Abstract
Despite a substantial amount of animal data linking deficits in memory inhibition to the development of overeating and obesity, few studies have investigated the relevance of memory inhibition to uncontrolled eating in humans. Further, although memory for recent eating has been implicated as an important contributor to satiety and energy intake, the possibility that variations in episodic memory relate to individual differences in food intake control has been largely neglected. To examine these relationships, we recruited ninety-three adult subjects to attend a single lab session where we assessed body composition, dietary intake, memory performance, and eating behaviors (Three Factor Eating Questionnaire). Episodic recall and memory inhibition were assessed using a well-established measure of memory interference (Retrieval Practice Paradigm). Hierarchical regression analyses indicated that memory inhibition was largely unrelated to participants’ eating behaviors; however, episodic recall was reliably predicted by restrained vs. uncontrolled eating: recall was positively associated with strategic dieting (β=2.45, p=0.02), avoidance of fatty foods (β=3.41, p=0.004), and cognitive restraint (β=1.55, p=0.04). In contrast, recall was negatively associated with uncontrolled eating (β= −1.15, p=0.03) and emotional eating (β= −2.46, p=0.04). These findings suggest that episodic memory processing is related to uncontrolled eating in humans. The possibility that deficits in episodic memory may contribute to uncontrolled eating by disrupting memory for recent eating is discussed.
Keywords: uncontrolled eating, disinhibition, memory for recent eating, inhibition, executive function
INTRODUCTION
Deficits in self-regulatory control are widely implicated in the etiology of overeating and obesity (for reviews, see Lavagnino, Arnone, Cao, Soares, & Selvaraj, 2016; Martin & Davidson, 2014). Several studies have shown that obese individuals often respond more impulsively (i.e., faster or more often) on behavioral tasks than normal weight individuals (for recent systematic reviews, see Bartholdy, Dalton, O’Daly, Campbell, & Schmidt, 2016; McClelland et al., 2016). This general failure of self-control is thought to underpin failures in food-intake control, thereby contributing to the disinhibition of eating and eventual weight gain. In line with this possibility, studies have shown that impulsivity predicts greater food intake (Guerrieri, Nederkoorn, & Jansen, 2007, 2012; Guerrieri, Nederkoorn, Schrooten, Martijn, & Jansen, 2009; Jansen et al., 2009), increased weight gain over time (Francis & Susman, 2009; Seeyave et al., 2009), and resistance to weight loss (Nederkoorn, Houben, Hofmann, Roefs, & Jansen, 2010; Nederkoorn, Jansen, Mulkens, & Jansen, 2007). Likewise, disinhibited eating behavior (as assessed by self-report questionnaires) has been associated with greater impulsivity and risk-taking on behavioral measures of self-control (Yeomans & Brace, 2015).
In rodents, these relationships between impulsivity, overeating, and obesity have been attributed to a common underlying deficit in memory inhibition (for reviews, see Davidson, Tracy, Schier, & Swithers, 2014; Kanoski & Davidson, 2011). Memory inhibition is the process which enables individuals to suppress or ignore unwanted or outdated associations from memory and, thus, helps to filter goal-relevant information from goal-irrelevant information (Levy & Anderson, 2002). As such, memory inhibition is critical for performing a variety of self-regulatory tasks, including most tasks associated with executive function such as working memory and cognitive flexibility (see Friedman & Miyake, 2017; Miyake et al., 2000; Akira Miyake & Friedman, 2012). Regarding food intake control, memory inhibition is critical for gating the incentive properties of food cues and, thus, our impetus to act on them (for a discussion of this account, see Martin & Davidson, 2014). Food and food cues are only capable of prompting us to eat if they remind us, via retrieval of learned associations, of food reward. Thus, food intake control depends not only upon the amount of behavioral control one possesses--food intake control also depends upon how well one can resist thinking about the rewarding aspects of food that motivate one to eat in the first place (i.e., memory inhibition).
Supporting this possibility, studies in rodents have consistently shown that manipulations that weaken memory inhibition also weaken rats’ self-control over their food intake and increase their susceptibility to overeat in response to external food cues. For instance, rats with deficits in memory inhibition exhibit greater food cue reactivity (i.e., “impulsive”, perseverative responding for food), overeating, and weight gain (for reviews, see Davidson et al., 2014; Kanoski, 2012; Kendig, 2014). Natural variations in memory inhibition have also been found to predict weight maintenance, with stronger memory inhibition predicting resistance to weight gain (Davidson et al., 2013; Davidson et al., 2012).
These findings implicate individual differences in memory inhibitory control as a predictor of uncontrolled eating. However, despite the links established in rodent studies, evidence examining the contribution of memory inhibition to human eating behavior is lacking. There is some evidence suggests that obese people and disinhibited eaters are more susceptible to food-related memory intrusions and are less effective at suppressing these food-related thoughts (Soetens & Braet, 2006; Soetens, Braet, Dejonckheere, & Roets, 2006; Soetens, Braet, & Moens, 2008; but see Erskine & Georgiou, 2010; Soetens & Braet, 2007) but these studies have relied predominantly on subjective thought-suppression paradigms wherein participants are asked to monitor and record their own food-related thoughts. Thus, although previous studies in this area are broadly suggestive that deficits in cognitive control relate to lower levels of food intake control, there is a need for further research on this problem using objective measures of cognitive performance.
While memory inhibition helps to control food intake by gating our recollection of the incentive properties of food that entice people to eat, memory retrieval is not uniformly detrimental to food intake control. Evidence suggests that episodic memory can also protect against overeating by helping individuals recall past eating episodes (i.e., portion size, time of last meal, etc.) (Brunstrom et al., 2012; Higgs, 2016; Robinson et al., 2013). This recall of past eating episodes enables individuals to make meal-to-meal compensatory adjustments in their food intake, and thus helps ensure that individuals do not overeat throughout the day--adjusting for a large breakfast by consuming a smaller lunch is difficult if one cannot accurately recall the size or nutritional content of one’s breakfast! By this account, individual differences in episodic recall might also predict tendencies towards uncontrolled eating in humans (i.e., weaker recall predicting a greater susceptibility to overeat).
In support for this idea, studies have shown that individuals who demonstrate severe episodic memory deficits (and, thus, cannot remember their recent meals) also exhibit aberrant eating behavior. For instance, amnesiac individuals will eat two meals in a row without exhibiting any loss of appetite, and will often begin eating a third meal if not stopped (Hebben, Corkin, Eichenbaum, & Shedlack, 1985; Rozin, Dow, Moscovitch, & Rajaram, 1998). In non-amnesiacs, manipulating participants’ ability to encode or retrieve the memory of a recent meal has also been shown to influence appetite and moderate eating at the next meal, with better recall being associated with less hunger and lower intakes (Brunstrom et al., 2012; Robinson et al., 2013; Higgs, Robinson, & Lee, 2012). Evidence from rodent studies suggest that this ability to episodically recall past eating occasions is mediated by the dorsal hippocampus, and is especially important for satiety processing and meal initiation (i.e., helping to delay the onset of the next meal) (Parent, 2016). For instance, disrupting memory consolidation after a feeding episode (by pharmacologically inhibiting dorsal hippocampal neurons) has been found to reduce the inter-meal interval and increase the size of the next meal (Henderson, Smith, & Parent, 2013; Parent, Darling, & Henderson, 2014). Together, these studies highlight the importance of memory for recent eating in appetite control, and implicate deficits in episodic memory as a contributor to overeating and obesity in humans.
Although memory for recent eating has been implicated as an important contributor to satiety and energy intake (Higgs, 2016), relatively few studies have experimentally examined whether general episodic recall ability is related to food intake control in normal (i.e., non-amnesic) individuals. As part of their research investigating the Western diet and its effects on hippocampal-function, Stevenson and colleagues have shown that verbal learning during a paired associates task is negatively associated with consumption of saturated fats and sugars (Francis & Stevenson, 2013) and that individuals with worse memory performance are resistant to the satiating effects of eating (i.e., exhibiting less reductions in ‘wanting’ food after consuming a standardized meal) (Attuquayefio et al., 2016). This finding was interpreted as evidence that consuming a Western diet disrupted memory, and thereby disrupted the ability to use satiety to inhibit pleasant food related memories (i.e., wanting) (for a review of this account, see Davidson, Kanoski, Walls, & Jarrard, 2005). Notably, that study (Attuquayefio et al., 2016) represents one of the few direct tests linking general (i.e., non-food) memory deficits in the development of eating dysregulation in humans, emphasizing the need for additional research in this area.
Here, we examined how natural variations in memory inhibitory control and episodic memory relate to individual differences in food intake control in healthy humans (N=93). Food intake control was assessed for each participant using a revised version of the original Three Factor Eating Questionnaire (Stunkard & Messick, 1985) called the TFEQ-R18-V2 (Cappelleri et al., 2009), which discriminates between cognitive restraint, uncontrolled eating, and emotional eating. We also included additional subtypes of “Restraint-related” and “Disinhibition-related” eating behaviors which have been suggested by others (Bond, McDowell, & Wilkinson, 2001; Westenhoefer, Stunkard, & Pudel, 1999). Individual differences in memory inhibition and episodic memory were assessed using a well-established, objective measure known as the Retrieval Practice (RP) (Anderson, Bjork, & Bjork, 1994). The RP paradigm includes both an episodic recall component (in which participants memorize a subset of word pairs), and a memory inhibitory component (a measurable amount of ‘forgetting’ that occurs for a subset of word pairs). Thus, the task allows us to measure the magnitude of memory inhibition in all subjects, and to relate the magnitude of that inhibitory effect to individual differences in disinhibited and restrained eating styles. We can also examine how individual differences in episodic recall relate to these eating behavior subtypes. We anticipated that disinhibitory tendencies towards eating behavior would be related to weaker memory inhibition and lower episodic recall, consistent with existing evidence linking impaired food intake control with deficits in memory control.
METHOD
Study Overview
Ninety-three (N=93) adult subjects attended a single laboratory session where we assessed memory performance (RP Paradigm), eating behaviors (TFEQ), body composition (% adiposity via BODPOD), and energy and macronutrient intake. A priori hypothesized relationships between memory performance and uncontrolled eating were tested using multiple hierarchical regression analyses. Dietary intake and body composition, which were collected as part of a larger ongoing study examining links between diet and chronic disease risk, were included as covariates in the models reported here. We predicted that higher scores on disinhibitory subscales (uncontrolled eating, emotional eating, habitual susceptibility, emotional susceptibility, situational susceptibility, rigid control) would be associated with weaker memory inhibition based on evidence that, a) deficits in memory inhibition are linked with overeating in rodents, and b) overeating is associated with tendencies towards uncontrolled eating in humans. We also anticipated that higher levels of disinhibition would be related to lower levels of episodic recall based on evidence linking memory for recent eating in the control of appetite and energy intake.
Participants
One-hundred and twenty-six participants were recruited from Purdue University and the surrounding community to participate in a 10-day study on diet and chronic disease risk. The analyses reported here focus on relationships with memory performance which were collected in a subset of participants (n=110) as part of their involvement in the larger, 10-day study. Generally healthy men and women were included into the study if they were aged 18–65y, had a BMI of 18.5–45 kg/m2 and a nonsmoker. Individuals were excluded from the study if they had any of the following: any condition or were taking medications or dietary supplements known to affect energy regulation or appetite (for example: lipid lowering medications, antihistamines, antibiotics, cardiovascular drugs, thyroid medications, ephedra, ginseng or guarana); chronic disease risk factors including systolic blood pressure ≥140 mm/Hg, diastolic blood pressure ≥90 mm/Hg, fasting values for glucose ≥126 mg/dL, high density lipoprotein cholesterol <40 mg/dL, low density lipoprotein cholesterol ≥90 mg/dL, total cholesterol ≥240 mg/dL or triglycerides ≥500 mg/dL; known food allergies/intolerances or dietary restrictions that would prohibit consumption of the study diets; were pregnant or lactating within the past 1 year; unstable body weight (gain or loss of >5 lb in the past 6 mo); performed physical activity or exercise >12 hours per week at moderate or greater intensity; consumed ≥ 3 alcoholic drinks per day; or were peri- or postmenopausal. Candidates who qualified for the study were invited to participate. Of the 110 participants who performed the memory tests, seventeen participants were dropped from the final analysis: nine participants were excluded for having incomplete data and eight participants were excluded for having unusually low recalls during the memory task (e.g., learning less than 50% of the memorized word pairs). All analyses were conducted on the final sample of N=93 (see Table 1 for Participant Characteristics). All subjects gave their informed consent prior to participation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board at Purdue University.
Table 1.
Participant characteristics
| M | SD | |
|---|---|---|
| Physical Characteristics | ||
| Age (years) | 28.83 | 11.92 |
| BMI (kg/m2) | 24.13 | 3.58 |
| Adiposity (%)a | 27.43 | 8.89 |
| Sex (F/M) | 56/37 | -- |
| Race | ||
| White | 70 | -- |
| Black | 7 | -- |
| Asian | 13 | -- |
| Other | 3 | -- |
| Eating Traits | ||
| TFEQ-R subtypes (Bond et al., 2001) | ||
| Strategic Dieting | 1.30 | 1.33 |
| Attitude to Self-Regulation | 1.60 | 1.05 |
| Avoidance of Fat. | 2.37 | 1.28 |
| TFEQ-D subtypes (Bond et al., 2001) | ||
| Habitual Susceptibility | 1.12 | 1.17 |
| Emotional Susceptibility | 0.87 | 1.13 |
| Situational Susceptibility | 2.62 | 1.16 |
| TFEQ-R18-V2 (Cappelleri et al., 2009). | ||
| Cognitive Restraint | 2.55 | 1.94 |
| Uncontrolled Eating | 2.61 | 2.54 |
| Emotional Eatingb | 0.87 | 1.13 |
| Restraint subtypes (Westehoefer et al., 1999) | ||
| Flexible Control | 2.80 | 1.64 |
| Rigid Control | 2.43 | 1.91 |
| Dietary Factorsc | ||
| Total Energy Intake | 2259.84 | 701.14 |
| Carbohydrate (% energy) | 49.91 | 7.99 |
| Fat (% energy) | 33.96 | 7.60 |
| Protein (% energy) | 16.03 | 3.93 |
| Added Sugars (% energy) | 12.87 | 6.26 |
| SFA (% energy) | 10.92 | 3.08 |
Mean (SD) for the 93 participants included in this study.
Adiposity calculated as percent of fat mass (g) to total mass (g) by air displacement plethysmography
Note: The emotional eating scores are identical for Cappelleri et al. and Bond et al. because both factors are comprised of the same three items.
Total energy intake was obtained from a 3-day diet recall and represents the average calories consumed by the participants, calculated as a percentage of resting energy expenditure. Macronutrients reported as a percentage of total calories. Added sugars and saturated fatty acids were included a priori based on prior literature linking the Western diet (high in saturated fats and added sugars) to impairments in memory and cognitive function.
SFA, saturated fatty acids; BMI, body mass index
Procedure
Prior to the start of the study, informed consent was obtained and the participant was told about the objectives of the study. Participants were told that the study aimed to investigate relationships between “diet and chronic disease risk” but no explicit objectives regarding the memory test were given. On the day of the memory test, participants attended a 1-hour laboratory session at The Department of Nutrition Science at Purdue University. Sessions were scheduled in the morning with the participant fasted overnight for a minimum of 10 hours. Participants were shown to a private room where they were given instructions on how to complete the memory task on the computer and were administered the first two portions of the memory task (see Methods details, below). After finishing the initial study and practice phases of the memory task, the participants were given questionnaires to complete during a 20-minute delay period before taking a final memory test. Afterwards, the participant was given a snack and thanked for their participation.
Anthropometry and Body Composition
Fasting body weight and body composition were assessed with BOD POD air displacement plethysmography (Model 2000, Cosmed USA, Concord, CA) with measured thoracic gas volume (Dempster & Aitkens, 1995; McCrory, Gomez, Bernauer, & Molé, 1995). Measurements were taken with the participant wearing minimal clothing and a swimcap, according to manufacturer’s instructions, until two values for percentage body fat agreed within 2.0% body fat. Body composition was calculated from body density (BOD POD software, version 1.69) using the Schutte equation (Schutte et al., 1984) for black males, the Ortiz equation (Ortiz et al., 1992) for black females, and the Siri equation (Siri, 1961) for all other participants. Height was measured in duplicate to the nearest 0.1 cm using a wall-mounted stadiometer, and additional measures were taken as necessary until two values agreed within 0.25 cm. BMI was calculated as weight (in kg) divided by height2 (in m).
Dietary Intake
Three, multiple-pass 24-hour dietary recalls were used to assess participants’ typical energy and macronutrient intake (Conway, Ingwersen, & Moshfegh, 2004; Conway, Ingwersen, Vinyard, & Moshfegh, 2003) over the 10-day study period. Dietary recalls were conducted with the assistance of visual aids including two-dimensional food models (Nutrition Consulting Enterprises) and portion size tools (i.e., household measuring cups and spoons). For each recall, participants were first asked to list what they ate the day before in chronological order (i.e., number and type of meals) and were then guided by the experimenter to provide explicit details for each eating occasion (i.e., portion sizes, ingredients, cooking preparations, ‘missed’ calories and nutrients in the form of added sugar, salt, and other condiments, etc.). Participants were also prompted to remember commonly forgotten foods (e.g., “Did you have any coffee, tea, or soft drinks? Did you add any sugar or milk to your tea?”) (for full procedure, see Conway et al., 2003). Finally, the total recall was summarized for participants. The first recall was completed in person, and the final two were completed by telephone. Efforts were made to collect dietary recalls for two weekdays and one weekend day. The dietary recalls were entered and analyzed using Nutrition Data System for Research (NDSR) database developed by the Nutrition Coordinating Center (University of Minnesota, Minneapolis, MN). The NDSR database provides an accurate estimate of each foods’ nutritional and energy content based on brand-, manufacturer-, and restaurant-specific formulations, as well as nutritional estimates for unlabelled foods.
Assessment of Uncontrolled Eating
Eating behavior was assessed with the Three Factor Eating Questionnaire (Stunkard & Messick, 1985) using the updated factors suggested by Cappelleri et al. (2009): uncontrolled eating, emotional eating, and cognitive restraint. Our analysis also included specific subtypes of uncontrolled eating that have been identified by Bond et al. (2001) and Westenhoefer et al. (1999). The Bond et al. scores identified three disinhibitory subtypes (situational/habitual/emotional susceptibility to disinhibition) and three restrictive subtypes (strategic dieting behavior, avoidance of fattening foods, attitude to self-regulation). We omitted the hunger constructs from our analysis on the basis that they overlapped with the disinhibition construct (Cappelleri et al., 2009) and are more likely to measure state-based changes in appetite rather than a stable trait (Yeomans & McCrickerd, 2017). Thus, we included 6 factors from the Bond scales: Strategic dieting behavior refers to actions used to control one’s weight (e.g., ‘deliberately take small helpings’, ‘consciously holding back at meals’); Attitude to self-regulation (of eating) refers to one’s general perspective on eating and weight control (e.g. ‘life is too short to worry about dieting’, ‘I eat anything I want, anytime I want’); Avoidance of fattening foods refers to one’s likelihood of buying and eating palatable foods (e.g., ‘How frequently do you avoid stocking up on tempting foods?’, ‘How likely are you to shop for low calorie foods?’); Habitual susceptibility (to disinhibition) represents the foundational construct of disinhibition, which is the recurrent loss of control of eating (e.g., ‘I start dieting in the morning, but because of any number of things that happen during the day, by evening I have given up and eat what I want, promising myself to start dieting again tomorrow’); Emotional susceptibility (to disinhibition) refers to comfort eating (i.e., ‘I feel anxious’, ‘I feel blue’); and Situational susceptibility (to disinhibition) refers to eating in response to environmental cues (e.g., ‘at social occasions’, ‘with someone else who is overeating’). Lastly, we included two subtypes of restraint suggested by Westenhoefer et al. (1999): flexible restraint which represents a balanced approach to food restriction and an overall more successful dieting strategy, and rigid restraint which refers to ‘all-or-nothing’ approaches to self-restriction that often result in loss of control over eating and disinhibition. Score validity was ensured by only including participants who answered at least 85% of the questions from each scale and calculating proportional scores according to procedures described previously (Hays et al., 2002). For all scales, higher scores refer to higher levels of restraint or disinhibition.
Assessment of Memory Inhibition and Episodic Recall
Episodic and inhibitory memory were assessed using the RP paradigm according to the procedures described in Anderson, Bjork, & Bjork (1994). The RP paradigm involves both an episodic recall component (in which participants memorize a subset of word pairs), as well as a memory inhibitory component (a measurable amount of ‘forgetting’ that occurs for a subset of word pairs). To produce memory inhibition, the RP paradigm capitalizes on a cognitive phenomenon known as retrieval induced forgetting (RIF)—when multiple items are associated together in memory, the act of memorizing one item will result in competing items being temporarily inhibited or ‘forgotten’ (for in-depth reviews of this inhibitory phenomenon, see Anderson, 2003; Anderson & Green, 2001; Storm & Levy, 2012). Thus, trying to learn a subset of Fruits (Apple, Banana) might lead to the temporary suppression of other, non-practiced items in that category (Orange, Cherry). The RP paradigm deliberately produces this memory inhibitory effect by forcing some items into competition with each other during a memorization procedure--the memorized items that are practiced by the participant (RP+) inadvertently compete with other items within the same category (RP−); these non-practiced RP− items are consequentially ‘forgotten’ or inhibited. This effect is observed at a final recall test when participants are asked to recall all of the words from the initial study phase--participants tend to recall fewer RP− words than a set of matched ‘control’ items (NP) that also did not undergo retrieval practice (Anderson et al., 1994; Levy & Anderson, 2002). The difference in recall calculated between these two conditions is the RIF score, our measure of memory inhibition. In support of the idea that RIF is a memory inhibitory effect, studies have shown that RIF is predicted by performance on other cognitive inhibitory tasks, such as measures of executive function (Román, Soriano, Gómez-Ariza, & Bajo, 2009) and working memory capacity (Aslan & Bäuml, 2012), and that RIF is impaired in individuals characterized by other inhibitory deficits—such as individuals with attention deficit hyperactivity disorder (ADHD) (Storm & White, 2010), and very young children whose frontal-inhibitory regions have not yet matured (Aslan & Bäuml, 2010).
The RP paradigm consists of three phases—a study phase, a practice phase, and a final test phase. During the study phase, participants viewed 48 category-exemplar pairs; there were eight categories containing six exemplars per category (e.g., FRUIT – orange, FRUIT – pineapple, INSECT – beetle, INSECT – hornet). Participants were instructed to study each pair in preparation for a later memory test. Each pair was presented for five seconds in a randomized order. After completing this initial study phase of all the items, participants practiced retrieving a subset of the pairs from memory (items that receive retrieval practice; RP+). In this ‘practice phase’, participants were given 3 cued-recall tests on twelve of the category-exemplar pairs. For each pair, the category and a two-letter stem was presented to the participants to help them recall the words (e.g., Fruit: ba_____, Fruit: ap_____) and participants typed their responses using a keyboard. After a 20 minute delay, participants were then given a final cued-recall test over the original 48 exemplars. For this final test, participants were presented with a category name and were asked to type as many exemplars as they could remember from the original study list (i.e. participant is shown “FRUIT - _____” and types “orange, pineapple, apple....”). They were given thirty seconds to enter their responses for each category.
The key manipulation of the RP paradigm is that the participant only practices retrieving 12 of the 48 exemplars during the practice phase (RP+); the remaining items receive no practice. Importantly, some of the unpracticed items come from categories for which none of the items were practiced (NP), and the other items come from the same category as the RP+ items but received no practice themselves--these are the items that become inhibited as a result of the practice procedure (RP−). For instance, the participant studied “Fruit: apple” which is in the RP+ condition but did not study “Fruit: orange” which is in the RP− condition. The act of practicing the RP+ items results in the inhibition of the associated RP− items. This is determined at the final recall test by calculating the percentage of practiced items (RP+), unpracticed items from the same category as the practiced items (RP−), and items from unpracticed categories (NP) that were recalled by the participant.
Statistical Analyses
Percent recall was calculated for each participant on each of the three recall conditions (RP+, RP−, NP) using a computerized scoring algorithm, which tallied the number of words the participant correctly recalled and converted them to a percentage score. To confirm whether the RP paradigm was effective in promoting retrieval induced forgetting in our sample, we first conducted a repeated measures ANOVA comparing percent recall across the three recall conditions: RP+ (practiced items), RP− (unpracticed items which should become inhibited as a function of the learning procedure), and NP (control items that receive the same amount of retrieval practice as the RP− items but which aren’t subject to the intracategory interference that leads to retrieval induced forgetting). Mauchley’s test from this analysis indicated that the assumption of sphericity had been violated, X2(2)=8.06, p=0.02; thus, the degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = 0.92). If our task was successful at generating retrieval induced forgetting, then participants should exhibit lower recall of RP− items compared to the matched NP items. Recall of the RP+ items should be the greatest as these were the items which were actually practiced during the task.
After confirming that the task was effective at promoting retrieval induced forgetting, we sought to determine whether individual differences in memory performance were related to individual differences in self-reported food intake control. This was accomplished with hierarchical linear regression models predicting percent recall in the RP+ condition (our measure of episodic memory) and RIF (our measure of memory inhibition). RIF was calculated by subtracting percent recall of RP− items from percent recall of NP items. A priori covariates included sex, age, self-reported energy intake (as a percentage of resting energy expenditure), and percent body fat (BODPOD). Pearson correlation coefficients conducted during data exploration indicated that many of the restraint-related subscales were related to significantly lower intakes of added sugars (largest r = −0.21, p<0.05) and saturated fats (largest r = −0.36, p<0.001) so these factors were also included as covariates in order to account for these relationships, and to address associations that have been described linking Western diets (high in saturated fats and sugars) to impairments in memory and cognitive function (Hsu & Kanoski, 2014; Kanoski & Davidson, 2011; Kendig, 2014). We focused on ‘added sugars’ rather than ‘total sugars’ because added sugars more directly capture the sources of sugar at have been implicated in obesity (e.g., sugar-sweetened beverages, sugary snacks and candy).
Thus, Step 1 of the hierarchical model consisted of our base model of covariates (sex, age, reported energy intake, percent body fat, percent calories from added sugars, percent calories from saturated fatty acids) and Step 2 introduced the particular eating style of interest (e.g., emotional susceptibility; avoidance of fattening foods; rigid restraint). For analyses of RIF, we also included recall of NP items as a covariate to ensure that baseline levels of recall did not drive the RIF difference scores. This approach allowed us to examine the relationship between food intake control and memory after controlling for factors known to impact learning and memory, and to show convergence across several widely-used questionnaires regarding the relationship of restraint- and disinhibition-related eating traits to the two memory phenomena under investigation. All assumptions of multiple linear regression were satisfied. Results are presented as the mean ± standard error (M ± SD) unless otherwise stated. Analyses were performed in SPSS version 24 with a priori significance level set at p < 0.05 (SPSS, IBM, IL, USA).
RESULTS
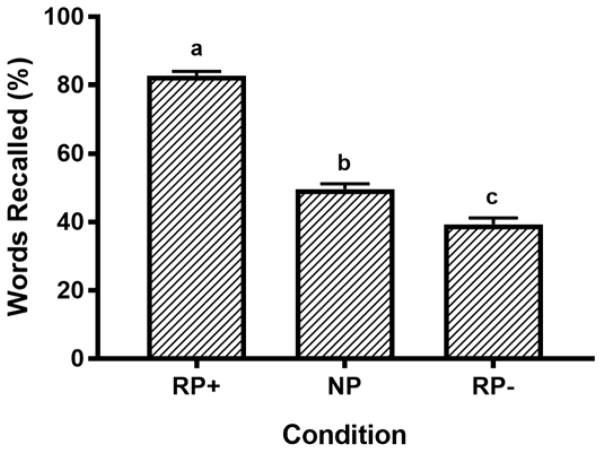
Repeated measures ANOVA with a Greenhouse-Geisser correction confirmed that the memory task was effective in producing retrieval induced forgetting. As shown in Figure 1, participants recalled fewer items from the RP− condition (the ‘inhibition’ condition) compared to the NP control condition (Main effect of Condition, F(1.844, 169.617)=318.88, p<0.001, ηp2=.78), indicating retrieval induced forgetting. Participants also recalled nearly all of the RP+ items, indicating good episodic recall in our sample. Posthoc tests using the Bonferroni correction confirmed that all three recall conditions were significantly different from each other at p<0.001.
Figure 1. The retrieval practice paradigm was effective in promoting retrieval-induced forgetting.
Practicing the RP+ items caused the forgetting of competing RP− items, as evidenced by poorer recall of RP− items compared to NP control items. This effect suggests the task was successful at engaging memory inhibitory processes, and at generating strong episodic recall in our sample. Note: Bars with different superscripts were significantly different at p<0.001
Having confirmed that the RP paradigm was effective in producing an episodic and inhibitory memory effect in our participants, we next examined whether memory performance was related to individual differences in food intake control. The results of the hierarchical regression analyses are displayed in Table 2. Contrary to our hypothesis that RIF would be weaker in individuals with tendencies towards uncontrolled eating, no significant inverse relationships were observed with any of the disinhibitory subtype scales (e.g., situational susceptibility), nor with the emotional or uncontrolled eating scales. In fact, significant positive associations were observed with rigid control (β=2.39, p=0.02) and habitual susceptibility to disinhibition (β=3.89, p=0.01) predicting greater RIF. Attitude to self-regulation was not significantly associated with lower RIF (β= −2.62, p=0.10). No other significant relationships were observed for RIF.
Table 2.
Relationships between food intake control and memory performance
| Memory Outcome | Model | β | ΔR2 | ΔR2 p value |
|---|---|---|---|---|
| Recall RP+a | Base Model | 0.04 | 0.73 | |
|
| ||||
| + Strategic Dieting | 2.45* | 0.06 | 0.02 | |
| + Attitude to Self-Regulation | 1.43 | 0.01 | 0.26 | |
| + Avoidance of Fat. | 3.41† | 0.09 | 0.004 | |
| + Habitual Susceptibility | −0.53 | 0.00 | 0.66 | |
| + Emotional Susceptibility | −2.46 | 0.05 | 0.04 | |
| + Situational Susceptibility | −2.10 | 0.04 | 0.07 | |
| + Flexible Control | 1.97* | 0.06 | 0.03 | |
| + Rigid Control | 2.22† | 0.08 | 0.008 | |
| + Cognitive Restraint | 1.55 | 0.05 | 0.04 | |
| + Uncontrolled Eating | −1.15 | 0.05 | 0.03 | |
| + Emotional Eating | −2.46 | 0.05 | 0.04 | |
|
|
||||
| RIFb | Base Modelc | 0.21 | 0.004 | |
|
| ||||
| + Strategic Dieting | 0.22 | 0.00 | 0.87 | |
| + Attitude to Self-Regulation | −2.62 | 0.03 | 0.10 | |
| + Avoidance of Fat. | 1.02 | 0.004 | 0.52 | |
| + Habitual Susceptibility | 3.89† | 0.06 | 0.01 | |
| + Emotional Susceptibility | 2.00 | 0.02 | 0.19 | |
| + Situational Susceptibility | −0.09 | 0.00 | 0.95 | |
| + Flexible Control | 1.05 | 0.008 | 0.35 | |
| + Rigid Control | 2.39* | 0.05 | 0.02 | |
| + Cognitive Restraint | 1.26 | 0.02 | 0.19 | |
| + Uncontrolled Eating | 0.20 | 0.00 | 0.78 | |
| + Emotional Eating | 2.00 | 0.02 | 0.20 | |
Hierarchical linear regression was used to examine the change in R2 with the addition of each eating style. The base model included sex, age, reported energy intake as a percentage of resting energy expenditure, percent body fat from the air displacement plethysmography, percent calories from added sugars, and percent calories from saturated fatty acids. R2 is reported for the base model and the change in R2 is reported from each univariate analysis in response to adding each eating style to the base model.
The percent of words recalled from the practiced category; a measure of episodic memory.
RIF assessed as the difference in the percent of words recalled (NP condition minus RP− condition). Higher scores indicate greater retrieval induced forgetting and are interpreted as evidence of better memory inhibition.
In addition to the other covariates, recall of NP items was included in the base model to control for baseline differences in memory of the control items
β, unstandardized regression coefficient; RIF, retrieval induced forgetting; RP+, items that received retrieval practice; NP, control items that received no retrieval practice.
p<0.05,
p<0.01
Strikingly, episodic recall performance was consistently predicted by controlled versus uncontrolled eating styles—higher scores on restraint-related scales were associated with better recall performance, whereas higher scores on disinhibition-related scales were associated with poorer recall. As shown in Table 2, recall of RP+ items was positively related to strategic dieting (β=2.45, p=0.02), avoidance of fatty foods (β=3.41, p=0.004), flexible control (β=1.97, p=0.03), rigid control (β=2.22, p=0.008), and cognitive restraint (β=1.55, p=0.04). In contrast, recall of RP+ items was negatively associated with emotional susceptibility (β= −2.46, p=0.04), uncontrolled eating (β= −1.15, p=0.03), and emotional eating (β= −2.46, p=0.04). There was a trend towards recall being negatively associated with situational susceptibility (β= −2.10, p=0.07) but this effect did not reach significance. A similar pattern was observed for items in the control condition, with uncontrolled eating relating to poorer recall of NP items (see Supplementary Table 1).
Pearson correlations controlling for age and sex indicated that memory inhibitory performance was significantly related to certain covariates. As shown in Table 3, higher energy intakes (r= −0.21, p=0.05) and higher intakes of saturated fatty acids (r= −0.21, p=0.02) were both associated with lower RIF (i.e., weaker memory inhibition). No significant relationships were observed for episodic recall performance (i.e., RP+ condition). As expected, RIF performance was significantly related to recall of the NP condition (on which the RIF difference score is calculated) (r= 0.33), supporting our rationale for including NP recall as a covariate in the hierarchical analyses involving RIF. The same pattern of results were observed in the model parameters of the hierarchical regression analyses--greater intake of saturated fatty acids trended towards predicting lower RIF scores (β= −0.88, p=0.11) and higher NP scores predicted significantly higher RIF difference scores (β= 0.33, p=0.003). With the exception of the NP recall variable, none of the base model covariates significantly contributed to performance on either memory outcome.
Table 3.
Pearson correlations between memory performance and covariatesa
| RIF | RP+ | NP | |
|---|---|---|---|
| Energy Intake | −0.21* | 0.10 | −0.11 |
| Adiposity (%) | 0.17 | −0.11 | 0.07 |
| SFA (%) | −0.25* | −0.02 | −0.18 |
| Added Sugars (%) | 0.13 | 0.05 | −0.13 |
| RIF | --- | 0.07 | 0.33† |
| RP+ | 0.07 | --- | 0.32 |
| NP | 0.33† | 0.32† | --- |
Correlation coefficients reported for each relationship, after controlling for age and sex. RIF, retrieval induced forgetting; RP+, items that received retrieval practice; NP, control items that received no retrieval practice; SFA, saturated fatty acids; BMI, body mass index
p<0.05,
p<0.01
DISCUSSION
Although deficits in behavioral inhibition have been studied as risk factors for uncontrolled eating (Bartholdy et al., 2016), studies examining the relationship between memory inhibition and eating behavior in humans are lacking. Further, although episodic memory has been implicated as a contributor to meal size, hunger, and overall intake (Higgs, 2016), few studies have examined how individual differences in general episodic recall relate to eating behavior in healthy (i.e., non-amnesic) human subjects. Here, we sought to address these gaps in the literature by examining whether individual differences in episodic recall and memory inhibitory control were related to self-reported food intake control in a sample of healthy young adults.
The major finding of this study was that general episodic recall was reliably (and oppositionally) predicted by un/-controlled eating styles--individuals who reported being more susceptible to overeating (i.e., disinhibitory subtypes) exhibited poorer episodic recall, whereas individuals who reported greater control over their intake (i.e., restrictive subtypes) exhibited better recall. This pattern was observed for items in the RP+ condition as well as the NP control condition, demonstrating convergence across multiple measures of episodic memory. Given that interference control plays a role in memory retrieval (i.e., successfully recalling one item necessitates the ability to prevent irrelevant items from interfering with retrieval of the ‘correct’ or sought-after memory (see Levy & Anderson, 2002)), it is possible that these relationships observed for episodic recall are indirectly indicative of underlying differences in cognitive-inhibitory control. However, this possibility remains to be empirically tested in follow-up studies. Regardless, the observation that restrictive vs. disinhibited eating styles are oppositionally associated with episodic recall performance is intriguing, as this result suggests that poor episodic recall may be a cognitive risk factor for overeating. This finding adds to emerging evidence that deficits in learning and memory processing might be associated with uncontrolled eating (e.g., Attuquayefio et al., 2016; Calvo, Galioto, Gunstad, & Spitznagel, 2014; Maayan, Hoogendoorn, Sweat, & Convit, 2011) and extends research linking memory impairments to the consumption of obesogenic diets (i.e., high in saturated fats and sugars) (e.g., Francis & Stevenson, 2013; Hsu & Kanoski, 2014; Kanoski & Davidson, 2011).
The most likely way in which weak episodic memory might promote uncontrolled eating is by compromising memory for recent eating. Convergent evidence from studies conducted in humans (Higgs, 2016) and non-human animals (Parent, 2016) suggests that episodic recall is critically important for the inhibitory control of intake. Without the ability to adequately encode and retrieve memories of recent meals, individuals are less capable of judging their feelings of hunger and fullness, and are less successful at regulating their energy intake throughout the day (e.g., adjusting how often they eat, or the size of their meals). To date, most studies in humans have explored this relationship by conducting meal-based assessments of food intake, either in individuals with severe episodic memory loss (e.g., Hebben et al., 1985; Rozin et al., 1998) or in normal individuals whose memory for recent eating has been manipulated (e.g., Brunstrom et al., 2012; Higgs, Robinson, & Lee, 2012). Thus, the focus has been specific to memories for eating (rather than general episodic recall), and eating behavior assessment has been restricted to a single eating occasion (rather than a more global, trait-based tendency to overeat across multiple meals). Our results extend this literature by showing--in a non-clinical, community-dwelling sample of healthy adults--that natural variations in episodic memory significantly relate to individual differences in food intake control, as assessed by trait-based measures of ‘typical’ eating behavior.
In addition to episodic memory, we also examined whether memory inhibition was related to individual differences in eating behavior. On the basis that overeating is attributed, in part, to failures to inhibit the thoughts about food that motivate us to eat (Martin & Davidson, 2014), we predicted that disinhibitory traits representing a loss of control over eating would be negatively associated with RIF, our primary measure of memory inhibition. Previous studies have shown that individuals who exhibit greater amounts of RIF also exhibit better working memory (Aslan & Bäuml, 2012) and faster reaction times in behavioral measures of impulsivity (Schilling, Storm, & Anderson, 2014). Neuroimaging studies suggest that brain regions associated with inhibitory control (e.g., dorsolateral prefrontal cortex) play an active role in determining RIF (Kuhl, Dudukovic, Kahn, & Wagner, 2007; Penolazzi, Stramaccia, Braga, Mondini, & Galfano, 2014). This evidence is consistent with the idea that RIF reflects a common underlying deficit in cognitive-inhibitory/executive functioning, and would be sensitive to detecting memory inhibitory deficits in our participants (see Levy & Anderson, 2002; Storm & Levy, 2012). On this basis, we anticipated that individuals who report being less able to suppress their intake would be less capable of suppressing their memories, and that this implicit deficit in executive-inhibitory functioning would manifest as less forgetting in our RP paradigm. However, counter to our predictions, none of the disinhibitory eating styles we assessed were inversely related to RIF. In fact, two unexpected relationships were observed wherein both habitual susceptibility and rigid restraint both predicted higher rates of forgetting in our task (i.e., ‘better’ memory inhibition).
At present, the basis for these results are unclear. Our study was not the only one to observe relationships for episodic memory but not inhibitory performance—Attuquayefio et al. (2016) similarly failed to observe any relationship between TFEQ-Disinhibition and memory inhibitory performance on a “Think/No Think” task despite observing a significant relationship with a measure of episodic recall (albeit, in the opposite direction reported here). In that study, the authors concluded that episodic measures may simply be more robust than measures of memory inhibition. However, the fact that episodic memory performance was more robust than RIF does not seem to be a convincing explanation for why we did not observe relationships between RIF and uncontrolled eating in our study, given that we observed a significant RIF effect in our sample.
One possible explanation for why we failed to observe relationships between RIF and uncontrolled eating is that the deficits in episodic memory observed in uncontrolled eaters masked the associations with RIF. According to interference theory, the degree to which items will become inhibited or ‘forgotten’ in the RP paradigm depends upon, a) participants’ ability to memorize a subset of items (necessitating the inhibition of competing items that are disruptive to recall) and, b) the ‘retrievability’ of the competing items (i.e., items only need to be inhibited if they interfere with recall; weak associates – or impairments in recall ability--might reduce the likelihood that these items will be retrieved and, thereby, undermine any need to ‘inhibit’ them) (for reviews, see Levy & Anderson, 2002; Storm & Levy, 2012). On this basis, the deficits in episodic recall observed in uncontrolled eaters may have disrupted the conditions necessary for generating a strong retrieval-induced forgetting effect, and thereby minimized our ability to observe relationships with uncontrolled eating. At present, this is only speculation and remains to be empirically tested. Our findings highlight an important point—namely, that the memory processes involved with food intake regulation do not function independently but are likely to interact, sometimes in unexpected ways. This is especially true for executive functions, which typically encompass a host of overlapping cognitive and inhibitory processes (Rabbit, 1997). Thus, studies examining the cognitive control of intake must consider the possibility that the outcome of a given learning and memory test may be depend on an interaction involving multiple memory processes or systems. Ultimately, further studies are needed exploring the relationship between memory functions and uncontrolled eating in order to elucidate these relationships, as it is only by administering these tasks and teasing apart their outcomes that we can begin to understand how complex higher-order processes, like memory inhibition, contribute to the complexities of eating behavior.
Although we observed a strong RIF effect in our sample (representing a 10% reduction in recall between the RP− and NP conditions) consistent with the idea that our task was sensitive to measuring memory inhibition, we cannot rule out the possibility that processes other than inhibition contributed to this result. Like all measures of inhibition and executive function, the RP paradigm is limited by the ‘task impurity problem’ (Rabbitt, 1997)–because higher-order cognitive-inhibitory tasks often involve many overlapping and interactive cognitive processes, it can often be difficult to determine which process is uniquely responsible for differences in performance. In other words, since the RP paradigm is not a ‘pure’ measure of memory inhibition (if such a one can even be said to exist), performance on the task might have been explained by other, non-inhibitory, cognitive processes—this could have played a role in why we did not observe relationships with uncontrolled eating. This explanation seems unlikely given that the RP paradigm is widely utilized in the field of cognition and neuroscience to assess memory inhibition in humans (for a recent meta-analysis, see Murayama, Miyatsu, Buchli, & Storm, 2014), and there is strong evidence that memory inhibition is a major cognitive process underlying RIF (for a detailed review, see Storm & Levy, 2012). However, task-sensitivity is always a concern in these studies and cannot be ruled out as an explanation for why we failed to observe effects between uncontrolled eating and RIF here.
It is worth noting that although RIF was not associated with uncontrolled eating in this study, RIF was significantly correlated with dietary factors that have been implicated in the development of memory inhibitory deficits—higher total energy intakes and higher intakes of saturated fatty acids were both associated with lower levels of forgetting in our study, consistent with what has been reported in rodents (Davidson et al., 2014). A variety of evidence suggests that consumption of ‘obesogenic’ diets high in fats and sugars contributes to neuroinflammation in brain regions which underlie memory inhibitory control, thereby contributing to a “Vicious Cycle” of disinhibited eating and obesity (Davidson et al., 2005; Hsu & Kanoski, 2014). Memory disruptions have also been linked to saturated fat intake in humans (Francis & Stevenson, 2013). Our observation that forgetting was associated with intake of saturated fats suggests that the RP Paradigm may, in fact, have been sensitive to (diet-induced) deficits in memory inhibition, even though relationships were not observed for uncontrolled eating.
Strengths of our study include the use of an objective (rather than subjective, self-report based) measure of memory inhibition, the use of a within-subjects design with carefully implemented control conditions to rule out non-specific differences in performance (i.e., inattention), and the assessment of two major types of memory performance using a novel, well-established measure of memory interference. There were also some limitations to our study. One is that we relied on self-reported measures of uncontrolled eating and dietary intake. While these behavioral questionnaires are commonly used in the field and have high predictive validity in discriminating individuals of various levels of food intake control, dieting success, and weight status (e.g., Bryant, King, & Blundell, 2008), it is nevertheless ideal to have an objective measure of food intake control (e.g., food cue reactivity; preload compensation) and this was lacking in this experiment. Second, although our results link deficits in episodic recall with failures to regulate one’s intake, we did not assess either memory for recent eating or energy intake in this study; thus, we cannot make any causal claims about the mechanisms underlying this relationship. Indeed, uncontrolled eating is often associated with greater consumption of the ‘obesogenic’ maconutrients that have been shown to disrupt memory performance (i.e., saturated fats and sugars); thus, it is possible that uncontrolled eating is related to memory deficits via consumption of an obesogenic diet (Davidson et al., 2005). Follow-up studies that assess these phenomena (i.e., episodic memory, memory for recent eating, and energy intake) in the same cohort will be critical for establishing the exact nature and direction of these relationships. Third, our study utilized only one measure of memory inhibition. Future work in this field would benefit from studies explicitly contrasting uncontrolled eating in relation to a variety of memory inhibitory tasks (e.g., directed forgetting; working memory; task switching or reversal learning). This kind of work will be key for determining which tasks are sensitive to differences in memory inhibition (i.e., convergent validity), and for establishing common deficits in memory inhibition as a risk factor for overeating in humans.
CONCLUSION
This study is one of the few to directly examine whether deficits in memory inhibition contribute to overeating in humans. Although our results did not reveal strong evidence that uncontrolled eating is mediated by memory inhibitory deficits, we did uncover a novel relationship between food intake control and episodic recall ability that has not been reported previously--across several diagnostic eating behavior instruments, susceptibilities to overeat were associated with reductions in episodic memory. This finding highlights the novel possibility that episodic memory deficits may contribute to a loss of control over eating, potentially (albeit speculatively) by compromising important food-related memory phenomena, such as memory for recent eating. On this basis, cognitive interventions aimed at improving episodic recall, in concert with other cognitive-inhibitory functions like working memory (Houben, Dassen, & Jansen, 2016; Verbeken, Braet, Goossens, & van der Oord, 2013) and selective attention (Boutelle, Monreal, Strong, & Amir, 2016) might be an effective way of increasing food-intake control in individuals at-risk for obesity (see Boutelle & Bouton, 2015; Jansen, Houben, & Roefs, 2015). Future studies are needed in order to causally establish whether weaker recall performance indeed relates to weaker food-related memory, as has been suggested here, and to establish whether cognitive interventions directed at improving episodic memory might be a novel therapeutic target for offsetting overeating and weight gain (see Higgs, Robinson, & Lee, 2012).
Supplementary Material
Acknowledgments
All authors have read and approved the manuscript as submitted. All authors declare no conflict of interest. This research was funded by the National Institutes of Health (P01HD052112-6; R01DK075862).
Footnotes
AUTHORS CONTRIBUTIONS
AM, TD, and MM designed the study; AM and MM conducted the research; AM analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson M. Rethinking interference theory: Executive control and the mechanisms of forgetting. Journal of Memory and Language. 2003;49 https://doi.org/10.1016/j.jml.2003.08.006. [Google Scholar]
- Anderson MC, Bjork Ra, Bjork EL. Remembering can cause forgetting: retrieval dynamics in long-term memory. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1994;20(5):1063–87. doi: 10.1037//0278-7393.20.5.1063. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7931095. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410(6826):366–9. doi: 10.1038/35066572. https://doi.org/10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Aslan A, Bäuml KHT. Retrieval-induced forgetting in young children. Psychonomic Bulletin & Review. 2010;17(5):704–709. doi: 10.3758/PBR.17.5.704. https://doi.org/10.3758/PBR.17.5.704. [DOI] [PubMed] [Google Scholar]
- Aslan A, Bäuml KHT. Retrieval-induced forgetting in old and very old age. Psychology and Aging. 2012;27(4):1027–1032. doi: 10.1037/a0028379. https://doi.org/10.1037/a0028379. [DOI] [PubMed] [Google Scholar]
- Attuquayefio T, Stevenson RJ, Boakes RA, Oaten MJ, Yeomans MR, Mahmut M, Francis HM. A High-Fat High-Sugar Diet Predicts Poorer Hippocampal-Related Memory and a Reduced Ability to Suppress Wanting Under Satiety. Journal of Experimental Psychology: Animal Learning and Cognition. 2016;42(4):415–428. doi: 10.1037/xan0000118. https://doi.org/10.1037/xan0000118. [DOI] [PubMed] [Google Scholar]
- Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neuroscience & Biobehavioral Reviews. 2016;64:35–62. doi: 10.1016/j.neubiorev.2016.02.010. https://doi.org/10.1016/j.neubiorev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Bond MJ, McDowell AJ, Wilkinson JY. The measurement of dietary restraint, disinhibition and hunger: an examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ) International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2001;25(6):900–6. doi: 10.1038/sj.ijo.0801611. https://doi.org/10.1038/sj.ijo.0801611. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Bouton ME. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015 doi: 10.1016/j.appet.2015.05.013. https://doi.org/10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed]
- Boutelle KN, Monreal T, Strong DR, Amir N. An open trial evaluating an attention bias modification program for overweight adults who binge eat. Journal of Behavior Therapy and Experimental Psychiatry. 2016;52:138–46. doi: 10.1016/j.jbtep.2016.04.005. https://doi.org/10.1016/j.jbtep.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom JM, Burn JF, Sell NR, Collingwood JM, Rogers PJ, Wilkinson LL, … Ferriday D. Episodic Memory and Appetite Regulation in Humans. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0050707. https://doi.org/10.1371/journal.pone.0050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant EJ, King Na, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2008;9(5):409–19. doi: 10.1111/j.1467-789X.2007.00426.x. https://doi.org/10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Calvo D, Galioto R, Gunstad J, Spitznagel MB. Uncontrolled eating is associated with reduced executive functioning. Clinical Obesity. 2014;4(3):172–179. doi: 10.1111/cob.12058. https://doi.org/10.1111/cob.12058. [DOI] [PubMed] [Google Scholar]
- Cappelleri J, Bushmakin A, Gerber R, Leidy N, Sexton C, Lowe M, Karlsson J. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants. International Journal of Obesity. 2009;33:611–620. doi: 10.1038/ijo.2009.74. https://doi.org/10.1038/ijo.2009.74. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. Journal of the American Dietetic Association. 2004;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. https://doi.org/10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. The American Journal of Clinical Nutrition. 2003;77(5):1171–8. doi: 10.1093/ajcn/77.5.1171. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12716668. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. https://doi.org/10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86(5):731–46. doi: 10.1016/j.physbeh.2005.09.004. https://doi.org/10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & Behavior. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. https://doi.org/10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Tracy AL, Schier LA, Swithers SE. Journal of Experimental Psychology: Animal Learning and Cognition A View of Obesity as a Learning and Memory Disorder A View of Obesity as a Learning and Memory Disorder. 2014 doi: 10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMPSTER P, AITKENS S. A new air displacement method for the determination of human body composition. Medicine & Science in Sports & Exercise. 1995;27(12):1692–1697. https://doi.org/10.1249/00005768-199512000-00017. [PubMed] [Google Scholar]
- Erskine JAK, Georgiou GJ. Effects of thought suppression on eating behaviour in restrained and non-restrained eaters. Appetite. 2010;54(3):499–503. doi: 10.1016/j.appet.2010.02.001. https://doi.org/10.1016/j.appet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–28. doi: 10.1016/j.appet.2012.12.018. https://doi.org/10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Francis La, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Archives of Pediatrics & Adolescent Medicine. 2009;163(4):297–302. doi: 10.1001/archpediatrics.2008.579. https://doi.org/10.1001/archpediatrics.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. https://doi.org/10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007;48:119–122. doi: 10.1016/j.appet.2006.06.004. https://doi.org/10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. Disinhibition is easier learned than inhibition. The effects of (dis)inhibition training on food intake. Appetite. 2012;59:96–99. doi: 10.1016/j.appet.2012.04.006. https://doi.org/10.1016/j.appet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Schrooten M, Martijn C, Jansen A. Inducing impulsivity leads high and low restrained eaters into overeating, whereas current dieters stick to their diet. Appetite. 2009;53:93–100. doi: 10.1016/j.appet.2009.05.013. https://doi.org/10.1016/j.appet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. The American Journal of Clinical Nutrition. 2002;75(3):476–83. doi: 10.1093/ajcn/75.3.476. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11864852. [DOI] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behavioral Neuroscience. 1985;99(6):1031–1039. doi: 10.1037//0735-7044.99.6.1031. https://doi.org/10.1037/0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23(1):100–107. doi: 10.1002/hipo.22062. https://doi.org/10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- Higgs S. Cognitive processing of food rewards. Appetite. 2016;104:10–17. doi: 10.1016/j.appet.2015.10.003. https://doi.org/10.1016/j.appet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Higgs S, Robinson E, Lee M. Learning and memory processes and their role in eating: implications for limiting food intake in overeaters. Current Obesity Reports. 2012;1:91–98. [Google Scholar]
- Houben K, Dassen FCM, Jansen A. Taking control: Working memory training in overweight individuals increases self-regulation of food intake. Appetite. 2016;105:567–574. doi: 10.1016/j.appet.2016.06.029. https://doi.org/10.1016/j.appet.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Frontiers in Aging Neuroscience. 2014;6(88):1–6. doi: 10.3389/fnagi.2014.00088. https://doi.org/10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Houben K, Roefs A. A Cognitive Profile of Obesity and Its Translation into New Interventions. Frontiers in Psychology. 2015;6:1807. doi: 10.3389/fpsyg.2015.01807. https://doi.org/10.3389/fpsyg.2015.01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Nederkoorn C, van Baak L, Keirse C, Guerrieri R, Havermans R. High-restrained eaters only overeat when they are also impulsive. Behaviour Research and Therapy. 2009;47(2):105–10. doi: 10.1016/j.brat.2008.10.016. https://doi.org/10.1016/j.brat.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiology & Behavior. 2012:1–8. doi: 10.1016/j.physbeh.2012.01.007. https://doi.org/10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiology & Behavior. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. https://doi.org/10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig MD. Cognitive and behavioural effects of sugar consumption in rodents. A review. Appetite. 2014;80:41–54. doi: 10.1016/j.appet.2014.04.028. https://doi.org/10.1016/j.appet.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nature Neuroscience. 2007;10(7):908–914. doi: 10.1038/nn1918. https://doi.org/10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2016;68:714–726. doi: 10.1016/j.neubiorev.2016.06.041. https://doi.org/10.1016/j.neubiorev.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences. 2002;6(7):299–305. doi: 10.1016/s1364-6613(02)01923-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12110363. [DOI] [PubMed] [Google Scholar]
- Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited Eating in Obese Adolescents Is Associated With Orbitofrontal Volume Reductions and Executive Dysfunction. Obesity. 2011;19:1382–1387. doi: 10.1038/oby.2011.15. https://doi.org/10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiology & Behavior. 2014 doi: 10.1016/j.physbeh.2014.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, Schmidt U. A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neuroscience & Biobehavioral Reviews. 2016;71:506–528. doi: 10.1016/j.neubiorev.2016.09.024. https://doi.org/10.1016/j.neubiorev.2016.09.024. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Gomez TD, Bernauer EM, Molé PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Medicine and Science in Sports and Exercise. 1995;27(12):1686–91. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8614326. [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The Nature and Organization of Individual Differences in Executive Functions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. https://doi.org/10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. https://doi.org/10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Murayama K, Miyatsu T, Buchli D, Storm BC. Forgetting as a Consequence of Retrieval: A Meta-Analytic Review of Retrieval-Induced Forgetting. Psychological Bulletin. 2014;140(5):1383–1409. doi: 10.1037/a0037505. https://doi.org/10.1037/a0037505. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2010;29(4):389–93. doi: 10.1037/a0019921. https://doi.org/10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy. 2007;45(5):1071–1075. doi: 10.1016/j.brat.2006.05.009. https://doi.org/10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Ortiz O, Russell M, Daley TL, Baumgartner RN, Waki M, Lichtman S, … Heymsfield SB. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. The American Journal of Clinical Nutrition. 1992;55(1):8–13. doi: 10.1093/ajcn/55.1.8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1728823. [DOI] [PubMed] [Google Scholar]
- Parent MB. Dorsal Hippocampal–Dependent Episodic Memory Inhibits Eating. Current Directions in Psychological Science. 2016;25(6):461–466. https://doi.org/10.1177/0963721416665103. [Google Scholar]
- Parent MB, Darling JN, Henderson YO. Remembering to eat: hippocampal regulation of meal onset. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2014;306(10):R701–13. doi: 10.1152/ajpregu.00496.2013. https://doi.org/10.1152/ajpregu.00496.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penolazzi B, Stramaccia DF, Braga M, Mondini S, Galfano G. Human Memory Retrieval and Inhibitory Control in the Brain: Beyond Correlational Evidence. The Journal of Neuroscience. 2014;34(19):6606–6610. doi: 10.1523/JNEUROSCI.0349-14.2014. https://doi.org/10.1523/JNEUROSCI.0349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P. Introduction: Methodologies and models in the study of executive function. In: Rabbitt P, editor. Methodology of frontal and executive function. Hove, UK: Psychology Press; 1997. pp. 1–38. [Google Scholar]
- Robinson E, Aveyard P, Daley A, Jolly K, Lewis A, Lycett D, Higgs S. Eating attentively: a systematic review and meta-analysis of the effect of food intake memory and awareness on eating. American Journal of Clinical Nutrition. 2013;97(4):728–742. doi: 10.3945/ajcn.112.045245. https://doi.org/10.3945/ajcn.112.045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román P, Soriano MF, Gómez-Ariza CJ, Bajo MT. Retrieval-Induced Forgetting and Executive Control. Psychological Science. 2009;20(9):1053–1058. doi: 10.1111/j.1467-9280.2009.02415.x. https://doi.org/10.1111/j.1467-9280.2009.02415.x. [DOI] [PubMed] [Google Scholar]
- Rozin P, Dow S, Moscovitch M, Rajaram S. What Causes Humans to Begin and End a Meal? A Role for Memory for What Has Been Eaten, as Evidenced by a Study of Multiple Meal Eating in Amnesic Patients. Psychological Science. 1998;9(5):392–396. https://doi.org/10.1111/1467-9280.00073. [Google Scholar]
- Schilling CJ, Storm BC, Anderson MC. Examining the costs and benefits of inhibition in memory retrieval. Cognition. 2014;133(2):358–370. doi: 10.1016/j.cognition.2014.07.003. https://doi.org/10.1016/j.cognition.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, Blomqvist CG. Density of lean body mass is greater in blacks than in whites. Journal of Applied Physiology. 1984;56(6) doi: 10.1152/jappl.1984.56.6.1647. [DOI] [PubMed] [Google Scholar]
- Seeyave DM, Coleman S, Appugliese D, Corwyn RF, Bradley RH, Davidson NS, … Lumeng JC. Ability to delay gratification at age 4 years and risk of overweight at age 11 years. Archives of Pediatrics & Adolescent Medicine. 2009;163(4):303–8. doi: 10.1001/archpediatrics.2009.12. https://doi.org/10.1001/archpediatrics.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri W. Body composition from fluid spaces and density: Analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC, US: NAS/NRC; 1961. [Google Scholar]
- Soetens B, Braet C. “The weight of a thought”: Food-related thought suppression in obese and normal-weight youngsters. Appetite. 2006;46(3):309–317. doi: 10.1016/j.appet.2006.01.018. https://doi.org/10.1016/j.appet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Soetens B, Braet C. Information processing of food cues in overweight and normal weight adolescents. British Journal of Health Psychology. 2007;12(Pt 2):285–304. doi: 10.1348/135910706X107604. https://doi.org/10.1348/135910706X107604. [DOI] [PubMed] [Google Scholar]
- Soetens B, Braet C, Dejonckheere P, Roets A. When Suppression Backfires. Journal of Health Psychology. 2006;11(5):655–668. doi: 10.1177/1359105306066615. https://doi.org/10.1177/1359105306066615. [DOI] [PubMed] [Google Scholar]
- Soetens B, Braet C, Moens E. Thought suppression in obese and non-obese restrained eaters: Piece of cake or forbidden fruit? European Eating Disorders Review. 2008;16:67–76. doi: 10.1002/erv.771. https://doi.org/10.1002/erv.771. [DOI] [PubMed] [Google Scholar]
- Storm BC, Levy BJ. A progress report on the inhibitory account of retrieval-induced forgetting. Memory & Cognition. 2012;40(6):827–843. doi: 10.3758/s13421-012-0211-7. https://doi.org/10.3758/s13421-012-0211-7. [DOI] [PubMed] [Google Scholar]
- Storm BC, White HA. ADHD and retrieval-induced forgetting: Evidence for a deficit in the inhibitory control of memory. Memory. 2010;18(3):265–271. doi: 10.1080/09658210903547884. https://doi.org/10.1080/09658210903547884. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. https://doi.org/10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Goossens L, van der Oord S. Executive function training with game elements for obese children: A novel treatment to enhance self-regulatory abilities for weight-control. Behaviour Research and Therapy. 2013;51:290–299. doi: 10.1016/j.brat.2013.02.006. https://doi.org/10.1016/j.brat.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. International Journal of Eating Disorders. 1999;26(1):53–64. doi: 10.1002/(sici)1098-108x(199907)26:1<53::aid-eat7>3.0.co;2-n. https://doi.org/10.1002/(SICI)1098-108X(199907)26:1<53::AID-EAT7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Brace A. Cued to Act on Impulse: More Impulsive Choice and Risky Decision Making by Women Susceptible to Overeating after Exposure to Food Stimuli. PLOS ONE. 2015;10(9):e0137626. doi: 10.1371/journal.pone.0137626. https://doi.org/10.1371/journal.pone.0137626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, McCrickerd K. Acute hunger modifies responses on the Three Factor Eating Questionnaire hunger and disinhibition, but not restraint, scales. Appetite. 2017;110:1–5. doi: 10.1016/j.appet.2016.12.008. https://doi.org/10.1016/j.appet.2016.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.