Abstract
Background
Rates of adolescent obesity and overweight are high. The offspring of overweight parents are at increased risk of becoming obese later in life. Investigating neural correlates of familial obesity risk and current overweight status in adolescence could help identify biomarkers that predict future obesity and that may serve as novel targets for obesity interventions.
Objective
Our primary aim was to use functional MRI to compare neural responses to words denoting high or low energy density (ED) foods and non-foods, in currently lean adolescents at higher compared with lower familial risk for obesity, and in overweight compared with lean adolescents. Secondary aims were to assess group differences in subjective appetite when viewing food and non-food words, and in objective ad libitum intake of high-ED foods in a laboratory setting.
Design
We recruited 36 adolescents (14–19y), of whom 10 were overweight, 16 lean with obese/overweight mothers (lean high-risk, “lean-HR”), and 10 lean with lean mothers (lean low-risk, “lean-LR”). All underwent fMRI scanning while they viewed words representing either high-ED foods, low-ED foods, or non-foods, and while they provided appetitive ratings in response to each word stimulus. They then consumed a multi-item ad libitum buffet meal.
Results
Food compared with non-food words activated a distributed emotion/reward system including insula and pregenual anterior cingulate cortex (ACC). Participants who were at increasing risk for obesity exhibited progressively weaker activation of an attentional/regulatory system including dorsolateral PFC, dorsal ACC, and basal ganglia nuclei (activation was greatest in lean-LR, intermediate in lean-HR, and weakest in the overweight group). These group differences were most apparent for neural responses to high- compared with low-ED foods. Lean-HR (compared with lean-LR and overweight) adolescents reported greater desire for high-ED foods. Meal intake was greatest for the overweight, then lean-HR, then lean-LR groups.
Conclusions
Adolescents at higher obesity risk exhibited reduced neural responses to high-ED food cues in a neural system that prototypically subserves attention and self-regulation. They also reported heightened appetitive responses to high-ED cues. Future interventions that promote the capacity for self-regulation could prevent youth who have a familial predisposition for obesity from translating risk into reality.
Keywords: neuroimaging, obesity genetics, obesity, wanting and liking, cognitive control, food stimuli
Introduction
In the U.S., 35% of adolescents are overweight (BMI [Body Mass Index] percentile ≥ 85th) and a further 21% are obese (BMI percentile ≥ 95th) (1). Children or adolescents with high BMI percentiles are more likely to have excess weight as adults (2). Further, the offspring of obese and overweight parents are significantly more likely either to have excess weight currently (3) or to develop excess weight in later childhood (4, 5) or as adults (6, 7). Familial risk for obesity may derive in part from a greater appetite for high energy-density (ED) foods in the offspring of heavier parents, as shown previously in meals consumed in a laboratory setting (8, 9).
Neuroimaging studies using food images in youth and in groups at increased risk of obesity have reported in heavier youth a greater responsivity of neural systems subserving reward and emotion (orbitofrontal cortex [OFC], putamen, anterior and mid insula) (10–12) and in obese adolescents at increased genetic risk for obesity in the basal ganglia (dorsal and ventral striatum) (13). They have also reported less feeding-associated attenuation of activation in a reward and emotion system (insula) in adults at increased risk of weight gain based on prior obesity status (14). These findings suggest that food cues trigger heightened appetitive responses in those at increased obesity risk, potentially predisposing these individuals to excessive food intake and weight gain.
Studies have also demonstrated greater responses to food images in neural systems subserving attention and self-regulation in adults who have successfully maintained weight loss (left superior frontal region) (15), in obese cancer patients who have successfully followed a behavioral healthy lifestyle intervention (anterior and superior frontal gyrus) (16), and treatment-seeking obese youth (dorsolateral prefrontal cortex [PFC]) (17). These results suggest that, in addition to emotion/reward systems, food cues may activate attentional/regulatory systems in participants who either automatically or willfully regulate their appetitive responses to food stimuli. Engaging these systems may therefore protect against excessive food intake and weight gain.
Little is currently understood about the neural mechanisms underlying the transmission of the familial risk for obesity, particularly the roles of neural systems that subserve either appetitive tendencies or their regulation. Greater activation of reward systems (in anterior insula, OFC, striatum) to small tastes of milkshake have been observed in lean adolescents with obese or overweight parents (18), suggesting heightened consummatory reward responses in youth at increased familial risk for obesity; that study, however, did not assess neural responses to food images, and did not obtain researcher-measured parental weights and heights for all subjects, which is a more reliable method than obtaining reported data (19, 20). Although a substantial behavioral literature in children reports appetitive differences in offspring according to parent weight (8, 9), no studies have reported associations of parental weight status with appetitive tendencies in adolescence, a time when greater environmental variation and opportunities for environmental selection may enable fuller expression of genetic tendencies towards excess weight (21). Investigating the neural correlates of appetite in adolescents who differ in familial risk for obesity may help to identify neural endophenotypes underlying familial obesity risk without confounding from the effects that long-term obesity may have on the brain.
Task-based fMRI studies examining food cue responsiveness have been scientifically informative, but they have been constrained by certain limitations in design of the fMRI activation paradigm. For example, most have used passive viewing paradigms, making neural activations hard to interpret. Obtaining and evaluating appetitive ratings ‘online’ for each stimulus presented during the fMRI scan allows the assessment of associations of neural responses with subjective appetitive responses. Individual variation in the patterns of neural activation elicited by such a design may have more direct implications for food-related decision-making and actual eating behavior, and may be particularly useful for differentiating between high and low risk groups in adolescence, when general self-regulation skills and the neural circuits underlying decision-making are still developing (22–24).
Most studies examining food cue responsiveness in obesity have used food images. However, food words (alone or in combination with images) are found in print, radio, and television food advertising, and in food menus of restaurants. Simple words or phrases can elicit powerful emotional responses (25), and written food words have been shown to elicit appetitive responses (26). An advantage of word stimuli is that they allow participants to picture their own food/object exemplars. Word stimuli also permit optimal matching for stimulus features (e.g. word length, number of syllables) across active and control stimuli in task-based fMRI studies – a critically important feature of task design that is considerably more challenging for image stimuli.
To address these research gaps, we examined neural responses to, and online appetitive ratings of, words representing high-ED foods, low-ED foods, and non-foods in adolescents who were currently lean but differed in the measured weight status of their biological mother, and in adolescents who were currently obese/overweight. We hypothesized that lean adolescents at higher compared with lower familial obesity risk, and obese/overweight compared with lean adolescents, would: (a) activate more to food compared with non-food stimuli, and to high-ED compared with low-ED food stimuli, in areas subserving emotion and reward (e.g. insula, ventral striatum, OFC, ventral anterior cingulate cortex [ACC]); and (b) activate less to food compared with non-food stimuli, and to high-ED vs. low-ED food stimuli, in areas subserving attention and self-regulation (e.g. dorsolateral and inferolateral PFC, dorsal ACC, basal ganglia nuclei, thalamus). Secondary predictions were that lean adolescents at higher compared with lower familial obesity risk, and obese/overweight compared with lean adolescents, would exhibit greater appetite on behavioral indices, namely greater subjective ratings of wanting to eat in relation to high-ED compared with low-ED food words, and greater ad libitum intake of high-ED foods in a laboratory multi-item buffet meal. We also explored associations of neural responses to food stimuli with both subjective appetitive ratings and ad libitum intake of high-ED foods.
Participants and Methods
Participant recruitment
Adolescents and their biological mothers were recruited to one of three groups based on current weight and familial risk: (1) a lean low-risk group (lean adolescent with lean mother; “Lean-LR”); (2) a lean high-risk group (lean adolescent with obese/overweight mother; “Lean-HR”); and (3) an obese/overweight group (“Overweight”; no requirement regarding maternal weight status). Recruitment was via flyers posted at and around St. Luke’s Hospital, Columbia University, and Columbia University Medical Center in New York City, as well as via Craigslist volunteer advertisements, from November 2010 to August 2012. Both mothers and adolescents underwent a telephone screening and, provided they were not excluded at this point, further screening at an initial consultation at the lab. Based on the telephone screen, adolescents were excluded if they reported any previous or current significant health problems, were on any medication affecting body weight and appetite or currently participating in a structured weight loss program, or would be outside our target age range (14–18 y) at the time of scanning. They were also excluded if they had food or nut allergies, claustrophobia, or metal implants. Mothers were excluded if they were pregnant or breastfeeding, currently experiencing significant health problems, on any medications affecting body weight and appetite, or currently participating in a structured weight loss program. Both adolescents and mothers were required to be fluent in English. If families met eligibility criteria on the basis of telephone screening, both mother and adolescent were invited to an initial consultation at the lab. At this visit, a Kiddie Schedule for Affective Disorders and Schizophrenia [KSADS] was administered to adolescents, and a Structured Clinical Interview for DSM Disorders [SCID-1] to mothers, in order to exclude those with psychiatric conditions, including depression, eating disorders and substance use or dependence, and to exclude current smokers and those consuming >2 alcoholic drinks per day. Participants who met all recruitment criteria were invited back for a Test Day. All procedures followed were in accordance with the Institutional Review Boards of St. Luke’s-Roosevelt Hospital and New York State Psychiatric Institute.
Overview of protocol
The Initial Consultation, attended by both the child and biological mother, took 3 hours and occurred between 9 am and 5 pm, with the time determined by participant availability, in a test room at the lab. Upon arrival, the details of the study were explained, informed consent was obtained, anthropometric measures were taken, and a trained research assistant administered the SCID-1 and KSADS for further screening. Adolescents also completed the Self-Administered Rating Scale for Pubertal Development (27); adolescent boys were required be at least midpubertal and girls to be at least in late pubertal stages (including menarche). All adolescents underwent a body metal screening to ensure MRI safety. Mothers completed a demographic questionnaire in which they reported their own and their child’s ethnicity and race, as well as their own education level and annual household income. Adolescents and mothers both completed a packet including questionnaires assessing eating behavior and general behavior traits, and adolescents underwent a taste test to determine which flavor (vanilla, chocolate, strawberry) of Boost to use for the pre-fast meal. Adolescents also rated their liking of each food item to be presented at the ad libitum meal on a VAS scale ((0=Not at all, 100=Extremely) to ensure that they did not dislike (i.e. score < 50) more than 20% of the foods/beverages provided.
Eligible adolescents then participated in a Test Day at the MRI suite, on which they were instructed to consume their regular breakfast, followed by 2 bottles (474 ml i.e. 474 kcal in total) of their preferred flavor of BOOST (Novartis Nutrition; 24 % protein, 55 % carbohydrate, 21 % fat; 1 kcal/ml) at their regular lunchtime (c. 12 pm), and then to not eat or drink anything except water until they reported to the lab for the study c. 3:15 pm. On arrival at the lab, adolescents underwent a blood draw (14 ml) c. 3:45 pm (3:49 pm ± 0:12) (i.e. c. 3 h 45 post-prandial), to obtain samples for DNA and hormone analysis. Adolescents were then escorted to the Columbia University Brain Imaging Lab, where they participated in the food cue paradigm in the fMRI scanner (5:14 pm ± 0:24; i.e. about 5 h post-prandial) (see Measures). Following the scan, at 6:00 pm (6:15 pm ± 0:22, i.e. about 6 h post-prandial), adolescents were given a multi-item ad libitum buffet meal.
Measures
Anthropometric measures
Anthropometric indices were assessed in both adolescents and mothers by a trained research assistant. To measure height, participants were asked to take their shoes off and stand straight up against a stadiometer, looking straight ahead. To measure weight and body fat, participants were asked to remove socks and step onto a TANITA scale [TBF-300A, Tanita corp], which measures body weight value, and estimates body fat percentage via Bio-electrical Impedance Analysis [BIA]. BMI values (kg/m2) were calculated, and BMI z scores and percentiles in comparison to an age- and sex-matched US reference population were derived for adolescents, based on Center for Disease Control (CDC) growth charts for 2000 (28). Those at the 85th percentile or above were considered overweight, and those at the 95th percentile or above obese. Among mothers, a BMI of 25 or more was considered overweight, and a BMI of 30 or more obese.
Questionnaires
To characterize habitual eating behaviors, adolescents completed the 33-item Dutch Eating Behavior Questionnaire [DEBQ (29)], which assesses External Eating (EX; e.g. If food smells and looks good, do you eat more than usual?), Emotional Eating (EE; e.g. Do you have the desire to eat when irritated?), and Restrained Eating (RS; e.g. If you have put on weight, do you eat less than you usually do?). Adolescents also completed the 21-item Power of Food Scale [PFS (30)], which explores appetitive drive in today’s food environment (e.g. I find myself thinking about food even when I’m not physically hungry).
fMRI protocol
Food word paradigm
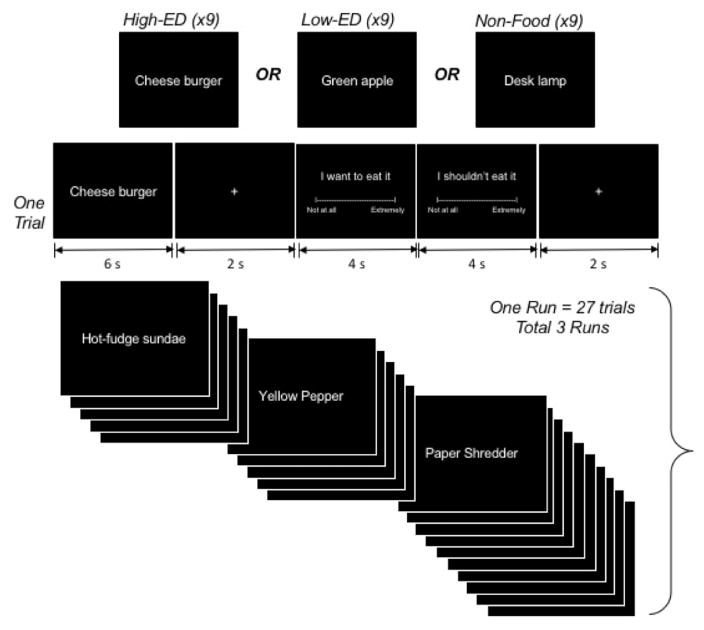
For the fMRI protocol, participants underwent an event-related paradigm containing food-denoting words (Figure 1), programmed in E-Prime (Psychology Software Tools, Inc., Sharpsburg, Pennsylvania). The paradigm comprised 3 runs, each beginning with a ‘block’ rating period, during which participants used a mouse to respond on a VAS scale (end-points Not at all, Extremely) to the following statements, each of which was presented for 4 sec: I feel hungry, I feel full, I feel stressed, I feel pain, and I feel thirst.
Figure 1.
Food word paradigm.
Following the block rating period, in each run, were 27 trials composed of 9 High-ED food trials, 9 low-ED food trials and 9 non-food trials. Each trial consisted of a stimulus presentation (6 seconds), a central cross-hair fixation period (2 seconds) and a stimulus rating period (13 seconds), which included a wanting rating (4 sec), and a restraint rating (4 sec), each followed by a fixation period (central crosshair jittered with ISI of 1–3 sec). High-ED, Low-ED, and Non-Food trials were cycled within each run of 27 trials so that the same stimulus category was never presented consecutively. This design was based loosely on the paradigm we have used previously in studies assessing neural responses to emotion-denoting words (25). High-ED food stimuli were two-word names representing high ED foods (e.g. Frosted cupcake, Chocolate spread, Grilled cheese, Chicken wings, Salted peanuts); Low-ED food stimuli were two-word names representing low energy-density foods (e.g. Cherry tomatoes, Brussels sprouts, Green beans, Mixed berries, Black cherries); and non-food stimuli were two-word names representing office supplies (e.g. Rubber bands, Plastic ruler, Post-it notes, Bulletin board, Staple remover). By design, stimulus categories did not differ significantly in number of letters, syllables, or hyphenated words. To increase ecological validity, all words were presented in a white handwriting font on a black background, emulating a menu-board. During the ‘wanting’ rating period following food words, participants saw a screen reading ‘I want to eat it’. For the wanting rating period following non-food words, the screen read ‘I want to use it’, and participants responded on a VAS scale with the end-points Not at all and Extremely. For the ‘restraint’ rating following food words, participants saw a screen reading ‘I shouldn’t eat it’ while for that following non-food words, the screen read ‘I shouldn’t use it’, and VAS end-points were Disagree and Agree
Prior to the scan, adolescents were trained by a research assistant on task performance, using a desktop computer located outside the scanning room. To promote elaborative imagery, participants were instructed, when they saw a food word, to focus on the food word and think about how the food looks, smells, and tastes, and how it would feel to eat it at that moment, and, when seeing an object word, to focus on the object word and think about how it looks and how it would feel to use it at that moment. When answering the rating questions, adolescents were instructed to rate how much they wanted to eat the food or use the object at the moment, if it were to be placed in front of them, and to rate how much they felt they shouldn’t eat/use something, even if they might like or want the item.
At the end of Test Day, adolescents completed a word stimuli familiarity/liking questionnaire with the following response options for each food and object word stimulus that appeared while they were in the scanner: Never had/use it, Don’t know what it is, I dislike it extremely, I dislike it, Neutral, I like it, I like it extremely.
Image acquisition
Images were acquired on a GE Signa 3 Tesla LX scanner (Milwaukee, WI) with a 55 cm diameter bore equipped with an 8-channel receive-only head coil. A three-plane localization scan was used to verify head position, and motion was minimized with restraint pads around the head. A T1-weighted sagittal localizing scan was used to position the axial functional images parallel to the anterior commissure-posterior commissure [AC-PC] line. A high-resolution 3D spoiled gradient recall [SPGR] anatomical image was acquired for coregistration with the axial functional images and with the standard MNI [Montreal Neurological Institute] coordinate system. Functional images were obtained using a T2*-sensitive gradient-recalled, single-shot, echo-planar imaging [EPI] pulse sequence with a 2800 msec repetition time (TR), 25 msec echo time [TE], 90° flip angle, 24 × 24 cm field of view [FOV] and 64 × 64 voxel matrix. Forty-three oblique slices positioned parallel to the AC-PC line were acquired per volume, providing whole brain coverage with 185 volumes (preceded by 6 dummy volumes) per run, and 3 runs per participant. Slice thickness was 3.0mm throughout, with 0.5mm spacing between slices. Effective spatial resolution was therefore 3.75 × 3.75 × 3.5 mm.
Multi-item ad libitum meal
Post-task, adolescents were presented with 3 Domino’s 12″ medium-sized hand-tossed pizzas cut into 8 slices (cheese: 673 g, 1510 kcal; pepperoni: 655 g, 1560 kcal; vegetable: 627 g, 1242 kcal), and large quantities of Doritos chips (150g, 756 kcal), Chips Ahoy cookies (200g, 1000 kcal), M&Ms (210g, 1052 kcal), cherry tomatoes (200g, 36 kcal), baby carrots (200g, 88 kcal), celery sticks (200g, 44 kcal), green grapes (700g, 497 kcal), Sabra plain hummus (100g, 260 kcal), Wishbone ranch dressing (480ml, 2080 kcal), water (1L, 0 kcal), Coke (0.5L, 202 kcal), and Diet Coke (0.5L, 0 kcal). To encourage ad libitum eating, participants were asked to “treat the meal like your dinner” and “not eat for 5 hours following the buffet meal”. They were advised that they would be left for 30 minutes to eat as much of the meal as they would like, but they could inform the research assistants if they finished early. Participants ate in private to ensure the subject was not disturbed during the meal. Food was weighed prior to and following the meal (out of sight of the participant) to determine amount consumed. Leftover food items were measured and subtracted from the amount consumed and intake values were generated for total food and beverages.
Statistical analysis
Behavioral analyses
Univariate ANOVAs were used to compare sample characteristics and questionnaire scores, and Chi squared tests to compare gender, ethnicity, education and income, between groups. Univariate ANOVAs were also used to compare in-scanner ratings of hunger, fullness, stress, pain and thirst, averaged across runs. For food word paradigm data, we calculated overall mean wanting and restraint scores (in scanner, 100 point VAS scale ranging from Not at all to Extremely) and liking scores (out of scanner, 5-point likert scale I dislike it extremely, I dislike it, Neutral, I like it, I like it extremely) for each stimulus type (high-ED, low-ED, non-food), then conducted mixed model ANOVAs with stimulus type as the within-subjects factor and risk group as the between-subjects factor. Chi squared tests were used to compare the rate of <Never had/use it> or <Don’t know what it is> (i.e. familiarity) responses across groups, for each stimulus type separately. For the multi-item ad libitum meal data (total food and beverage intake, total pizza and snack (high-ED) intake, total fruits and vegetables (low-ED) intake, liking ratings) and questionnaire data, univariate ANOVAs including tests for linear trend were used to compare groups, with post-hoc tests (Bonferroni) to further investigate differences where the overall F was significant. To calculate scores for behavioral variables we required that no more than 25% of items were missing; this was true for all variables. All analyses were conducted using SPSS 20 with p<0.05 considered significant, and p>0.05≤0.06 marginal.
Imaging analyses
Image preprocessing and statistical analysis was performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), run using MATLAB 2009B. Functional images were first corrected for timing differences between slices using a windowed Fourier interpolation to minimize their dependence on the reference slice. Images were then motion-corrected and realigned to the first image within each run, or discarded if estimates for peak motion exceeded 3mm translation or 2 degrees rotation. The corrected images were resampled to a resolution of 3×3×3 mm and then spatially coregistered by warping each subject’s SPGR image to the MNI template ICBM152 and then warping each functional image to the subject-specific SPGR image. Images were then spatially smoothed using a Gaussian-kernel filter with a full width at half maximum of 8 mm.
In first-level analyses we detected task-related activity within each individual participant by applying a general linear model (GLM) to each participant’s data that included 6 independent functions: the canonical hemodynamic response function (HRF) convolved with a box car function (BCF) representing the onsets and durations of the presentation of i) the high-ED stimuli, ii) the low-ED stimuli, iii) the non-food stimuli, iv) on-line wanting ratings for each stimulus, v) on-line restraint ratings for each stimulus, vi) fixation. The model was estimated using the Restricted Maximum Likelihood (ReML) algorithm, and task-related T contrast images (high-ED vs. low-ED, high-ED vs. non-food, low-ED vs. non-food) were generated using the SPM8 contrast manager.
In second-level analyses that detected random effects of task-related activity within and between groups (overweight vs. lean-LR; lean-HR vs. lean-LR, overweight vs. lean-HR), we implemented Bayesian posterior inference (31) applied to the contrast images generated from the first-level analysis. Unlike more conventional second-level analyses, which use classical parametric inference to detect group effects by disproving the null hypothesis (β = 0) at each voxel in a statistical parametric map, and therefore require correction for multiple comparisons, the Bayesian method infers the posterior probability of detecting the observed group effects (β ≠ 0) given the observed activation map; it therefore does not generate false positives requiring adjustment of p-values (32, 33). Following (34), we report voxels that were identified as having a posterior probability of 98.75% using a p-value threshold of <0.0125 as well as a cluster filter of at least 8 adjacent voxels, to strengthen the biological (as opposed to statistical) validity and relevance of our findings (35). Based on an approximation formula (36), this conjoint requirement yields a conservative effective p value of <0.000005.
To explore associations of contrast images with behavioral indices across the whole sample, we conducted GLM-based multiple regressions using a p-value threshold of <0.0125 combined with a cluster extent threshold of 30 voxels, determined by Monte Carlo simulation, to obtain an effective p-value of 0.05 corrected. For analyses of online appetitive ratings (wanting, restraint), we calculated difference scores (mean rating for food stimuli – mean rating for non-food stimuli; mean rating for high-ED food stimuli – mean rating for low-ED food stimuli) for each individual, then regressed brain activation maps for each contrast on the relevant difference score. For analyses of food intake, we regressed brain activation maps for food vs. non-food on total intake, and maps for high-ED vs. low-ED on percentage of total intake from high-ED foods.
Results
Participant characteristics
Two families failed to meet criteria at the Initial visit, three families could not be reached following the Initial visit, one family dropped out, and three overweight/obese families were excluded due to the required n for that group having been met. A total of 36 adolescents completed the study, 10 of whom were obese or overweight (6 obese, 4 overweight; overweight group), and 26 of whom were lean. Of the lean adolescents, 10 had lean biological mothers (i.e. low familial risk for overweight, lean-LR group), and 16 had obese or overweight biological mothers (9 had obese mothers, 7 had overweight mothers; high familial risk for overweight, lean-HR group). Seven of the adolescents in the overweight group had an obese mother, 1 an overweight mother, and 2 a lean mother. Available paternal height and weight as reported by mothers (8/10 available for overweight, 12/16 available for lean-HR, 8/10 available for lean-LR), supported a higher rate of overweight in the fathers of adolescents in the overweight group (89%), than in those of adolescents in the lean-HR (58%) and lean-LR (38%) groups.
Completer characteristics and questionnaire scores are given in Table 1. Reflecting the study design, overweight adolescents had higher BMI percentiles, waist circumferences, and body fat percentages than lean adolescents, with no differences apparent between the lean-LR and lean-HR groups. Adolescents were all aged 14 to 18 y, with the exception of one adolescent who was 19 y old at the time of scanning. Maternal age was 33 to 59 y, with no significant differences between groups. Chi square analyses revealed that the groups did not differ by gender, ethnicity, race, education, or income. Univariate ANOVA showed no significant group differences for EE, EX or RS scores on the DEBQ, but a significant group difference for PFS scores (F[2,35]=3.798, p =0.033), with post hoc tests indicating higher PFS scores for the lean-HR compared to the overweight group.
Table 1.
Sample characteristics and eating trait questionnaire scores
| Lean-LR (n=10) | Lean-HR (n=16) | Obese/overweight (n=10) | Total (n=36) | |
|---|---|---|---|---|
| Adolescents | ||||
|
| ||||
| Age (y) | 16.0 ± 1.9 (14 – 19) | 15.5 ± 1.4 (14 – 18) | 15.8 ± 1.8 (14 – 18) | 15.7 ± 1.6 (14 – 19) |
| Sex | ||||
| Female | 7 (70%) | 8 (50%) | 5 (50%) | 20 (56%) |
| BMI (kg/m2) | 21.0 ± 1.7 a (18.9 – 23.3) | 20.9 ± 2.2 a (16.5 – 24.1) | 32.6 ± 7.1 b (24.3 – 44.0) | 24.2 ± 6.6 * (16.5 – 44.0) |
| BMI percentile | 51 ± 23 a (23 – 84) | 53 ± 23 a (12 – 84) | 95 ± 4 b (88 – 99) | 64 ± 27 * (12 – 99) |
| % body fat | 22 ± 7 a (10 – 33) | 18 ± 7 a (7 – 30) | 34 ± 13 b (12 – 51) | 24 ± 11 * (7 – 51) |
| Waist (cm) † | 75.1 ± 6.7 a (66.0 – 83.8) | 74.5 ± 6.2 a (62.2 – 86.4) | 101.2 ± 20.9 b (76.2 – 132.1) | 82.3 ± 17.0 * (62.2 – 132.1) |
| Ethnic group | ||||
| Black/African-American | 2 (20%) | 8 (50%) | 4 (40%) | 14 (39%) |
| White | 5 (50%) | 4 (25%) | 2 (20%) | 11 (31%) |
| Asian | 1 (10%) | 1 (6%) | 0 (0%) | 2 (6%) |
| More than one race | 2 (20%) | 1 (6%) | 2 (20%) | 5 (14%) |
| Other | 0 (0 %) | 2 (13%) | 2 (20%) | 4 (11%) |
|
| ||||
| Questionnaire scores | ||||
|
| ||||
| DEBQ Emotional Eating | 2.1 ± 0.7 | 1.9 ± 0.5 | 1.7 ± 0.4 | 1.9 ± 0.5 |
|
| ||||
| DEBQ External Eating | 2.9 ± 0.7 | 2.9 ± 0.4 | 2.7 ± 0.4 | 2.8 ± 0.5 |
|
| ||||
| DEBQ Restrained Eating | 2.6 ± 1.2 | 1.9 ± 0.7 | 2.6 ± 0.5 | 2.3 ± 0.8 |
|
| ||||
| Power of Food Scale | 2.1 ± 0.7 | 2.4 ± 0.7 a | 1.8 ± 0.4 b | 2.2 ± 0.7 * |
|
| ||||
| Hunger ratings (mean across 3 runs) | 36.0 ± 23.8 | 58.0 ± 29.9 | 56.3 ± 20.4 | 51.4 ± 27.0 |
|
| ||||
| Fullness ratings (mean across 3 runs) | 21.8 ± 15.8 | 19.6 ± 16.5 | 28.0 ± 18.5 | 22.5 ± 16.8 |
|
| ||||
| Thirst ratings (mean across 3 runs) | 56.4 ± 21.3 | 62.1 ± 30.8 | 60.8 ± 19.1 | 60.2 ± 24.9 |
|
| ||||
| Mothers | ||||
|
| ||||
| Age (y) | 47.1 ± 7.8 (33 – 59) | 43.3 ± 7.8 (33 – 55) | 43.5 ± 7.3 (34 – 58) | 44.4 ± 7.6 (33 – 59) |
| BMI (kg/m2) | 21.2 ± 1.4 a (18.9 – 23.4) | 31.6 ± 6.6 b (25.7 – 51.9) | 34.3 ± 9.2 b (21.9 – 46.5) | 29.5 ± 8.3 * (18.9 – 51.9) |
| % Body Fat † Δ | 25 ± 6 a (13 – 31) | 40 ± 6 b (30 – 55) | 41 ± 11 b (23 – 56) | 36 ± 10 * (13 – 56) |
| Waist (cm) † Δ | 76.5 ± 7.0 a (68.6 – 92.7) | 104.1 ± 17.8 b (78.7 – 152.4) | 103.4 ± 19.1 b (68.6 – 129.5) | 96.6 ± 19.9 * (68.6 – 152.4) |
| Educational level | ||||
| ≤High school | 0 (0%) | 5 (31%) | 4 (40%) | 9 (25%) |
| Household income | ||||
| Below 40k | 4 (40%) | 8 (50%) | 8 (80%) | 20 (56%) |
Lean-LR = lean adolescent with lean mother; Lean-HR = lean adolescent with obese/overweight mother; Obese/overweight = obese/overweight adolescent
Values for continuous variables are mean ± standard deviation, with range in parentheses.
Values for frequencies are n (%).
Significant group difference, ANOVA main effect, p <0.001
Different letters indicate significant differences based on post-hoc testing (Bonferroni), p < 0.05
n=9 for lean adolescents with lean mothers,
n=15 for lean adolescents with obese/overweight mothers
fMRI protocol
Behavioral results
Univariate ANOVA revealed no group differences in ratings for current health (84.2 ± 15.2), previous night’s sleep (8:08 hours ± 1:30). Groups also did not differ in mean hunger (51.4 ± 27.0), fullness (22.5 ± 16.8) or thirst (60.2 ± 24.9) ratings averaged across all three runs of the scan (Table 1).
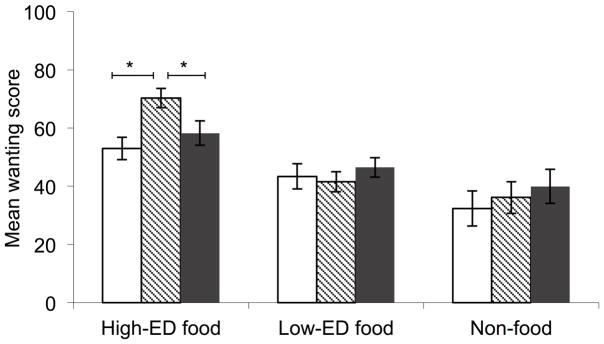
Repeated measures ANOVA for wanting ratings (Figure 2) revealed an effect of stimulus type (F[2,66]=31.4, p<0.001) and a marginal interaction with risk group (F[4,66]=2.50, p=0.051). Post hoc paired t-tests indicated that the main effect arose from greater wanting scores for the high-ED vs. low-ED (p<0.001), high-ED vs. non-food (p<0.001) and low-ED vs. non-food (p=0.036) cues. Univariate ANOVAs with post-hoc tests indicated that the marginal interaction was driven by a group difference for the high-ED cues (F[2,35]=7.79, p=0.002), such that wanting scores were greater for lean-HR compared to both lean-LR (p=0.002) and overweight (p=0.041), with no difference between the latter two. There were no group differences in wanting for either the low-ED or the non-food cues. In addition, to control for non-significant variations in BMI z-score between the lean-LR and lean-HR groups, we conducted a post-hoc analysis comparing these groups only, controlling for BMI z-score. For this analysis, the interaction with risk group was significant (F[2,46]=3.827, p=0.029). Repeated measures ANOVA for restraint ratings (Supplementary Figure 1) showed a significant effect of stimulus type (F[2,66]=16.25, p<0.001), but no interaction with risk group. Scores were greater for the high-ED cues than for both the low-ED (p<0.001) and non-food (p=0.001) cues.
Figure 2.
Wanting scores for high-ED, low-ED and non-food stimuli. Left panel gives wanting scores for lean-LR (□), lean-HR (
 ) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), high-ED>non-food (p<0.001) and low-ED>non-food (p=0.036). Asterisks over lines indicate significant group difference for the high-ED cues (p=0.002), such that lean-HR>lean-LR (p=0.002) and lean-HR> overweight (p=0.041).
) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), high-ED>non-food (p<0.001) and low-ED>non-food (p=0.036). Asterisks over lines indicate significant group difference for the high-ED cues (p=0.002), such that lean-HR>lean-LR (p=0.002) and lean-HR> overweight (p=0.041).
Repeated measures ANOVA for liking ratings revealed an effect of stimulus type (F[2,66]=31.79, p<0.001), but no interaction with risk group. Post hoc paired t-tests indicated liking was greater for high-ED than low-ED cues (p<0.001), while liking for non-food cues was higher than for both high-ED (p=0.001) and low-ED (p<0.001) cues. Chi-square tests comparing the rate of <Never had/use it> or <Don’t know what it is> (i.e. familiarity) for each stimulus type separately indicated no significant differences between groups, with each group showing a mean familiarity rate of 99–100% with stimuli in the high-ED, low-ED and non-food word categories, respectively.
Group comparisons did not change substantively when controlling for non-significant group imbalances in either child sex or maternal education.
Imaging results
Group effects for food vs. non-food contrast
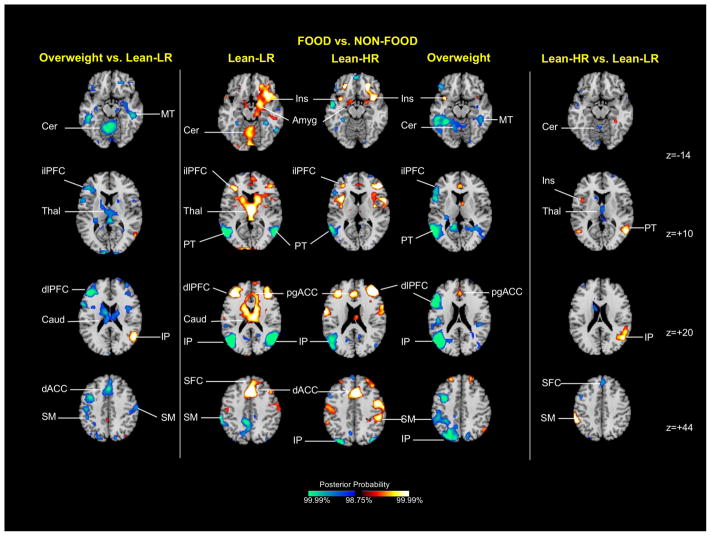
Group average activation maps (Figure 3, central column)
Figure 3.
Group average activation maps and between-group differences for food vs. non-food contrast. The central column shows representative 2-dimensional axial slices highlighting areas showing differential activation for food (high-ED+low-ED) compared with non-food cues. The side columns illustrate group effects for the same stimulus contrast, with the overweight vs. lean-LR effect shown on the left and the lean-HR vs. lean-LR effect on the right. Key: Amyg amygdala, Cer cerebellum, Caud caudate, dACC anterior cingulate cortex, dlPFC dorsolateral prefrontal cortex, ilPFC inferolateral prefrontal cortex, IP inferior parietal cortex, Ins insula, MT middle temporal cortex, pgACC pregenual anterior cingulate cortex, PT posterior temporal cortex, SFC superior frontal cortex, SM sensorimotor cortex, Thal thalamus
Several food vs. non-food differences were detected across all three subject groups. For all three groups, relatively greater food-related activation was detected in the insula (z=−14) and pregenual ACC (z=+20), and relatively less activation in the posterior temporal cortex (z=+10) and inferior parietal cortex (z=+20). Greater amygdala (z=−14), inferolateral (z=+10) and dorsolateral PFC, (z=+20) and dorsal ACC (z=+20) activation was observed in both lean groups.
Overweight vs. lean-LR (Figure 3, left column)
Relatively less activation in overweight compared with lean-LR adolescents was detected in a number of areas. Clusters in the dorsal ACC (z=+44), caudate (z=+20) and thalamus (z=+10) were driven by activations to food cues in the lean-LR group. Clusters in the inferolateral (z=+10) and dorsolateral PFC (z=+20) and cerebellum (z=−14) derived from activations to food cues in lean-LR, as well as food-related deactivations in the overweight group, and a cluster in the middle temporal cortex (z=−14) was driven by deactivation to food cues in the overweight group. A right inferior parietal cluster of relatively greater activation in overweight vs. lean-LR subjects was driven by deactivation in the lean-LR group (z=+20).
Lean-HR vs. lean-LR (Figure 3, right column)
Relatively less activation in lean-HR compared with lean-LR adolescents (Fig 1, right column) was detected in the superior frontal cortex (z=+44), cerebellum (z=−14) and thalamus (z=+10); these clusters were driven by activation to food cues in the lean-LR group. A cluster of relatively greater activation in lean-HR vs. lean-LR in the insula (z=+10) derived from activation to food cues in the lean-HR group, while two right posterior temporal (z=+10) and inferior parietal (z=+20) clusters of greater lean-HR activation were driven by deactivation to food cues in the lean-LR group.
Overweight vs. lean-HR (Supplementary Figure 2)
Results for the overweight vs. lean-HR comparison resembled those for overweight vs. lean-LR, with relatively less activation in obese compared with lean-HR adolescents in a number of areas. Clusters in the OFC (z=−10) and dorsal ACC (z=+46) were driven by activations to food cues in the lean-HR group, and clusters in the inferolateral PFC (z=+16), mid-cingulate cortex (z=+32), inferior temporal cortex (z=−10) and cerebellum (z=−10) were driven by a combination of activation in lean-HR and deactivation in the overweight group. Clusters in the sensorimotor cortex (z=+32) and cuneus (z=+32) were driven by deactivation in the overweight group. A cluster in the inferior parietal cortex (z=+46) demonstrating relatively greater activation in overweight vs. lean-HR subjects was driven by activation in the overweight group paired with deactivation in the lean-HR group.
Group effects for high-ED vs. low-ED food contrast
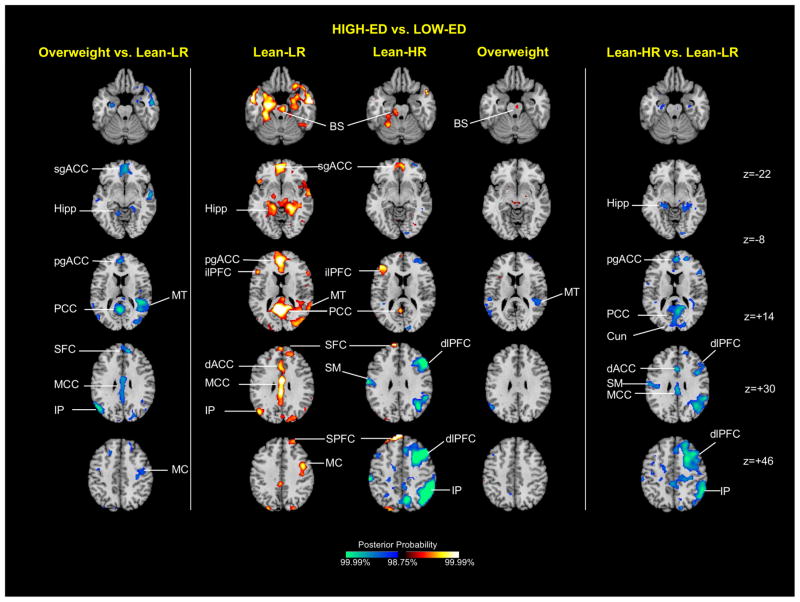
Group average activation maps (Figure 4, central column)
Figure 4.
Group average activation maps and between-group differences for high-ED food vs. low-ED food contrast. The central column shows representative 2-dimensional axial slices highlighting areas showing greater activation to high-ED vs. low-ED food cues. The side columns illustrate group effects for the same stimulus contrast, with the obese/overweight vs. lean-LR effect shown on the left, and the lean-HR vs. lean-LR effect on the right. Key: BS brainstem, Cun cuneus, dACC dorsal anterior cingulate cortex, dlPFC dorsolateral prefrontal cortex, Hipp hippocampus, ilPFC inferolateral prefrontal cortex, IP inferior parietal cortex, MC motor cortex, MT middle temporal cortex, MCC middle cingulate cortex, PCC posterior cingulate cortex, pgACC pregenual anterior cingulate cortex, sgACC subgenual anterior cingulate cortex, SFC superior frontal cortex, SPFC superior prefrontal cortex
Several high-ED vs. low-ED differences were detected in more than one subject group. For all three groups, relatively greater activation to high-ED food stimuli was detected in the brainstem (z=−22). Greater activation in the inferolateral PFC (z=+14), superior frontal (z=+30), subgenual ACC (z=−8) and PCC (z=+14) was observed for the lean groups only.
Overweight vs. lean-LR (Figure 4, left column)
Relatively less activation in overweight compared with lean-LR adolescents was detected in the pregenual ACC (z=+14), mid cingulate (z=+30) PCC (z=+14) and subgenual ACC (z=−8) cortex, the superior frontal cortex (z=+30), the inferior parietal cortex (z=+30), middle temporal cortex (z=+14), motor cortex (z=+46) and hippocampus (z=−8). These clusters were all driven by activation to the high-ED food cues in the lean-LR group.
Lean-HR vs. lean-LR (Figure 4, right column)
Relatively less activation in the lean-HR compared with lean-LR adolescents was also detected in a number of areas. Clusters in the pregenual ACC (z=+14), dorsal anterior cingulate (z=+30), mid cingulate cortex (z=+30) and PCC (z=+14), as well as the cuneus (z=+14) and hippocampus (z=−8), were driven by activation to high-ED cues in the lean-LR participants. Clusters in the dorsolateral PFC (z=+30, +46), motor cortex (z=+30) and inferior parietal cortex (z=+46), were driven by deactivation to high-ED cues in the lean-HR group.
Overweight vs. lean-HR (Supplementary Figure 3)
Comparisons of the overweight vs. lean-HR group did not closely resemble those between the overweight vs. lean-LR, and lean-HR vs. lean-LR groups. A cluster of relatively less activation in the overweight compared with the lean-HR group observed in the subgenual ACC (z=−6) was driven by activation to high-ED cues within the lean-HR group, while a cluster in the middle temporal cortex (z=+16) was driven primarily by deactivation in the overweight group. However, additional clusters of relatively greater activation to high-ED food stimuli, detected in the dorsolateral PFC (z=+30, +52), as well as the sensorimotor (z=+30) and dorsal parietal cortex (z=+52), were driven by deactivation within the lean-HR group.
To ensure that the group effects did not derive from non-significant group imbalances in potential confounding factors, we repeated all group comparisons controlling for child sex and maternal education. We also repeated the comparison between lean-LR and lean-HR controlling for BMI z-score. In all cases contrast maps were nearly identical to the unadjusted results.
Multi-item ad libitum meal intake
Univariate ANOVA revealed a significant difference between lean-LR, lean-HR and obese/overweight groups for total food and beverage energy intake (868 ± 414 kcal vs. 1274 ± 512 kcal vs. 1739 ± 763 kcal; F[2,35]=5.85, p=0.007, Figure 5), and total pizza and snack (i.e. high-ED food) intake (708 ± 402 kcal vs. 1056 ± 502 kcal vs. 1526 ± 734 kcal; F[2,35]=5.53, p=0.008, Figure 5), but not fruit and vegetable (i.e. low-ED food) intake (Figure 5). Post-hoc tests revealed that these differences were largely driven by greater intake in the overweight vs. lean-LR group (p=0.005 total, p=0.007 pizza and snacks), although there was also a significant linear trend across groups for both measures (p=0.002 total, p=0.002 pizza and snacks). This pattern of significant differences remained the same even when repeating the ANOVAs using caloric intakes represented as a percentage of individual children’s daily metabolic needs based on age, sex, body weight, height and physical activity levels (assumed to be low) (37). Results were also unchanged when controlling for child sex and maternal education.
Figure 5.
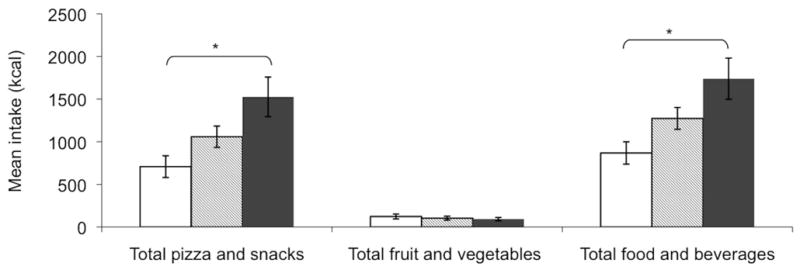
Meal intake by food category. Cluster shows total food and beverage intake, total pizza and snack intake, and fruit and vegetable intake for lean-LR (□), lean-HR (
 ) and overweight (■) groups. Asterisks indicate significant group differences and linear trends across groups (p=0.007, p for trend=0.002)
) and overweight (■) groups. Asterisks indicate significant group differences and linear trends across groups (p=0.007, p for trend=0.002)
Brain-behavior correlations
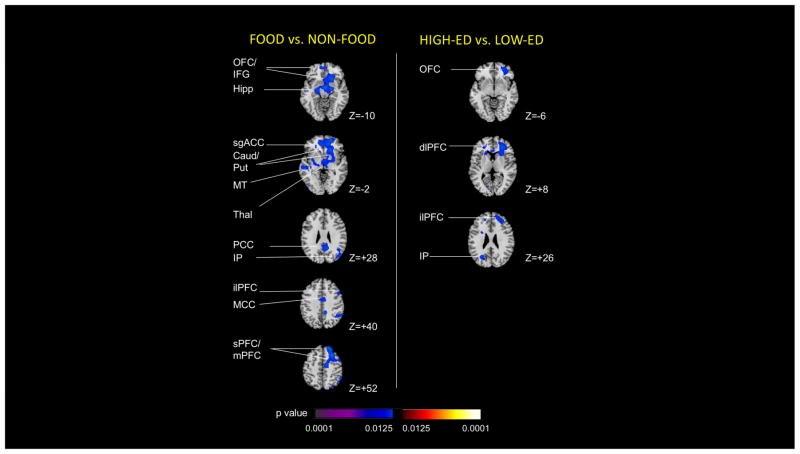
Regressing wanting difference scores on contrast maps (Figure 6) revealed that higher wanting difference scores for food vs. non-food (mean 16.6 ± 18.4, range 16.6–57.95) were associated with lower activation to food vs. non-food cues in the OFC/inferior frontal gyrus (z=−10), hippocampus (z=−10), subgenual ACC (z=−2), caudate/putamen (z=−2), ACC (z=−2), middle temporal cortex (z=−2), thalamus (z=−2), PCC (z=+28), inferior parietal cortex (z=+28), inferolateral PFC (z=+40), mid-cingulate cortex (z=+40), superior/medial PFC (z=+40). Higher wanting difference scores for high-ED vs. low-ED foods (mean 18.7 ± 16.8, range −4.7 – 61.3) associated with lower activation to high-ED vs. low-ED foods in the OFC (z=−6), dorsolateral PFC (z=+8), inferolateral PFC (z=+26) and inferior parietal cortex (z=+26). Restraint difference scores for food vs. non-food were uncorrelated with food vs. non-food brain activation maps, and restraint difference scores for high-ED vs. low-ED were uncorrelated with high-ED vs. low-ED brain activation maps. Total intake and percentage intake of high-ED foods at the ad libitum meal did not correlate significantly with either food vs. non-food or high-ED vs. low-ED brain activation maps, respectively.
Figure 6. Association of activations with wanting scores.
The left-hand column depicts regions showing an inverse correlation between magnitude of activation to food (high-ED+low-ED) compared with non-food cues, and scores representing the difference in wanting ratings for food (high-ED+low-ED) compared with non-food cues (positive difference score indicates relatively greater wanting for food cues). The right-hand column depicts regions showing an inverse correlation between magnitude of activation to high-ED compared with low-ED food cues, and scores representing the difference in wanting ratings for high-ED compared with low-ED food cues (positive difference score indicates relatively greater wanting for high-ED food cues). Key: sgACC subgenual anterior cingulate cortex, Caud caudate, dlPFC dorsolateral prefrontal cortex, Hipp hippocampus, IFG inferior frontal gyrus, ilPFC inferolateral prefrontal cortex, IP inferior parietal cortex, MCC middle cingulate cortex, mPFC medial prefrontal cortex, MT middle temporal cortex, OFC orbitofrontal cortex, PCC posterior cingulate cortex, Put putamen, sPFC superior prefrontal cortex, Thal thalamus
Discussion
This investigation of neural responses to food cues in adolescents who were at varying familial risk for obesity employed a novel fMRI paradigm that enabled us to contrast responses to words denoting foods with words denoting non-food objects, and responses to words denoting high-ED compared with low-ED foods. The food stimuli were presented in the context of an evaluative task that required participants to rate their desire for each item and their sense that they should restrain desire for the item. Our findings revealed patterns of similar, and also differential, activation across groups in three putative neural systems. The first is an emotion/reward system comprising the insula, OFC, pregenual and subgenual ACC, PCC, and amygdala (38–41). The second is an attentional/self-regulatory system comprising the dorsolateral, inferolateral, and superior PFC, superior frontal cortex, dorsal ACC, caudate, thalamus, and cerebellum. This system is activated in tasks involving attention and inhibitory control, such as the Stroop (42, 43), Simon spatial incompatibility (42–44), go/no-go (45), and continuous performance tasks (46). The third is a higher-order sensory/memory system comprising inferior and dorsal parietal cortex, posterior, inferior and middle temporal cortex, cuneus, and hippocampus (47–50).
Key components of the emotion/reward system activated in all three groups in response to food vs. non-food cues (insula, pregenual ACC, Figure 3) and in response to high-ED vs. lowED food cues (brainstem, Figure 4). We also detected evidence for group differences in activation within this system. For example, lean-HR adolescents more strongly activated the insula (Figure 3) in response to food vs. non-food cues than did lean-LR adolescents, consistent with greater food-related engagement of emotion/reward systems in those at higher obesity risk. In addition, the lean-LR group more strongly activated some regions within this system (pregenual and subgenual ACC, PCC, Figure 3) in response to high- compared with low-ED foods, which in the context of other activated areas (below), suggests that the lean-LR group may generate a more robust distributed neural response to high-ED foods than do the higher risk groups. Consistent with this interpretation, lean-HR activated the OFC (Supplementary Figure 2) more to food vs. non-food stimuli than did overweight adolescents, perhaps representing greater reward valuation and salience of food stimuli in this risk group (51).
The attentional/self-regulatory system demonstrated a decreasing activation gradient with increasing obesity risk, such that activation was greatest in the lowest risk group (lean-LR), lower in the intermediate risk group (lean-HR), and least in the highest risk group (overweight). The activation gradient was evident for both stimulus contrasts, but particularly for high-ED vs. lowED cues. Group differences in activation within this circuit (in superior frontal cortex, dorsal ACC, thalamus, cerebellum, and caudate, Figure 3) were driven substantially by food-related activations in the lean-LR group, but several of the PFC differences derived from deactivations to foods compared with non-foods (i.e. greater responses to non-foods) in the overweight group (inferolateral PFC, dorsolateral PFC, Figure 3) or to high-ED compared with low-ED foods (i.e. greater responses to low-ED foods) in the lean-HR group (dorsolateral PFC, Figure 4). These group differences suggest that lower risk adolescents more strongly activate this regulatory system, perhaps conferring neural responses to salient food cues that protect against the development of obesity, whereas higher risk adolescents fail to engage this system fully, perhaps owing to lack of food-related restraint.
Group differences in activation within the higher order sensory/memory system (inferior parietal and posterior temporal cortex, Figure 3, inferior parietal and sensorimotor cortex, Figure 4) were largely driven by deactivations in response to the more salient stimulus within the contrast (i.e. in response to foods compared with non-foods, or to high-ED compared with lowED foods). This pattern is similar to the well documented suppression of the default mode network in the presence of a salient task (52–55), suggesting that the observed deactivations could reflect a reduced recruitment or suppression of activity in circuits involved in the explicit processing of the more salient stimulus, which was food in the food vs. non-food contrast and high-ED foods in the high- vs low-ED food contrast. If this interpretation of deactivations is correct, then suppression of activity for food stimuli was greater in the lean-LR group, and for high-ED food stimuli was greater in the lean-HR group. This interpretation of the deactivations in terms of suppression is consistent with the greater activity of self-regulatory control circuits in the low risk groups, as well as with the analogous and well described anti-correlation of activity in the default mode and task-positive networks (56). Other group differences derived from differences in the degree of activation to the more salient stimulus. For example, the lean-LR group more strongly activated some regions to high-ED cues than did the overweight group (inferior parietal, middle temporal, and motor cortex, and hippocampus, Figure 4) or lean-HR group (hippocampus, Figure 4). The ultimate cause of these group differences is not clear, but could represent a relatively greater need for explicit retrieval of past encounters with high-ED foods in the lean-LR group, due to the lower frequency of intake of those foods in those participants.
One of our primary hypotheses was that currently lean youth who are at higher familial risk for obesity would demonstrate heightened responses to salient food cues in reward-associated regions, including the ventral striatum and ventral tegmental area, which previously have distinguished between lean and obese children (10–12, 57) and adults (58, 59). Lean-HR vs. lean-LR differences, however, were significant only within other components of a broader emotion/reward system (insula, pregenual and subgenual ACC and PCC, Figures 3 & 4). Our word-denoting stimuli may have been less salient reward triggers than the food pictures used in most previous studies. Our behavioral findings, however, did demonstrate higher ‘wanting’ ratings in the lean-HR group than in the lean-LR group for high- compared with low-ED foods. Moreover, word stimuli have been used successfully to activate reward areas in response to words presented aurally in combination with pictures (58), as well as to trigger emotions (25).
In contrast, most of the lean-HR vs. lean-LR differences were entirely consistent with our second hypothesis, that the lean-HR participants would less strongly activate a widespread attentional/self-regulatory system. Group differences within this system may have derived in part from eliciting subjective ratings of ‘wanting’ and ‘restraint’ in response to each food stimulus, which may have preferentially engaged neural systems involved in the evaluation of whether or not to consume each food. These design elements notwithstanding, our combined results suggest that activation of a neural system subserving attention and self-regulation could potentially be the most salient predictor of non-obesogenic, food-directed behavior and food choices in the obesogenic food environment of this age group.
Our behavioral analyses also provided evidence for the presence of a greater subjective appetite in lean-HR compared with lean-LR adolescents, with greater ‘wanting’ scores following high-ED food words (Figure 2). Further, higher wanting difference scores for food vs. non-food (indicating more desire for food items) and for high-ED vs. low-ED foods (indicating more desire for high-ED foods) were associated with less activation to food cues in a number of brain regions (Figure 6). These included regions within the default mode network, consistent with a task-positive allocation of greater attention to foods than to non-foods, and suggesting that more wanting is associated with a proportional deactivation of this circuit. The observed locations of inverse correlation also included regions implicated in attention/self-regulation (e.g. PFC and caudate), consistent with reduced regulation of desire for the foods denoted by the cues in participants who expressed greater wanting for those foods. Inverse correlation was also observed within our putative emotion/reward circuit (OFC), suggesting that greater wanting was associated with less, rather than more engagement of emotion/reward-related regions. However, activation of this latter circuit was not detected in our primary between-group comparisons and should therefore be interpreted with caution. Notably, overweight adolescents reported wanting levels similar to those of the lean-LR group (Figure 2), a finding that may represent the social desirability of the response, given that obese/overweight individuals underreport food intake particularly of high-ED snack-foods (60). Alternatively, printed food words may have been insufficient to trigger a subjective desire to eat in overweight individuals, who previously have shown blunted reward responses to food and food cues, possibly a consequence of their excessive intake of highly-palatable foods (61, 62).
We also observed greater ad libitum intake of high-ED foods in the lean-HR compared with the lean-LR group during a multi-item buffet meal, with overweight adolescents consuming the most. Body composition and weight were similar between the lean-HR and lean-LR groups, and group differences in intake persisted in analyses adjusted for metabolic needs, suggesting that group differences in food intake reflect a differing propensity for excess weight gain rather than differences in underlying metabolic needs. Our findings agree with other reports of increased intake with raised familial risk, controlling for current adiposity (8).
One limitation of our study is the absence of a prospective assessment of weight gain, precluding us from relating the neural profiles we identified to future weight. Our definition of familial risk is also unable to distinguish between genetic, epigenetic, and social determinants of the intergenerational transmission of obesity. Another limitation is our relatively small sample size, which limits statistical power and biases findings toward the null. Small sample size generally does not bias toward false positive findings, yet to limit further the likelihood of spurious results, we assessed our data carefully to ensure the absence of activation outliers. Nevertheless, our findings should be considered preliminary and in need of replication.
A final limitation is the use of only maternal weight in assigning risk status, as it provides an incomplete operationalization of familial risk. Prior evidence, however, suggests that maternal, not paternal, weight supplies most of the familial loading for risk (3), likely because of differences in parental feeding practices and epigenetic contributions. In addition, maternal reports of paternal BMIs in our study confirmed a progressively greater prevalence of paternal overweight across the lean-LR, lean-HR, and overweight groups, suggesting that our familial risk assignments also reflected obesity risk from the paternal side. In fact, the vast majority of obese/overweight adolescents had obese/overweight mothers and obese/overweight fathers, so that the obese/overweight group represented the highest risk group not only in terms of their manifest weight, but also in terms of familial risk.
Our findings thus suggest that a progressively greater risk for obesity, as conferred by both familial obesity risk in currently lean adolescents, and current overweight/obese status in adolescents, is associated with a progressively weaker activation of neural systems subserving attention and self-regulation in response to food-denoting words, as well as an increased subjective appetite for high-ED foods. Food words may be considered relatively minimal food cues compared with the food images more commonly used in studies of food cue responsiveness. Our findings therefore suggest that familial risk differences in neural responsivity may be present across a wide range of food stimuli. Future work should attempt to extend our findings by addressing the limitations described above. Such studies could also employ alternative tasks designed to tap food-related self-regulation, and directly test task-related functional connectivity within the systems we uncovered here. Nevertheless, our results have important implications for the development of novel and more individualized prevention and treatment interventions for adolescent obesity. Interventions designed to stimulate the self-regulatory systems that are most active in lean low-risk youth may be especially promising.
Supplementary Material
Restraint scores for high-ED, low-ED and non-food stimuli. Left panel gives wanting scores for lean-LR (□), lean-HR (
 ) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), and high-ED>non-food (p<0.001).
) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), and high-ED>non-food (p<0.001).
Overweight vs. lean-HR differences for food vs. non-food contrast. The left hand column illustrates obese vs. lean-HR group differences for the food (high-ED+low-ED) vs. non-food contrast, and the right hand column shows this contrast in the same slice for Obese and lean-LR groups separately. Key: Cer cerebellum, Cun cuneus, dACC dorsal anterior cingulate cortex, ilPFC inferolateral prefrontal cortex, IP inferior parietal cortex, MCC mid-cingulate cortex, IT middle temporal cortex, OFC orbitofrontal cortex, SM sensorimotor cortex
Overweight vs. lean-HR differences for high-ED vs. low-ED food contrast. The left hand column illustrates overweight vs. lean-HR group differences for the highED vs. low-ED food contrast, and the right hand column shows this contrast in the same slice for overweight and lean-LR groups separately. Key: sgACC subgenual anterior cingulate cortex, dlPFC inferolateral prefrontal cortex, DP dorsal parietal cortex, MT middle temporal cortex, SM sensorimotor cortex
Acknowledgments
Funding: This work was supported by the National Institute of Diabetes & Digestion & Kidney Disease (NIDDK) (K99R00DK088360 [PI: SC] and R01DK074046 and DK080153 [PI: AG])).
This work was conducted when the authors were located at the New York Obesity Nutrition Research Center at St. Luke’s-Roosevelt Hospital and Columbia University College of Physicians & Surgeons (S.C., L.B., K.C., A.G.), and the New York State Psychiatric Institute and Columbia University College of Physicians & Surgeons (Z.W., B.S.P., S.C., B.S.P. and A.G. formulated hypotheses and designed the study. K.C. and L.B. ran the study and Z.W. programmed the fMRI paradigm. Z.W., S.C and L.B. analyzed the functional imaging data. S.C. and L.B. analyzed behavioral data. S.C. wrote the first draft of the paper and B.S.P and L.B. contributed to subsequent drafts. All authors reviewed and approved the submitted manuscript. We are grateful for partial support from NIDDK (K99R00DK088360 [PI: SC]) and R01DK074046 and DK080153 [PI: AG]. No authors declared a conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Uncategorized References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. The American journal of clinical nutrition. 2002;76(3):653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. The American journal of clinical nutrition. 2010;91(6):1560–7. doi: 10.3945/ajcn.2009.28838. [DOI] [PubMed] [Google Scholar]
- 4.Semmler C, Ashcroft J, van Jaarsveld CH, Carnell S, Wardle J. Development of overweight in children in relation to parental weight and socioeconomic status. Obesity. 2009;17(4):814–20. doi: 10.1038/oby.2008.621. [DOI] [PubMed] [Google Scholar]
- 5.Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord. 2001;25(2):147–57. doi: 10.1038/sj.ijo.0801538. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. The New England journal of medicine. 1997;337(13):869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 7.Lake JK, Power C, Cole TJ. Child to adult body mass index in the 1958 British birth cohort: associations with parental obesity. Arch Dis Child. 1997;77(5):376–81. doi: 10.1136/adc.77.5.376. [DOI] [PubMed] [Google Scholar]
- 8.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity. 2006;14(1):131–8. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 9.Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters’ increase in BMI and disinhibited overeating from 5 to 13 years. Obesity. 2007;15(6):1544–53. doi: 10.1038/oby.2007.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International journal of obesity. 2010;34(10):1494–500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PloS one. 2009;4(7):e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. The American journal of clinical nutrition. 2009;90(4):928–34. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nock NL, Dimitropolous A, Tkach J, Frasure H, von Gruenigen V. Reduction in neural activation to high-calorie food cues in obese endometrial cancer survivors after a behavioral lifestyle intervention: a pilot study. BMC Neurosci. 2012;13:74. doi: 10.1186/1471-2202-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30(1):91–8. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(12):4360–6. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pursey K, Burrows TL, Stanwell P, Collins CE. How Accurate is Web-Based Self-Reported Height, Weight, and Body Mass Index in Young Adults? J Med Internet Res. 2014;16(1) doi: 10.2196/jmir.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer C, McPartlan L, Sines J, Waller G. Accuracy of self-reported weight and height: relationship with eating psychopathology among young women. Int J Eat Disord. 2009;42(4):379–81. doi: 10.1002/eat.20618. [DOI] [PubMed] [Google Scholar]
- 21.Dubois L, Ohm Kyvik K, Girard M, Tatone-Tokuda F, Perusse D, Hjelmborg J, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an international study of over 12,000 twin pairs. PLoS One. 2012;7(2):e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–68. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–63. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posner J, Russell JA, Gerber A, Gorman D, Colibazzi T, Yu S, et al. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Human brain mapping. 2009;30(3):883–95. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakes ME, Slotterback CS. Self-reported measures of appetite in relation to verbal cues about many foods. Curr Psychol. 2000;19:137–42. [Google Scholar]
- 27.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. The Journal of adolescent health: official publication of the Society for Adolescent Medicine. 1993;14(3):190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 28.Center for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts: United States. 2000. [Google Scholar]
- 29.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- 30.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–8. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Neumann J, Lohmann G. Bayesian second-level analysis of functional magnetic resonance images. NeuroImage. 2003;20(2):1346–55. doi: 10.1016/S1053-8119(03)00443-9. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. NeuroImage. 2002;16(2):484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Penny W. Posterior probability maps and SPMs. NeuroImage. 2003;19(3):1240–9. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 34.Peterson BS, Wang Z, Horga G, Warner V, Rutherford B, Klahr KW, et al. Discriminating risk and resilience endophenotypes from lifetime illness effects in familial major depressive disorder. JAMA psychiatry. 2014;71(2):136–48. doi: 10.1001/jamapsychiatry.2013.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 36.Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human brain mapping. 1994;1(3):210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Medicine of the National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 38.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and biobehavioral reviews. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 39.Paret C, Ruf M, Gerchen MF, Kluetsch R, Demirakca T, Jungkunz M, et al. fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. NeuroImage. 2016;125:182–8. doi: 10.1016/j.neuroimage.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and biobehavioral reviews. 2006;30(2):173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry research. 2013;213(1):24–30. doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13(3):427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22(3):1097–106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 44.Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168(11):1210–20. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human brain mapping. 2001;12(3):131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 47.Goodman J, Marsh R, Peterson BS, Packard MG. Annual research review: The neurobehavioral development of multiple memory systems--implications for childhood and adolescent psychiatric disorders. J Child Psychol Psychiatry. 2014;55(6):582–610. doi: 10.1111/jcpp.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goh S, Peterson BS. Imaging evidence for disturbances in multiple learning and memory systems in persons with autism spectrum disorders. Dev Med Child Neurol. 2012;54(3):208–13. doi: 10.1111/j.1469-8749.2011.04153.x. [DOI] [PubMed] [Google Scholar]
- 49.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, Javitt DC, et al. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc Natl Acad Sci U S A. 2008;105(11):4399–404. doi: 10.1073/pnas.0708418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 52.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 54.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human brain mapping. 2009;30(2):625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International journal of obesity. 2010;34(1):94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- 58.Carnell S, Benson L, Pantazatos SP, Hirsch J, Geliebter A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity. 2014;22(11):2370–8. doi: 10.1002/oby.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tooze JA, Subar AF, Thompson FE, Troiano R, Schatzkin A, Kipnis V. Psychosocial predictors of energy underreporting in a large doubly labeled water study. The American journal of clinical nutrition. 2004;79(5):795–804. doi: 10.1093/ajcn/79.5.795. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42(4):1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Current drug abuse reviews. 2011;4(3):182–9. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Restraint scores for high-ED, low-ED and non-food stimuli. Left panel gives wanting scores for lean-LR (□), lean-HR (
 ) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), and high-ED>non-food (p<0.001).
) and overweight groups (■). There was a significant main effect of cue-type (p<0.001), such that high-ED>low-ED (p<0.001), and high-ED>non-food (p<0.001).
Overweight vs. lean-HR differences for food vs. non-food contrast. The left hand column illustrates obese vs. lean-HR group differences for the food (high-ED+low-ED) vs. non-food contrast, and the right hand column shows this contrast in the same slice for Obese and lean-LR groups separately. Key: Cer cerebellum, Cun cuneus, dACC dorsal anterior cingulate cortex, ilPFC inferolateral prefrontal cortex, IP inferior parietal cortex, MCC mid-cingulate cortex, IT middle temporal cortex, OFC orbitofrontal cortex, SM sensorimotor cortex
Overweight vs. lean-HR differences for high-ED vs. low-ED food contrast. The left hand column illustrates overweight vs. lean-HR group differences for the highED vs. low-ED food contrast, and the right hand column shows this contrast in the same slice for overweight and lean-LR groups separately. Key: sgACC subgenual anterior cingulate cortex, dlPFC inferolateral prefrontal cortex, DP dorsal parietal cortex, MT middle temporal cortex, SM sensorimotor cortex