Abstract
Recombinase responsive mouse lines expressing diphtheria toxin subunit A (DTA) are well established tools for targeted ablation of genetically defined cell populations. Here we describe a new knock-in allele at the Gt(Rosa)26Sor locus that retains the best features of previously described DTA alleles—including a CAG promoter, attenuated mutant DTA cDNA, and ubiquitous EGFP labeling—with the addition of a Cre-dependent FLEx switch for tight control of expression. The FLEx switch consists of two pairs of antiparallel lox sites requiring Cre-mediated recombination for inversion of the DTA to the proper orientation for transcription. We demonstrate its utility by Cre-dependent ablation of both a broad domain in the embryonic nervous system and a discrete population of cells in the fetal gonads. We conclude that this new DTA line is useful for targeted ablation of genetically-defined cell populations.
Keywords: DTA, Rosa26, loxP, DIO, En1, Dppa3
Introduction
Targeted ablation is a well-established method for investigating the role of individual cells and cell populations in the development and function of complex organs or whole organisms. In laboratory mice, expression of genetically encoded diphtheria toxin subunit A (DTA) permits efficient ablation of genetically defined cell lineages by inactivation of elongation factor 2 (EEF2), preventing protein synthesis and rapidly leading to cell death (Breitman et al., 1987; Collier, 2001; Palmiter et al., 1987). This ablation is highly specific, because mice lack a functional receptor for diphtheria toxin (Mitamura et al., 1995), and DTA lacks the B subunit required for penetration of cell membranes (Collier, 2001). Therefore, ablation is restricted to cells that actively synthesize DTA protein, and cells that have not expressed DTA are unaffected by toxin released from adjacent apoptotic cells.
Numerous transgenic and knock-in lines have been generated to take advantage of the efficiency and specificity of ablation offered by DTA in the mouse. Of these, the most experimentally flexible are several lines in which expression of DTA is dependent on the activity of Cre recombinase (Brockschnieder et al., 2004; Brockschnieder et al., 2006; Ivanova et al., 2005; Marques et al., 2009; Voehringer et al., 2008; Wu et al., 2006; Yoshikawa et al., 2011). These alleles can be used to ablate any cell lineage for which a Cre recombinase driver line is available, and if an inducible Cre driver is used, ablation can be temporally controlled
Given the extreme toxicity of DTA, expression of even an attenuated mutant form of the protein (Maxwell et al., 1987) must be very tightly regulated. In Cre-dependent DTA alleles, this control is achieved using loxP-flanked transcriptional stop cassettes placed upstream of the DTA cDNA to terminate transcription and block expression in the absence of Cre recombination. Unfortunately, comparison of several different stop cassettes has demonstrated that they vary in their ability to tightly control marker expression (Dymecki et al., 2010), and even the most efficient permits low-level “leaky” expression of fluorescent proteins under certain conditions (Plummer et al., 2015). Leaky expression of DTA is more difficult to detect, but some evidence suggests that it can be a problem with significant experimental implications. For example, when expression of Cre-dependent DTA transgenes was driven by the highly active CAG promoter (Niwa et al., 1991), very few founder animals survived (Matsumura et al., 2004; Sato and Tanigawa, 2005). The established transgenic lines exhibited unexpectedly low levels of expression (Sato and Tanigawa, 2005) or incomplete ablation of Cre-expressing cells (Matsumura et al., 2004), consistent with selection against those founders exhibiting the expected robust CAG promoter activity and survival of mice carrying the transgene in a genomic context that suppresses the promoter. These results suggest that low-level leak through the stop cassette is lethal when combined with a highly active promoter. In a moderately expressed DTA allele, leak through a stop cassette could cause sporadic loss of cells at any developmental time point and in any cell-type. Such sporadic ablation would be very difficult to detect in the absence of a gross phenotype, but the loss of relatively few cells could complicate interpretation of more subtle phenotypes.
An alternative to a transcriptional stop cassette is the Cre-dependent flip-excision (FLEx) switch (Schnutgen et al., 2003) which uses two pairs of antiparallel lox sites to permanently invert a DNA segment. Initial characterization in vitro and in cell culture demonstrated that recombination of a FLEx switch is highly efficient, and subsequent testing in mice showed it to be functionally indistinguishable from simple recombination of a single pair of loxP sites (Schnutgen et al., 2003). Although originally designed to reveal Cre recombination by turning on expression of a reporter gene while a second is simultaneously turned off, FLEx switches (also called DIO, Double-floxed Inverted Open reading frame) are now widely used in viral constructs for tight control of gene expression (Atasoy et al., 2008; Sohal et al., 2009). The FLEx switch offers a similar advantage in a transgenic DTA construct, because the DTA cDNA can be placed in an antisense orientation relative to the promoter, providing tighter control of DTA expression relative to stop cassettes in the absence of Cre activity.
Here, we describe a new Cre-responsive DTA allele that is the first to utilize a FLEx switch to minimize the possibility of off-target ablation. In addition, this new allele combines features that are found individually in the most frequently utilized of the previously published DTA alleles. As in the Gt(ROSA)26Sortm1(DTA)Mrc allele (Wu et al., 2006), we use the attenuated Tox176 mutant cDNA (Maxwell et al., 1987) to further reduce the impact of off-target expression. An enhanced green fluorescent protein (EGFP) cDNA expressed in the absence of Cre recombination enables rapid visual genotyping and confirmation that the allele is expressed in the target cell population, similar to the Gt(ROSA)26Sortm1(DTA)Jpmb allele (Ivanova et al., 2005). To confirm that our new allele permits efficient ablation of targeted cell populations, we have tested it using two different Cre lines: An En1Cre knock in allele (Kimmel et al., 2000) expressed in the embryonic midbrain and rostral hindbrain (Li et al., 2002), and a tamoxifeninducible Dppa3-cre/Esr1* transgene that is expressed in germ cells (Hirota et al., 2011). These results demonstrate that this new DTA allele is useful for ablation of genetically defined cell populations within and outside the nervous system.
Results and Discussion
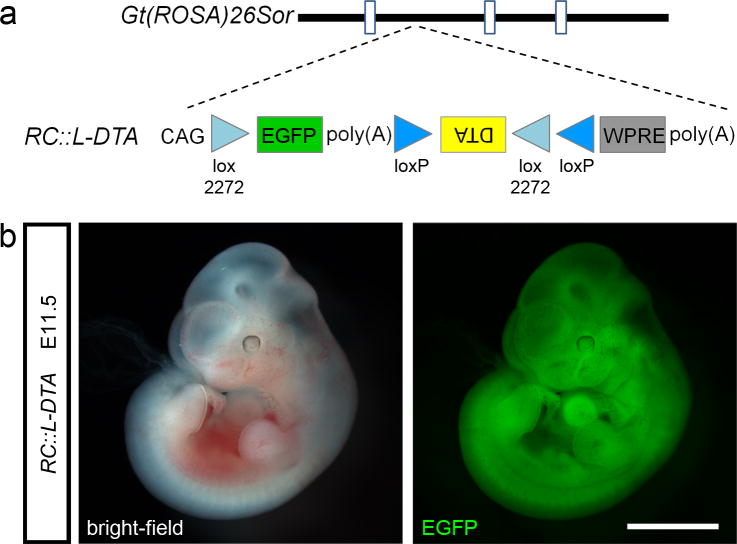
To ensure that the new Cre-responsive DTA allele, Gt(ROSA)26Sortm2.1(CAG-EGFP,-DTA*G128D)Pjen (hereafter designated RC∷L-DTA; Rosa-CAG-lox-DTA), is expressed in the widest range of cells types throughout development, we targeted the Gt(ROSA)26Sor locus (Friedrich and Soriano, 1991) and used a synthetic CAG promoter (Niwa et al., 1991) to drive expression. Following the CAG promoter is a FLEx switch with EGFP between the first two lox sites and DTA between the second and third, a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) (Zufferey et al., 1999), and a bovine growth hormone poly(A) cassette (Fig. 1a). The Cre-responsive FLEx switch (Schnutgen et al., 2003) controls DTA expression. In the absence of Cre activity, EGFP is expressed and the DTA cDNA is in an antisense orientation relative to the CAG and Gt(ROSA)26Sor promoters, greatly reducing the likelihood of off-target expression. RC∷L-DTA homozygotes are viable and born at Mendelian ratios (13/53 offspring of heterozygote intercrosses). Male homozygotes appear to be infertile. The cause of this infertility is unknown, but females are fully fertile and neither males nor females exhibit any other abnormal phenotypes. These results confirm tight control of DTA expression in the presence of the highly active CAG promoter. We observed bright, ubiquitous EGFP fluorescence in heterozygous embryos (Fig. 1b), demonstrating that the allele is broadly expressed and will be useful for ablating cells of any type for which a Cre driver line is available.
Figure 1. RC∷L-DTA allele.
(a) Schematic diagram of the Gt(ROSA)26Sor locus showing insertion of the CAG promoter, EGFP, and DTA cDNA. Open rectangles represent noncoding exons of Gt(ROSA)26Sor, and solid lines represent introns. DTA is oriented antisense to the CAG promoter and is located between two pairs of antiparallel lox sites (wildtype loxP and variant lox2272) which form the Cre-dependent FLEx Switch. WPRE, woodchuck hepatitis virus post-transcriptional regulatory element (Zufferey et al., 1999); poly(A), rabbit β-globin polyadenylation cassette (following EGFP) or bovine growth hormone polyadenylation cassette (following WPRE). (b) In the absence of Cre recombinase, ubiquitous EGFP expression is observed in RC∷L-DTA heterozygous embryos at E11.5. Image shows native fluorescence in a fixed embryo. Scale bar, 2 mm.
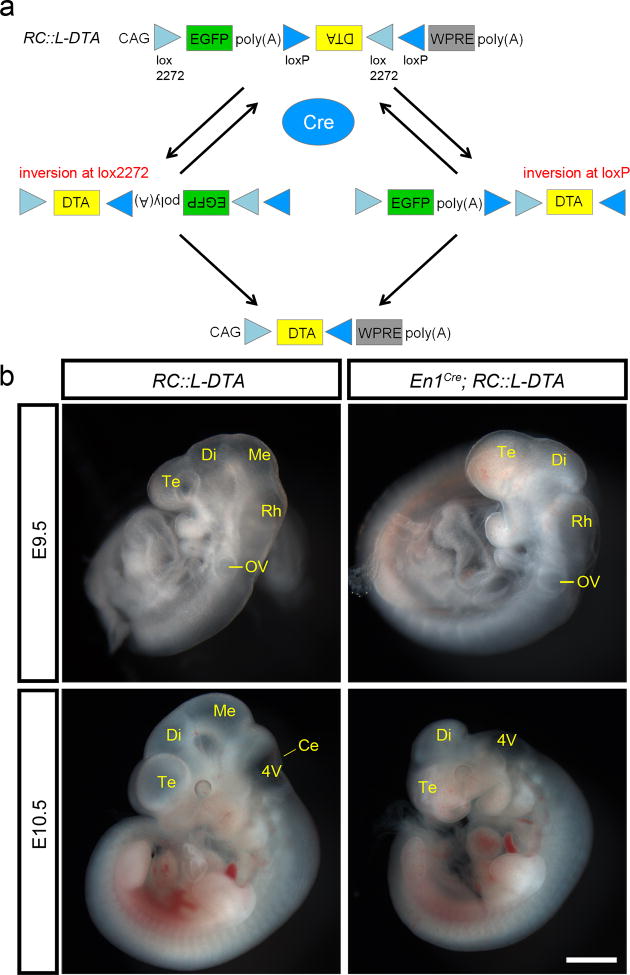
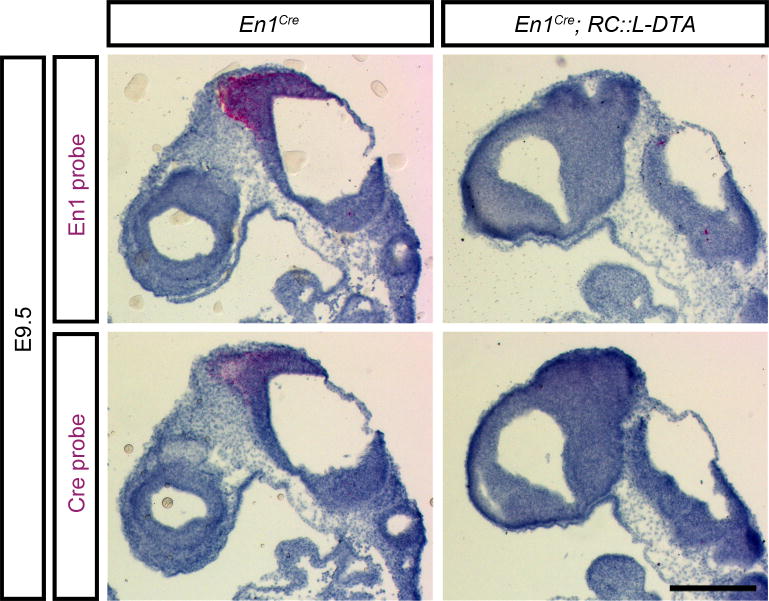
To confirm that Cre recombination of RC∷L-DTA mediates a switch from EGFP to DTA expression (Fig. 2a) and to test its ability to ablate cells within a relatively broad expression domain in the central nervous system, we crossed RC∷L-DTA heterozygotes with En1Cre heterozygous mice (Kimmel et al., 2000). In En1Cre mice, Cre activity is observed starting at ∼E8 and by E9.5 is detected in virtually all cells of the midbrain and anterior hindbrain (Li et al., 2002). In En1cre; RC∷L-DTA double heterozygotes, ablation of this En1-expressing domain was obvious at E9.5 (Fig. 2b). At E10.5, development of forebrain structures such as the telencephalic vesicles appeared to be stunted relative to wild-type controls, perhaps due to loss of signaling from neurons in the midbrain and hindbrain (Fig. 2b). To examine the efficiency of ablation, we performed RNA in situ hybridization using a probe against En1. At E9.5, we detected En1 transcripts in the midbrain/hindbrain of control embryos but not En1Cre; RC:L-DTA double heterozygotes (Fig. 3, top), suggesting complete ablation of the En1 domain. Further analysis using a probe against Cre confirmed complete loss of Cre-expressing cells in the midbrain/hindbrain (Fig. 3, bottom). Thus, RC∷L-DTA can ablate a broad domain of cells approximately one day after initiation of Cre expression. This result is in line with the time course of ablation observed for other Cre-responsive DTA alleles (Ivanova et al., 2005; Wu et al., 2006).
Figure 2. Ablation of embryonic midbrain and hindbrain tissue following Cre-mediated recombination of RC∷L-DTA in the En1 expression domain.
(a) Cre-mediated recombination of RC:L-DTA results in sequential inversion and deletion events which delete EGFP and invert DTA to the correct orientation for transcription. (b) Ablation of cells in mesencephalon (Me) and anterior rhombencephalon (Rh), including the embryonic cerebellum (Ce), is observed in whole-mount En1Cre; RC∷L-DTA double heterozygous embryos at E9.5 and E10.5. Cells outside the midbrain/hindbrain En1Cre expression domain are not ablated, but expansion of the telencephalic vesicles (Te) appears to be delayed at E10.5. Di, diencephalon; OV, otic vesicle; 4V, fourth ventricle. Scale bar, 500 μm (E9.5) or 961 μm (E10.5).
Figure 3. Loss of Cre expressing cells in midbrain and hindbrain following En1Cre-mediated expression of DTA.
In situ hybridization demonstrates overlapping expression of En1 (top) and Cre (bottom) transcripts in heterozygous En1Cre embryos at E9.5 (left), but expression is absent in En1Cre; RC∷L-DTA double heterozygotes (right). Scale bar, 500 μm
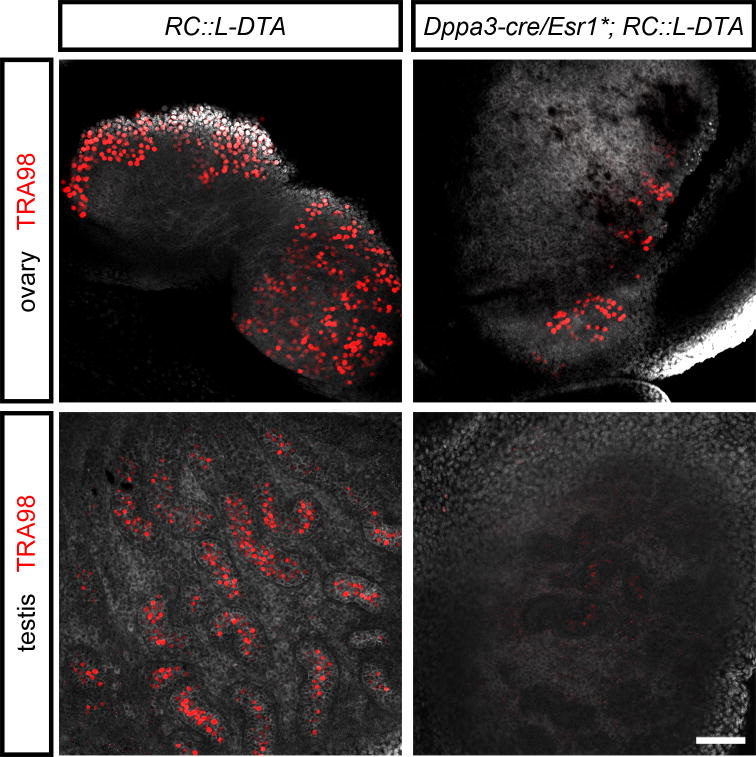
As a further test of RC∷L-DTA, we examined its ability to ablate a small, unipotent cell population using the Dppa3-cre/Esr1* transgene (Hirota et al., 2011) to target embryonic germ cells. A tamoxifen-inducible transgene was required due to early, ubiquitous expression of Dppa3 (PGC7) in the pre-implantation mouse embryo (Sato et al., 2002). After dosing pregnant dams with tamoxifen (5 mg/kg i.p. daily) between E10.5 and E12.5, we observed an approximate 70-90% reduction in the number of TRA98-positive germ cells in the testes and ovaries of Dppa3-cre/Esr1*; RC∷L-DTA double heterozygous embryos at E18.5, relative to littermate controls (Fig. 4). The recombination rate is consistent with previously published results using this Cre transgene (Hirota et al., 2011)
Figure 4. Ablation of germ cells in Dppa3-cre/Esr1*; RC∷L-DTA mice after prenatal treatment with tamoxifen.
In embryonic ovary (top left) and testis (bottom left) of tamoxifen-treated RC∷L-DTA mice at E18.5, nuclei of germ cells (red) are labeled by an antibody against TRA98 (Gcna) (Carmell et al., 2016). The number of surviving germ cells is greatly reduced in ovary and testis tamoxifen-treated Dppa3-cre/Esr1*; RC∷L-DTA double heterozygotes (right, top and bottom). DAPI staining (gray) shows tissue not stained by the TRA98 antibody. Scale bar, 100 μm.
Taken together, these results demonstrate that this new allele represents an improvement over existing Cre-responsive DTA alleles. RC∷L-DTA retains the best features of previously published alleles—including CAG promoter, EGFP, and attenuated DTA—with the addition of a FLEx switch for tight control of DTA expression. Our successful production of DTA mice with a CAG promoter, along with WPRE sequence which may further enhance DTA expression, inserted into a locus known to be permissive of robust CAG activity is strong evidence that the FLEx switch efficiently suppresses DTA expression in the absence of Cre. We conclude that RC∷L-DTA will be valuable for ablation of genetically defined cell populations and will make it available to other researchers through the Jackson Laboratory mouse repository (Stock# 026944).
Methods
Generation of RC∷L-DTA mice
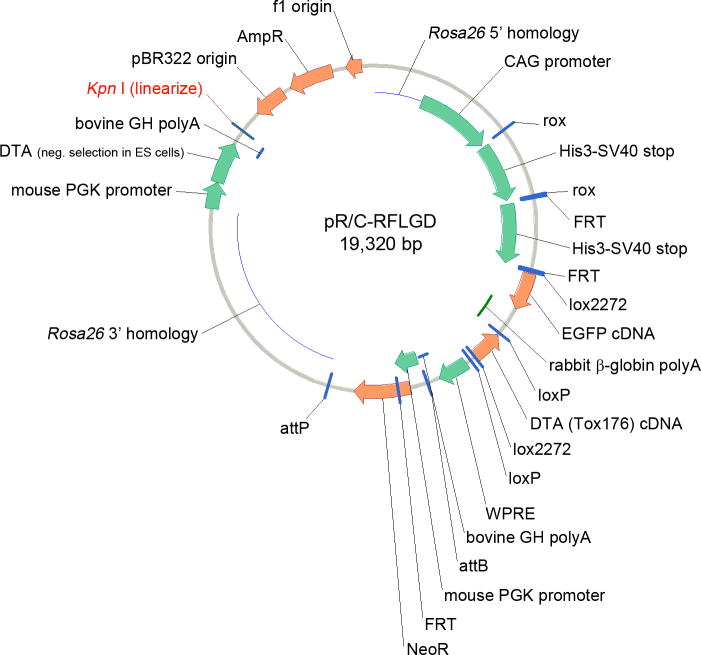
A targeting vector for homologous recombination in embryonic stem cells was generated as previously described (Sciolino et al., 2016). This targeting vector contains 5′ and 3′ homology to the Gt(ROSA)26Sor locus, CAG promoter (Niwa et al., 1991), rox-flanked His3-SV40 transcriptional stop cassette (Sauer, 1993), FRT-flanked His3-SV40 cassette, modified FLEx switch (Schnutgen et al., 2003) containing an EGFP cDNA, woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) (Zufferey et al., 1999), bovine growth hormone poly(A) cassette, and attB/attP-flanked Neomycin resistance cassette. A cDNA encoding the Tox176 (G128D) mutant DTA (Maxwell et al., 1987) was cloned into the center of the FLEx switch, in antisense orientation relative to the CAG promoter and EGFP cDNA to produce the final targeting vector (Fig. 5).
Figure 5. Targeting vector for generation of recombinase-responsive DTA allele at the Gt(ROSA)26Sor locus.
Prior to electroporation of embryonic stem cells, the vector was linearized with Kpn I restriction enzyme. Two segments of genomic DNA (Rosa26 5′ homology and Rosa26 3′ homology) target the intervening expression construct to intron 1 of Gt(ROSA)26Sor. The vector backbone, including a PGK-DTA-bGHpolyA cassette for negative selection, was eliminated during homologous recombination.
Linearized vector was electroporated into G4 embryonic stem cells (B6129 F1 genetic background), and homologous recombinants were identified by long range PCR and Southern blotting. A recombinant clone was transiently transfected with pPGFKPhiC31obpa (Raymond and Soriano, 2007) to remove the attB/attP-flanked Neo cassette before cells were injected into B6(Cg)-Tyrc-2J/J blastocysts to produce chimeric mice. This new DTA allele was designed to be responsive to Dre (rox), Flp (FRT), and Cre (lox) recombinases, allowing intersectional genetic control of DTA expression similar to a previously described hM3Dq allele (Sciolino et al., 2016). However, when these mice were crossed to a ubiquitous Cre driver, placing the DTA in sense orientation relative to the CAG promoter, we observed no live-born Cre+; DTA+ double heterozygotes (0/21 offspring of heterozygote intercross, p=0.0104 χ2). Furthermore, when using tissue-specific Dre, Flp, and Cre drivers for intersectional crosses, we observed phenotypes consistent with sporadic DTA expression in cells that had expressed Cre but not Dre or Flp (data not shown). These results are consistent with leaky expression of DTA through the transcriptional stop cassettes. Therefore, we decided to remove the two stop cassettes, generating a new Cre-responsive allele in which DTA expression is controlled solely by the FLEx switch. The original chimeras were bred with B6;129-Tg(CAG-dre)1Afst mice (Anastassiadis et al., 2009) and B6.Cg-Tg(ACTFlpe)9205Dym/J mice (Rodriguez et al., 2000) to excise the rox- and FRT-flanked transcriptional stop cassettes and generate the RC∷L-DTA line. This new strain was maintained by backcrossing to C57BL/6J mice and intercrossed to confirm that homozygotes are viable. RC∷L-DTA mice will be available to the research community through the Jackson Laboratory (Stock# 026944).
Experimental crosses and tamoxifen treatment
All mouse experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory animals of the National Institutes of Health. The protocols were approved by the Animal Care and Use Committee (ACUC) of the National Institute of Environmental Health Sciences. Mice were maintained on a 12-hour light/dark cycle at 72±2 °F and given water and food ad libitum. All mice used in experiments were bred in-house at NIEHS.
RC∷L-DTA mice were intercrossed with En1tm2(cre)Wrst (Kimmel et al., 2000) and Tg(Dppa3-cre/Esr1*)9Sait (Hirota et al., 2011) mice. For timed matings and embryo collection, noon of the day on which plugs were observed was taken to be E0.5, and pregnant dams were euthanized by CO2 inhalation before embryo dissection. Pregnant dams from the cross with Tg(Dppa3-cre/Esr1*)9Sait were treated with tamoxifen (Sigma-Aldrich) diluted in corn oil (Sigma-Aldrich) injected intraperitoneally (i.p.) at 5 mg/kg once daily at E10.5. E11.5, and E12.5. Tamoxifen treated embryos at E18.5 were euthanized by decapitation after removal from the uterus.
In Situ Hybridization
Mouse embryos were fixed overnight at 4 °C in 4% paraformaldehyde (PFA) diluted in 0.01M phosphate buffered saline (PBS). After fixation, embryos were equilibrated in 10%, 20%, and finally 30% sucrose diluted in PBS. The cryoprotected embryos were embedded in Tissue Freezing Medium (General Data Company) and sectioned on a Leica CM305 S cryostat (Leica Biosystems, Buffalo Grove, IL). 14-μm thick sections were mounted on Superfrost Plus microscope slides (Thermo Scientific, Waltham, MA), and stored at −80 °C after air drying. RNA in situ hybridization was performed using RNAscope technology (Wang et al., 2012) according to manufacturer's instructions for the RNAscope 2.5 HD Reagent Kit-Red (Advanced Cell Diagnostics, Hayward, CA). Sections were hybridized with probes against En1 (Cat# 442651, Advanced Cell Diagnostics) and Cre (Cat# 312281, Advanced Cell Diagnostics). Hybridized RNA was detected with Fast Red dye, and the tissue was counterstained with hematoxylin.
Immunohistochemistry
For immunohistochemistry on frozen sections, gonads were fixed in 4% PFA in PBS overnight at 4 °C, dehydrated through a sucrose gradient, embedded in O.C.T. Compound (Sakura Finetek, Torrance, CA) and cryosectioned at 10 μm increments. Tissue sections were preincubated with 5% normal donkey serum in PBS for 1 hour, then incubated with anti-TRA98 (1:1000, MBL International, Woburn, MA) in PBS-Triton X-100 (0.1%) solution with 5% normal donkey serum at 4 °C overnight. The antibody-labeled tissue sections were washed three times in PBS and incubated in Alexa 594 goat anti-rat secondary antibody (1:500; Invitrogen, Carlsbad, CA) for one hour at room temperature, before a final three washes in PBS and mounting in Vector Mount with DAPI (Vector Laboratories, Burlingame, CA).
Imaging
Fluorescent and bright-field images of PFA-fixed whole-mount embryos were collected on a SteREO Lumar.V12 stereomicroscope (Carl Zeiss Microscopy, Thornwood, NY). Bright-field images of embryo sections labeled by in situ hybridization were collected on an Olympus IX70 inverted microscope (Olympus Corporation, Center Valley, PA). Immunolabeled slides of embryonic gonads were imaged on a Leica DMI4000 confocal microscope. Images were modified only by using Photoshop software (Adobe Systems, San Jose, CA) to adjust brightness and contrast across the entire image.
Acknowledgments
NIH Support: This research was supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Environmental Health Sciences (ES102805 to P.J. and ES102965 to H.H.-C.Y.).
We thank D. D'Agostin for technical assistance. Valuable support was provided by the NIEHS Comparative Medicine Branch, Knockout Mouse Core, and Fluorescence Microscopy and Imaging Core.
References
- Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech. 2009;2:508–515. doi: 10.1242/dmm.003087. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave KA, Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–7642. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D, Pechmann Y, Sonnenberg-Riethmacher E, Riethmacher D. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 2006;44:322–327. doi: 10.1002/dvg.20218. [DOI] [PubMed] [Google Scholar]
- Carmell MA, Dokshin GA, Skaletsky H, Hu YC, van Wolfswinkel JC, Igarashi KJ, Bellott DW, Nefedov M, Reddien PW, Enders GC, Uversky VN, Mello CC, Page DC. A widely employed germ cell marker is an ancient disordered protein with reproductive functions in diverse eukaryotes. eLife. 2016;5:e19993. doi: 10.7554/eLife.19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Hirota T, Ohta H, Shigeta M, Niwa H, Saitou M. Drug-inducible gene recombination by the Dppa3-MER Cre MER transgene in the developmental cycle of the germ cell lineage in mice. Biol Reprod. 2011;85:367–377. doi: 10.1095/biolreprod.110.090662. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Li JY, Lao Z, Joyner AL. Changing requirements for Gbx2 in development of the cerebellum and maintenance of the mid/hindbrain organizer. Neuron. 2002;36:31–43. doi: 10.1016/s0896-6273(02)00935-2. [DOI] [PubMed] [Google Scholar]
- Marques R, Williams A, Eksmond U, Wullaert A, Killeen N, Pasparakis M, Kioussis D, Kassiotis G. Generalized immune activation as a direct result of activated CD4+ T cell killing. J Biol. 2009;8:93. doi: 10.1186/jbiol194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Hasuwa H, Inoue N, Ikawa M, Okabe M. Lineage-specific cell disruption in living mice by Cre-mediated expression of diphtheria toxin A chain. Biochem Biophys Res Commun. 2004;321:275–279. doi: 10.1016/j.bbrc.2004.06.139. [DOI] [PubMed] [Google Scholar]
- Maxwell F, Maxwell IH, Glode LM. Cloning, sequence determination, and expression in transfected cells of the coding sequence for the tox 176 attenuated diphtheria toxin A chain. Mol Cell Biol. 1987;7:1576–1579. doi: 10.1128/mcb.7.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Plummer NW, Evsyukova IY, Robertson SD, de Marchena J, Tucker CJ, Jensen P. Expanding the power of recombinase-based labeling to uncover cellular diversity. Development. 2015;142:4385–4393. doi: 10.1242/dev.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Sato M, Kimura T, Kurokawa K, Fujita Y, Abe K, Masuhara M, Yasunaga T, Ryo A, Yamamoto M, Nakano T. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech Dev. 2002;113:91–94. doi: 10.1016/s0925-4773(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Sato M, Tanigawa M. Production of CETD transgenic mouse line allowing ablation of any type of specific cell population. Mol Reprod Dev. 2005;72:54–67. doi: 10.1002/mrd.20323. [DOI] [PubMed] [Google Scholar]
- Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- Schnutgen F, Doerflinger N, Calleja C, Wendling O, Chambon P, Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, Jensen P. Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep. 2016;15:2563–2573. doi: 10.1016/j.celrep.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol. 2008;180:4742–4753. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Kode A, Xu L, Mosialou I, Silva BC, Ferron M, Clemens TL, Economides AN, Kousteni S. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26:2012–2025. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]