Abstract
Purpose
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease. Clinical follow-up of incidental findings in ARVC-associated genes is recommended. We aimed to determine the prevalence of disease thus ascertained.
Methods
30,716 individuals underwent exome sequencing. Variants in PKP2, DSG2, DSC2, DSP, JUP, TMEM43, or TGFβ3 that were database-listed as pathogenic or likely pathogenic were identified and evidence-reviewed. For subjects with putative loss-of-function (pLOF) variants or variants of uncertain significance (VUS), electronic health records (EHR) were reviewed for ARVC diagnosis, diagnostic criteria, and International Classification of Diseases (ICD-9) codes.
Results
18 subjects had pLOF variants; none had an EHR diagnosis of ARVC. Of 14 patients with an electrocardiogram (ECG), one had a minor diagnostic criterion, 13 were normal. 184 subjects had VUSs; none had an ARVC diagnosis. In subjects with VUSs, there was no difference in the proportion with major (4%) or minor (13%) ECG diagnostic criteria compared to variant-negative controls. ICD-9 codes showed no difference in defibrillator utilization, electrophysiologic abnormalities or non-ischemic cardiomyopathies in patients with pLOF or VUSs compared to controls.
Conclusion
pLOF variants in an unselected cohort were not associated with ARVC phenotypes based on EHR review. The negative predictive value of EHR review remains uncertain.
Keywords: arrhythmogenic right ventricular cardiomyopathy, exome sequencing, genotype-phenotype association, electronic health record, incidental findings
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease characterized by ventricular dysfunction and arrhythmias.1 ARVC has an estimated phenotypic prevalence of 1:1000–1:50002 and is among the leading causes of sudden cardiac death in people under 35 years old, especially young athletes.3,4 Clinical symptoms are frequently absent before a sudden death event,5 so improved methods of screening and early diagnosis are needed to provide an opportunity for life-saving prophylaxis. ARVC is primarily attributed to genetic variants in cardiac desmosome genes,6,7 so a “genome-first” approach to patient identification is a promising option for earlier diagnosis. This paradigm is a natural extension of recommendations from the American College of Medical Genetics and Genomics (ACMG) to report specific incidental findings from clinical genetic sequencing to patients because of the potential for medical benefit.8 Indeed, ARVC was one of the few selected conditions included with these guidelines, representing five of the 56 genes recommended for screening.
Recent studies have called into question the efficacy of incidental genetic findings for ARVC and other inherited cardiac diseases.9–13 Most notably, the study by Van Driest and colleagues11 concluded that there was no abnormal phenotype in individuals carrying potentially pathogenic variants for select genes associated with long QT or Brugada syndromes, suggesting that notifying patients of incidental findings was unwarranted. While striking, this study was limited by the facts that only 2022 individuals were studied, and only two genes—accounting for approximately 38% of long QT cases14 and 16% of Brugada syndrome cases15—were screened. Moreover, the observed variants were almost exclusively missense/variants of uncertain significance (VUS). Missense variants have previously been shown to be both common10,16 and difficult to evaluate for pathogenicity,17 as further evidenced by the lack of consensus among clinical laboratory reviews in Van Driest’s study. Based on disease prevalence,11 the likelihood of identifying even one patient with a causal mutation linked to either syndrome in that cohort was only 34%. Larger numbers are clearly needed to sufficiently power such analyses for rare diseases.
In this study, we used a fifteen fold larger cohort (30,716 subjects with exome sequencing), reviewed more genes (7), and focused on “radical” putative loss-of-function (pLOF) variants, which have a higher probability of disease association,9 to provide a more comprehensive analysis of the genotypic prevalence of ARVC. Furthermore, since no other study has evaluated phenotypes of individuals with pLOF ARVC variants ascertained through population sequencing, we reviewed electronic health records (EHR) for identified subjects to establish a genotype-phenotype association. While full disease penetrance was not expected, we hypothesized that a discernible EHR phenotype (such as the presence of ARVC diagnostic criteria, arrhythmias, or other primary cardiomyopathies) would be present in individuals with pLOF variants.
PATIENTS AND METHODS
Study Information
The MyCode® Community Health Initiative of Geisinger Health System (GHS) is an IRB-approved research biorepository and precision medicine project. MyCode participants provide samples for research, including permission to link samples and associated data with information in their EHR.18 Through the DiscovEHR collaboration between GHS and the Regeneron Genetics Center (Tarrytown, NY), DNA samples from MyCode participants are used to generate exome sequence data to populate this database.18,19 Details of the exome sequencing and postprocessing have been described elsewhere;19 with additional detail provided in the appendix. Briefly, sequencing was performed on an Illumina v4 HiSeq 2500 to a coverage depth such that over 95% of samples have greater than 85% of the target bases covered with more than 20x read depth. At the time of this study, the MyCode repository comprised 30,716 subjects of predominantly European ancestry. No exclusions were made for relatedness or ancestry.
Variant Evaluation
We reviewed the ClinVar (ncbi.nlm.nih.gov/clinvar) and ARVC (arvcdatabase.info20) databases for all ARVC-associated variants in PKP2, DSP, DSC2, DSG2, JUP, TMEM43, and TGFβ3 that were classified as “pathogenic” (P) or “likely pathogenic” (LP) as of June 2, 2015. ClinVar search criteria included both the gene name and “Arrhythmogenic right ventricular cardiomyopathy”. For conflicting classifications, ClinVar superseded the ARVC database, and conflicting interpretations in ClinVar were resolved based on the most recent submission. The variants observed in the MyCode cohort were then further classified by expert review according to published methods21 consistent with the 2015 ACMG/AMP guideline for sequence variant interpretation.22 Of note, LOF is the predominant mechanism of pathogenicity for the desmosome genes in ARVC,9 so the evidence assertion for radical pLOF variants was generally very strong. This expert review was conducted by staff at the Laboratory for Molecular Medicine at Partners Personalized Medicine (CAT, HMS, ML). To account for continuous, ongoing improvements in the bioinformatics pipeline with time, variant calls were rechecked against the most recent quality control filtered data at the time of manuscript preparation. This pipeline included GATK best practices for variant calling,23 and filtering with GATK for Genotype Quality (GQ) with a threshold of 20.
Subject Identification and Phenotype Analysis
All subjects carrying at least one potential ARVC variant (database reported P/LP) were identified. Age (±5 years) and sex-matched subjects (5:1 match) were randomly ascertained from MyCode subjects lacking ARVC-associated variants.
For all subjects with potential variants and a random subset (10%) of controls, we completed a blinded expert review of primary data, including:
most recent non-paced electrocardiograms (ECG) (initial review by CAJ, CT; any abnormal finding read by HC for classification),
most recent echocardiograms for which the right ventricular function and size were not explicitly reported as normal (VCM, DJM). A random 20% of the “normal” cases were also reviewed to ensure accuracy.
Data from the most recent Holter monitoring were also reviewed. Findings were compared against the diagnostic Task Force criteria for ARVC, which include right ventricular dysfunction and depolarization/repolarization abnormalities on ECG.24 International Classification of Disease, Ninth Revision (ICD-9) codes in patient records as of July 21, 2015 were reviewed for specific (Table S1) and non-specific (Table S2) codes. As appropriate, cause of death (i.e., cardiac, non-cardiac, or unknown) was assessed from a blinded physician chart review (BKF) of death certificates or contemporaneous physician notes.
Separately, the GHS EHR was reviewed for individuals having any medical encounter with an associated diagnosis of “arrhythmogenic right ventricular cardiomyopathy/dysplasia” (Table S1). Physician chart review (BKF) of the two most recent cardiology, internal/family medicine, and discharge notes (or any two sub-specialty notes when other notes were not available) was performed for affirmative documentation of disease. This blinded procedure was also used to confirm diagnostic status for a random subset (33%) of subjects with potential variants, plus any subject with an external cardiology referral.
Statistical Analysis
Statistical testing was performed using R (version 3.3.2; Vienna, Austria). Descriptive statistics are reported as mean ± standard deviation. Group differences in phenotypes were compared using Fisher’s Exact tests; the log-rank test was used to compare survival, using the OIsurv package in R.25 Reported p-values were not adjusted for multiple comparisons.
RESULTS
Missense Variants are Common; Previously Reported pLOF Variants are Rare
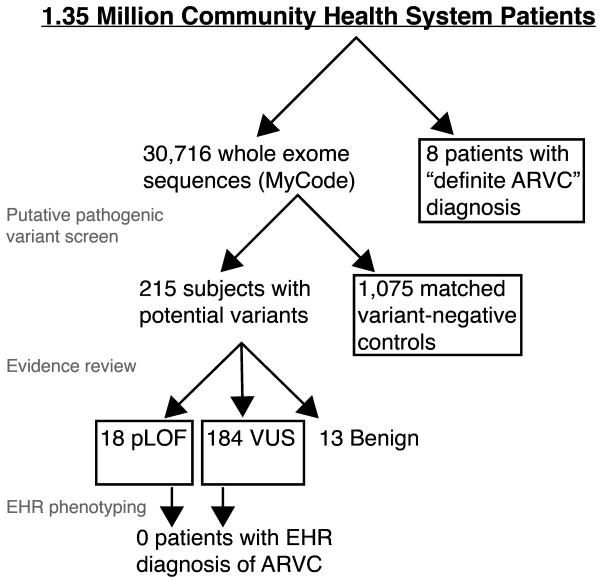
A total of 323 rare variants were database listed as “P/LP” in ClinVar and/or the ARVC database (Table S3). Of these, 45 potentially pathogenic variants were identified in 301 MyCode participants. One variant (TMEM43 c.705+7G>A) was observed in 76 subjects and immediately re-classified as likely benign due to the high allele frequency (> 0.075%).21 Furthermore, the variant calls for seven subjects did not pass GQ filtering, and were excluded. The remaining 215 patients (0.7% of the population) comprised the potentially pathogenic variant group (Figure 1; Table 1). Average age was 61 ± 17 years (Table 2). The distribution with respect to affected gene is shown in Table S4. Missense variants were most prevalent with respect to observed variants (30/44 (excluding TMEM43 c.705+7G>A); 68%) and number of subjects (191/215; 89%).
Figure 1.
Overview of study design and patient group identities.
Table 1.
Details of variants database listed as “P/LP” and identified in 30,716 patients
| Gene | HGVS.c | HGVS.p | Variant Type | Patient count | Expert review |
|---|---|---|---|---|---|
| DSC2 | c.2587G>A | p.Gly863Arg | Missense | 30 | VUS |
| c.2368_2370delGGA | p.Gly790del | Deletion | 1 | LB | |
| c.1048G>T | p.Asp350Tyr | Missense | 1 | VUS | |
| c.1034T>C | p.Ile345Thr | Missense | 16 | VUS | |
| c.394C>T | p.Arg132Cys | Missense | 1 | VUS | |
| c.4G>A | p.Glu2Lys | Missense | 15 | VUS | |
| DSG2 | c.260A>G | p.Tyr87Cys | Missense | 1 | VUS |
| c.437G>A | p.Arg146His | Missense | 7 | VUS | |
| c.523+1G>A | Splice | 1 | LP | ||
| c.523+2T>C | Splice | 3 | LP | ||
| c.961T>A | p.Phe321Ile | Missense | 2 | VUS | |
| c.991G>A | p.Glu331Lys | Missense | 9 | VUS | |
| c.1520G>A | p.Cys507Tyr | Missense | 4 | VUS | |
| DSP | c.478C>T | p.Arg160* | Nonsense | 1 | LP |
| c.688G>A | p.Asp230Asn | Missense | 9 | VUS | |
| c.699G>A | p.Trp233* | Nonsense | 1 | LP | |
| c.1333A>G | p.Ile445Val | Missense | 2 | VUS | |
| c.2360A>G | p.Tyr787Cys | Missense | 1 | VUS | |
| c.2423G>A | p.Arg808His | Missense | 2 | VUS | |
| c.3764G>A | p.Arg1255Lys | Missense | 5 | VUS | |
| c.4117A>G | p.Thr1373Ala | Missense | 27 | VUS | |
| c.4961T>C | p.Leu1654Pro | Missense | 1 | VUS | |
| c.5513G>A | p.Arg1838His | Missense | 6 | VUS | |
| c.6496C>T | p.Arg2166* | Nonsense | 1 | VUS | |
| c.7622G>A | p.Arg2541Lys | Missense | 1 | VUS | |
| c.7784C>T | p.Thr2595Ile | Missense | 8 | VUS | |
| c.7916G>A | p.Arg2639Gln | Missense | 4 | B | |
| JUP | c.2078A>G | p.Tyr693Cys | Missense | 5 | VUS |
| c.1280C>T | p.Thr427Met | Missense | 3 | VUS | |
| c.1219G>A | p.Val407Ile | Missense | 2 | VUS | |
| c.475G>T | p.Val159Leu | Missense | 6 | VUS | |
| c.56C>T | p.Thr19Ile | Missense | 4 | VUS | |
| PKP2 | c.2578-3A>G | Splice | 1 | VUS | |
| c.2489+1G>A | Splice | 1 | P | ||
| c.2484C>T | Splice | 1 | VUS | ||
| c.2203C>T | p.Arg735* | Nonsense | 5 | LP | |
| c.2146-1G>C | Splice | 4 | LP | ||
| c.1904G>A | p.Arg635Gln | Missense | 3 | VUS | |
| c.1379-1G>A | Splice | 2 | VUS | ||
| c.1237C>T | p.Arg413* | Nonsense | 1 | P | |
| c.1093A>G | p.Met365Val | Missense | 8 | LB | |
| c.895C>T | p.Arg299Cys | Missense | 4 | VUS | |
| c.337-2A>T | Splice | 1 | LP | ||
| TMEM43 | c.169G>A | p.Ala57Thr | Missense | 4 | VUS |
| c.705+7G>A† | Splice | 76 | LB |
re-classified as likely benign based on observed allele frequency; VUS – variant of uncertain significance; P/LP – pathogenic/likely pathogenic; B/LB – benign/likely benign; HGVS.c – Human Genome Variation Society cDNA change; HGVS.p – Human Genome Variation Society amino acid change.
Table 2.
Patient demographic details by group.
| Age (years) | Sex (% female) | |
|---|---|---|
| pLOF (n = 18) | 59 ± 18 | 56 |
| VUS (n = 184) | 62 ± 17 | 58 |
| Variant-negative control (n = 1075) | 61 ± 17 | 58 |
| Definite ARVC (n = 8) | 41 ± 16 | 63 |
After evidence review, nine radical pLOF variants (nonsense and splice site) met criteria to be classified as P/LP. Supporting evidence for other variants, including all missense, were insufficient and they were scored as VUS or likely benign (Table 1). Of note, eight of the nine pLOF variants were documented in the ClinVar database; only one of the 32 observed variants unique to the ARVC database was confirmed.
The nine pLOF variants were identified in 18 subjects (1:1706). Average age of this group was 59 ± 18 years (Table 2), and the average body mass index (BMI) was 32 ± 8.
No Increase in the Prevalence of ARVC Diagnostic Criteria in Patients with pLOF Variants
The 18 subjects with pLOF variants had a median of 9.5 years of EHR data (range: 0–16 years). Based on ICD-9 coding and randomized chart review, none had a documented diagnosis of ARVC. Fourteen subjects (78%) had a previous ECG, which were manually reviewed for diagnostic depolarization/repolarization criteria (Table 3). One of the fourteen subjects satisfied a minor criterion (inverted T-waves in V5 and V6), which qualifies for a “borderline” diagnosis with the addition of the LP variant. Separately, echocardiograms were reviewed for the eight subjects (44%) with previous studies, and right ventricular function was reported as “normal” for all. Four of the 18 subjects with pLOF variants (22%) had neither an ECG nor echocardiogram—including three individuals younger than 37 years.
Table 3.
Summary findings of 2010 Task Force Criteria by Group
| pLOF (n =18) | Rare VUS (n = 184) | Variant-negative controls (n = 110) | p-value (VUS/Control) | ||
|---|---|---|---|---|---|
| ECG | Subjects with ECG | 14 (78%) | 160 (87%) | 93 (85%) | 0.60 |
| Major criterion | 0 | 6 (4%) | 5 (5%) | 0.54 | |
| Minor criterion | 1 (7%)‡ | 20 (13%) | 16 (17%) | 0.35 | |
|
| |||||
| Echo | Subjects with Echo | 8 (44%) | 109 (59%) | 63 (57%) | 0.81 |
| Major criterion | 0 | 1 (1%) | 2 (3%) | 0.56 | |
| Minor criterion | 0 | 2 (2%) | 0 | 0.53 | |
|
| |||||
| Holter monitor | Subjects with Holter | 0 | 23 (13%) | 13 (12%) | 1 |
| Major criterion | 0 | 0 | 0 | N/A | |
| Minor criterion | 0 | 7 (30%) | 5 (38%) | 0.72 | |
|
| |||||
| Mixed | Major + minor † | 0 | 1 (1%)§ | 1 (1%)*‡ | 1 |
| >1 Minor criteria † | 0 | 2 (1%) | 2 (2%) | 0.60 | |
Data presented as number (percentage);
Echo dysfunction (major) and repolarization findings from ECG (minor);
Subjects with multiple criteria were also separately accounted with the individual criteria;
Satisfies criteria for borderline diagnosis;
Repolarization (major) and depolarization (minor) criteria from ECG
Given the uncertain importance of a rare VUS in ARVC-associated genes, primary EHR data for the 184 individuals with such variants were also reviewed (Table 2). Average BMI for these subjects was 31± 7. Based on ICD-9 coding and randomized chart review, none of these individuals had a documented diagnosis of ARVC. ECGs were available for 160 individuals (87%); 83% satisfied no diagnostic criteria, 4% satisfied a major criterion, and 13% satisfied a minor criterion (Table 3). These frequencies were comparable to those of the variant-negative control group, from which 5% and 17% were found to have major and minor ECG criteria, respectively. Hence, the findings in the VUS group were statistically similar to the variant-negative control group (p = 0.54 and 0.35, respectively). The same trends were observed with respect to echocardiographic and Holter monitoring criteria (Table 3).
Non-Specific Phenotypes Distinguish Known ARVC Cases, but not Potential Variant Groups
Thirty patients with at least one instance of an ARVC diagnostic code in their EHR were identified out of 1.35 million subjects. Physician chart review confirmed an ARVC diagnosis in only eight subjects (1:168,750; Table 2); diagnostic codes were determined to be inappropriately applied in the remaining 22 subjects. No exome sequence data were available for these individuals.
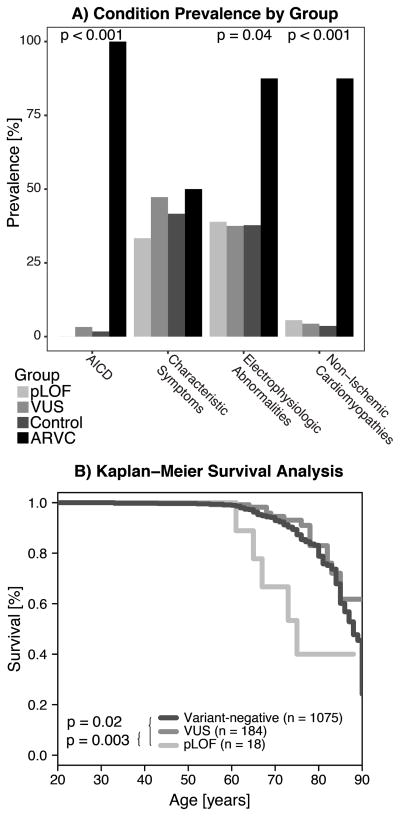
Non-specific ICD-9 composite phenotypes—non-ischemic cardiomyopathies, cardiac electrophysiologic abnormalities, ARVC “characteristic symptoms”, and automatic implantable cardioverter defibrillator (AICD) use—for the pLOF, VUS, variant-negative controls, and definite ARVC study groups are compared in Figure 2A. The prevalence of non-ischemic cardiomyopathies, electrophysiologic abnormalities, and AICD use was significantly higher in the definite ARVC group, while the patients with a pLOF variant or VUS showed prevalences similar to the negative control group.
Figure 2.
A) Prevalence of the composite ICD-9 categories within each study group. P-values denote Fisher’s exact test for a given category. AICD- automatic implantable cardioverter defibrillator. B) Kaplan-Meier survival estimates for each study group, showing that the pLOF group had significantly reduced survival by log-rank test.
Increased All-Cause Mortality in pLOF Group
We compared all-cause mortality (age at death) between groups (pLOF, VUS, and variant-negative controls). For the control and VUS groups, 89% and 92% of subjects were alive at the time of analysis, respectively (p = 0.19; Figure 2B). By comparison, only 72% (13/18) of subjects in the pLOF group were alive, representing significantly increased mortality (p = 0.003 compared with VUS). However, from chart review, the causes of death for these five subjects were non-cardiac related in four cases, and unknown in one.
DISCUSSION
Our major findings appear to parallel recent studies in cardiac genetics with respect to the high prevalence of rare variants once thought to be disease causing, but a weak association with classic disease symptoms.10–13,16 However, the present work specific to ARVC represents a significant advance for several reasons. This study represents the largest analysis to date of next-generation sequencing data to evaluate the presence of putative genetic variants related to ARVC. This size and scope (inclusive of all primary ARVC genes) provided a 98–99% likelihood of finding at least one subject with ARVC and a causal variant. This high probability contrasts with previous studies—such as Van Driest, et al.—which have been drastically under-powered to study rare diseases and their associated radical genetic variants.11 Indeed, we observed previously reported pLOF variants in 18 (1:1706) individuals, which were further adjudicated as P/LP following expert evidence review. This study also represents the first population-based analysis of ARVC genetics with linked EHR data to evaluate genotype-phenotype associations. With these linked data, we reviewed diagnostic codes for all subjects, ECGs for 86% of the pLOF and rare VUS groups, and right ventricular function via echocardiogram in 58%. Our findings suggest that, in unselected individuals with incidentally detected pLOF variants, genetic penetrance may be much lower than estimates (40–60%) from familial studies,5 as no individual had a documented diagnosis of disease, and only one subject satisfied any additional minor diagnostic criteria. Moreover, our findings demonstrate that individuals with a rare VUS also have an unremarkable phenotype, with no existing diagnoses and diagnostic criteria frequencies in line with the observed false positive rate in variant-negative controls. Several studies have documented the considerable “background noise” in our understanding of putative pathogenic ARVC variants,9,10 but none has previously demonstrated that this noise has no phenotypic consequence.
Vigorous exercise is the most significant known modulator of genetic penetrance for ARVC.26,27 In fact, current guidelines restrict athletic participation following even a “possible” diagnosis.28 Considering the obese average BMI of our cohort (>32 in the pLOF group), it is likely that few subjects, if any, regularly participate in the level of vigorous physical activity typically associated with ARVC. Such a lifestyle likely confers a protective effect in the setting of genetic predisposition to ARVC and may have played a role in the low penetrance we observed in our study.
While our phenotype determination was not made from prospective patient evaluations, 86% of the individuals studied had a non-paced ECG available for review. ECG abnormalities comprise two of the six elements of ARVC diagnosis, and the negative predictive value of a normal ECG is very high, as past studies have shown that over 80% of diagnosed ARVC patients have ECG abnormalities.29,30 Hence, while the available data are insufficient to definitively determine the status for the 13% of subjects with observed abnormalities or the 14% without ECGs, there is a high degree of certainty that the 73% of subjects whose ECG did not satisfy Task Force criteria do not have ARVC.
These results are not an inherent indictment of clinical follow-up for incidental genetic findings of ARVC, as there was at least one case of suggestive findings discovered through a genome-first ascertainment. The increased all-cause mortality in the small group of subjects with pLOF variants was also suggestive of a meaningful finding, although confirmation with a larger sample size is necessary. Instead, our findings strongly indicate that such efforts should be managed conservatively and with stringent evaluation of observed genetic variants based on current standards. Even for individuals identified with radical pLOF variants through genome-first ascertainment, low penetrance should be assumed because such screening is likely to maximize the probability of false positive findings. Furthermore, this low penetrance may be exacerbated in the absence of environmental or lifestyle factors, such as a vigorously active lifestyle. Thus, the incidental identification of a pathogenic variant does not appear to warrant automatic acceptance as a major criterion for ARVC diagnosis, as it is for patients ascertained through clinical presentation or familial testing.24 Similar modification of family history criteria for arrhythmic risk stratification has recently been proposed.30 Ultimately, more research is needed to develop evidence-based guidelines for clinical management of patients with incidentally identified pLOF variants in ARVC-associated genes.
Other Potential Mechanisms to Explain the Results
Genetic Modulation of Penetrance
Additional unknown genetic factors may also explain the low penetrance we observed. For example, there has been some debate about the importance of compound or digenic heterozygosity to ARVC,9,31,32 which may pre-dispose patients to a more severe phenotype.5,32 Furthermore, since only 50–70% of diagnosed cases have been attributed to a genetic cause, unrecognized genes may be playing critical roles. More extensive use of next generation sequencing may help to address such questions.33 None of the subjects in our cohort had more than one known variant, although potential novel variants were not assessed.
Phenotype mis-classification
ARVC is a relatively new and evolving clinical entity that may be underrecognized in clinical populations. The observed prevalence of diagnosed ARVC in our large community health system was 1:168,750, which is two orders of magnitude below reported prevalence and supports this assertion. Since our evaluation was based on EHR data, we cannot rule out clinical phenotype mis-classification in the 27% of our cohort who did not have a documented normal ECG. Moreover, without a formalized set of evidence-based diagnostic criteria for the expanding phenotypic spectrum of ARVC (e.g., bi-ventricular and left-dominant forms),34 our ability to rule out these less common manifestations of the disease from this cohort is limited.
Sampling bias
Given the advanced mean age of the sequenced cohort, it is possible that sampling was biased for survivors. Since ARVC often presents with sudden death in early adulthood, truly affected individuals may have died at a younger age and are therefore not represented in the data. Furthermore, the presence of other age-related co-morbidities, such as coronary artery disease, in this older cohort may also contribute to underdiagnosis of ARVC. Future studies should seek to incorporate younger individuals into penetrance estimates.
Limitations
This study was retrospective and used data derived from our institutional EHR, which had a median of 12 years of longitudinal data per patient for the entire MyCode cohort.18 Prospective targeted phenotyping of pLOF subjects is forthcoming through our institution’s GenomeFIRST return of results initiative, which will provide additional information on genotype/phenotype relationships and better clarify the predictive value of EHR phenotyping.35 However, the only other study using next-generation sequencing to study ARVC variants in 6,354 unselected individuals did not have linked EHR data.10 With a fivefold larger sequenced population and linkage to EHR, our study offers substantially more comprehensive results.
ARVC exhibits age-dependent penetrance, with symptoms and diagnostic criteria developing with time.30 Therefore, without completed life-long follow-up, we cannot definitively say that none of the subjects in our cohort will develop new symptoms. However, previous studies have reported that the cumulative prevalence of ARVC is essentially flat after age 60,36 suggesting that few of our subjects could be expected to develop new symptoms, given the mean age of the cohort.
We did not evaluate novel variants, instead focusing on previously observed variants, in a similar approach to the recent work of Ghouse and colleagues.12,13 The addition of novel pLOF variants will increase the likelihood of identifying affected individuals, but will also increase the genotype prevalence estimates. Future studies will include potentially novel variants.
CONCLUSION
In a large, unselected cohort with exome sequencing data linked to EHR, rare variants listed as P/LP in database resources are common (1:143 genotypic prevalence); however, only 20% of the observed variants (9/44) were putative loss-of-function. Of the 18 individuals (1:1706) identified with a putative loss-of-function variant, the majority (72%) had a normal ECG and are likely unaffected while only 6% (1 subject) satisfied a minor ARVC diagnostic criterion. Because of the importance of environmental factors on ARVC pathogenesis, identification of radical variants alone does not provide a genome-first solution to the identification of ARVC. A conservative approach to clinical return of incidental genetic findings associated with ARVC with further in-person condition specific phenotyping is warranted.
Supplementary Material
Acknowledgments
This work was supported in part by Regeneron Genetics Center, LLC.; Geisinger Health System; and the National Institutes of Health [grant number DP5 OD012132]. We acknowledge all participants in the Geisinger MyCode Community Health Initiative.
Footnotes
Supplementary information is available at the Genetics in Medicine website.
CONFLICTS OF INTEREST
Lebo, Mason-Suares, Austin-Tse, and Rehm are employees of the Laboratory for Molecular Medicine at Partners HealthCare. Murray has consulted for Invitae and Merck. Calkins has received research support from Medtronic and St. Jude Medical. All other authors have nothing to disclose.
This work was financially supported by Regeneron Genetics Center, LLC.; Geisinger Health System; and the National Institutes of Health [grant number DP5 OD012132].
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: A report of 24 adult cases. Circulation. 1982;65(2):384–398. doi: 10.1111/j.1542-474X.1999.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373(9671):1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339(6):364–369. doi: 10.1056/NEJM199808063390602. [DOI] [PubMed] [Google Scholar]
- 4.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318(3):129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 5.Groeneweg Ja, Bhonsale A, James Ca, et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet. 2015;8(3):437–446. doi: 10.1161/CIRCGENETICS.114.001003. [DOI] [PubMed] [Google Scholar]
- 6.McKoy G, Protonotarios N, Crosby a, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355(9221):2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 7.Campuzano O, Alcalde M, Allegue C, et al. Genetics of arrhythmogenic right ventricular cardiomyopathy. J Med Genet. 2013;50(March):280–289. doi: 10.1136/jmedgenet-2013-101523. [DOI] [PubMed] [Google Scholar]
- 8.Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapplinger JD, Landstrom AP, Salisbury Ba, et al. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia- associated mutations from background genetic noise. J Am Coll Cardiol. 2011;57(23):2317–2327. doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreasen C, Nielsen JB, Refsgaard L, et al. New population-based exome data are questioning the pathogenicity of previously cardiomyopathy-associated genetic variants. Eur J Hum Genet. 2013;21(9):918–928. doi: 10.1038/ejhg.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Driest SL, Wells QS, Stallings S, et al. Association of Arrhythmia-Related Genetic Variants With Phenotypes Documented in Electronic Medical Records. JAMA. 2016;315(1):47–57. doi: 10.1001/jama.2015.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghouse J, Have CT, Skov MW, et al. Numerous Brugada syndrome–associated genetic variants have no effect on J-point elevation, syncope susceptibility, malignant cardiac arrhythmia, and all-cause mortality. Genet Med. 2016 Aug;:1–8. doi: 10.1038/gim.2016.151. [DOI] [PubMed] [Google Scholar]
- 13.Ghouse J, Have CT, Weeke P, et al. Rare genetic variants previously associated with congenital forms of long QT syndrome have little or no effect on the QT interval. Eur Heart J. 2015;36(37):2523–2529. doi: 10.1093/eurheartj/ehv297. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg I, Zareba W, Moss AJ. Long QT Syndrome. Curr Probl Cardiol. 2008;33(11):629–694. doi: 10.1016/j.cpcardiol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Crotti L, Marcou CA, Tester DJ, et al. Spectrum and Prevalence of Mutations Involving BrS1- Through BrS12-Susceptibility Genes in a Cohort of Unrelated Patients Referred for Brugada Syndrome Genetic Testing: Implications for Genetic Testing. J Am Coll Cardiol. 2012;60(15):1410–1418. doi: 10.1016/j.jacc.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bick AG, Flannick J, Ito K, et al. Burden of Rare Sarcomere Gene Variants in the Framingham and Jackson Heart Study Cohorts. Am J Hum Genet. 2012;91(3):513–519. doi: 10.1016/j.ajhg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapa S, Tester DJ, Salisbury BA, et al. Genetic testing for long-qt syndrome: Distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752–1760. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey DJ, Fetterolf SN, Davis FD, et al. The Geisinger MyCode community health initiative: an electronic health record–linked biobank for precision medicine research. Genet Med 2016. 2015 Aug;:1–8. doi: 10.1038/gim.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016 doi: 10.1056/NEJMoa1510926. NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazzarini E, Jongbloed JDH, Pilichou K, et al. The ARVD/C genetic variants database: 2014 update. Hum Mutat. 2015;36(4):403–410. doi: 10.1002/humu.22765. [DOI] [PubMed] [Google Scholar]
- 21.Duzkale H, Shen J, Mclaughlin H, et al. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 2013;84(5):453–463. doi: 10.1111/cge.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants : a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GATK. [Accessed January 30, 2017];Best Practices. https://software.broadinstitute.org/gatk/best-practices/
- 24.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur Heart J. 2010;31(13):806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez DM. OIsurv: Survival analysis supplement to OpenIntro guide. R package version 0.2. 2013 https://cran.r-project.org/package=OIsurv.
- 26.Ruwald AC, Marcus F, Estes NAM, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopath. Eur Heart J. 2015:1735–1743. doi: 10.1093/eurheartj/ehv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62(14):1290–1297. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis. J Am Coll Cardiol. 2015:c. doi: 10.1016/j.jacc.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 29.te Riele ASJM, James CA, Bhonsale A, et al. Malignant Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy with a normal 12-lead electrocardiogram: A rare but underrecognized clinical entity. Hear Rhythm. 2013;10(10):1484–1491. doi: 10.1016/j.hrthm.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 30.te Riele ASJM, James CA, Groeneweg JA, et al. Approach to family screening in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur Heart J. 2015:ehv387. doi: 10.1093/eurheartj/ehv387. [DOI] [PubMed] [Google Scholar]
- 31.Xu T, Yang Z, Vatta M, et al. Compound and Digenic Heterozygosity Contributes to Arrhythmogenic Right Ventricular Cardiomyopathy. J Am Coll Cardiol. 2010;55(6):587–597. doi: 10.1016/j.jacc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhonsale A, Groeneweg Ja, James Ca, et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J. 2015:847–855. doi: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
- 33.Medeiros-Domingo A, Saguner AM, Magyar I, et al. Arrhythmogenic right ventricular cardiomyopathy: implications of next-generation sequencing in appropriate diagnosis. Europace. 2016:euw098. doi: 10.1093/europace/euw098. [DOI] [PubMed] [Google Scholar]
- 34.Sen-Chowdhry S, Syrris P, Prasad SK, et al. Left-Dominant Arrhythmogenic Cardiomyopathy. An Under-Recognized Clinical Entity. J Am Coll Cardiol. 2008;52(25):2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Murray MF. Your DNA is not your diagnosis : getting diagnoses right following secondary genomic findings. Genet Med. 2015;18:1–3. doi: 10.1038/gim.2015.134. [DOI] [PubMed] [Google Scholar]
- 36.Quarta G, Muir A, Pantazis A, et al. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: Impact of genetics and revised task force criteria. Circulation. 2011;123(23):2701–2709. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.