasticity in the Corticospinal Tract of Musicians: A DiffusionTensor Imaging Study. NeuroImage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 100.Bengtsson SL, Nagy Z, Skare S, Ullen F. Extensive Piano Practicing has Regionally Specific Effects on White Matter Development. Nat. Neuroscience. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 101.Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci. 2013;33(3):1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teki S, von Kriegstein K, Stewart L, Lyness CR, Moore BC, Capleton B, Griffiths TD. Navigating the auditory scene; an expert role for the hippocampus. J Neurosci. 2012;32(35):12251–12257. doi: 10.1523/JNEUROSCI.0082-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Metzler-Baddeley C, Cantera J, Coulthard E, Rosser A, Jones DK, Baddeley RJ. Improved executive function and callosal white matter microstructure after rhythm exercise in Huntington’s disease. J Huntingtons Dis. 2014;3(3):273–283. doi: 10.3233/JHD-140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gebauer D, Fink A, Filippini N, Johansen-Berg H, Reishofer G, Koschutnig K, Kargl R, Purgstaller C, Fazekas F, Enzinger C. Differences in integrity of white matter and changes with training in spelling impaired children: a diffusion tensor imaging study. Brain Struct Funct. 2012;217(3):747–760. doi: 10.1007/s00429-011-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gebauer D, Fink A, Kargl R, Reishofer G, Koschutnig K, Purgstaller C, Fazekas F, Enzinger C. Differences in brain function and changes with intervention in children with poor spelling and reading abilities. PLoS One. 2012;7(5):e38201. doi: 10.1371/journal.pone.0038201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24(8):1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 107.Carreiras MM, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461(7266):983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- 108.Sampaio-Baptista C, Khrapitch A, Foxley S, Johansen-Berg H. Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. J Neuroscience. 2013;33(50):19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao L, Ohayon D, McKenzie I, Sinclair-Wilson A, Wright J, Fudge A, Emery B, Li H, Richardson D. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nature Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Envig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reichman WE, Rose NS. History and experience: the direction of Alzheimer’s disease. Menopause. 2012;19(7):724–734. doi: 10.1097/gme.0b013e31825a28f2. [DOI] [PubMed] [Google Scholar]
- 112.Cramer H, Hall H, Leach M, Frawley J, Zhang Y, Leung B, Adams J, Lauche R. Prevalence, patterns, and predictors of meditation use among US adults: a nationally representative survey. Sci Rep. 2016;6:36760. doi: 10.1038/srep36760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Epel ES, Puterman E, Lin J, Blackburn EH, Lum PY, Beckmann ND, Zhu J, Lee E, Gilbert A, Rissman RA, Tanzi RE, Schadt EE. Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Transl Psychiatry. 2016;6(8):e880. doi: 10.1038/tp.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang YT, Holzel B, Posner M. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 115.Posner MI, Tang YY, Lynch G. Mechanisms of white matter change induced by meditation training. Front Psychol. 2012;5:1220. doi: 10.3389/fpsyg.2014.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- 117.Tang YT, Lu Q, Fan M, Posner M. Mechanisms of White Matter Changes Induced by Meditation. PNAS. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang DH, Jo HJ, Jung WH, Kwon JS. The Effect of Meditation on Brain Structure: Cortical Thickness Mapping and Diffusion Tensor Imaging. SCAN. 2013;(8):27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen K, Zhang L, Tan M, Lai CS, Li A, Ren C, So KF. Treadmill exercise suppressed stress-induced dendritic spine elimination in mouse barrel cortex and improved working memory via BDNF/TrkB pathway. Transl Psychiatry. 2017;7(3):e1069. doi: 10.1038/tp.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith KC. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845–856. doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim TW, Sung YH. Regular exercise promotes memory function and enhances hippocampal neuroplasticity in experimental autoimmune encephalomyelitis mice. Neuroscience. 2017;346:173–181. doi: 10.1016/j.neuroscience.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 122.Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function. Brain. 2016;139(Pt 3):662–73. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 124.Krityakiarana W, Espinosa-Jeffrey A, Ghiani CA, Zhao PM, Gomez-Pinilla F, Yamaguchi M, Kotchabhakdi N, de Vellis J. Voluntary exercise increases oligodendrogenesis in spinal cord. Int J Neurosci. 2010;120(4):280–290. doi: 10.3109/00207450903222741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oberlin L, Verstynen T, Burzynska A, Erickson K. White Matter Microstructure Mediates the Relationship between Cardiorespiratory Fitness and Spatial Working Memory in Older Adults. NeuroImage. 2016;131:91–101. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burzynska A, Chaddock-Heyman L, Voss M, Kramer A. Physical Activity and Cardiovascular Fitness are Beneficial for White Matter in Low-Fit Older Adults. PLoS ONE. 2014;9(9):e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian Q, Erickson KI, Simonsick EM, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris TB, Rosano C. Physical activity predicts microstructural integrity in memory-related networks in very old adults. J Gerontol A Biol Sci Med Sci. 2014;69(10):1284–1290. doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin X, Dong G, Wang Q, Du X. Abnormal gray matter and white matter volume in ‘internet gaming addicts’. Addict Behav. 2015;40:137–142. doi: 10.1016/j.addbeh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 129.Weinstein A, Livny A, Weizman A. New developments in brain research of internet and gaming disorder. Neurosci Biobehav Rev. 2017;75:314–330. doi: 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 130.Hampton W, Alm K, Venkatraman V, Nugiel T, Olson I. Dossociable frontostriatal white matter connectivity underlies reward and motor impulsivity. NeuroImage. 2016;150:336–343. doi: 10.1016/j.neuroimage.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chamberlain SR, Derbyshire K, Daws RE, Odlaug BL, Leppink EW, Grant JE. White matter tract integrity in treatment-resistant gambling disorder. Br J Psychiatry. 2016;208(6):579–584. doi: 10.1192/bjp.bp.115.165506. [DOI] [PubMed] [Google Scholar]
- 132.Grant J, Correira S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Research: Neuroimaging. 2006;147:233–237. doi: 10.1016/j.pscychresns.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu J, Li Y, Lin H, Sinha R, Potenza MN. Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum; a diffusion tensor imaging study. Hum Brain Mapp. 2013;34(5):1044–1052. doi: 10.1002/hbm.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoon H, Kleven A, Paulsen A, Kleppe L, Wu J, Ying Z, Gomez-Pinalla F, Scarisbrick I. Interplay between exercise and dietary fat modulates myelinogenesis in the central nervous system. Biochimica et Biophysica Acta. 2016;1862(4):545–555. doi: 10.1016/j.bbadis.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fortier C, Leritz E, Salat D, Lindemer E, Maksimovsky A, Shepel J, Williams V, Venne J, Milberg W, McGlinchey R. Widespread effects of alcohol on white matter microstructure. Alcohol Clin Exp Res. 2014;38(12):2925–2933. doi: 10.1111/acer.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Squeglia L, Tapert S, Sullivan E, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy drinking adolescents. Am J Psychiatry. 2015;172(6):531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Donald KA, Roos A, Fouche JP, Koen N, Howells FM, Woods RP, Zar HJ, Narr Kl, Stein DJ. A study of the effects of prenatal alcohol exposure on white matter microstructural integrity at birth. Acta Neuropsychiatr. 2015;27(4):197–205. doi: 10.1017/neu.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baeza-Loya S, Velasquez K, Molfese D, Viswanath H, Curtis K, Thompson-Lake D, Baldwin P, Ellmore T, Garza R, Salas R. Anterior cingulum white matter is altered in tobacco smokers. Am J Addict. 2016;25:210–214. doi: 10.1111/ajad.12362. [DOI] [PubMed] [Google Scholar]
- 139.Yu R, Deochand C, Krotow A, Leao R, Tong M, Agarwal A, Cadenas E, de la Monte S. Tobacco smoke-induced brain white matter myelin dysfunction: potential co-factor role of smoking in neurodegeneration. J Alz Dis. 2016;50(1):133–148. doi: 10.3233/JAD-150751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cog Neurosci. 2015;16:23–35. doi: 10.1016/j.dcn.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morie KP, Yip SW, Zhai ZW, Xu J, Hamilton KR, Sinha R, Mayes LC, Potenza MN. White-matter crossing-fiber microstructure in adolescents prenatally exposed to cocaine. Drug Alcohol Depend. 2017;174:23–29. doi: 10.1016/j.drugalcdep.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lederer K, Fouche JP, Wilson D, Stein DJ, Uhlmann A. Frontal white matter changes and aggression in methamphetamine dependence. Metab Brain Dis. 2016;31(1):53–62. doi: 10.1007/s11011-015-9775-9. [DOI] [PubMed] [Google Scholar]
- 143.Sun Y, Wang GB, Lin QX, Lu L, Shu N, Meng SQ, Wang J, Han HB, He Y, Shi J. Disrupted white matter structural connectivity in heroin abusers. Addict Biol. 2017;22(1):184–195. doi: 10.1111/adb.12285. [DOI] [PubMed] [Google Scholar]
- 144.Dauncey MJ. Recent advances in nutrition, genes, and brain health. Proc Nutr Soc. 2012;71(4):581–591. doi: 10.1017/S0029665112000237. [DOI] [PubMed] [Google Scholar]
- 145.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelin. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 146.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 147.Todorich B, Pasquini J, Garcia C, Paez P, Connor J. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 148.Gupta P, Perrine Z, Mei Z, Scanlon K. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8:330. doi: 10.3390/nu8060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Algarin C, Karunakaran KD, Reyes S, Morales C, Lofoff B, Peirano P, Biswal B. Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Front Aging Neurosci. 2017;9:54. doi: 10.3389/fnagi.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 151.Stephenson E, Nathoo N, Mahjoub Y, Dunn J, Yong V. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol. 2014;10:459–468. doi: 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- 152.Steiger TK, Weiskopf N, Bunzeck N. Iron level and myelin content in the ventral striatum predict memory performance in the aging brain. J Neurosci. 2016;36(12):3552–3558. doi: 10.1523/JNEUROSCI.3617-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yehuda S, Rabinovitz S, Mostofsky D. Essential fatty acids and the brain: from infancy to aging. Neurobiol Aging. 2005;26(Suppl 1):98–102. doi: 10.1016/j.neurobiolaging.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 154.Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obes Rev. 2015;16(4):273–281. doi: 10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- 155.Hackman D, Farah M, Meaney M. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neuro. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Piccolo L, Merz E, He X, Sowell E, Noble K. Age-related differences in cortical thickness vary by socioeconomic status. PLoS ONE. 2016;11(9):e0162511. doi: 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harlow H, Dodsworth R, Harlow K. Total social isolation in monkeys. Proc Natl Acad Sci U.S.A. 1965;54(1):90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol Mood Anxiety Disord. 2013;3(1):21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kumar A, Behen M, Singsoonsud P, Veenstra A, Wolfe-Christiansen C, Helder E, Chugani H. Microstructural abnormalities in language and limbic pathways in orphanage-reared children: a diffusion tensor imaging study. J Child Neurol. 2014;29(3):318–325. doi: 10.1177/0883073812474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Howson CP, Kinney MV, McDougall L, Lawn JE the Born Too Soon Preterm Birth Action Group. Born too soon: preterm birth matters. Reprod Health. 2013;10(Suppl 1):S1. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Allin MP, Kontis D, Walshe M, Wyatt J, Barker G, Kanaan R, McGuire P, Rifkin L, Murray R, Nosarti C. White matter and cognition in adults who were born preterm. PLoS ONE. 2011;6:e24525. doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Van Tilborg E, Heijnen C, Benders M, van Bel F, Fleiss B, Gressens P, Nijboer C. Impaired oligodendrocyte maturation in preterm infants: potential therapeutic targets. Prog in Neurobiol. 2016;136:28–49. doi: 10.1016/j.pneurobio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 163.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71(1):93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coq J, Delcour M, Massicotte V, Baud O, Barbe M. Prenatal ischemia deteriorates white matter, brain organization, and function: implications for prematurity and cerebral palsy. Dev Med Child Neurol. 2017;58(4):7–11. doi: 10.1111/dmcn.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;(81924):2082–2090. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schindler T, Gilbert Y, Jayatilake S, Stevenson G, Oei JL, Welsh A. Basal ganglia perfusion in the preterm infant. Pediatr Res. 2016;80:573–576. doi: 10.1038/pr.2016.106. [DOI] [PubMed] [Google Scholar]
- 167.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacuad A, Saliba E, Dammann O, Gallego J. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:846–856. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 168.Reid M, Murray K, Marsh E, Golden J, Simmons R, Grinspan J. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenic protein 4. J Neuropathol Exp Neurol. 2012;71(7):640–653. doi: 10.1097/NEN.0b013e31825cfa81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Volpe J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.White-Traut R, Norr K, Fabiyi C, Rankin K, Li Z, Li L. Mother-infant interaction improves with developmental intervention for mother-preterm infant dyads. Infant Behav Dev. 2013;36:694–706. doi: 10.1016/j.infbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kotey FO, Spatz DL. White matter injury in preterm infants: could human mil play a role in its prevention? Adv Neonatal Care. 2013;13(2):89–94. doi: 10.1097/ANC.0b013e31827bfead. [DOI] [PubMed] [Google Scholar]
- 173.Pineda RG, Neil J, Dierker D, Inder T. Alterations in Brain Structure and Neurodevelopmental Outcome in Preterm Infants Hospitalized in Different Neonatal Intensive Care Unit Environments. J Pediatrics. 2014;164(1):52–60. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santos J, Pearce S, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27:254–260. doi: 10.1097/MOP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McCann ME, Schouten An. Beyond survival; influences of blood pressure, cerebral perfusion, and anesthesia on neurodevelopment. Paediatr Anaesth. 2014;24(1):68–73. doi: 10.1111/pan.12310. [DOI] [PubMed] [Google Scholar]
- 176.Doesburg SM, Chau SM, Cheung TP, Moiseev A, Ribary U, Herdman AT, Miller SP, Cepeda IL, Synnes A, Grunae RE. Neonatal pain-related stress, functional cortical activity and visual-perceptal abilities in school-age children born at extremely low gestational age. Pain. 2013;154(10):1946–1952. doi: 10.1016/j.pain.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson S, Hollis C, Kochhar, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal findings at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. [PubMed] [Google Scholar]
- 178.Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, Roberts G, Doyle LW, Neil JJ, Inder TE. Regional cerebral development at term relates to school-age social-emotional development in preterm children. J Am Acad Child Adolesc Psychiatry. 51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Als H, Duffy F, McAnulty G, Rivkin M, Vajapeyam S, Eichenwald E. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 180.Wong HS, Edwards P. Nature or nurture: a systematic review of the effect of socio-economic status on the development and cognitive outcomes of children born preterm. Matern Child Health. 2013;17(9):1689–1700. doi: 10.1007/s10995-012-1183-8. [DOI] [PubMed] [Google Scholar]