Abstract
Transcatheter mitral valve replacement (TMVR) procedures can be an alternative to surgical valve replacement for high surgical risk patients with bioprosthetic mitral valves, annuloplasty rings, or severe mitral annular calcification (MAC). TMVR can trigger acute left ventricular outflow tract (LVOT) obstruction from permanent displacement of the native anterior mitral leaflet toward the left ventricular septum, more often among patients undergoing valve-in-ring and valve-in-MAC procedures. Although acute LVOT obstruction is well described in the literature, there are important additional complications of TMVR related to the length and/or redundancy of the anterior mitral valve that have been recognized after mitral valve surgery and have not been previously reported in the setting of TMVR. These additional complications include acute mitral regurgitation secondary to prolapsing native leaflet through the TMVR, frozen TMVR leaflet secondary to overhanging native leaflet and late LVOT obstruction in the neo-LVOT secondary to long native leaflet. Preprocedural planning with imaging (echocardiography and computed tomography) and measurement of anterior mitral leaflet length is critical important in understanding the risk for these complications. As transcatheter mitral valve technology proliferates, interactions with the anterior mitral leaflet after TMVR may be more frequent than initially anticipated. We believe that there is no advantage to an intact anterior leaflet and advocate removal or reduction of the leaflet prior to TMVR.
Keywords: transcatheter mitral valve replacement, valve-in-valve, valve-in-ring, valve-in-native, valve-in-MAC, redundant anterior leaflet, complications
INTRODUCTION
Transcatheter mitral valve replacement (TMVR) procedures can be an alternative to surgical valve replacement for high surgical risk patients with bioprosthetic mitral valves, annuloplasty rings, or severe mitral annular calcification (MAC) [1–4]. TMVR can trigger acute left ventricular outflow tract (LVOT) obstruction from permanent displacement of the native anterior mitral leaflet toward the left ventricular septum, more often among patients undergoing valve-in-ring and valve-in- MAC procedures [5]. Although acute LVOT obstruction is well described in the literature, there are important additional complications of TMVR related to the length and/or redundancy of the anterior mitral valve that have been recognized after mitral valve surgery [6] and have not been previously reported in the setting of TMVR. We describe three cases of life-threatening dysfunction of transcatheter mitral valves caused by unexpected interactions with the native anterior mitral valve leaflet.
CASE #1
A 65-year-old male was referred for TMVR after having recurrent symptomatic and severe mitral regurgitation (MR). One-year earlier, he underwent a surgical mitral valve repair with a 32-mm Carpentier-Edwards Physio Annuloplasty Ring (Edwards Lifesciences, Irvine, CA). His MR recurred after surgery and two additional surgical attempts to correct his regurgitation were aborted because of severe and diffuse chest fibrosis (via both re-do sternotomy and right lateral thoracotomy approaches). Baseline 3D transesophageal echocardiogram (TEE) confirmed mitral annuloplasty ring diameters of 21 mm by 29 mm.
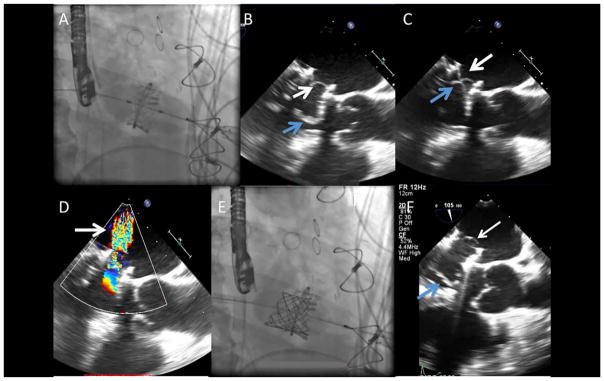
Valve-in-ring TMVR was performed via a transapical approach with a 26-mm Edwards Sapien 3 valve (Edwards Lifesciences) (Fig. 1A). Immediately after deployment, intraoperative TEE revealed severe central MR caused by a prolapsing native anterior mitral leaflet mechanically interfering with closure of a leaflet of the Sapien valve during systole (Fig. 1B–D). This iatrogenic MR was corrected by implanting a transcatheter Melody Valve (Medtronic, Minneapolis, MN) mounted on a 24-mm balloon-in-balloon, (NuMED, Inc., Hopkinton, NY) inside the Sapien valve, farther into the atrium away from the prolapsing leaflet (Fig. 1E and F). TEE images revealed normal transcatheter leaflet motion and coaptation, and the patient was discharged with marked clinical improvement.
Fig. 1.
Transcatheter mitral valve-in ring. A: A 26-mm Edwards Sapien valve was deployed at the level of the mitral valve ring using a transapical approach. B: Intraoperative transesophageal echocardiogram (TEE) after mitral valve-in-ring replacement revealed native valve leaflets (blue arrow) overhanging and interfering (C) with closure of the prosthetic leaflets (white arrow), and resulting severe central MR (D) by color Doppler (arrow). D: A 22-mm Melody valve was deployed farther into the atrium away from the prolapsing leaflet. E: TEE after second transcatheter valve showing functioning transcatheter valve leaflets (white arrow) that are a significant distance from native valve leaflets (white arrow). [Color figure can be viewed at wileyonlinelibrary.com]
CASE #2
A 74-year-old male underwent TMVR for recurrent severe symptomatic MR 13 years after surgical mitral valve repair by placement of a 28-mm model 4400 Edwards Classic ring (Edwards Lifesciences). He was deemed to have prohibitive surgical risk due to severe obstructive lung disease, severe pulmonary hypertension, and frailty. Preprocedural computed tomography (CT) scanning also revealed partial dehiscence of the anterior portion of the ring with ring diameters of 17 mm by 27 mm.
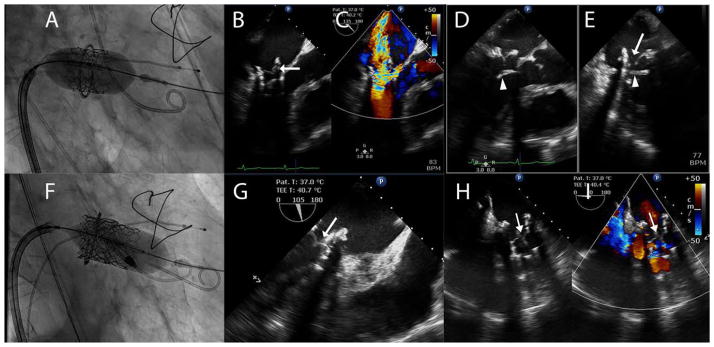
He initially underwent TMVR using a 23-mm Edwards Sapien S3 valve (Edwards Lifesciences) via a transeptal approach using a wire rail through the left ventricular apex (Fig. 2A). A 23-mm S3 valve was selected over a 26 mm S3 to avoid severe distortion of the leaflets secondary to the oval-shape annular ring (area of ring 339 mm2). Immediately after deployment there was severe central MR through the THV and TEE revealed abnormal motion of one leaflet of the THV (Fig. 2B and C), accompanied by cardiogenic shock. In an attempt to address a suspected “frozen leaflet” phenomenon, a second 23-mm Edwards Sapien S3 valve (Edwards Lifesciences) was deployed within the originally placed S3 without improvement in MR and with similar abnormal leaflet motion. Continued deterioration required placement of a transcatheter mechanical circulatory support device (Impella CP, Abiomed, Danvers, MA), which stabilized blood pressure. Further examination of the valve by TEE revealed the native anterior leaflet to be overlying the medial aspect of the THV altering flow during ventricular systole. The resultant low-pressure jet impaired systolic coaptation via a Bernoulli effect of the THV leaflets (Fig. 2D and E). A third Edwards Sapien S3 THV (Edwards Lifesciences) was subsequently deployed much farther apically (Fig. 2F) causing immediate resolution of the MR, stabilization of blood pressure, and normal THV leaflet motion (Fig. 2G–I). The patient was discharged with significant clinical improvement.
Fig. 2.
A: A 23-mm Edwards Sapien S3 valve (Edwards Lifesciences, Irvine, CA) was deployed within a 28-mm model 4400 Edwards Classic Ring (Edwards Lifesciences, Irvine CA). B, C: Transesophageal echocardiography (TEE) of the newly deployed Edwards Sapien S3 valve with simultaneous color comparison showed abnormal leaflet motion (arrow) and corresponding severe mitral regurgitation secondary to leaflet malcoaptation. D, E: An X-plane image of the Edwards Sapien 3 prosthetic mitral valve depicted the native anterior mitral leaflet (arrowhead) overlaying the prosthesis and the obstruction from the anterior leaflets was the cause of the malcoaptation of one of the prosthesis leaflets (arrow). F: A third Sapien 3 valve was implanted in a telescoping fashion with significant ventricular bias in attempts to force the anterior mitral leaflet aside. G: TEE demonstrated the symmetrical coaptation and functioning of the Sapien 3 leaflets (arrow). H, I: TEE again showed normal leaflet function (arrow) with simultaneous color flow imaging (I) demonstrating resolution of the mitral regurgitation (arrow). [Color figure can be viewed at wileyonlinelibrary.com]
CASE #3
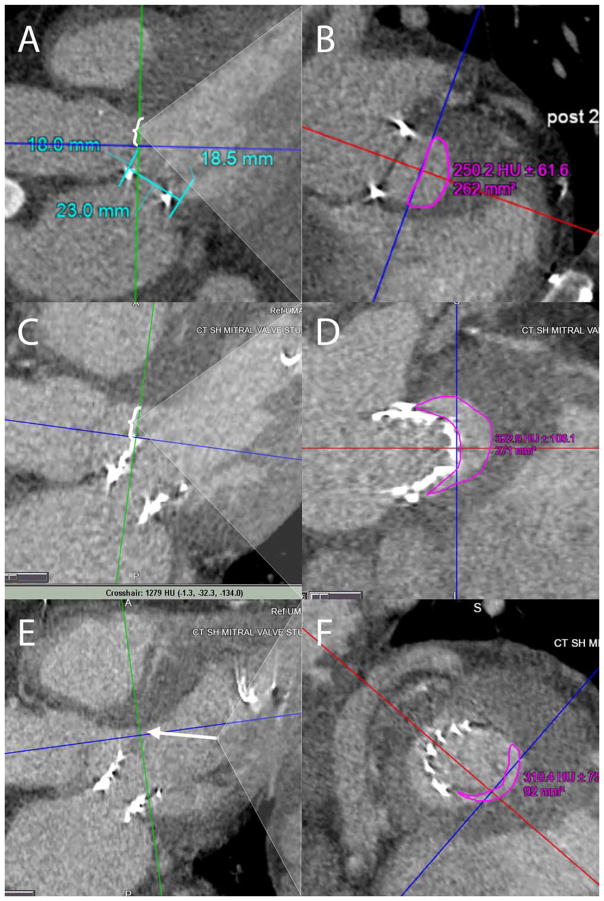
A 65-year-old male on renal replacement therapy had severe mitral stenosis (19.0 mm Hg mean gradient by transthoracic echocardiography) and New York Heart Association class IV symptoms 7 years s/p surgical coronary artery bypass grafting and mitral valve repair using a 28-mm Edwards ET Logix annuloplasty ring (Edwards Lifesciences). The heart team determined that TMVR was feasible and preferred in this high surgical risk patient. A 23-mm S3 valve was selected over a 26-mm S3 to avoid severe distortion of the leaflets secondary to the oval-shape annular ring (area of ring 317 mm2). Predicted neo-LVOT post valve deployment was 262 mm2 (Fig. 3A and D).
Fig. 3.
A, B: Pre-procedural computed tomography (CT) analysis was used to simulate the 23-mm Sapien 3 valve superimposed in the mitral space and the cross-sectional area remaining in the left ventricular outflow tract (LVOT) area was estimated (bracket) at 262 mm2 in the green plane depicted in panel (B). C, D: Post-Sapien 3 implantation CT LVOT measurement (bracket) demonstrated an adequate area of 271 mm2 when measured at the Sapien 3 stent frame. However, since the “neo-LVOT” comprises of the stent frame and anterior mitral leaflet, it was more accurate to measure the “neo- LVOT” at the tip of the anterior mitral leaflet (arrow). This “neo-LVOT” measured 92 mm2 (E and F), which could explain the flow acceleration in the LVOT after preload reduction from a dialysis session. [Color figure can be viewed at wileyonlinelibrary.com]
At the time of TMVR, baseline procedural peak LVOT gradients were 5.5 and 6.0 mm Hg by TEE and invasive measurements, respectively. Immediately following uncomplicated TMVR using a 23-mm Edwards Sapien 3 valve, peak LVOT gradients were 12 and 14.0 mm Hg by TEE and invasive measurements, respectively. During routine 1-month follow-up, echocardiography revealed a peak LVOT velocity of 4.3 m/s and peak gradient of 75.0 mm Hg. The patient was diagnosed with dynamic LVOT obstruction precipitated by volume shifts during hemodialysis. Post procedure CT confirmed a neo-LVOT of 270 mm2 when measured from the edge of the THV frame (Fig. 3B and E), which is unlikely to cause LVOT obstruction. However, the anterior mitral valve leaflet was significantly longer than the THV, draped around it, and created a neo-LVOT during systole of only 92 mm2 (Fig. 3C and F). Over time, this patient has reported some improvement in dyspnea, but LVOT tract obstruction persisted.
DISCUSSION
In our series, we describe important complications of TMVR that are caused by a long and/or redundant native anterior leaflet of the mitral valve. These complications can be equally as devastating as acute LVOT obstruction and should be considered in planning TMVR procedures and in discussion with the patients regarding risk. The pre-procedure analysis should incorporate anterior mitral leaflet length and redundancy, both on CT and echocardiography, especially in patients undergoing valve-in-ring and valvein-MAC TMVR. While limited evidence exists regarding the efficacy of CT vs. echocardiography in the evaluation of the mitral valve anatomy prior to TMVR, the use of both techniques if possible is recommended to obtain the best characterization of the landing zone.
In the first two cases, the anterior mitral leaflet’s length/redundancy, or lack of tethering by the anterior chordal apparatus created significant MR immediately post TMVR. The leaflet in Case 1 had frank prolapse through the THV and direct mechanical interference with the closure of the THV leaflets. The long leaflet in Case 2 covered the medial aspect of the THV, preventing pressurization of the leaflets and resulting severe central MR. In both patients, the MR resolved with placement of an additional THV. We suspect that placing the additional valve either more atrial (away from the prolapsing leaflet) or more ventricular (pinning the native leaflet in the open position) would be equally effective. It does not appear that depth of implantation of the initial THV would have prevented the complication, and the first THV served as an anchor for the subsequent ones. The decision to place the subsequent THV more atrial or ventricular should be based on the risk of LVOT obstruction and remain individualized. In Case 3, the anterior leaflet that was displaced by the THV elongated the neo-LVOT. Although initially there was no LVOT obstruction during the procedure, preload reduction by hemodialysis unmasked significant late LVOT obstruction.
As transcatheter mitral valve technology proliferates, interactions with the anterior mitral leaflet after TMVR may be more frequent than initially anticipated. We believe that there is no advantage to an intact anterior leaflet and advocate removal or reduction of the leaflet prior to TMVR. Prophylactic surgical strategies for removal or reduction of the anterior mitral leaflet during TMVR are under investigation [7]. Recently, intentional transcatheter laceration of the anterior leaflet to prevent LVOT obstruction (LAMPOON) was described in animal models [8] and introduces the potential for transcatheter modification of the anterior mitral valve leaflet prior to TMVR in humans.
CONCLUSIONS
TMVR is a promising strategy for treatment of patients at high surgical risk. Operators performing this procedure should be aware that the anterior mitral leaflet can cause significant mortality and morbidity by interfering with the THV and late LVOT obstruction. In these patients, concomitant procedures to remove or modify the anterior leaflet should be considered.
Footnotes
Conflict of interest: Adam Greenbaum is a proctor for Edwards Lifesciences and Abiomed. Stamatios Lerakis is a consultant for Abbott Vascular. Gaetano Paone is a proctor for Edwards Lifesciences. Vinod Thourani is a consultant for Edwards Lifesciences, Maquet, St Jude Medical, and Sorin. He is also co-founder and stock holder of Apica. Vasilis Babaliaros MD, FACC is a consultant for Abbott Vascular and Edwards Lifesciences. The other authors have nothing to disclose.
References
- 1.Shuto T, Kondo N, Dori Y, Koomalsingh KJ, Glatz AC, Rome JJ, Gorman JH, III, Gorman RC, Gillespie MJ. Percutaneous transvenous Melody valve-in-ring procedure for mitral valve replacement. J Am Coll Cardiol. 2011;58:2475–2480. doi: 10.1016/j.jacc.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Weger A, Ewe SH, Delgado V, Bax JJ. First-in-man implantation of a trans-catheter aortic valve in a mitral annuloplasty ring: Novel treatment modality for failed mitral valve repair. Eur J Cardiothorac Surg. 2011;39:1054–1056. doi: 10.1016/j.ejcts.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Petronio A, Giannini C, De Carlo M, Guarracino F. Antegrade percutaneous valve implantation for mitral ring dysfunction, a challenging case. Catheter Cardiovasc Interv. 2012;80:700–703. doi: 10.1002/ccd.24307. [DOI] [PubMed] [Google Scholar]
- 4.Seiffert M, Conradi L, Baldus S, Schirmer J, Knap M, Blankenberg S, Reichenspurner H, Treede H. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv. 2012;5:341–349. doi: 10.1016/j.jcin.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Guerrero M, Dvir D, Himbert D, Urena M, Eleid M, Wang DD, Greenbaum A, Mahadevan VS, Holzhey D, O’Hair D, Dumonteil N, Rodés-Cabau J, Piazza N, Palma JH, DeLago A, Ferrari E, Witkowski A, Wendler O, Kornowski R, Martinez- Clark P, Ciaburri D, Shemin R, Alnasser S, McAllister D, Bena M, Kerendi F, Pavlides G, Sobrinho JJ, Attizzani GF, George I, Nickenig G, Fassa AA, Cribier A, Bapat V, Feldman T, Rihal C, Vahanian A, Webb J, O’Neill W. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: From the first multicenter global registry. JACC Cardiovasc Interv. 2016;9:1361–1371.11. doi: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Esper E, Ferdinand FD, Aronson S, Karp RB. Prosthetic mitral valve replacement: Late complications after native valve preservation. Ann Thorac Surg. 1997;63:541–543. doi: 10.1016/s0003-4975(96)01107-1. [DOI] [PubMed] [Google Scholar]
- 7.Surgical Implantation of TRAnscatheter vaLve in Native Mitral Annular Calcification (SITRAL) Study (SITRAL) NCT02830204 Available at: https://ClinicalTrials.Gov.
- 8.Khan JM, Rogers T, Schenke WH, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: Pre-clinical findings. JACC Cardiovasc Interv. 2016;9:1835–1843. doi: 10.1016/j.jcin.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]