Abstract
Semantically rich learning contexts facilitate semantic, phonological, and articulatory aspects of word learning in children with typical development (TD). However, because children with autism spectrum disorder (ASD) show differences at each of these processing levels, it is unclear whether they will benefit from semantic cues in the same manner as their typical peers. The goal of this study was to track how the inclusion of rich, sparse, or no semantic cues influence semantic, phonological, and articulatory aspects of word learning in children with ASD and TD over time. Twenty-four school-aged children (12 in each group), matched on expressive vocabulary, participated in an extended word learning paradigm. Performance on five measures of learning (referent identification, confrontation naming, defining, phonetic accuracy, and speech motor stability) were tracked across three sessions approximately one week apart to assess the influence of semantic richness on extended learning. Results indicate that children with ASD benefit from semantically rich learning contexts similarly to their peers with TD; however, one key difference between the two groups emerged—the children with ASD showed heightened shifts in speech motor stability. These findings offer insights into common learning mechanisms in children with ASD and TD, as well as point to a potentially distinct speech motor learning trajectory in children with ASD, providing a window into the emergence of stereotypic vocalizations in these children.
Keywords: autism spectrum disorder, word learning, semantic richness, speech motor stability, echolalia
Words provide the foundation for human thought (Sapir, 1949; Waxman & Leddon, 2011; Whorf, 1956). These fundamental linguistic units are essential for conveying communicative intent, establishing categorical concepts (Booth & Waxman, 2002; Fulkerson & Waxman, 2007), acquiring the syntax of one’s native language (Gillette, Gleitman, Gleitman, & Lederer, 1999; Mok & Kipka, 2009), developing early literacy skills (Spencer, Kaschak, Jones, & Lonigan, 2015), and participating in social routines. For many parents, the production of their child’s first word is cause for celebration. For children with autism spectrum disorder (ASD), a delay in the emergence of spoken words is often a parent’s earliest concern (Herlihy, Knoch, Vibert, & Fein, 2015). In fact, many of these children go on to show delays in vocabulary learning (Loucas et al., 2008) and, by age nine, approximately 20% fail to use more than five words on a given day (Lord, Risi, & Pickles, 2004).
Word-learning is a non-trivial task (McGregor, Sheng, & Ball, 2007). To learn a new word, one has to acquire the phonological form, pair it with a representation, and derive the meaning of the word. To later produce this newly acquired word, the speaker must map phonetic features and articulatory movements to the meaning. Over time and following multiple experiences with new words, the learner augments and refines semantic meanings (Kucker, McMurray, & Samuelson, 2015) and phonological forms (McGregor, 2014) within their memory.
For typical language learners, presenting semantic cues within the learning context and enriching lexical-semantic representations in memory has a myriad of beneficial effects in word learning, such as increasing the number of accurate semantic features that are recalled later (McGregor et al., 2007), enhancing the retrieval of the phonological form (McGregor, Friedman, Reilly, & Newman, 2002; McGregor, Newman, Reilly, & Capone, 2002; Rabovsky, Schad, & Rahman, 2016), and improving phonetic accuracy (Gladfelter & Goffman, 2013) and speech motor stability (Heisler, Goffman, & Younger, 2010). However, for children with ASD, it is questionable whether the same benefits may be derived from the inclusion of enhanced semantic cues. The primary goal of the current study is to investigate the potential influence of semantic richness on newly learned word productions in children with ASD.
Semantically Enriching the Learning Context in Typical Learners
One method of promoting the formation of more robust semantic representations in memory is to enrich the semantic context of the new words to be learned. Semantic richness incorporates additional information regarding the meaning of the referent and may be enhanced by presenting the semantic features of the new word through any sense, usually auditory and visual. Using manipulations of semantic richness, McGregor and her colleagues (2007) taught 8-year-olds with typical development (TD) unfamiliar real words in either a semantically informative context (e.g., “A sphinx has a lion’s body and human head” while viewing a photo of the referent) or without any additional semantic information (e.g., “A sphinx is on the screen” with the same photo of the referent) over the course of two weeks. The words taught in the informative context were more accurately defined than the words taught without the additional semantic cues; both sets of words had similarly high exposure rates. The inclusion of semantically rich information facilitated learning in these children.
In another study, Justice, Meier, and Walpole (2005) demonstrated that semantically elaborating upon words during a naturalistic learning task (i.e., repeated storybook reading over a 10-week course of intervention) led to greater gains in word learning compared to reading the words in non-elaborated contexts in typically developing kindergarteners, as well as in children at-risk for developing language disorders. Although the influence of semantic richness during tasks of word learning has been explored in children with TD, it has not been studied in children with ASD.
Both of these studies raise one other important point on the use of semantic richness as a facilitative learning strategy—it requires an extended time course before any beneficial effects are observed. During the initial encoding phase of word learning, semantic representations are presumed to be fragile, with limited mappings between the referent and the phonological form (Kucker et al., 2015). Subsequent experiences with the new words are necessary for the learner to augment and refine their semantic representations. This enhancement and stabilization of the newly learned word is thought to occur during consolidation (Munro, Baker, McGregor, Docking, & Arciuli, 2012), which is most apparent following periods of sleep (Diekelmann & Born, 2010). To capture the facilitative role of semantic richness, measures of word learning likely need to be collected across multiple days and learning opportunities.
Different Learning Trajectories in Children with ASD
When each level of processing (semantic, phonological, and articulatory) is examined individually in children with ASD, it seems likely these children will show a different constellation of learning trajectories than would be expected in their typically developing peers. Even in their earliest learning, children with ASD do not always process semantic information similarly to their typical peers. For example, by age 3 years, children with ASD do not show sensitivity to the shape bias (Potrzeba, Fein, & Naigles, 2015; Tek, Jaffery, Fein, & Naigles, 2008). This preference to apply a newly learned word to an object with a similar shape is a facilitative learning mechanism present in typically developing 2-year-olds (Landau, Smith, & Jones, 1988).
Later in development, children with ASD continue to exhibit weak semantic representations as indicated by their inclusion of fewer semantic features in their definitions of words compared with their peers (Norbury, Griffiths, & Nation, 2010). On picture naming tasks, children with pragmatic language difficulties (including those with ASD) produce significantly more errors that are semantically nonrelated (e.g., saying “grass” rather than “bow” for the target arrow) than their peers without pragmatic impairments (Ketelaars, Hermans, Cuperus, Jansonius, & Verhoeven, 2011). This lack of recognizing the defining semantic characteristics of words is further exemplified in word fluency tasks. On these tasks, typical learners cluster their productions based on semantic relations. For example, when asked to name as many animals as possible, typical learners list farm animals together. The organization of words produced by individuals with ASD is idiosyncratic, rather than semantically clustered (Bowler, Gaigg, & Gardiner, 2008). Also, children with ASD produce fewer prototypical exemplars than their peers (Dunn, Gomes, & Sebastian, 1996). Prototypical exemplars are words that have many semantic features associated with a semantic category (e.g., robins are prototypic exemplars of the category “bird,” whereas penguins are not because they do not fly; Rosch, 1975). Children with ASD show difficulty accessing pertinent semantic information (Dunn et al., 1996). Collectively, these studies reveal that children with ASD often do not exploit similar strategies to their typical peers to access semantic information.
In contrast to their semantic skills, phonological skills in children with ASD appear to be a relative area of strength, possibly serving as a scaffold for word learning. In an effort to compare whether phonological aspects of novel words are learned more effectively than semantic, Norbury, Griffiths, and Nation (2010) taught novel word and object mappings to school-aged children with ASD. Using an extended word learning paradigm, these investigators assessed the proportion of phonemes and semantic features children with ASD learned in comparison to their typical peers. Children with ASD learned a greater proportion of phonemes than semantic features, signifying they may be more reliant on phonological than semantic aspects of words. This interpretation is plausible, given that preschoolers with ASD have been reported to produce more words than they comprehend (Charman, Drew, Baird, & Baird, 2003). Interestingly, this “sound before meaning” strategy is the opposite pattern to that utilized by typically developing learners (Norbury et al., 2010, p. 4012); this is one indication that word learning may follow an alternative trajectory in children with ASD.
Speech motor (i.e. articulation) skills are generally thought to be spared in children with ASD (Cleland, Gibbon, Peppe, O’Hare, & Rutherford, 2010; Kjelgaard & Tager-Flusberg, 2001; Schoen, Paul, & Chawarska, 2011). Even as toddlers, children with ASD produce similar speech sounds to their typical peers (Schoen et al., 2011). However, though articulation is relatively intact, speech may be stereotypic or echolalic, and children may not be accessing deeper phonological, semantic or pragmatic knowledge of the words they produce (Lanovaz & Sladeczek, 2012; Mancina, Tankersley, Kamps, Kravits, & Parrett, 2000). While it may be assumed that stereotyped behaviors lack variability (Rapp & Vollmer, 2005), such variability has never been directly measured in children with ASD. Even though stereotypic speech may be indexed by articulatory measures, to our knowledge, the structure and stability of articulatory movements have not been investigated in children with ASD. Therefore, it is difficult to predict whether children with ASD rely on a different speech motor learning trajectory during tasks of word learning than their typically developing peers.
Capturing Interactions between Semantic, Phonological, and Articulatory Processing in Tasks of Word Learning
In classic models of speech production, each level of processing (semantic/conceptual, lexical, phonological, and articulatory) is posited to function discretely and independently (Levelt, Roelofs, & Meyer, 1999). More recent investigators (e.g., Baese-Berk & Goldrick, 2009; Goffman, 2010; Goldrick, Baker, Murphy, & Baese-Berk, 2011; McMillan, Corley, & Lickley, 2009; Rapp & Goldrick, 1997; Smith & Goffman, 2004) have argued for increased interactivity among these levels of processing. Following from these more recent, interactive models, it is possible that enhancing semantic processing may facilitate downstream processing at the phonological or articulatory levels. Furthermore, it is also possible that differences in processing at the phonological or articulatory levels may have upstream implications for semantic learning. Because of these interdependent relations between semantic, phonological, and articulatory levels of processing, it is essential to track learning at each level to identify differences in the learning trajectories between children with ASD and TD.
Learning can be indexed by changes in accuracy or automaticity (Seger, 1998). In the speech production realm, measures such as phonetic accuracy and kinematic stability may be used to quantify changes in word learning based on higher order processing factors (Gladfelter & Goffman, 2013; Heisler et al., 2010). At the phonological level, Storkel (2001) demonstrated that children learn new words with higher phonotactic probability more readily than words with low phonotactic probability, as indicated by increases in phonetic accuracy. Using measures of kinematic stability, frequency of prosodic structures have been shown to impact word learning in children with typical language; low frequency prosodic structures show more rapid improvements in articulatory stability during tasks of word learning than high frequency prosodic structures (Gladfelter & Goffman, 2013).
Measures of speech production also have been applied to assess changes in word learning based on lexical-semantic processing (Gladfelter & Goffman, 2013; Heisler et al., 2010; Storkel & Adlof, 2009). Heisler, Goffman, and Younger (2010) used kinematic measures of speech motor stability to index the influence of lexical and lexical-semantic cues during novel word learning. In this study, children with TD and children with language impairments were taught four novel phonetic strings. Two of the phonetic strings were presented with a visual referent to provide a lexical representation and thus to attain word-status; the other two phonetic strings were not paired with a visual referent. Both groups of children showed increased articulatory stability after a short-term perceptual learning experience when novel phonetic strings were paired with a visual referent (i.e., their lexical-semantic information was enriched); when no lexical information was provided, no speech motor learning was observed. From these results, it cannot be determined whether lexicalization alone influenced increases in stability, or whether other semantic factors may be implicated. In addition, it is possible that the changes in articulatory consistency also related to attentional factors. A consistent (and less engaging) checkerboard pattern was presented during the no lexical cue condition compared to a dynamic and changing visual referent in the lexical cue condition. It is therefore difficult to confirm whether the increased stability in the lexical cue condition was purely due to the lexical cue (i.e., mapping) or the increased attentional engagement.
A later study by Gladfelter and Goffman (2013) began to differentiate word learning within semantically rich or semantically sparse contexts in children with TD. Phonetic accuracy consistently improved in the semantically rich context, but not in the semantically sparse context. Storkel and Adlof (2009) found that children more accurately produced nonwords taught with smaller semantic set sizes (i.e., with fewer semantically related competitors) than those with larger semantic set sizes (i.e., with many semantic competitors). All of these studies highlight how phonetic accuracy and speech motor stability interact closely with aspects of lexical-semantic processing, such as semantic richness or semantic competition, in children. These measures have not been applied to word learning in children with ASD.
In summary, children with ASD often struggle to learn new words. For typical learners, the addition of semantically rich information facilitates word learning over a protracted time course; however, due to differences in semantic, phonological, and articulatory skills in children with ASD, these facilitative learning cues may lead to alternative learning trajectories in these children. In the current study, we asked the following questions: 1) does semantic richness influence word learning in children with ASD over time; and 2) do children with ASD show different semantic, phonological, and/or articulatory learning trajectories compared with their peers with TD over the course of extended word learning? Because enriched semantic contexts have been found to facilitate learning in children with TD (Capone & McGregor, 2005; McGregor et al., 2007) and children at risk for developing language impairments (Justice et al., 2005), we predicted that children with TD would benefit from the additional semantic cues over time. For the children with TD, we predicted that the semantically richer cues would lead to enhanced learning at the semantic level (McGregor et al., 2007), phonological level (Gladfelter & Goffman, 2013; McGregor et al., 2007; Rabovsky et al., 2016; Singleton, 2012), and articulatory level (Heisler et al., 2010) of word learning.
For the children with ASD, the predictions at each level over time were more varied. Because segmental phonology is generally found a strength in children with ASD (Kjelgaard & Tager-Flusberg, 2001), and because children with ASD show intact recognition and retrieval of phonological forms following consolidation (Henderson, Powell, Gaskell, & Norbury, 2014), we anticipated that their performance on the measures of phonological learning would follow the same trajectory as their peers with TD. However, because children with ASD rely more heavily on phonological forms, rather than semantic features, during tasks of word learning (Norbury et al., 2010), and because they show lexical competition between novel and existing words immediately after learning in contrast to typically developing peers (Henderson et al., 2014), we predicted that phonological learning would initially outpace semantic learning in these children. Finally, because of the prevalence of motor stereotypies in children with ASD, we predicted that the children with ASD would show higher levels of speech motor stability on the articulatory learning measures than their typically developing peers.
Method
Participants
Twelve children with ASD (mean age = 7;9 [years;months]) and 12 with TD (mean age = 5;11) participated in this study. Groups were matched on their expressive vocabulary using the Expressive Vocabulary Test-2nd Edition (Williams, 2007), with no significant group effect on a one-way ANOVA comparing raw scores, F(1, 22) = .49, p = .49 (see participant summary in Table 1). Expressive vocabulary was used because it is more reliably measured than receptive vocabulary in children with ASD (Luyster, Kadlec, Carter, & Tager-Flusberg, 2008). In addition, the experimental task focused on the production of novel words. To be included in the ASD group, each participant had an independent diagnosis of ASD (disclosed via parent report) and obtained a score on the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; Lord et al., 2012) that met cutoffs for either autism or autism spectrum (McGregor & Bean, 2012; Olu-Lafe, Liederman, & Tager-Flusberg, 2014). To be included in the TD group, each child attained a standard score of 85 or higher on the Structured Photographic Expressive Language Test-3rd Edition (Dawson, Stout, & Eyer, 2003) or the core battery of the Clinical Evaluation of Language Fundamentals—4 (Semel, Wiig, & Secord, 2003), whichever was age appropriate. Also, each child with TD scored within the “Minimal-to-No Symptoms” of ASD on the Childhood Autism Rating Scale – 2nd Edition (Schopler, Van Bourgondien, Wellman, & Love, 2010).
Table 1.
Participant Information
| ASD (n = 12)
|
TD (n = 12)
|
|||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Test Statistic | p | |
| Age in Months | 93.83 (22.54) |
55–135 | 71.83 (8.85) |
52–88 | 9.90 | <.01 |
| Sex | 3 Females | 6 Females | 1.60 | .20 | ||
| EVT Raw Scores | 88.67 (23.15) |
53–120 | 94.5 (17.34) |
68–128 | 0.48 | .49 |
| EVT Standard Scores | 95.75 (7.57) |
79–112 | 114.83 (13.38) |
91–135 | 18.49 | <.001 |
| Nonverbal IQ Standard Scores | 96.6 (6.54) |
85–106 | 121.5 (16.96) |
96–149 | 19.50 | <.001 |
Note. EVT = Expressive Vocabulary Test. Nonverbal IQ Standard Scores were from either the Primary Test of Nonverbal Intelligence, the Columbia Mental Maturity Scale, or the Test of Nonverbal Intelligence.
Children in both groups passed an oral-mechanism examination (Robbins & Klee, 1987) and a bilateral pure tone hearing screening. All participants obtained a standard score of 85 or higher on a standardized nonverbal intelligence measure (Test of Nonverbal Intelligence, Fourth Edition; Brown, Sherbenou, & Johnsen, 2010; Columbia Mental Maturity Scale; Burgemeister, Blum, & Lorge, 1972; Primary Test of Nonverbal Intelligence; Ehrler & McGhee, 2008). Although participants had a standard score of 85 or higher, the groups were not matched on nonverbal IQ. A nonverbal IQ score could not be obtained from one participant with ASD because she could not be trained to the task. All participants were monolingual English speakers. Participant recruitment and testing followed the approved Purdue University IRB protocols for the treatment of human subjects.
Because of the significant time commitment from participants and their families to attend numerous sessions over the course of several weeks, the challenges of finding and matching participants on vocabulary, and the intensive nature of analyzing the speech motor stability measures, the size of the sample included in this study is smaller than desired. However, prior to initiating this study, power calculations based on similar studies conducted using the same kinematic methodology and similar learning measures with children with typical development and those with language impairments (e.g., Heisler et al., 2010) indicated that this sample size would provide a minimum of 85% power to obtain significant effects at p < .05. As an additional measure, effect sizes are provided for all non-significant, as well as significant, results.
Procedure
Auditory stimuli
Six 2-syllable nonsense phonetic strings (/fʌ∫pəm/, /pʌvgəb/, /bʌpkəv/, /mʌfpəm/, /fʌspəb/, and /pʌbtəm/) were presented in the word learning task. For the analysis of upper and lower lip movement, it was essential to constrain the novel words to labial consonants in initial, medial, and final word positions. All novel words had low neighborhood density and low positional phonotactic probability (Munson, Swenson, & Manthei, 2005; Storkel, 2001; Storkel & Hoover, 2010). The six novel words were split into three word pairs, with one pair for each semantic cue learning condition (described below). Because of the multiple repetitions necessary to capture the speech motor stability of each word, only two words were presented within each learning condition to prevent participant fatigue. These word pairs were counter-balanced across all three semantic cue conditions.
Instrumentation
Eight infrared light emitting diodes (IREDs) were placed on each child’s face to capture lip and jaw movement (see Figure 1). Participants were positioned in front of a 3D Investigator camera (Northern Digital Inc., Waterloo, Ontario, Canada). Directly below the 3D Investigator, a 76.2 cm Dell monitor was used to display the visual stimuli. This monitor was connected to a laptop controlled by the experimenter, and stimuli were delivered using Microsoft PowerPoint at a comfortable pace for each child. The auditory stimuli were played from a set of external speakers placed next to the monitor. A Marantz CD recorder and a Panasonic DVD camcorder were used to obtain acoustic and video recordings. The kinematic signal was collected at a rate of 250 samples/second and a time locked acoustic signal, at a rate of 16,000 samples/second. Following data collection, kinematic trajectories were analyzed using the Matlab signal processing program (Mathworks, 2009). The displacement signal was low pass filtered in the forward and backward directions using a Butterworth filter with a cut-off frequency of 10 Hz.
Figure 1.
IRED placement.
Session structure
To track learning over time, all six words were taught across three experimental sessions, and each session occurred approximately one week apart, dependent on family schedule. Each individual word pair was presented through a pre-test, an exposure phase, and finally a post-test within each session. Each session had three pre-tests, three exposure phases (one for each semantic cue condition), and three post-tests (see an example session order in Table 2; these orders were counter-balanced across children).
Table 2.
Example Session Structure
| Task |
|---|
| No Semantic Cues Pre-test |
| No Semantic Cues Exposure Phase |
| No Semantic Cues Post-test |
|
|
| Sparse Semantic Cues Pre-test |
| Sparse Semantic Cues Exposure Phase |
| Sparse Semantic Cues Post-test |
| Referent Identification Task |
| Confrontation Naming Task |
| Definition Task |
|
|
| Rich Semantic Cues Pre-test |
| Rich Semantic Cues Exposure Phase |
| Rich Semantic Cues Post-test |
| Referent Identification Task |
| Confrontation Naming Task |
| Definition Task |
Pre-test
Random, nonsense images were presented to hold the participants’ visual attention toward the 3D Investigator and to control for interest level of the stimuli (see Figure 2 for an example nonsense (no semantic cue) image). Each of the two novel words per semantic condition was presented 12 times in quasi-random order. The participants were instructed to imitate each word they heard. These imitations were used to assess phonetic accuracy and speech motor stability prior to exposure to the word referents.
Figure 2.

Example nonsense image.
Exposure Phase
The degree of semantic richness within the exposure phase was the primary manipulation in this study. During each exposure phase, one of the three semantic cue learning conditions was presented (no semantic cues, sparse semantic cues, or rich semantic cues). Four semantically distinct visual images served as referents for the novel phonetic strings that attained word status (i.e., were paired with visual referents) during the exposure phase. These consisted of child friendly cartoon-like pictures drawn by a professional illustrator (Pounders, unpublished). Because the number of words that are meaningfully related to a given object influences the learnability of that new word (Borovsky, Ellis, Evans, & Elman, 2015; Rabovsky et al., 2016; Storkel & Adlof, 2009), the visual referents paired with the novel words resided in different superordinate semantic categories. One was an instrument, one was a tool, one was a vehicle, and one was an animal (Figure 3).
Figure 3.

Visual referents paired with the novel phonetic strings that attained word status.
To determine whether there was an inherent learning advantage to any image prior to training in the semantically varied learning contexts, all four visual referents were tested in the context of another study (Gladfelter, Goffman, & Steeb, in prep.). Data were obtained from 13 children with TD and 14 with language impairment who were taught all four visual referents, counterbalanced across the novel phonetic strings. All images showed similar levels of learnability on a referent identification task, F (3, 75) = 0.06, p = 0.98, a confrontation naming task, F (3, 75) = 1.01, p = 0.39, the degree of improvement in phonetic accuracy, F (3, 75) = 0.27, p = 0.84, and the degree of change in kinematic stability, F (3, 75) = 1.61, p = 0.19, using repeated measures ANOVAs. Based on these findings, it was judged that no referent-phonetic string pairing was inherently more learnable (or preferred), and that any advantages in learning within the current study could be attributed to the semantic learning context.
Within an exposure phase, participants heard each novel word seven times in quasi-random order (never occurring consecutively more than twice). The session order of each exposure phase was quasi-randomized; the same semantic cue condition could not occur in the same order in two consecutive sessions, and the semantically rich cue condition occurred once in each of the three order positions across the three sessions. The three semantic cue learning conditions were:
No Semantic Cues
For stimulus pairs trained in the no semantic cues condition, the auditory presentations of the novel phonetic strings were presented with different colorful and visually engaging nonsense images and lacked a consistent visual referent, never attaining word-status. It was essential to include a condition without any semantic information to determine the degree to which improvements in phonetic accuracy and speech motor stability were purely due to practice effects (Walsh, Smith, & Weber-Fox, 2006) compared to benefits from the lexical-semantic cues within the learning contexts (Heisler et al., 2010). Further, a constantly changing nonsense image was employed to better hold attention to ensure that any learning advantage of the lexical-semantic cues over the no semantic cue conditions could not be attributed to decreased attention, a potential limitation identified in previous work (e.g., Heisler et al., 2010).
Sparse Semantic Cues
Novel word pairs in the sparse semantic cues condition were auditorily presented in synchrony with two matching visual referents. The visual referents used in this condition included the musical instrument and the tool.
Rich Semantic Cues
In the rich semantic cues condition, the novel words were embedded in a children’s story with semantic descriptors and visual referents. Similar to the Sparse Semantic Cues condition, stimuli were presented auditorily and visually—pictures were presented and described. The same female talker was used across cue conditions. No orthographic text was provided during the presentation of the story. The story was controlled for number of semantic attributes and functions within a stimulus pair, as well as for syntactic complexity. Because of the narrative nature of this semantic cue condition, the words were not presented in random order. The vehicle and animal visual referents were used in this condition. The entire story script with the child-friendly images is available in the supplemental materials associated with this article. Central to the Rich Semantic Cues Condition is that a story script is included and that information about the attributes and functions of the novel objects are also incorporated. For example, in one picture depicting a boy riding the novel vehicle, the story text is: “My _______ is blue and big. I ride my _____. My ________ drives fast!” The semantically enriched information was presented both visually and auditorily, allowing the participants to extract meaningful semantic features either explicitly or through their own inferences.
Post-test
The post-test was identical to the pre-test. The participants’ novel word imitations during the post-test were used to assess learning following the exposure phase.
Learning Measures
Measures of word learning were collected in each of the three sessions to determine whether semantic richness influenced word learning over time in children with ASD and TD. Three measures of word learning were collected following the post-tests for the sparse and the rich semantic cue conditions. These measures included a referent identification task, a confrontation naming task, and a definition task (described below). Two measures of learning (i.e., phonetic accuracy and speech motor stability, described below) were collected for all three semantic cue conditions during the imitated productions in the pre- and post-tests. For these measures, imitated productions of each novel phonetic string were extracted for analysis. To ensure that all productions could be adequately transcribed and that factors extraneous to the task (e.g., laughter, yawning) did not artificially influence variability, productions judged to contain laughter, dysfluencies, whispering, singing, or yawning were excluded. To be consistent across participants, the first 10 usable productions of each novel word were analyzed. The average number of usable productions across participants was 9.79 out of 10 (for ASD, M = 9.74, range of 8.55 – 10; for TD, M = 9.86, range of 9.44 – 10). The same productions were included in the phonetic transcription and kinematic analyses.
Referent identification task
A digital recording of the word was played for the participant while he or she was shown an array of four pictures. The participant was asked to select the picture that matched the word’s visual referent. Of the four presented pictures, one item was the target, one was the visual referent for the second word within the same stimulus pair, and the remaining two were random foils to which the child had been exposed during the experimental task. Accuracy was rated on a three-point scale; selection of the target referent was scored as a “2,” selection of the competitor referent was scored as a “1,” and selection of either of the two foils was scored as a “0.” A mean accuracy level was derived. Because each child had only one opportunity to demonstrate comprehension, more detailed analysis of error patterns was precluded. To prevent each child’s confrontation naming accuracy from differentially influencing the number of correct target productions they were exposed to prior to the referent identification task, this learning probe was given first, and all probes were administered in the same order across conditions and across participants.
Confrontation naming task
Participants were shown a picture of the referent and asked to name it. Each segment from the participants’ responses was scored using a method developed by Edwards, Beckman, and Munson (2004). Each consonant was worth a maximum of three points: one point for place (bilabial, labiodental, alveolar, palatal, or velar); manner (fricative, stop, or nasal); and voicing (voiced or voiceless). The scores for all four consonants within each word were summed, and scores of 9 or higher out of the 12 possible phonetic features were considered “learned” words.
Definition task
Knowledge of word meanings was assessed using a definition task, in which the examiner asked the participant, “What does ___ mean?” and followed up with one prompt, “What else can you tell me about___?” The participants’ definitions were scored for number of accurate units of information (McGregor et al., 2007). Any inferred information judged to be at least plausible within the presented semantic context was treated as an accurate unit of information. For example, in the definition for the word paired with the musical instrument referent, one participant with ASD stated, “It makes music,” even though this word was taught using only a still image without sound. Because this was a plausible inference given the image of the referent, this was coded as an accurate unit of information. Because the primary goal of the current study was to assess the degree of learning with varying levels of semantic cues (either rich or sparse), only the number, but not the type, of semantic features is assessed here.
Semantic feature reliability
An additional coder was trained to calculate the reliability for the number of plausible semantic features produced during the definition task. A set of definitions from 25% of all sessions equally distributed across groups was assembled using a random number generator (random.org) to select the participant numbers. The total number of semantic features identified by the first author was 159 and by the second coder was 165, with an overlap of 158 semantic features. Reliability was then judged to be between 95.76% (159/165) and 99.37% (158/159).
Phonetic accuracy
Digital audio recordings of the participants’ imitated speech productions were phonetically transcribed, and a Percent of Consonants Correct (PCC) was derived. Omission and substitution errors were weighted equally. The critical measure of learning was the change in phonetic accuracy from the pre-test to the post-test following the exposure phase.
Phonetic transcription reliability
A second trained coder phonetically transcribed the pre- and post-test productions in a randomly selected (again using random.org) 25% of all sessions. An equal number of sessions were transcribed for each group. To calculate reliability, all disagreements were treated equally, and a percentage of agreements was derived. The overall transcription reliability was 98%. Reliability ranged from 96% to 99% for participants with ASD and from 97% to 100% for participants with TD.
Speech motor stability
Upper lip, lower lip, and jaw signals were recorded. Following Smith and colleagues (Smith, Goffman, Zelaznik, Ying, & McGillem, 1995; Smith, Johnson, McGillem, & Goffman, 2000), the lower lip signal was used to extract 10 repetitions of the movement sequences associated with each novel word from the long data files. When productions lacked an initial or final labial consonant, or contained inconsistent labial closure in the medial consonant cluster, they were excluded. For each participant’s production of a novel word, a minimum of five repetitions and a maximum of ten during each pre- or post-test were included in analyses. Movement onsets corresponded to the peak velocity as the lips opened for the initial vowel, and offsets corresponded to the peak velocity as the lips closed for the final consonant. If an initial or final labial was consistently omitted, then only one syllable was trimmed, using the peak velocity of the medial labial as the corresponding onset or offset, and this was done for all 10 productions of the word. Out of the 36 possible pre- and post-tests for each participant, only one syllable could be trimmed for 18 pre/post-tests from one participant with TD, 2 pre/post-tests from another, and 6 pre-/post-tests from a third participant with TD. Movement onsets and offsets associated with each target word production were initially identified by visual inspection of the displacement record, and the corresponding velocity peaks were selected. An algorithm determined the peak velocity value within a 25-point (100-ms) window of the experimenter-selected point. Synchronized acoustic signals were used to confirm selections of the kinematic records.
After movement trajectories for each word were extracted, point-by-point subtraction of the lower lip motion from the upper lip motion was used to derive lip aperture. The stability of this difference signal was assessed using the spatiotemporal index (STI; Smith et al., 1995). First the waveforms were amplitude- and time-normalized to remove absolute differences in duration (e.g., speech rate) and amplitude (e.g., loudness). A spline function (Mathworks, 2009) was used to interpolate each aperture record onto a common time base of 1000 points (see Smith et al., 1995; Smith et al., 2000). For amplitude normalization, the mean was set to zero and the standard deviation to one. Following time- and amplitude-normalization, standard deviations were computed at 2% intervals across all of the records. The STI is the sum of these 50 standard deviations. A higher STI value indicates increased variability, whereas a lower STI indicates enhanced stability. An STI of 0 would reflect perfect stability. See Figure 4 for an example of the STI obtained from a child with ASD.
Figure 4.
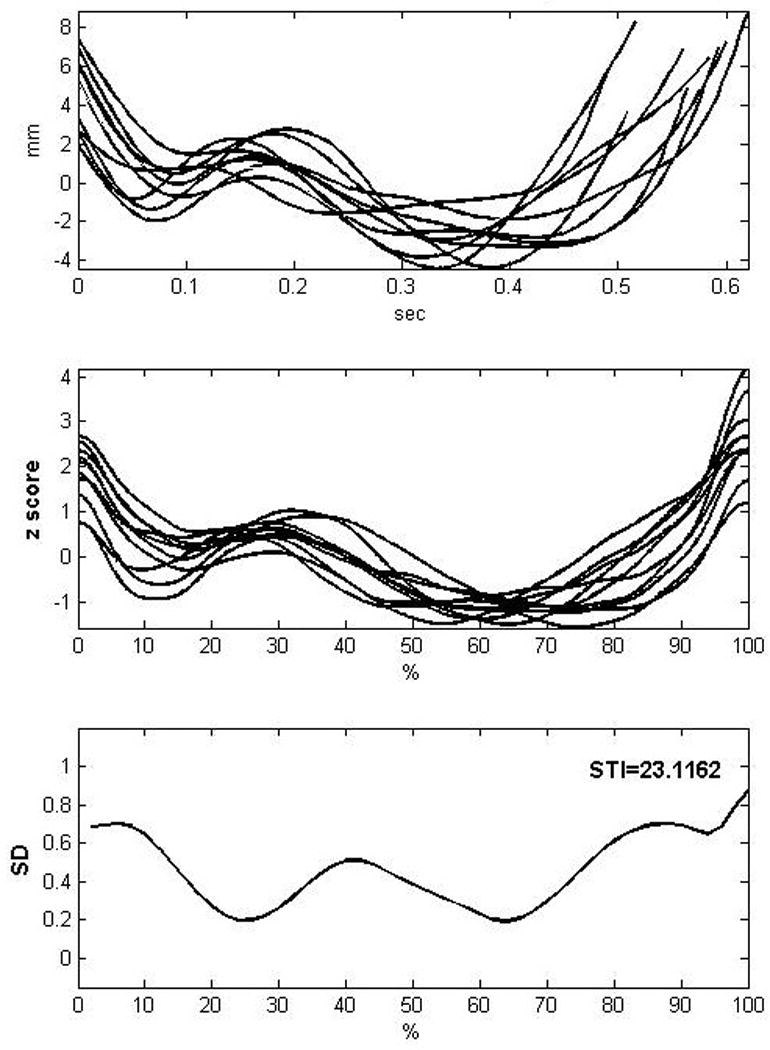
Illustration of the Spatiotemporal Index (STI). The top panel shows 10 non-normalized productions from a child with ASD producing the novel word /bʌpkəv/. The middle panel shows the same productions now time- and amplitude normalized. The bottom panel shows the standard deviations obtained across the same productions and the resulting STI.
Training Procedures for Children with ASD
Because this research involved the placement of light emitting diodes (IREDS) on the face, and children with ASD are likely to show sensitivities to tactile input, we included two additional training procedures. First, at the end of the initial inclusionary testing session, the examiner read a social story, similar to those developed by Gray and Garand (1993), that outlined the experimental task and the procedure for wearing the IREDs to each participant with ASD. We then sent home the social story book with the caregiver to read at home. Second, we provided families with the same adhesive stickers used to attach the IREDs to practice wearing at home before participating in the experiment.
Statistical Analyses
The overall statistical design was a mixed, repeated measures ANOVA with group (ASD and TD) as the between-subjects factor. The within-subjects factors were semantic cue condition (no semantic cues, sparse semantic cues, and rich semantic cues) and session (Session 1, Session 2, and Session 3). Separate ANOVAs were completed for all learning measures. For the phonetic accuracy and speech motor stability measures, both raw scores and difference scores were used to assess changes in learning over time. A .05 alpha level was considered significant.
For the speech motor stability measures, one child with ASD and one child with TD provided fewer than five useable productions on three (out of 36 total) of the pre-tests, and their data were removed for those three cells for the subsequent analyses. Because the identical trials from the kinematic analyses were also used for calculating the phonetic accuracy, the same cells were removed in the phonetic analyses as well. Also, due to an equipment malfunction, the kinematic data for two words within one pre-test for another participant with TD were not collected, and therefore are not included.
Results
This study aimed to answer two questions: 1) does semantic richness influence word learning in children with ASD over time; and 2) do children with ASD show different semantic, phonological, and/or articulatory learning trajectories than their peers with TD over the course of extended word learning? To address these questions, each group’s performance on five learning measures derived from three different semantic cue learning conditions obtained across three sessions were analyzed. The key findings are summarized in Table 3 and detailed below.
Table 3.
Summary of Key Findings
| Learning Measure | Rich Semantic Cue Benefit? (Collapsed across Groups) | Group Difference in Learning Trajectory? |
|---|---|---|
| Referent Identification Task1 | F(1, 21) = .52, p = .48, ηp2 = .02 | F(1, 21) = .05, p = .82, ηp2 < .01 |
| Confrontation Naming Task | * F(1, 22) = 5.13, p = .03, ηp2 = .19 | F(1, 22) = 0.19, p = .67, ηp2 < .01 |
| Definition Task | * F(1, 22) = 6.27, p = .02, ηp2 = .22 | F(1, 22) = .25, p = .62, ηp2 = .01 |
| Change in Phonetic Accuracy | F(2, 40) = .75, p = .48, ηp2 = .02 | F(1, 20) = 1.06, p = .31, ηp2 = .05 |
| Change in Speech Motor Stability | * F(2, 40) = 4.06, p = .02, ηp2 = .16 | * F(1, 20) = 6.39, p = .02, ηp2 = .24 |
Note:
= significant effect.
Lack of differences could be because performance was at ceiling.
Referent identification task
Both groups showed evidence of learning on the referent identification task, with mean accuracy in all sessions well above the level of chance, but no significant session learning effects, F (2, 42) = 2.69, p = .08, ηp2 = .11. There were no significant differences between groups, F(1, 21) = .05, p = .82, ηp2 < .01 (for ASD, M = .79, SD = .22; for TD, M = .81, SD = .21). No semantic cue effects were observed, F(1,21) = .52, p = .48, ηp2 = .02, and there was not a group by cue interaction, F(1, 21) < .01, p = .98, ηp2 < .0001, or a group by session interaction, F(2,42) = .57, p = .57, ηp2 = .03.
Confrontation naming task
A significant learning effect was observed across sessions, F(2, 44) = 12.60, p < .0001, ηp2 = .36. A Tukey HSD test revealed that more words were learned in Session 2 than Session 1, p < .01 (for Session 1, M = .88, SD = .87; for Session 2, M = 1.35, SD = .84), and learning was maintained in Session 3, p = .37 (M = 1.54, SD = .80). On the production probes, the semantically rich cues (M = 1.42, SD = .82) showed a learning advantage over the sparse semantic cues (M = 1.10, SD = .91), F(1, 22) = 5.13, p = .03, ηp2 = .19. There was not a significant difference between the two groups on this confrontation naming task, F(1, 22) = 0.19, p = .67, ηp2 < .01 (for ASD, M = 1.21, SD = .89; for TD, M = 1.34, SD = .85), and there was not a group by cue interaction, F(1, 22) = .09, p = .77, ηp2 < .01, or a group by session interaction, F(2,44) = 2.79, p = .07, ηp2 = .11.
Definition task
On the definition task, learning across sessions was significant, F(2, 44) = 5.51, p < .01, ηp2 = .20. A Tukey HSD test indicated that the greatest gains occurred from Session 1 to Session 2, p < .03 (for Session 1, M = 2.00, SD = 3.07; for Session 2, M = 4.00, SD = 3.81) and then were maintained in Session 3, p = .93 (M = 4.00, SD = 3.67). Significantly more semantic features were produced for the words taught in the rich semantic cue condition (M = 4.14, SD = 4.18) than the sparse cue condition (M = 2.63, SD = 2.76), F(1, 22) = 6.27, p = .02, ηp2 = .22. No group differences were observed F(1, 22) = .25, p = .62, ηp2 = .01 (for ASD, M = 10.83, SD = 7.72; for TD, M = 9.46, SD = 8.77), and there was not a group by cue interaction, F(1, 22) = .23, p = .63, ηp2 = .01, or a group by session interaction, F(2,44) = .17, p = .85, ηp2 < .01. For both groups, the rich cues led to the formation of more semantically detailed definitions.
Phonetic Accuracy
Within each session, both groups showed evidence of learning through increases in phonetic accuracy from pre- to post-test, F(1, 20) = 5.80, p = .03, ηp2 = .23. There was no significant effect for group, F(1, 20) = .43, p = .52, ηp2 = .02, (for ASD, pre-test M = 92.64, pre-test SD = 11.31, post-test M = 92.83, post-test SD = 11.63; for TD, pre-test M = 90.81, pre-test SD = 13.52, post-test M = 91.64, post-test SD = 12.72), session F(2, 40) = .15, p = .86, ηp2 < .01, or semantic cue condition F(2, 40) = .63, p = .54, ηp2 = .03, and there was not a group by cue interaction, F(2, 40) = .44, p = .65, ηp2 = .02, or a group by session interaction, F(2,40) = .41, p = .67, ηp2 = .02, for the raw PCC scores. To directly index changes in phonetic accuracy, the PCC difference scores between the pre- and post-tests were analyzed across all three sessions. The two groups did not differ in their amount of change from pre- to post-test across sessions, F(1, 20) = 1.06, p = .31, ηp2 = .05 (for ASD, M = .32, SD = 4.24; for TD, M = .80, SD = 5.37). Both groups improved in their phonetic accuracy to a similar extent within each session.
Speech motor stability
Both groups showed evidence of learning through increases in speech motor stability, both from pre- to post-test within each session, F(1, 19) = 7.27, p = .01, ηp2 = .28, as well as across sessions, F(2, 38) = 4.28, p = .02, ηp2 = .18; there was not a group by session interaction, F(2,38) = 1.24, p = .30, ηp2 = .07. Although an overall semantic cue condition effect was not statistically significant, F(2, 38) = .11, p = .90, ηp2 < .01, and there was not a group by cue interaction, F(2, 38) = 1.38, p = .27, ηp2 = .06, an interaction between semantic cue condition and pre- and post-test emerged, F(2, 38) = 3.53, p = .04, ηp2 = .16. A Tukey HSD indicated that, in the rich semantic cue condition, speech motor stability in the post-test was significantly higher than in the pre-test. As a direct measure of change in speech motor stability, the difference scores between the pre- and post-test were analyzed. A significant effect was observed based on the semantic cue condition, F(2, 40) = 4.06, p = .02, ηp2 = .16 (No Semantic Cues M = −.41, SD = 5.17; Sparse Semantic Cues M = .50, SD = 4.55; Rich Semantic Cues M = 1.10, SD = 4.57; see Figure 5). A Tukey HSD revealed that the gains in stability in the rich semantic cues condition were greater than in the no semantic cues condition.
Figure 5.
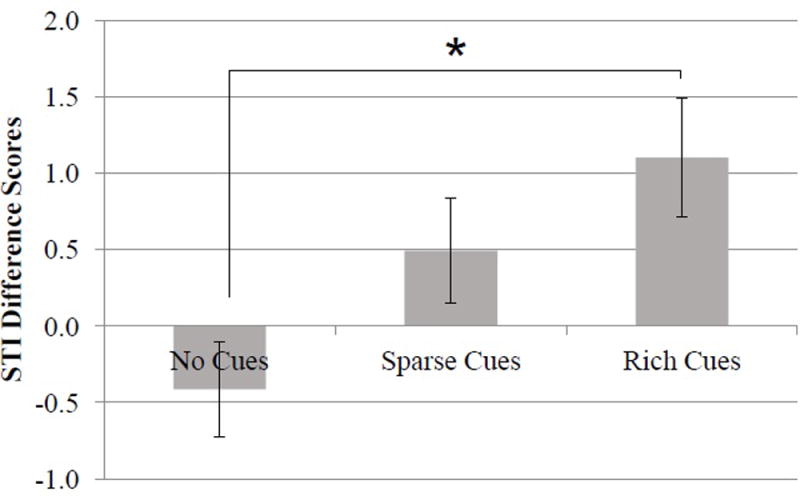
Change in speech motor stability by condition collapsed across groups. Larger difference scores indicate enhanced stability. Error bars show standard error.
When considering actual levels of speech motor stability, rather than cue specific learning, there were no statistically significant differences between groups, F(1, 19) = 0.02, p = .90, ηp2 < .01 (see Figure 6); both groups of children showed similar overall levels of speech motor stability. However, there was a significant interaction between group and pre-/post-test, F(1, 19) = 4.93, p = .04, ηp2 = .20. A Tukey HSD test revealed that, for the children with ASD, speech motor stability was significantly greater in the post-tests than in the pre-tests, and no other differences were significant (all p values above .05). It was important to rule out whether this result related to initial differences in speech motor stability, potentially due to differences in age, or to learning. For this reason, a follow-up repeated measures ANOVA was conducted, revealing that there were no significant differences between the two groups during the pre-tests, F(1, 19) = .17, p = .68, ηp2 < .01.
Figure 6.
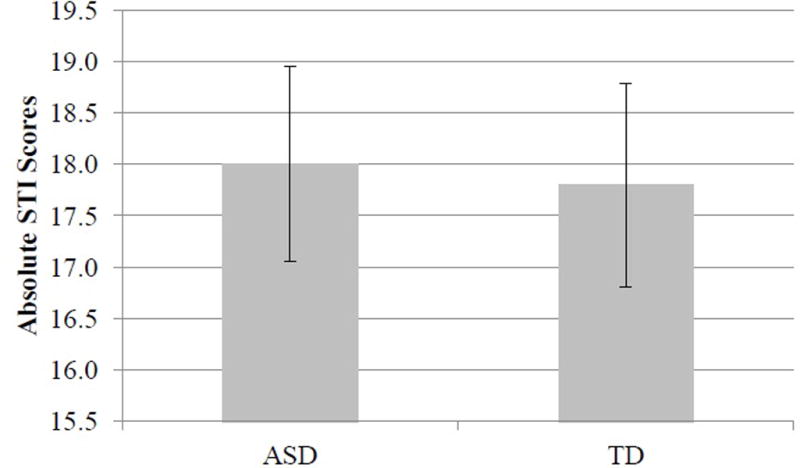
Overall speech motor stability between the two groups collapsed across pre- and post-tests and sessions. Error bars represent standard error.
To further evaluate this result, we examined the STI difference scores directly, finding there was a significant effect based on group, F(1, 20) = 6.39, p = .02, ηp2 = .24; the children with ASD showed larger gains in stability from pre- to post-test than their typically developing peers (see Figure 7). This heightened increase in motor stability from pre- to post-test in children with ASD was apparent even within the very first session, F(1, 21) = 6.00, p = .02, ηp2 = .22. In sum, the children with ASD were not inherently more stable in their production of speech, but rather they showed greater shifts in motor stability from pre- to post-test than their peers with TD.
Figure 7.
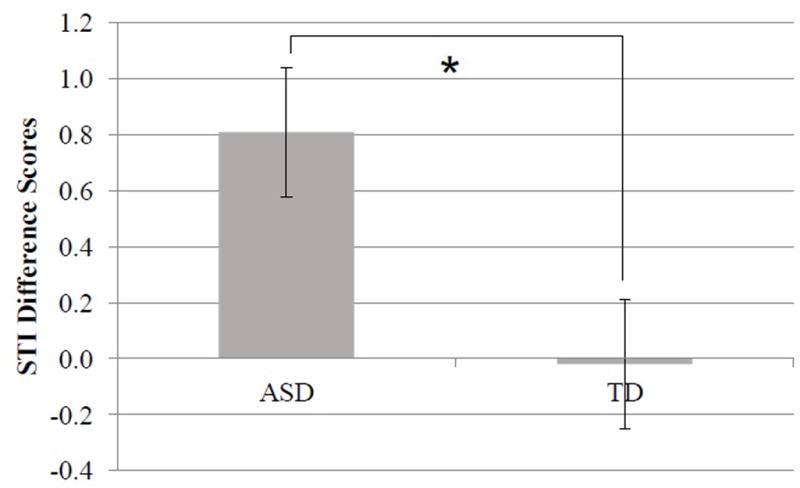
The degree of change in speech motor stability from pre- to post-test (i.e., difference scores) for both groups. Error bars indicate standard error.
Discussion
Increasing the richness and complexity of semantic contexts helps children with ASD and TD learn new words over time. This semantically rich advantage was observed on the confrontation naming task, the definition task, and the speech motor stability measures. The children with ASD were able to use the semantically rich contexts to enhance their retrieval of phonological forms, establish more robust semantic representations in memory, and increase their articulatory stability. As in typical language learners, richer semantic contexts facilitated multiple components of word learning in children with ASD. However, one key difference in the learning trajectories for these two groups did emerge: the children with ASD showed heightened gains in speech motor stability compared with the typical learners.
Semantic Richness Impacts How Words Are Learned Over Time
In the current study, the production and semantic probes did not show any advantage for words learned with rich cues during the initial session; however, after extended experience with the words in the following sessions approximately one week later, the beneficial role of semantically deep cues was evident. Studies of word learning have conventionally split the learning process into two sequential stages: a fast-mapping phase, which establishes the initial link between a word and its referent, and a subsequent slow-mapping phase, which uses later experiences with the word to build on the initial representation in memory (e.g., Carey, 1978; Carey & Bartlett, 1978).
A recent theoretical account of word learning has shifted from a two stage model to instead focus on the underlying processes that support word learning across varying timescales (Kucker et al., 2015). In Kucker, McMurray, and Samuelson’s account of word learning (2015), a network of mappings between words and their related concepts are created and modified following repeated encounters with a word over time. The formation and strength of these networks are based on association learning rules – whenever a word and its referent co-occur, a link in the network is established. This slow, associative learning allows the learner to gradually establish more nuanced, refined meanings of these word-referents in memory. This process of augmenting distinctive semantic information to one’s semantic representation of a word facilitates its later retrieval (Cree, McNorgan, & McRae, 2006; McRae, deSa, & Seidenberg, 1997; Rogers & McClelland, 2004; Rogers & McClelland, 2008). Through repeated and semantically relevant experiences with new words over a protracted time span, learners efficiently and effectively learn new words.
One key point from this account of word learning is that while initial learning may be fast, it is incomplete; deeper learning progresses slowly (Kucker et al., 2015). For example, typically developing two-year-olds who successfully map word-referents fail to retain their lexical meanings five minutes later (Horst & Samuelson, 2008). School-aged children require multiple exposures to successfully retain new lexical information (McGregor et al., 2007). For typical learners, learning experiences spaced further apart promote more accurate retention of the verbal information (Cepeda, Pashler, Vul, Wixted, & Rohrer, 2006; McGregor, 2014). In the current study, the children required additional time and exposure for a semantic cue effect to emerge. This more protracted course of acquisition may explain why previous researchers investigating the utility of semantic cues within fast mapping paradigms fail to find their advantageous effects (e.g., Gray & Brinkley, 2011), while those that evaluate word learning over a more extended timeframe find this facilitative outcome (Angwin, Phua, & Copland, 2014; Capone & McGregor, 2005; Henderson, Weighall, & Gaskell, 2013; Justice et al., 2005; McGregor et al., 2007; Rabovsky, Sommer, & Rahman, 2012). On tasks of learning that involve active retrieval, such as confrontation naming and defining, but not referent identification, more robust consolidation of the information is required before the benefit of rich semantic contexts is evident. A similar protracted time scale was reported in adults retrieving newly acquired semantic information (Tamminen & Gaskell, 2013). It is important to note that semantic richness is a complex construct and that many factors many contribute to the findings, such as the inclusion of linguistic as well as visual referent information. Future research is needed to further evaluate what aspects of semantic richness, from perceptual, conceptual, and linguistic domains, may influence word learning.
Differences in Semantic and Phonological Processing Are not Obstacles to Word Learning in Children with ASD
Although word learning differences are widely documented in children with ASD, some of the same word learning strategies observed in children with TD have also been reported in children with ASD. For example, children with ASD do demonstrate a noun bias, or an inclination to assign novel phonological forms to objects rather than actions (Swensen, Kelley, Fein, & Naigles, 2007). Even toddlers with ASD ascribe a novel word to an object that does not already have a name as effectively as their peers with TD (Preissler & Carey, 2005). In follow-up post-tests that occur only 24 hours after initial training, children with ASD improve in their ability to recognize and recall newly learned, novel words as well as their typical peers (Henderson et al., 2014). Children with ASD have also been shown to develop broad semantic categories within word learning tasks in a manner similar to their peers with TD (McGregor & Bean, 2012; Tager-Flusberg, 1985). The current findings show that the addition of semantically rich cues facilitates multiple levels of word learning in children with ASD in a manner similar to their typically-developing peers.
Even though this is the first study we are aware of that directly manipulates the level of semantic input on word learning in children with ASD, this is not the only study that has found a beneficial effect of semantic cues on the production of words in children with ASD. In a study by Ketelaars and her colleagues (2011), the number of accurate naming and definition responses by children with pragmatic language impairments (including some with ASD) increased when the examiners provided the children with semantic cues, indicating that these specific cues help compensate for weak semantic representations during tasks of active word retrieval.
Factors associated with phonological processing also have been implicated as interacting with semantic learning in children with ASD. In a study by Norbury and her colleagues (2010), children with ASD learned the phonemic features of novel words prior to the semantic features. This over-reliance on phonological features, rather than semantic (and social) cues, was interpreted as a compensatory mechanism employed by children with ASD to support performance on word learning tasks that require only minimal phonological form to picture mappings (e.g., referent identification tasks). This phonemic over semantic learning pattern is the reverse of that observed in typically developing children (Norbury et al., 2010).
Contrary to the findings in the Norbury study, the children with ASD in the current study did not show different phonological and semantic learning trajectories than their peers with TD. The novel phonetic strings constructed for this study had low phonotactic probability, low neighborhood density, and were phonological neighbors; these high phonological demands may have prevented the children with ASD from relying on the phonological forms as rapidly as the semantic features. In addition, the Norbury study incorporated a longer time course of learning (i.e., one month)—this longer delay may have resulted in difficulties in consolidation. However, other research has shown that individuals with ASD, much like their typical peers, rely on semantic features more heavily than phonological features when recalling words from memory (Whitehouse, Maybery, & Durkin, 2007), which falls in line with the current findings.
Alternatively, the discrepancy with the Norbury results could be due to varying degrees of social demands in the two studies. In the current study, the children with ASD mapped the word-referent pairs by viewing still-frame images on a computer, reducing the social demands of the word learning task. In contrast, the novel referents in the Norbury study were linked to a referential gaze shift by a woman in a video. Even though the children with ASD were able to use the social cues to form the word-referent pairs, many other researchers have reported weaknesses in children with ASD’s ability to capitalize on social cues during tasks of word learning (Baron-Cohen, Baldwin, & Crowson, 1997; McDuffie, Yoder, & Stone, 2006; Parish-Morris, Hennon, Hirsh-Pasek, Golinkoff, & Tager-Flusberg, 2007). Perhaps the children with ASD in the Norbury and colleagues study were able to follow the social cues, but in their effort to overcome these referential gaze shift challenges, they failed to acquire the semantic aspects of the novel words as well as their typically developing peers.
Clearly future work is needed to disentangle the role of semantic and phonological factors on word learning in children with ASD. Based on the current study, when children with ASD are given rich semantic cues during encoding, and social demands are minimal, they can efficiently establish semantic representations in the face of increased phonological demands.
Heightened Shifts in Speech Motor Stability in Children with ASD
To classify as having an autism spectrum disorder, an individual must also present with “restricted, repetitive patterns of behavior, interests, or activities” (American Psychiatric Association, 2013). This may manifest as stereotyped or repetitive movements, such as vocal stereotypies or echolalia. The stereotypical nature of these speech productions suggests that they follow a more stable, perhaps even an inflexible or rigid motor pattern; however, to our knowledge, the speech motor stability of children with ASD has not been previously assessed. Interestingly, the overall speech motor stability of the children with ASD did not differ from that of their peers with TD. But, the children with ASD were found to more dramatically shift towards elevated levels of motor stability following additional exposures to the new words than their typical peers. These increases in speech motor stability may provide a window into how stereotypical speech productions emerge in children with ASD. In this case, the gains in speech motor stability may show how the speech motor patterns become more repetitive during the child with ASD’s earliest experiences with new words.
This pattern of increased motor stability, rather than variability, during the early phases of word learning in children with ASD may be detrimental to subsequent language learning. A growing body of evidence indicates that variability during tasks of motor learning (Herzfeld & Shadmehr, 2014; James & Conatser, 2014) and language learning (Childers & Tomasello, 2001; Gomez, 2002; Richtsmeier, Gerken, Goffman, & Hogan, 2009; Twomey, Ranson, & Horst, 2014) leads to greater gains in skill learning and generalization. If children with ASD rapidly become reliant on more stable, repetitive motor patterns during tasks of word learning, they may be at a disadvantage during later stages of language learning.
Clinical Implications for Children with ASD
One of the most clinically important findings from this study is that children with ASD who are able to produce multi-word speech do not differ from their typically developing peers in whether they benefit from a semantically rich word learning context. In the semantically rich condition, novel words were embedded within a children’s story, and children with ASD have been shown to struggle when segmenting words from the speech stream (Scott-Van Zeeland et al., 2010) and when processing complex information (Minshew, Goldstein, & Siegel, 1997; Williams, Goldstein, & Minshew, 2006). In spite of these documented difficulties, the children with ASD in the current study still showed enhanced learning in the words taught with rich and varied, rather than sparse or no, semantic cues.
A prominent current approach to language intervention in children with ASD is discrete trial training (Delprato, 2001; Granpeesheh, Tarbox, & Dixon, 2009; Vismara & Rogers, 2010). Discrete trial training, first put forth by Lovass (1981 Lovass (1987), applies the principles of operant learning from Skinner (1957) and “involves breaking down complex skills and teaching each subskill through a series of highly adult-structured, massed teaching trials” (Vismara & Rogers, 2010, p. 449). This approach aligns with the evidence that children with ASD have difficulty processing complex information and would more readily learn if the information was presented in a more simplified context (Minshew et al., 1997; Williams et al., 2006). For children who are minimally verbal, discrete trial training has been shown to help develop functional language skills, especially in children with poor receptive language (Paul, Campbell, Gilbert, & Tsiouri, 2013). However, because of the highly structured and adult-led teaching context, discrete trial training lags behind more naturalistic approaches to language intervention in the generalization of spontaneous language skills in children with ASD (Delprato, 2001; Koegel, Camarata, Koegel, Ben-Tall, & Smith, 1998). The present study offers more evidence that verbal children with ASD show enhanced word learning when exposed to more semantically rich language input, rather than simplified learning contexts.
A study by Naigles has also shown that children with ASD respond to language input in a similar manner to children developing language typically (2013). In this longitudinal study, Naigles sought to determine whether maternal speech input influenced language acquisition in young children with ASD. The results revealed that children with ASD showed greater language gains when they received more complex language input from their mothers (e.g., more diverse vocabulary use, expansions of the child’s utterances), and that complex input incorporating a variety of lexical and grammatical structures was even more facilitative than simple, highly repetitive input (Naigles, 2013). For the development of effective interventions for children with ASD, there is evidence that clinicians may apply typical language input approaches. Specifically, complex and semantically rich input facilitates language learning in children with ASD.
Conclusion
By embedding new words within a semantically rich context during the encoding phase of learning, both children with ASD and TD were able to form deeper semantic representations in their memory. These rich semantic representations influenced language production processes at semantic, phonological, and articulatory levels. In future work, it will be essential to delineate the sorts of semantic and contextual information that facilitate learning.
To understand the language deficits in children with ASD, it is necessary to identify not only what is impaired, but also what is spared in their language learning. Surprisingly, more similarities than differences emerged, with one key exception—speech motor stability. The results of this study highlight the importance of studying multiple aspects of language learning to better identify effective learning strategies that may also be applied to children with ASD.
Research Highlights.
During the learning of new words, children with ASD are as sensitive to semantically rich cues in the input as their typical peers. This finding has significant implications for theoretical accounts of the weighting of semantic and phonological cues during extended word learning and for clinical approaches.
This study is the first to directly assess changes in speech motor stability in children with ASD as they acquire a new word. These results show how, even during their earliest experiences with new words, children with ASD converge onto a more stable speech motor pattern than their peers with typical development, providing a window into how vocal stereotypies or echolalic productions may first manifest in children with ASD.
Acknowledgments
We would like to thank the participants and their families for their involvement in this study. We would also like to extend our gratitude to Janna Berlin, Barbara Brown, Meredith Saletta, Janet Vuolo, Sara Benham, Amanda Steeb, and Mitchell Barna for their assistance throughout several stages of this project. This research was supported by NIH grants R01DC004826 and 2T32DC000030.
Contributor Information
Allison Gladfelter, Allison Gladfelter, Department of Speech, Language, and Hearing Sciences, Purdue University.
Lisa Goffman, Department of Speech, Language, and Hearing Sciences, Purdue University.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Angwin AJ, Phua B, Copland DA. Using semantics to enhance new word learning: An ERP investigation. Neuropsychologia. 2014;59(0):169–178. doi: 10.1016/j.neuropsychologia.2014.05.002. http://dx.doi.org/10.1016/j.neuropsychologia.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Baese-Berk M, Goldrick M. Mechanisms of interaction in speech production. Language and Cognitive Processes. 2009;24(4) doi: 10.1080/01690960802299378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Development. 1997;68(1):48–57. [PubMed] [Google Scholar]
- Booth AE, Waxman S. Object names and object functions serve as cues to categories for infants. Developmental Psychology. 2002;38(6):948–957. doi: 10.1037//0012-1649.38.6.948. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Ellis EM, Evans JL, Elman JL. Lexical leverage: category knowledge boosts real-time novel word recognition in 2-year-olds. Developmental Science. 2015 doi: 10.1111/desc.12343. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM, Gaigg SB, Gardiner JM. Subjective organisation in the free recall learning of adults with Asperger’s syndrome. Journal of Autism and Developmental Disorders. 2008;38(1) doi: 10.1007/s10803-007-0366-4. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence. Fourth. Austin, TX: Pro-Ed; 2010. [Google Scholar]
- Burgemeister BB, Blum LH, Lorge I. Columbia Mental Maturity Scale. New York: Harcourt Brace Jovanovich; 1972. [Google Scholar]
- Capone NC, McGregor KK. The effect of semantic representation on toddlers’ word retrieval. Journal of Speech Language and Hearing Research. 2005;48(6) doi: 10.1044/1092-4388(2005/102). [DOI] [PubMed] [Google Scholar]
- Carey S. The child as word learner. In: Halle M, Bresnan J, Miller GA, editors. Linguistic theory and psychological reality. Cambridge, MA: MIT Press; 1978. pp. 264–293. [Google Scholar]
- Carey S, Bartlett E. Acquiring a single new word. Papers and Reports on Child Language Development. 1978;15:17–29. [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychological Bulletin. 2006;132(3):354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) Journal of Child Language. 2003;30(1):213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Childers JB, Tomasello M. The role of pronouns in young children’s acquisition of the English transitive construction. Developmental Psychology. 2001;37(6):739–748. doi: 10.1037//0012-1649.37.6.739. [DOI] [PubMed] [Google Scholar]
- Cleland J, Gibbon FE, Peppe SJE, O’Hare A, Rutherford M. Phonetic and phonological errors in children with high functioning autism and Asperger syndrome. International Journal of Speech-Language Pathology. 2010;12(1):69–76. doi: 10.3109/17549500903469980. [DOI] [PubMed] [Google Scholar]
- Cree GS, McNorgan C, McRae K. Distinctive features hold a privileged status in the computation of word meaning: Implications for theories of semantic memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(4):643–658. doi: 10.1037/0278-7393.32.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Stout C, Eyer J. Structured Photographic Expressive Language Test. 3rd. Dekalb, IL: Janelle Publications Inc; 2003. [Google Scholar]
- Delprato DJ. Comparisons of discrete-trial and normalized behavioral language intervention for young children with autism. Journal of Autism and Developmental Disorders. 2001;31(3):315–325. doi: 10.1023/a:1010747303957. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. SLEEP The memory function of sleep. Nature Reviews Neuroscience. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dunn M, Gomes H, Sebastian MJ. Prototypicality of responses of autistic, language disordered, and normal children in a word fluency task. Child Neuropsychology. 1996;2(2):99–108. doi: 10.1080/09297049608401355. [DOI] [Google Scholar]
- Edwards J, Beckman ME, Munson B. The interaction between vocabulary size and phonotactic probability effects on children’s production accuracy and fluency in nonword repetition. Journal of Speech Language and Hearing Research. 2004;47(2):421–436. doi: 10.1044/1092-4388(2004/034). [DOI] [PubMed] [Google Scholar]
- Ehrler DJ, McGhee RL. Primary Test of Nonverbal Intelligence. Austin, TX: Pro-Ed Inc; 2008. [Google Scholar]
- Fulkerson AL, Waxman SR. Words (but not Tones) facilitate object categorization: Evidence from 6-and 12-month-olds. Cognition. 2007;105(1):218–228. doi: 10.1016/j.cognition.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette J, Gleitman H, Gleitman L, Lederer A. Human simulations of vocabulary learning. Cognition. 1999;73(2):135–176. doi: 10.1016/s0010-0277(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Gladfelter A, Goffman L. The Influence of Prosodic Stress Patterns and Semantic Depth on Novel Word Learning in Typically Developing Children. Language Learning and Development. 2013:1–24. doi: 10.1080/15475441.2012.684574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman L. Dynamic interaction of motor and language factors in development in normal and disordered development. In: Maassen B, Lieshout PHHMv, Kent R, Hulstijn W, editors. Speech Motor Control: New Developments in Applied Research. Oxford, UK: Oxford University Press; 2010. pp. 137–152. [Google Scholar]
- Goldrick M, Baker HR, Murphy A, Baese-Berk M. Interaction and representational integration: Evidence from speech errors. Cognition. 2011;121(1) doi: 10.1016/j.cognition.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RL. Variability and detection of invariant structure. Psychological Science. 2002;13(5):431–436. doi: 10.1111/1467-9280.00476. [DOI] [PubMed] [Google Scholar]
- Granpeesheh D, Tarbox J, Dixon DR. Applied behavior analytic interventions for children with autism: A description and review of treatment research. Annals of Clinical Psychiatry. 2009;21(3):162–173. [PubMed] [Google Scholar]
- Gray CA, Garand JD. Social Stories: Improving Responses of Students with Autism with Accurate Social Information. Focus on Autism and Other Developmental Disabilities. 1993;8(1):1–10. doi: 10.1177/108835769300800101. [DOI] [Google Scholar]
- Gray S, Brinkley S. Fast Mapping and Word Learning by Preschoolers With Specific Language Impairment in a Supported Learning Context: Effect of Encoding Cues, Phonotactic Probability, and Object Familiarity. Journal of Speech Language and Hearing Research. 2011;54(3):870–884. doi: 10.1044/1092-4388(2010/09-0285). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler L, Goffman L, Younger B. Lexical and articulatory interactions in children’s language production. Developmental Science. 2010;13(5) doi: 10.1111/j.1467-7687.2009.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L, Powell A, Gaskell MG, Norbury C. Learning and consolidation of new spoken words in autism spectrum disorder. Developmental Science. 2014;17(6):858–871. doi: 10.1111/desc.12169. [DOI] [PubMed] [Google Scholar]
- Henderson L, Weighall A, Gaskell G. Learning new vocabulary during childhood: Effects of semantic training on lexical consolidation and integration. Journal of Experimental Child Psychology. 2013;116(3):572–592. doi: 10.1016/j.jecp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Herlihy L, Knoch K, Vibert B, Fein D. Parents’ first concerns about toddlers with autism spectrum disorder: Effect of sibling status. Autism. 2015;19(1):20–28. doi: 10.1177/1362361313509731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Shadmehr R. Motor variability is not noise, but grist for the learning mill. Nature Neuroscience. 2014;17(2):149–150. doi: 10.1038/nn.3633. [DOI] [PubMed] [Google Scholar]
- Horst JS, Samuelson LK. Fast Mapping but Poor Retention by 24-Month-Old Infants. Infancy. 2008;13(2):128–157. doi: 10.1080/15250000701795598. [DOI] [PubMed] [Google Scholar]
- James EG, Conatser P. Effects of Practice Variability on Unimanual Arm Rotation. Journal of Motor Behavior. 2014;46(4):203–210. doi: 10.1080/00222895.2014.881314. [DOI] [PubMed] [Google Scholar]
- Justice LM, Meier J, Walpole S. Learning new words from storybooks: An efficacy study with at-risk kindergartners. Language Speech and Hearing Services in Schools. 2005;36(1) doi: 10.1044/0161-1461(2005/003). [DOI] [PubMed] [Google Scholar]
- Ketelaars MP, Hermans SIA, Cuperus J, Jansonius K, Verhoeven L. Semantic Abilities in Children With Pragmatic Language Impairment: The Case of Picture Naming Skills. Journal of Speech Language and Hearing Research. 2011;54(1) doi: 10.1044/1092-4388(2010/09-0116). [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Camarata S, Koegel LK, Ben-Tall A, Smith AE. Increasing speech intelligibility in children with autism. Journal of Autism and Developmental Disorders. 1998;28(3):241–251. doi: 10.1023/a:1026073522897. [DOI] [PubMed] [Google Scholar]
- Kucker SC, McMurray B, Samuelson LK. Slowing Down Fast Mapping: Redefining the Dynamics of Word Learning. Child Development Perspectives. 2015;9(2):74–78. doi: 10.1111/cdep.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Smith LB, Jones SS. The importance of shape in early lexical learning. Cognitive Development. 1988;3(3):299–321. doi: 10.1016/0885-2014(88)90014-7. [DOI] [Google Scholar]
- Lanovaz MJ, Sladeczek IE. Vocal Stereotypy in Individuals With Autism Spectrum Disorders: A Review of Behavioral Interventions. Behavior Modification. 2012;36(2):146–164. doi: 10.1177/0145445511427192. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22(1) doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Pickles A. Trajectory of language development in autistic spectrum disorders. In: Rice M, Warren SF, editors. Developmental language disorders: from phenotypes to etiologies. Mahwah, N.J.: Mahwah, N.J: Lawrence Erlbaum; 2004. pp. 7–29. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule. Second. Torrance, CA: Western Psychology Services; 2012. ADOS-2. [Google Scholar]
- Loucas T, Charman T, Pickles A, Simonoff E, Chandler S, Meldrum D, Baird G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. Journal of Child Psychology and Psychiatry. 2008;49(11):1184–1192. doi: 10.1111/j.1469-7610.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- Lovaas OI. Teaching developmentally disabled children: the me book. Austin, Tex.: Austin, Tex: PRO-ED; 1981. [Google Scholar]
- Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology. 1987;55(1):3–9. doi: 10.1037/0022-006X.55.1.3. [DOI] [PubMed] [Google Scholar]
- Luyster RJ, Kadlec MB, Carter A, Tager-Flusberg H. Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(8):1426–1438. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Mancina C, Tankersley M, Kamps D, Kravits T, Parrett J. Brief report: Reduction of inappropriate vocalizations for a child with autism using a self-management treatment program. Journal of Autism and Developmental Disorders. 2000;30(6):599–606. doi: 10.1023/a:1005695512163. [DOI] [PubMed] [Google Scholar]
- Mathworks I. Matlab: High performance numeric computation and visualization software. Natick, MA: 2009. [Google Scholar]
- McDuffie AS, Yoder PJ, Stone WL. Labels increase attention to novel objects in children with autism and comprehension-matched children with typical development. Autism. 2006;10(3):288–301. doi: 10.1177/1362361306063287. [DOI] [PubMed] [Google Scholar]
- McGregor KK. What a Difference a Day Makes: Change in Memory for Newly Learned Word Forms Over 24 Hours. Journal of Speech Language and Hearing Research. 2014;57(5):1842–1850. doi: 10.1044/2014_jslhr-l-13-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KK, Bean A. How Children With Autism Extend New Words. Journal of Speech Language and Hearing Research. 2012;55(1) doi: 10.1044/1092-4388(2011/11-0024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KK, Friedman RM, Reilly RM, Newman RM. Semantic representation and naming in young children. Journal of Speech Language and Hearing Research. 2002;45(2):332–346. doi: 10.1044/1092-4388(2002/026). [DOI] [PubMed] [Google Scholar]
- McGregor KK, Newman RM, Reilly RM, Capone NC. Semantic representation and naming in children with specific language impairment. Journal of Speech Language and Hearing Research. 2002;45(5):998–1015. doi: 10.1044/1092-4388(2002/081). [DOI] [PubMed] [Google Scholar]
- McGregor KK, Sheng L, Ball T. Complexities of expressive word learning over time. Language Speech and Hearing Services in Schools. 2007;38(4) doi: 10.1044/0161-1461(2007/037). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Corley M, Lickley RJ. Articulatory evidence for feedback and competition in speech production. Language and Cognitive Processes. 2009;24(1) doi: 10.1080/01690960801998236. [DOI] [Google Scholar]
- McRae K, deSa VR, Seidenberg MS. On the nature and scope of featural representations of word meaning. Journal of Experimental Psychology-General. 1997;126(2) doi: 10.1037//0096-3445.126.2.99. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society: JINS. 1997;3(4) [PubMed] [Google Scholar]
- Mok Z, Kipka PF. Lexical-semantic immaturities manifesting as grammatical disorders: Evidence from a child language sample. Clinical Linguistics & Phonetics. 2009;23(11):808–824. doi: 10.3109/02699200903242970. [DOI] [PubMed] [Google Scholar]
- Munro N, Baker E, McGregor K, Docking K, Arciuli J. Why word learning is not fast. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson B, Swenson CL, Manthei SC. Lexical and phonological organization in children: Evidence from repetition tasks. Journal of Speech Language and Hearing Research. 2005;48(1) doi: 10.1044/1092-4388(2005/009). [DOI] [PubMed] [Google Scholar]
- Naigles LR. Input and Language Development in Children with Autism. Seminars in Speech and Language. 2013;34(4):237–248. doi: 10.1055/s-0033-1353446. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Griffiths H, Nation K. Sound before meaning: Word learning in autistic disorders. Neuropsychologia. 2010;48(14):4012–4019. doi: 10.1016/j.neuropsychologia.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Olu-Lafe O, Liederman J, Tager-Flusberg H. Is the Ability to Integrate Parts into Wholes Affected in Autism Spectrum Disorder? Journal of Autism and Developmental Disorders. 2014;44(10):2652–2660. doi: 10.1007/s10803-014-2120-z. [DOI] [PubMed] [Google Scholar]
- Parish-Morris J, Hennon EA, Hirsh-Pasek K, Golinkoff RM, Tager-Flusberg H. Children with autism illuminate the role of social intention in word learning. Child Development. 2007;78(4):1265–1287. doi: 10.1111/j.1467-8624.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- Paul R, Campbell D, Gilbert K, Tsiouri I. Comparing Spoken Language Treatments for Minimally Verbal Preschoolers with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2013;43(2):418–431. doi: 10.1007/s10803-012-1583-z. [DOI] [PubMed] [Google Scholar]
- Potrzeba ER, Fein D, Naigles L. Investigating the shape bias in typically developing children and children with autism spectrum disorders. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler MA, Carey S. The role of inferences about referential intent in word learning: Evidence from autism. Cognition. 2005;97(1) doi: 10.1016/j.cognition.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rabovsky M, Schad DJ, Rahman RA. Language production is facilitated by semantic richness but inhibited by semantic density: Evidence from picture naming. Cognition. 2016;146:240–244. doi: 10.1016/j.cognition.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Rabovsky M, Sommer W, Rahman RA. Implicit Word Learning Benefits From Semantic Richness: Electrophysiological and Behavioral Evidence. Journal of Experimental Psychology-Learning Memory and Cognition. 2012;38(4) doi: 10.1037/a0025646. [DOI] [PubMed] [Google Scholar]
- Rapp B, Goldrick M. Interactivity? The relationship between semantics and phonology in speech production. Brain and Language. 1997;60(1) [Google Scholar]
- Rapp JT, Vollmer TR. Stereotypy I: A review of behavioral assessment and treatment. Research in Developmental Disabilities. 2005;26(6):527–547. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Richtsmeier PT, Gerken L, Goffman L, Hogan T. Statistical frequency in perception affects children’s lexical production. Cognition. 2009;111(3):372–377. doi: 10.1016/j.cognition.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Klee T. Clinical-assessment of oropharyngeal motor development in young-children. Journal of Speech and Hearing Disorders. 1987;52(3) doi: 10.1044/jshd.5203.271. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic cognition: A parallel distributed processing approach. Cambridge, Mass: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Précis of Semantic Cognition: A Parallel Distributed Processing Approach. Behavioral and Brain Sciences. 2008;31(6):689–714. [Google Scholar]
- Rosch E. Cognitive representations of semantic categories. Journal of Experimental Psychology: General. 1975;104(3):192–233. doi: 10.1037/0096-3445.104.3.192. [DOI] [Google Scholar]
- Sapir EMDG. Selected writings of Edward Sapir in language, culture and personality. Berkeley: University of California Press; 1949. etc. [Google Scholar]
- Schoen E, Paul R, Chawarska K. Phonology and Vocal Behavior in Toddlers With Autism Spectrum Disorders. Autism Research. 2011;4(3):177–188. doi: 10.1002/aur.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Van Bourgondien M, Wellman GJ, Love SR. Childhood Autism Rating Scale. Second. Western Psychological Services; 2010. [Google Scholar]
- Scott-Van Zeeland AA, McNealy K, Wang AT, Sigman M, Bookheimer SY, Dapretto M. No Neural Evidence of Statistical Learning During Exposure to Artificial Languages in Children with Autism Spectrum Disorders. Biological Psychiatry. 2010;68(4):345–351. doi: 10.1016/j.biopsych.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals–Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Singleton NC. Can Semantic Enrichment Lead to Naming in a Word Extension Task? American Journal of Speech-Language Pathology. 2012;21(4):279–292. doi: 10.1044/1058-0360(2012/11-0019). [DOI] [PubMed] [Google Scholar]
- Skinner BF. Verbal behavior. New York: New York, Appleton-Century-Crofts; 1957. [Google Scholar]
- Smith A, Goffman L. Interaction of motor and language factors in the development of speech production. In: Maasen B, Kent R, Peters H, Lieshout Pv, Hulstijn W, editors. Speech Motor Control in Normal and Disordered Speech. Oxford, UK: Oxford University Press; 2004. pp. 225–252. [Google Scholar]
- Smith A, Goffman L, Zelaznik HN, Ying GS, McGillem C. Spatiotemporal Stability and Patterning of Speech Movement Sequences. Experimental Brain Research. 1995;104(3) doi: 10.1007/BF00231983. [DOI] [PubMed] [Google Scholar]
- Smith A, Johnson M, McGillem C, Goffman L. On the assessment of stability and patterning of speech movements. Journal of Speech Language and Hearing Research. 2000;43(1) doi: 10.1044/jslhr.4301.277. [DOI] [PubMed] [Google Scholar]
- Spencer M, Kaschak MP, Jones JL, Lonigan CJ. Statistical learning is related to early literacy-related skills. Reading and Writing. 2015;28(4):467–490. doi: 10.1007/s11145-014-9533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkel HL. Learning new words: Phonotactic probability in language development. Journal of Speech Language and Hearing Research. 2001;44(6) doi: 10.1044/1092-4388(2001/103). [DOI] [PubMed] [Google Scholar]
- Storkel HL, Adlof SM. The Effect of Semantic Set Size on Word Learning by Preschool Children. Journal of Speech Language and Hearing Research. 2009;52(2) doi: 10.1044/1092-4388(2009/07-0175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkel HL, Hoover JR. An on-line calculator to compute phonotactic probability and neighborhood density based on child corpora of spoken American English. Behavior Research Methods. 2010;42(2):497–506. doi: 10.3758/BRM.42.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen LD, Kelley E, Fein D, Naigles LR. Processes of Language Acquisition in Children With Autism: Evidence from Preferential Looking. Child Development. 2007;78(2):542–557. doi: 10.1111/j.1467-8624.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Basic level and superordinate level categorization by autistic, mentally retarded, and normal children. Journal of Experimental Child Psychology. 1985;40(3):450–469. doi: 10.1016/0022-0965(85)90077-3. [DOI] [PubMed] [Google Scholar]
- Tek S, Jaffery G, Fein D, Naigles LR. Do Children With Autism Spectrum Disorders Show a Shape Bias in Word Learning? Autism Research. 2008;1(4):208–222. doi: 10.1002/aur.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey KE, Ranson SL, Horst JS. That’s More Like It: Multiple Exemplars Facilitate Word Learning. Infant and Child Development. 2014;23(2):105–122. doi: 10.1002/icd.1824. [DOI] [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral Treatments in Autism Spectrum Disorder: What Do We Know? Annual Review of Clinical Psychology. 2010;6(6):447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A, Weber-Fox C. Short-term plasticity in children’s speech motor systems. Developmental Psychobiology. 2006;48(8):660–674. doi: 10.1002/dev.20185. [DOI] [PubMed] [Google Scholar]
- Waxman SR, Leddon EM. Early word learning and conceptual development: Everything had a name, and each name gave birth to a new thought. In: Goswami U, editor. The Wiley-Blackwell Handbook of Childhood Cognitive Development. 2. Chichester: Wiley-Blackwell; 2011. pp. 180–208. [Google Scholar]
- Whitehouse AJO, Maybery MT, Durkin K. Evidence against poor semantic encoding in individuals with autism. Autism. 2007;11(3):241–254. doi: 10.1177/1362361307076860. [DOI] [PubMed] [Google Scholar]
- Whorf BLCJB. Language, thought, and reality: selected writings of Benjamin Lee Whorf MIT paperback series; Variation: MIT paperback series. 1956 Retrieved from WorldCat database Retrieved from http://hdl.handle.net/2027/heb.08469 Retrieved from Materials specified: ACLS Humanities E-Book http://hdl.handle.net/2027/heb.08469.
- Williams DL, Goldstein G, Minshew NJ. The profile of memory function in children with autism. Neuropsychology. 2006;20(1) doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Expressive Vocabulary Test-II. Circle Pines, MN: American Guidance Service; 2007. [Google Scholar]


