Objectives
Health outcomes, including chronic disease and mortality, attributed to or associated with alcohol abuse are discrepant between African Americans and Whites. To date, the topic is not fully understood and few studies conducted have used biomarker indicators of health. We investigated whether the association between alcohol abuse and biomarkers of the neuroendocrine system vary between black or African American and White respondents aged 34 to 84 from the Midlife in the United States Study (MIDUS) II (2004–2006) (n =1,129). Alcohol abuse was assessed with a modified version of the Michigan Alcohol Screening Test. Ordinary least squared (OLS) regression was used to evaluate whether race moderated the associations between alcohol abuse and four biomarkers—urinary cortisol and serum dehydroepiandrosterone sulfate (DHEA-S), epinephrine and norepinephrine—and two composite summary scores, each consisting of two components that characterize the hypothalamic pituitary adrenal (HPA)-axis and sympathetic nervous systems (SNS), respectively. Covariates included age, sex, education, income, current drinking, smoking, exercise, fast food consumption, heart disease, blood pressure, diabetes, body mass index, medication use, anxiety/depression, sleep duration, and cholesterol markers. Race significantly moderated the associations between alcohol abuse and norepinephrine concentration (χ2 [1] = 4.48, p=0.034) and the SNS composite score (χ2 [1] = 5.83, p=0.016). Alcohol abuse was associated with higher mean norepinephrine levels (b=0.26, standard error (SE)=0.12, p=0.034) and SNS composite score (b=0.23, SE=0.11, p=0.016) for African Americans compared to Whites. Interestingly, for Whites a paradoxical association between alcohol abuse, norepinephrine and SNS levels was observed; those who abused alcohol had lower mean norepinephrine levels than non-abusers. Race differences in neuroendocrine response could be biological pathways that contribute the excess risk of chronic disease and mortality attributed to alcohol abuse among African Americans compared to Whites. Replication of these analyses in larger cohorts are warranted in addition to further studies of underlying mechanisms among Blacks and Whites separately.
Keywords: alcohol abuse, neuroendocrine system, biological markers, race/ethnicity, chronic disease, MIDUS
1. Introduction
Alcohol abuse contributes to 5% of the global burden of disease (World Health Organization, 2011) and it is the third lifestyle-attributed cause of preventable mortality in the United States (US) (Centers for Disease Control and Prevention, 2011). Alcohol abuse is a major causal factor in over 60 chronic diseases (Bauer et al., 2014; Connor et al., 2015), including diabetes, hypertension, cirrhosis of the liver, breast and liver cancer, and early mortality (Boffetta and Hashibe, 2006; Centers for Disease Control and Prevention, 2011; Chen et al., 2011; Rehm et al., 2010; Rehm et al., 2014). Alcohol abuse acts a chronic stressor that can result in dysfunction of the neuroendocrine system, which drives poor physical and mental health, chronic diseases, and early mortality (Clarke et al., 2008; Cui et al., 2011; Dees et al., 2015; Yakovleva et al., 2011).
Blacks/African Americans compared to Whites have higher rates of many of the physical and chronic health outcomes, injury, and mortality that are, in part, attributed to alcohol abuse, dependence or excessive alcohol use (Polednak, 2008; Keyes et al., 2012; Stahre and Simon, 2010). That Black-White pattern in alcohol-related health outcomes is evident even after adjustment for socioeconomic status, social, and environmental covariates (Kerr et al., 2011; Chartier et al., 2013; Mulia et al., 2009), and ethanol concentrations in different alcoholic beverages (Witbrodt et al., 2014). Drinking patterns among African Americans are characterized by higher frequency of heavy drinking occassions (Sempos et al., 2003) while clinical-based assessments of alcohol use disorder—which include abuse—reveal small to no statistical differences in 12-month prevalence of alcohol abuse and/or dependence (Hasin et al., 2007; Grant et al., 2015).
In light of race differences in chronic disease and mortality, it is possible that the impact of alcohol abuse and diseases will differ between Blacks and Whites (Zapolski et al., 2014). For example, some studies found that for Blacks compared to Whites, alcohol abuse, dependence or excessive alcohol use had a stronger negative impact on cardiovascular-related diseases (Fuchs et al., 2004; Fuchs et al., 2001), breast cancer (Park et al., 2014), years of potential life lost (Shield et al., 2013), and mortality (Jackson et al., 2015; Williams et al., 2012).
However, one major gap in this topic of research so far is that little is known about racial differences the association between alcohol abuse and biological markers of health systems that underly disease and mortality. Environmental, psychosocial, and behavioral exposures drive race differences in chronic disease and mortality through complex interplays on multiple bio-physiological pathways that include the cardiovascular, immune, and metabolic systems, which can be represented through a measure of cumulative burden called allostatic load (Geronimus et al., 2006; Seeman et al., 2010). Despite the fact that multiple physiological systems contribute to health disparities, the scope of this study concerns the neuroendocrine system given the links to numerous diseases. Additionally, the topic is under-researched despite some evidence of Black-White differences in several hormones within this system, such as cortisol and norepinephrine (Cohen et al., 2006a; Ziegler et al., 1991).
Hormones such as epinephrine, norepinephrine, cortisol, and dehydroepiandrosterone sulfate (DHEA-S), play a central role in the pathogensis of physical and mental health, chronic disease, and premature death (Anand et al., 2003; Olff et al., 2006; Roggero et al., 2016; Schroeder and Jordan, 2012; Trivedi and Khaw, 2001; Zoccali et al., 2002). Specifically, epinephrine, norepinephrine, and cortisol are main effectors of the body’s response to stress and trigger a fight-or-flight response. Excessively high levels of these hormones are associated with high blood pressure, cancer tumor progression, and insomnia (Chrousos, 2009; Yang et al., 2009; Zijderveld et al., 1999). For example, one study of patients with type-2 diabetes found that a norepinephrine level ≥ 333 pg/ml was associated with a five-fold higher risk of incident adverse cerebral and cardiovascular events (Yufu et al., 2014) compared to levels below that threshold. Low levels of hormones such as norepinephrine has been linked to higher depressive symptoms (Moret and Briley, 2011) and lower cortisol has been linked to stress-related disorders through weakening the availability of glucocorticoid signaling (Raison and Miller, 2003).
Next, the hypothalamic pituitary adrenal (HPA)-axis is a major neuroendocrine signaling system that regulates physiological responses to stress through the hypothalamic release of corticotropin-releasing hormone (Smith and Vale, 2006). Dysregulation in the HPA-axis is associated with post-traumatic stress disorder (Olff et al., 2006) and likely development of cancers, although the impact of cancer growth as a function of lower or higher production of hormones, including cortisol and epinephrine, depends on the type of cancer (Armaiz-Pena et al., 2009; Sood et al., 2006; Yehuda, 2003).
Finally, DHEA-S declines with age and lower levels are associated with frailty and higher risk of mortality (Ohlsson et al., 2015) while some evidence suggest higher levels are protective of cardiovascular diseases (Savineau et al., 2013).
The links between alcohol abuse and dysregulation of the neuroendocrine system has been demonstrated in both animal and human studies. For instance, one experimental study in rats demonstrated that alcohol exposure at levels that reflect dependence, was associated with significant impairment of the HPA-axis and dampened neuroendocrine function — e.g., lower ability to cope with stress and heightened cortisol release (Richardson et al., 2008). One longitudinal observational study in humans found significant reductions (approximately 45%) in serotonergic neurotransmission in alcohol dependent individuals compared to controls (Fahlke et al., 2012). In addition, a population-based longitudinal study of adults from the Netherlands showed that heavy alcohol use (men: >3, women: >2 drinks/day) relative to moderate use (men: ≤3, women: ≤2 drinks/day) was associated with higher mean evening cortisol and lower mean cardiac sympathetic control, adjusting for sociodemographic, health and lifestyle, depression, and medication covariates (Boschloo et al., 2011).
To the best of our knowledge, no prior study has examined whether there are race differences in the association between alcohol abuse and physiological biomarkers of the neuroendocrine system. We hypothesize that dysregulation of the neuroendocrine system in association with alcohol abuse is pronounced among African Americans compared to Whites.
2. Methods
2.1. Sample
Data were from the second wave of the Midlife in the United States (MIDUS) biomarker sample. MIDUS is a longitudinal study designed to study social, psychological and behavioral factors in relation to physical and mental health (Radler and Ryff, 2010). MIDUS I enrolled 7108 individuals, including sibling and twins ages 25 to 74 years between January 1995 and September 1996 from a national sample of non-institutionalized adults living in 48 states, through random digit dialing (Love et al., 2010). Among the original sample, a second wave (MIDUS II) of (n = 4963, 70% response rate) was conducted between 2004 and 2006. At that time, an additional sample of (n = 592) African Americans was recruited from Milwaukee, WI to increase participation of African Americans in the study. Milwaukee is a highly segregated city, which was close to Madison, WI—one site where the biological data were collected. Respondents in the MIDUS II national sample and Milwaukee sample were eligible to participate in the biological assessments if they had completed the MIDUS II surveys, and lived in the contiguous US. Biomarker data were measured among individuals who stayed overnight at one of three General Clinical Research Centers (GCRC): the University of Wisconsin, Madison; University of California, LA; and Georgetown University. The institutional review boards at each university approved all data collection (Love et al., 2010). All participants provided informed consent. The final sample with biomarker data was 1,255 participants. The sample for this secondary analysis is (n = 1,129) black or African American and White respondents only, ages 35 to 84 years with no missing data on the exposure, outcomes, and covariates of interest below.
2.2. Measures
2.2.1. Neuroendocrine biomarkers
Urine cortisol adjusted for creatine, and DHEA-S were assayed using a Roche Modular Analytics E170 analyzer via an Elecsys kit (Roche Diagnostics, Indianapolis, IN). The intra-assay coefficient of variance was 2.9% for cortisol and between 0.8 to 6.5% for DHEA-S. Epinephrine and norepinephrine based on 12-hour overnight urine collections adjusted for urine creatine levels, were assayed using high-pressure liquid chromatography (HPLC). The intra-assay coefficient of variation was 7.9% for epinephrine and 6.0% for norepinephrine. In regression models, cortisol, DHEA-S, epinephrine and norepinephrine were modeled as continuous variables, and were log-transformed to correct a right-skewed distribution and satisfy normality assumptions for OLS regression. We also created composite scores to capture HPA-axis burden (consisting of cortisol and DHEA-S), and the SNS burden (consisting of epinephrine and norepinephrine). For both composite measures the range was 0 to 1, which indicates the average # of high-risk indicators (i.e., in the top quartile for each of the variables within that composite score).
Further details on the methodology of the biomarkers and composite summary score creation are published elsewhere (Duncan et al., 2003; Gruenewald et al., 2012).
2.2.2. Alcohol Abuse
was assessed using a modified version of the Michigan Alcoholism Screening Test (MAST), which showed adequate reliability and validity in population studies (Selzer et al., 1975; Shields et al., 2007). MAST is a diagnostic measure used in clinical settings and has demonstrated concurrent validity with other popular diagnostic indicators such as Alcohol Use Identification Test (AUDIT) and Cut-back, Annoyance by critics, Guilt about drinking, and Eye-opening morning drinking (CAGE) scale (Gibbs, 1983; Hays et al., 1995). The MAST questions were: (a) did you have any emotional or psychological problems from using alcohol, such as feeling depressed, being suspicious of people, or having strange ideas? (b) did you have such a strong desire or urge to use alcohol that you could not resist or could not think of anything else? (c) did you have a period of a month or more when you spent a great deal of time using alcohol or getting over its effects? (d) did you find that you had to use more alcohol than usual to get the same effect or that the same amount had less effect on you than before? The fifth question was not available in MIDUS II questionnaire: (e) were you ever, during the past 12 months, under the effects of alcohol or feeling its after-effects in a situation which increased your chances of getting hurt- such as when driving a car or boat, or using knives or guns or machinery? The response option for each question is yes or no.
The four MAST items were summed and dichotomized to 0 = no alcohol abuse and 1 = alcohol abuse if a participant responds positively to at least one of the four questions. The variable was only computed for cases that have at least one valid response to the four questions in the summary variable. The internal consistency coefficient (Cronbach's α) for the 5-item MAST based on MIDUS I was 0.67 for African Americans and 0.75 for Whites. The Cronbach's α in MIDUS I for the four items (a to d) was 0.68 for African Americans and 0.73 for Whites. The Cronbach's α in MIDUS II for the four items was 0.76 for African Americans and 0.70 for Whites.
2.2.3. Race
was operationalized via self-reported identification (black or African American vs. White, only).
2.2.4. Sociodemographic Covariates
included sex (men vs. women); mean centered age in years, and educational attainment in years, and household income categorized into three equal groups and a fourth group assigned for missing responses.
2.2.5. Health Status and Behavior Covariates
were selected based on their association with race, alcohol use, and with physiological and neuroendocrine biomarkers (Beulens et al., 2008; Boschloo et al., 2011; Cohen et al., 2006b; Galán et al., 2014; Thayer et al., 2006; Volpato et al., 2004). Current drinking (consuming at least one alcoholic beverage in the past month), smoking history (yes, ever smoked regularly—that is, a few cigarettes every day vs. no); exercise (defined as greater than or equal to 20 minutes three times per week vs. other exercise); body mass index (BMI) was calculated using height and weight measured by the GCRC staff (continuous variable, kg/m2); and fast food consumption (eating fast food greater than or equal to once per week vs. once per week vs. never).
Positive responses to self-reported physician-diagnosed history of diabetes mellitus, cardiovasular disease (CVD) (stroke, heart attack, angina, and chest pain), high blood pressure, and medications (anti-hypertensive, lipid-lowering, corticosteroid, and antidepressant) were included as dummy indicators. Anxiety and depression in the past 12 months were defined in accordance with criteria specified in the Diagnostic and Statistical Manual of Mental Disorders-third edition-revised (DSM-III-R), average sleep duration (seven hours or more vs. less than or equal to six hours) and cholesterol measures (high-density and low- density lipoprotein, and triglycerides) were also controlled for in analyses.
2.3. Statistical Analyses
STATA 14.0 software (StataCorp, 2015) was used to analyze the data. Means and standard deviations were calculated for continuous variables and number and percent for binary or categorical variables. A series of ordinary least square (OLS) regression models were computed to examine the independent association of race and alcohol abuse on each neuroendocrine biomarker and the HPA and SNS composite scores within the pooled African American and White sample. Effect modification by race was assessed via an interaction term and the significance of any interaction was assessed using test of contrasts that reports a Chi-Square value with one degree of freedom.
There were two effect modification models. The first adjusted for age, sex, education, income, current drinking, smoking, exercise, fast food consumption, medication use (blood pressure, cholesterol, steroid, and anti-depressant medications), anxiety/depressive symptoms, and average sleep duration (Model 2). The next model adds the following health variables to the prior model: body mass index, diabetes, heart disease, high blood pressure, high density lipoprotein, low density lipoprotein, and triglycerides. The purpose of adding those variables in a subsequent step was to examine whether any potential race differences in neuroendocrine dysregulation from the prior model operates through health status (Model 3).
For those interactions that were statistically significant, marginal mean scores and 95 % confidence intervals for the association between alcohol abuse and the biomarkers for African Americans and Whites were obtained and plotted on their untransformed metric. For all models, bootstrapped estimates (stratified by alcohol abuse) of the standard errors and confidence intervals were computed by generating 500 iterations using the bias corrected and accelerated (bca) method (Carpenter and Bithell, 2000). Robust standard errors were also obtained. Bootstrapping addresses potential non-normality of the error term and heteroscedasticity in OLS regression, which potentially may occur with the small sample size of African Americans compared to Whites with alcohol abuse. Although MIDUS II contained twins, we did not account for clustering with methods such as General Estimating Equations (GEE). This is because there were too few twin pairs among the African American sample, which caused the multivariable models to skip iterations and not converge during the bootstrapping.
3.0. Results
Supplement Table 1 shows the distribution of exposures and covariates between the analytic sample used in multivariable analysis and those excluded because of missing data on one or more covariates (N = 75). Ninety-four percent of the sample were included (i.e., N = 1,129). Respondents included were not statistically different from those excluded (i.e., p > 0.05) for alcohol abuse, nor any of the neuroendocrine markers except DHEA-S, nor any of the sociodemographic variables except age, nor any of the health status and behavior covariates except high blood pressure diagnosis and medication use. Moreover, among those excluded, there were no race differences in age, blood pressure diagnosis and medication use (all p > 0.10, results not displayed but available upon request).
Table 1 shows that the prevalence of 12-month alcohol abuse was higher for African Americans than Whites (7.9% vs. 4.4%, p = 0.03). The race-difference was significant among current drinkers (p = 0.008) but not among non-drinkers (p = 0.443), (results not displayed). For all neuroendocrine biomarkers, African American respondents had a lower mean value than Whites (all p values < 0.05). African Americans were younger, had lower mean levels of educational attainment and income, and vigorous exercise; however, African American respondents were more likely than White respondents to be women, smoke, consume fast-food, and have less than six hours of sleep (all p < 0.05). A lower proportion of African Americans compared to Whites had consumed alcohol in the past month and used medication for cholesterol, corticosteroid, and antidepressant medications (all p < 0.05).
Table 1.
Characteristics of White and African American respondents (n = 1,129); Midlife in the United States (MIDUS) Biomarker Study
| White Respondents (n=914)a |
African American Respondents (n=215)a |
p-value | |
|---|---|---|---|
| Mean (SD) or N (%) |
Mean (SD) or N (%) |
||
| Alcohol Abuse | |||
| Yes | 40 (4.4) | 17 (7.9) | =0.033 |
| Neuroendocrine system markers | |||
| Urine cortisol/creatine (ug/g) | 16.4 (15.23) | 10.4 (08.22) | <0.001 |
| Blood DHEA (ug/dL) | 104.7 (76.1) | 100.7 (77.1) | =0.047 |
| HPA-axis composite scoreb | 0.25 (00.3) | 0.18 (00.2) | =0.003 |
| Urine epinephrine/ creatine (ug/g) | 2.0 (01.3) | 1.7 (01.3) | =0.001 |
| Norepinephrine/ creatine (ug/g) | 27.7 (13.2) | 25.9 (16.7) | =0.007 |
| Sympathetic system composite scoreb | 0.24 (00.3) | 0.17 (00.03) | =0.001 |
| Age (years) | 55.5 (11.9) | 50.9 (10.6) | <0.001 |
| Sex | |||
| Men | 416 (45.5) | 72 (33.5) | 0.001 |
| Women | 498 (54.5) | 143 (66.5) | |
| Education (years)c | 7.78 (2.43) | 6.11 (2.48) | <0.001 |
| Income (dollars) | $77,519 ($60,730) | $38,607 ($34,888) | <0.001 |
| Health status and behaviors | |||
| Currently consume alcohol (% yes) | 622 (68.0) | 118 (54.9) | <0.001 |
| Smoked regularly (% yes) | 404 (44.2) | 131 (60.9) | <0.001 |
| Vigorous exercise (20 mins) ≥3 times/week (% yes) | 732 (80.9) | 138 (64.2) | <0.001 |
| Body mass index | 29.1 (5.9) | 32.8 (8.3) | <0.001 |
| Eat fast food (%> once per week) | 444 (48.6) | 105 (48.8) | =0.417 |
| CVD diagnosis (% yes) | 104 (11.3) | 25 (11.6) | =0.918 |
| Diabetes diagnosis (% yes) | 89 (09.7) | 51 (23.7) | <0.001 |
| High blood pressure diagnosis (% yes) | 300 (32.8) | 115 (53.5) | <0.001 |
| Current medications (% yes) | |||
| Blood pressure medication | 312 (34.1) | 100 (46.5) | =0.001 |
| Cholesterol medication | 268 (29.3) | 43 (20.0) | =0.006 |
| Corticosteroid medicationd | 115 (12.6) | 19 (8.8) | =0.127 |
| Anti-depressant medication | 144 (15.8) | 13 (06.0) | <0.001 |
| Anxiety/depression (% yes) | 175 (19.1) | 43 (20.0) | =0.775 |
| High-density lipoprotein | 54.9 (19.4) | 59.2 (19.4) | =0.002 |
| Low-density lipoprotein | 105.8 (34.9) | 101.8 (35.3) | =0.120 |
| Triglyceride | 130.9 (79.7) | 112.8 (73.3) | =0.001 |
| Average sleep duration | |||
| ≤ Six hours (%) | 232 (25.4) | 94 (43.7) | <0.001 |
Sample with no missing data on any of the covariates;
the composite summary score ranges from 0 to 1, which indicates the average # of high-risk indicators (i.e., in the top quartile for each of the variables within that composite score);
education (6=1 to 2 years of college no degree yet, 7= 3 or more years of college no degree yet;
Corticosteroid medication includes adrenals, estrogens, antiestrogens and estrogen agonists-antagonists. HPA is hypothalamic pituitary adrenal. DHEA is dehydroepiandrosterone sulfate, SNS is sympathetic nervous system.
Table 2 presents OLS regression results for the interaction between race and alcohol abuse with the biomarkers, adjusted for covariates. Model 1 shows the main effect for the independent associations of race and alcohol abuse on the neuroendocrine system markers and the composite scores, adjusted for basic covariates including age, gender, education and income. African American compared to White respondents had lower levels of cortisol (p < 0.001), epinephrine and norepinephrine, and SNS composite summary score (p < 0.001). Alcohol abuse, independently from race, was not statistically associated with any of the outcomes.
Table 2.
Ordinary least square regression assessing the main and interaction models of race and alcohol abuse in relation to biomarkers of the neuroendocrine system; Midlife in the United States (MIDUS) Biomarker Study (n=1,129)
| Log Cortisol b(SE) |
Log DHEA-S b(SE) |
HPA-axis compositea b(SE) |
Log Epinephrine b(SE) |
Log Norepinephrine b(SE) |
Sympathetic nervous system compositea b(SE) |
|
|---|---|---|---|---|---|---|
|
|
||||||
| Model 1. Baseline Modelsb | ||||||
| Alcohol Abuse: | ||||||
| Yes | −0.14 (0.10) | 0.05 (0.09) | −0.00 (0.03) | 0.03 (0.07) | −0.03 (0.05) | −0.01 (0.04) |
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Race: | ||||||
| African American | −0.40 (0.07)*** | −0.12 (0.06) | −0.04 (0.02) | −0.20 (0.05)*** | −0.17 (0.04)*** | −0.08 (0.03)** |
| White | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Model 2. Including Interaction Termb | ||||||
| Alcohol Abuse | ||||||
| Yes | −0.16 (0.12) | 0.11 (0.08) | −0.04 (.04) | 0.00 (0.08) | −0.09 (0.05) | −0.07 (0.04) |
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Race: | ||||||
| African American | −0.42 (0.06)*** | −0.10 (0.06) | −.07 (.02)** | −0.23 (0.05)*** | −0.18 (0.04)*** | −0.10 (0.03)*** |
| White (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Alcohol Use Disorders × Race | ||||||
| African American | −0.04 (0.25) | −0.23 (0.22) | 0.14 (0.08) | 0.21 (0.18) | 0.23 (0.12)* | 0.22 (0.11)* |
| White (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Model 3. Including Interaction Term + health variablesc | ||||||
| Alcohol Abuse | ||||||
| Yes | −0.14 (0.12) | 0.11 (0.08) | −0.04 (.04) | −0.02 (0.08) | −0.11 (0.05) | −0.08 (0.04) |
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Race: | ||||||
| African American | −0.43 (0.07)*** | −0.12 (0.06) | −.06 (.02)* | −0.22 (0.05)*** | −0.20 (0.04)*** | −0.11 (0.03)*** |
| White (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Alcohol Use Disorders × Race | ||||||
| African American | −0.01 (0.24) | −0.21 (0.22) | 0.14 (0.07) | 0.22 (0.16) | 0.26 (0.12)* | 0.23 (0.11)* |
| White (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
The composite summary score ranges from 0 to 1, which indicates the average # of high-risk indicators (i.e., in the top quartile for each of the variables within that composite score).
Models 1 and Model 2 include the covariates age, sex, education, income, current drinking, smoking, exercise, fast food consumption, medication use (blood pressure, cholesterol, steroid, and anti-depressant medications), anxiety/depressive symptoms, and average sleep duration.
Model 3 is built upon Model 2 + health variables: body mass index, diabetes, heart disease, high blood pressure, high density lipoprotein, low density lipoprotein, and triglycerides. Unstandardized coefficients and bootstrapped standard errors from 500 iterations using the bias corrected and accelerated (bca) method are from separate linear regression models.
P < .05;
P <.01;
P <.001
Model 2 builds on Model 1 to additionally include the interaction between race and alcohol abuse. Race moderated the associations between alcohol abuse and norepinephrine (χ2 [1] = 3.69, p = 0.054) and the sympathetic system composite summary score (χ2 [1] = 3.90, p = 0.048); we found no evidence of a significant interaction for the other outcomes.
Model 3 builds on Model 2 to add the health variables, including body mass index, diabetes, heart disease, high blood pressure, high density lipoprotein, low density lipoprotein, and triglycerides. The directions, point estimates and statistical significance of the outcomes were not materially altered by addition of those variables. Race significantly moderated the associations between alcohol abuse and norepinephrine (χ2 [1] = 4.48, p = 0.034) and the sympathetic system composite summary score (χ2 [1] = 5.83, p = 0.016).
Alcohol abuse was associated with higher mean levels of norepinephrine (b = 0.26, standard error (SE) = 0.12, p = 0.034) and SNS composite summary score (b = 0.23, SE = 0.11, p = 0.016) for African Americans compared to Whites.
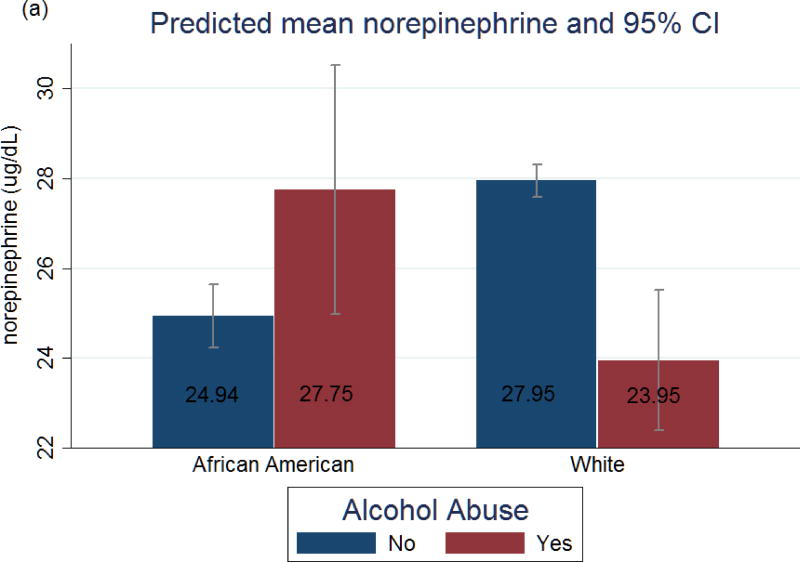
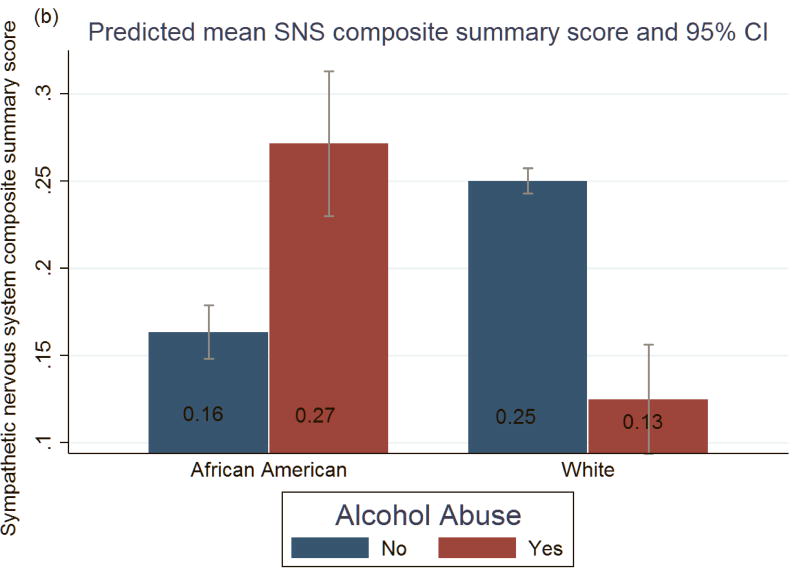
Figure 1 shows the marginal predicted means of norepinephrine and SNS composite score, respectively, from Model 3. Among African Americans, alcohol abuse compared to no abuse was associated with higher mean levels of norepinephrine (27.75 vs 24.94 ug/dL) and overall SNS composite score (0.27 vs 0.16 average # of high-risk indicators). Paradoxically, among Whites, alcohol abuse compared to no abuse was associated with lower mean levels of norepinephrine (23.95 vs 27.95 ug/dL) and SNS (0.13 vs 0.25 average # of high-risk indicators). Hormone levels between African Americans who abused alcohol and White non-abusers did not significantly differ.
Figure 1.
Predicted mean and 95 % Confidence Intervals of (a) norepinephrine and (b) sympathetic nervous system (SNS) composite summary score, by alcohol abuse for African American and White Respondents. Estimates were derived from OLS regression model as described in the text and results (Table 2, Model 3), which was adjusted for: age, sex, education, income, current drinking, smoking, exercise, fast food consumption, medication use (blood pressure, cholesterol, steroid, and anti-depressant medications), anxiety/depression, average sleep duration + health variables (body mass index, cardiovascular disease, diabetes, blood pressure and cholesterol measures (HDL, LDL, and Triglycerides)). IQR= Interquartile range, *for the entire sample
4.0. Discussion
Examining the role of alcohol abuse in the dysregulation of biomarkers within the neuroendocrine organ system can potentially elucidate the physiobiological mechanisms that can be intervened on within clinical settings (Freeman and Vrana, 2010; Schuckit, 2009). To the best of our knowledge, this is the first study to examine whether there were race differences in the association between alcohol abuse and biomarkers of the neuroendocrine organ system. As such, it would also be the first to show that alcohol abuse has an upregulating association of serum in norepinephrine and SNS composite for African Americans but downregulating association for Whites. Our findings contribute to the evidence of divergent health racial patterns in the association between alcohol use (abuse and disorders) and self-reported physical health and mortality (Chartier et al., 2013; Williams et al., 2012).
The main function of the sympathetic hormone norepinephrine is to mobilize the brain and body for action by increases in alertness, heart rate and blood pressure, and trigger release of glucose from energy stores. Higher norepinephrine is associated, at least partially, with high blood pressure, cardiovascular disease, depression, anxiety and other chronic diseases including diabetes (Montoya et al., 2016; Schroeder and Jordan, 2012; Thomas and Marks, 1978).
In this study, the levels of dysregulation in norepinephrine associated with alcohol abuse were not clinically significant given that normal range can span 15ug to 100ug/24hr (American Board of Internal Medicine, 2017). Nevertheless, our evidence suggest that dysregulation of norepinephrine and SNS could be components, which along with dysregulation of other serums from other biological systems, could contribute to a higher prevalence of chronic diseases and mortality for African Americans (Jackson et al., 2010; Williams, 2012). Although we focused on the neuroendocrine system for this study, it is well documented that multiple physiological systems interact in non-linear patterns in ways that underlie racial disparities in health (Geronimus et al., 2006; Seeman et al., 2010). Therefore, research on the topic going forward should examine measures such as allostatic load, which represents a more comprehensive view of bio-physiological risk profiles (Juster et al., 2010).
Given that we use cross-sectional data, it is plausible that poorer health profiles of African Americans drive the dysregulation between alcohol abuse and the outcome. For example, bivariate results showed that African Americans in this sample had higher prevalence of diabetes and blood pressure, and subsequently had higher medication use for blood pressure and cholesterol than Whites. However, our findings suggest that net of socioeconomic status and medication use, adding health variables including BMI, diabetes, and cardiovascular disease does not materially alter race differences in the association between alcohol abuse and norepinephrine and SNS serum.
Plasma norepinephrine levels reflect the spillover or clearance of the hormone from the system into the bloodstream (Goldstein et al., 2003). Previous experimental evidence showed race differences in the neuroendocrine system such that Blacks cleared infused norepinephrine from their plasma faster than Whites (Ziegler et al., 1991). A recent study showed that, adjusted for covariates, Black race predicted had higher plasma norepinephrine levels than Whites (Saxena et al., 2014). Other recent studies have also indicated that elderly African Americans had higher SNS control and responsiveness compared to Whites (Okada et al., 2012; Okada et al., 2016). Intriguingly, our results revealed that the norepinephrine levels are higher among White non-abusers compared to Black non-abusers as well as White persons who abuse alcohol. At the same time, there was no difference in serum levels between Black abusers and White non-abusers, which is equally perplexing.
The findings between White abusers and non-abusers, plausibly, may be due to lifestyle factors and socioeconomic status. For instance, some evidence show that alcohol abuse is associated with higher physical activity through pathways that appear to include common personality, biological, and social mechanisms (Lisha et al., 2013). Higher physical activity is in turn associated with reduced SNS and norepinephrine levels (Bote et al., 2014), although the extent of changes in the hormones varies by intensity of exercise (Greiwe et al., 1999). Higher socioeconomic status, specifically income, has also been associated with higher alcohol abuse (Keyes and Hasin, 2008) and with higher physical activity (Trost et al., 2002). Interestingly, in exploratory posthoc analyses (not shown), Whites with alcohol abuse had higher income than White non-alcohol abusers, but that income pattern was inverse among African Americans; and as shown in Table 1, Whites overall had higher physical activity rates than African Americans.
Race is a social construct that captures a set of social exposures environments including racism that influences gene-environment interactions (Jones, 2000; Lillie-Blanton and Laveist, 1996). Therefore, a combination of socioeconomic status and epigenetics could also plausibly explain why African Americans who abused alcohol had lower norepinephrine levels than White non-abusers. For instance, one population-based study showed that higher income-wealth ratio was associated with lower urinary cortisol and that inverse association was stronger among African Americans compared to Whites (Castro-Diehl et al., 2014). We adjusted for income and education and those measures did not account for race-differences in our study. However, it is possible that qualitative differences in effects of SES on health between Blacks and Whites (Williams et al., 2010) or other socioeconomic status markers such as wealth may play a greater role in health inequalities (Shapiro, 2004). In these data, we were not able to adjust for wealth.
One biologically plausible explanation for the paradoxical finding among Whites and compared to African Americans is racial/ethnic differences in genotypes of alcohol metabolizing enzymes (Chartier et al., 2014). For instance, ADH1B*3—the most widely replicated genetic variant of aldehyde dehydrogenase and primary enzyme responsible for metabolizing alcohol faster, is more prevalent among African Americans compared to Caucasians (Brennan et al., 2004; McCarthy et al., 2010).
However, evidence suggests that although ADH1B*3 is protective of alcoholism among African Americans; once they develop alcoholism, their health profile deteriorates dramatically because of higher risk for developing alanine and aspartate aminotransferase—biomarkers of liver disease (Ehlers et al., 2007). Higher risk for alcohol-related problems among African Americans has also been linked to higher sensitivity to the effects of alcohol compared to European Americans (Pedersen and McCarthy, 2013). On the basis of race differences in prevalence of ADH1B*3, ADH1C*1/2, ADH1B* 1/1, and the rapid health decline among African Americans with alcoholism; it is plausible to observe a lower overall norepinephrine level or no difference in SNS level between African Americans who abuse alcohol compared to White non-abusers. On that same basis discussed above, it is also possible to simultaneously observe higher norepinephrine and SNS level among African American abusers compared to Whites who abuse alcohol. Other, yet unknown, racial/ethnic differences in genetic expression and methylation of DNA (Zhang et al., 2011) may also play a role in our findings.
Despite the paradoxical association between alcohol abuse and norepinephrine and SNS among Whites, the association between race and alcohol abuse with health found in our study is consistent with the wider body of evidence on the topic. Two previous studies found that current alcohol use was associated with elevated levels of alanine and aspartate aminotransferase—biomarkers of liver disease among Black compared to their non-Hispanic White counterparts (Stewart, 2002; Stranges et al., 2004). One other study examined alcohol consumption in relation to breast cancer diagnosis using tumor biomarkers—e.g., estrogen receptor (ER) and human epidermal growth factor receptor 2—and found that African American women who drank greater than seven drinks per week had about 35% higher risk of breast cancer than their White counterparts (Williams et al., 2016). That study also found that the elevated risk of heavy alcohol use on ER negative and triple-negative breast cancers was significant for African American but not White women. Those studies differ from ours as they examined alcohol consumption and not alcohol abuse based on diagnostic indicators such as MAST.
We found that African Americans compared to Whites in MIDUS II had a higher 12-month prevalence of alcohol abuse. However, the higher prevalence was only significant among current drinkers. The higher prevalence of alcohol abuse in these data are in contrast to results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC wave 2, 2004–2005), which is one of the largest population-based studies in the United States and closest to the MIDUS time frame. NESARC data indicate that, in the general population, African Americans compared to Whites have lower 12-month prevalence of alcohol abuse (3.3% vs. 5.1%), however, NESARC assesses abuse using the Diagnostic Statistical Manual-IV (DSM-IV) criteria (Hasin et al., 2007). NESARC also sampled, especially among African Americans, from a more geographically diverse population, which is another plausible reason why prevalence estimates were different in our sample.
Our findings of higher alcohol abuse prevalence, however, is consistent with complementary evidence on race differences such that, among current drinkers, African Americans experience a greater number of alcohol abuse and dependence symptoms than Whites (Chartier and Caetano, 2010; Mulia et al., 2009; Witbrodt et al., 2014)
Some limitations of this study include sample representativeness. The African American sample in MIDUS II biomarker study was recruited primarily from Milwaukee, WI. While Milwaukee is a highly segregated city that reflects the living conditions of a large portion of urban Blacks, our sample is not representative of all African Americans in the US. A related issue is the relatively low sample number of African Americans in the study. The sample size of African Americans with alcohol abuse was almost half that compared to Whites. We attempted to adjust statistically for small sample size through bootstrapping the standard errors. Nonetheless, findings based on this sample of African Americans may have diminished our ability to detect effect modification by race across biomarkers other than norepinephrine and the SNS composite summary score. Next, although there was an approximately one year delay between when the alcohol questions were assessed and the biomarker data collected, the data are essentially cross-sectional, thus we cannot draw causal inferences about the associations found.
Another limitation is the measure of alcohol abuse. One of the five standard MAST questions was not available in MIDUS II. Although there are no previously published studies on the reliability of the MAST for the remaining four items at the present time, our study found that the reliability for both African Americans and Whites based on the four-item MAST was good (i.e., Cronbach's α 0.70 to 0.76) considering a scale with four items (Cortina, 1993). It is also noteworthy that the reliability for the four items for alcohol abuse according to criteria set forth in the Diagnostic and Statistical Manual fourth edition (DSM-IV) was 0.73 (Grant et al., 1995), which is within the range of the reliability found with the four-item MAST measure in our study. Nevertheless, further research that seeks to replicate our findings with a more geographically diverse and representative sample of African Americans and evaluation with other diagnostic measures or biomarkers of alcohol abuse is warranted.
5.0. Conclusion
The present study found that alcohol abuse is associated with upregulated norepinephrine and the SNS composite serum for African Americans but downregulated serum for Whites. Future studies incorporating biological markers of alcohol abuse are warranted to understand potentially paradoxical relationships between alcohol abuse and neuroendocrine markers of health, among Black and White persons separately. Future research should also examine whether there are race differences in the association between alcohol abuse and biological markers for other organ systems, which can inform research and interventions to eliminate racial disparities in health.
Supplementary Material
We examined the interaction of race*alcohol abuse on neuroendocrine system biomarkers
Blacks had lower mean cortisol, DHEA-S, epinephrine and norepinephrine than whites
Race moderated the association between alcohol abuse and norepinephrine, and SNS
Alcohol abuse upregulated norepinephrine and SNS for blacks compared to whites
White alcohol abusers exhibited lower norepinephrine and SNS than white non-abusers
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. Support was also provided by 1UL1RR025011 (UW) a grant from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. This research was also supported by the Alonzo Smythe Yerby Postdoctoral Fellowship from the Harvard T.H. Chan School of Public Health awarded to the first author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Board of Internal Medicine. ABIM laboratory test reference ranges. American Board of Internal Medicine; 2017. [Google Scholar]
- Anand IS, Fisher LD, Chiang Y-T, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- Beulens JW, Rimm EB, Hu FB, Hendriks HF, Mukamal KJ. Alcohol consumption, mediating biomarkers, and risk of type 2 diabetes among middle-aged women. Diabetes Care. 2008;31:2050–2055. doi: 10.2337/dc08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncology. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Licht CM, Vreeburg SA, Smit JH, van den Brink W, Veltman DJ, de Geus EJ, Beekman AT, Penninx BW. Heavy alcohol use, rather than alcohol dependence, is associated with dysregulation of the hypothalamic–pituitary–adrenal axis and the autonomic nervous system. Drug Alcohol Depend. 2011;116:170–176. doi: 10.1016/j.drugalcdep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Bote ME, García JJ, Hinchado MD, Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: Role of IL-8 and noradrenaline. Brain Behav Immun. 2014;39:107–112. doi: 10.1016/j.bbi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Brennan P, Lewis S, Hashibe M, Bell DA, Boffetta P, Bouchardy C, Caporaso N, Chen C, Coutelle C, Diehl SR, Hayes RB, Olshan AF, Schwartz SM, Sturgis EM, Wei Q, Zavras AI, Benhamou S. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. Am J Epidemiol. 2004;159:1–16. doi: 10.1093/aje/kwh003. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stats Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Castro-Diehl C, Roux AVD, Seeman T, Shea S, Shrager S, Tadros S. Associations of socioeconomic and psychosocial factors with urinary measures of cortisol and catecholamines in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychoneuroendocrinology. 2014;41:132–141. doi: 10.1016/j.psyneuen.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Excessive alcohol use: addressing a leading risk for death, chronic disease, and injury. National Center for Chronic Disease Prevention and Health Promotion; Hyattsvillle, MD: 2011. [Google Scholar]
- Chartier K, Caetano R. Ethnicity and health disparities in alcohol research. Alcohol Res Health. 2010;33:152–160. [PMC free article] [PubMed] [Google Scholar]
- Chartier KG, Hesselbrock MN, Hesselbrock VM. Ethnicity and gender comparisons of health consequences in adults with alcohol dependence. Subst Use Misuse. 2013;48:200–201. doi: 10.3109/10826084.2013.747743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier KG, Scott DM, Wall TL, Covault J, Karriker-Jaffe KJ, Mills BA, Luczak SE, Caetano R, Arroyo JA. Framing ethnic variations in alcohol outcomes from biological pathways to neighborhood context. Alc Clin Exp Res. 2014;38:611–618. doi: 10.1111/acer.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature reviews. Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Clarke T-K, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. REVIEW: HPA-axis activity in alcoholism: examples for a gene–environment interaction. Addiction biology. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2006a;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychol Med. 2006b;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Connor JP, Haber PS, Hall WD. Alcohol use disorders. Lancet. 2015;387:988–998. doi: 10.1016/S0140-6736(15)00122-1. [DOI] [PubMed] [Google Scholar]
- Cortina JM. What is coefficient alpha? An examination of theory and applications. J Appl Psychol. 1993;78:98. [Google Scholar]
- Cui C, Grandison L, Noronha A. Neuroimmune mechanisms of brain function and alcohol related disorders. Brain Behav Immun. 2011;25:S1–S3. doi: 10.1016/j.bbi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Dees W, Hiney J, Srivastava V. Alcohol alters hypothalamic glial-neuronal communications involved in the neuroendocrine control of puberty: In vivo and in vitro assessments. Alcohol. 2015;49:631–637. doi: 10.1016/j.alcohol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Montane-Jaime K, Moore S, Shafe S, Joseph R, Carr LG. Association of the ADHIB*3 Allele with alcohol-related phenotypes in Trinidad. Alc Clin Exp Res. 2007;31:216–220. doi: 10.1111/j.1530-0277.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Berggren U, Berglund KJ, Zetterberg H, Blennow K, Engel JA, Balldin J. Neuroendocrine assessment of serotonergic, dopaminergic, and noradrenergic functions in alcohol-dependent individuals. Alc Clin Exp Res. 2012;36:97–103. doi: 10.1111/j.1530-0277.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Vrana KE. Future prospects for biomarkers of alcohol consumption and alcohol-induced disorders. Alc Clin Exp Res. 2010;34:946–954. doi: 10.1111/j.1530-0277.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán I, Valencia-Martín J, Guallar-Castillón P, Rodríguez-Artalejo F. Alcohol drinking patterns and biomarkers of coronary risk in the Spanish population. Nutr Metab Cardiovasc Dis. 2014;24:189–197. doi: 10.1016/j.numecd.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs LE. Validity and reliability of the Michigan Alcoholism Screening Test: A review. Drug Alcohol Depend. 1983;12:279–285. doi: 10.1016/0376-8716(83)90071-6. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharm Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The alcohol use disorder and associated disabilities interview schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Hickner RC, Shah SD, Cryer PE, Holloszy JO. Norepinephrine response to exercise at the same relative intensity before and after endurance exercise training. J Appl Physiol. 1999;86:531–535. doi: 10.1152/jappl.1999.86.2.531. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hays RD, Merz JF, Nicholas R. Response burden, reliability, and validity of the CAGE, Short MAST, and AUDIT alcohol screening measures. Behav Res Methods Instrum Comput. 1995;27:277–280. [Google Scholar]
- Jackson CL, Hu FB, Kawachi I, Williams DR, Mukamal KJ, Rimm EB. Black–white differences in the relationship between alcohol drinking patterns and mortality among US men and women. Am J Public Health. 2015;105:S534–S543. doi: 10.2105/AJPH.2015.302615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100:933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CP. Levels of racism: a theoretic framework and a gardener's tale. Am J Public Health. 2000;90:1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosco Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Bond J, Ye Y, Rehm J. Racial and ethnic differences in all-cause mortality risk according to alcohol consumption patterns in the national alcohol surveys. Am J Epidemiol. 2011;174:769–778. doi: 10.1093/aje/kwr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hasin DS. Socio-economic status and problem alcohol use: The positive relationship between income and the DSM-IV alcohol abuse diagnosis. Addiction. 2008;103:1120–1130. doi: 10.1111/j.1360-0443.2008.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Liu XC, Cerda M. The role of race/ethnicity in alcohol-attributable injury in the United States. Epidemiol Rev. 2012;34:89–102. doi: 10.1093/epirev/mxr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie-Blanton M, Laveist T. Race/ethnicity, the social environment, and health. Social Science & Medicine. 1996;43:83–91. doi: 10.1016/0277-9536(95)00337-1. [DOI] [PubMed] [Google Scholar]
- Lisha NE, Sussman S, Leventhal AM. Physical activity and alcohol use disorders. Am J Drug Alcohol Abuse. 2013;39:115–120. doi: 10.3109/00952990.2012.713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Pedersen SL, Lobos EA, Todd RD, Wall TL. ADH1B*3 and response to alcohol in African Americans. Alc Clin Exp Res. 2010;34:1274–1281. doi: 10.1111/j.1530-0277.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A, Bruins R, Katzman MA, Blier P. The noradrenergic paradox: implications in the management of depression and anxiety. Neuropsychiatric disease and treatment. 2016;12:541–557. doi: 10.2147/NDT.S91311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric disease and treatment. 2011;7:9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulia N, Ye Y, Greenfield TK, Zemore SE. Disparities in alcohol-related problems among white, black, and Hispanic Americans. Alcohol Clin Exp Res. 2009;33:654–662. doi: 10.1111/j.1530-0277.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Vandenput L, Tivesten Å. DHEA and mortality: what is the nature of the association? J Steroid Biochem Mol Biol. 2015;145:248–253. doi: 10.1016/j.jsbmb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Okada Y, Galbreath MM, Jarvis SS, Bivens TB, Vongpatanasin W, Levine BD, Fu Q. Elderly blacks have a blunted sympathetic neural responsiveness but greater pressor response to orthostasis than elderly whites. Hypertension. 2012;60:842–848. doi: 10.1161/HYPERTENSIONAHA.112.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Jarvis SS, Best SA, Edwards JG, Hendrix JM, Adams-Huet B, Vongpatanasin W, Levine BD, Fu Q. Sympathetic neural and hemodynamic responses during cold pressor test in elderly Blacks and Whites. Hypertension. 2016;67:951–958. doi: 10.1161/HYPERTENSIONAHA.115.06700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Güzelcan Y, de Vries G-J, Assies J, Gersons BPR. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:1220–1230. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Pedersen SL, McCarthy DM. Differences in acute response to alcohol between African Americans and European Americans. Alc Clin Exp Res. 2013;37:1056–1063. doi: 10.1111/acer.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polednak AP. Temporal trend in the US black–white disparity in mortality rates from selected alcohol-related chronic diseases. J Ethn Subst Abuse. 2008;7:154–164. doi: 10.1080/15332640802055558. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, Grant B. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res. 2014;38:1068–1077. doi: 10.1111/acer.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggero E, Pérez AR, Pollachini N, Villar SR, Wildmann J, Besedovsky H, del Rey A. The sympathetic nervous system affects the susceptibility and course of Trypanosoma cruzi infection. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.07.163. Available Online. [DOI] [PubMed] [Google Scholar]
- Savineau J-P, Marthan R, Dumas de la Roque E. Role of DHEA in cardiovascular diseases. Biochemical pharmacology. 2013;85:718–726. doi: 10.1016/j.bcp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Saxena AR, Chamarthi B, Williams GH, Hopkins PN, Seely EW. Predictors of plasma and urinary catecholamine levels in normotensive and hypertensive men and women. J Hum Hypertens. 2014;28:292–297. doi: 10.1038/jhh.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. American journal of physiology. Heart and circulatory physiology. 2012;303:H1273–H1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, McEwen B, Schwartz J. Modeling multi-system biological risk in young adults: the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Hum Biol. 2010;22:463–472. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alc Drugs. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sempos CT, Rehm J, Wu T, Crespo CJ, Trevisan M. Average volume of alcohol consumption and all-cause mortality in African Americans: The NHEFS Cohort. Alc Clin Exp Res. 2003;27:88–92. doi: 10.1097/01.ALC.0000046597.92232.73. [DOI] [PubMed] [Google Scholar]
- Shapiro TM. The hidden cost of being African American: How wealth perpetuates inequality. Oxford University Press; New York, NY: 2004. [Google Scholar]
- Shield KD, Gmel G, Kehoe-Chan T, Dawson DA, Grant BF, Rehm J. Mortality and potential years of life lost attributable to alcohol consumption by race and sex in the United States in 2005. PLoS One. 2013;8:e51923. doi: 10.1371/journal.pone.0051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AL, Howell RT, Potter JS, Weiss RD. The Michigan Alcoholism Screening Test and its shortened form: A meta-analytic inquiry into score reliability. Subst Use Misuse. 2007;42:1783–1800. doi: 10.1080/10826080701212295. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone –mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahre M, Simon M. Alcohol-related deaths and hospitalizations by race, gender, and age in California. Open Epidemiol J. 2010;3:3–15. [Google Scholar]
- StataCorp. Stata statistical software: Release 14.0. StataCorp LP; College Station, TX: 2015. [Google Scholar]
- Stewart SH. Racial and ethnic differences in alcohol-associated aspartate aminotransferase and γ-glutamyltransferase elevation. Arc Intern Med. 2002;162:2236–2239. doi: 10.1001/archinte.162.19.2236. [DOI] [PubMed] [Google Scholar]
- Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, Trevisan M. Greater hepatic vulnerability after alcohol intake in African Americans compared with Caucasians: A population-based study. J Natl Med Assoc. 2004;96:1185. [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- Trivedi D, Khaw K-T. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86:4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Fellin R, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitior-1 in well-functioning older adults. The Health, Aging, and Body Composition Study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Williams DR. Miles to go before we sleep racial inequities in health. J Health Soc Behav. 2012;53:279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Bradley KA, Gupta S, Harris AH. Association between alcohol screening scores and mortality in black, Hispanic, and white male veterans. Alc Clin Exp Res. 2012;36:2132–2140. doi: 10.1111/j.1530-0277.2012.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LA, Olshan AF, Tse CK, Bell ME, Troester MA. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27:259–269. doi: 10.1007/s10552-015-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witbrodt J, Mulia N, Zemore SE, Kerr WC. Racial/ethnic disparities in alcohol-related problems: differences by gender and level of heavy drinking. Alcohol Clin Exp Res. 2014;38:1662–1670. doi: 10.1111/acer.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-κB system. Brain Behav Immun. 2011;25:S29–S38. doi: 10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EV, Kim S-j, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Hypothalamic–pituitary–adrenal alterations in PTSD: Are they relevant to understanding cortisol alterations in cancer? Brain Behav Immun. 2003;17:73–83. doi: 10.1016/s0889-1591(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Yufu K, Okada N, Ebata Y, Murozono Y, Shinohara T, Nakagawa M, Takahashi N. Plasma norepinephrine is an independent predictor of adverse cerebral and cardiovascular events in type 2 diabetic patients without structural heart disease. J Cardiol. 2014;64:225–230. doi: 10.1016/j.jjcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Zapolski TC, Pedersen SL, McCarthy DM, Smith GT. Less drinking, yet more problems: understanding African American drinking and related problems. Psychol Bull. 2014;140:188–223. doi: 10.1037/a0032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K, Vishwanatha JK, Santella RM, Morabia A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–629. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler MG, Mills PJ, Dimsdale J. The effects of race on norepinephrine clearance. Life sciences. 1991;49:427–433. doi: 10.1016/0024-3205(91)90584-x. [DOI] [PubMed] [Google Scholar]
- Zijderveld Gav, Veltman DJ, Dyck Rv, Doornen LJPv. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33:73–78. doi: 10.1016/s0022-3956(98)00051-x. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.