Abstract
Objective
The meta-analysis was used to evaluate the skeletal-related events (SREs) and efficacy of denosumab versus zoledronic acid (ZA) in patients with bone metastases.
Methods
The data of this meta-analysis study were searched from PUBMED, EMBASE, Cochrane Library, Web of Science with Conference Proceedings, Elsevier and China National Knowledge Infrastructure (CNKI) databases till August 2017. Two independent reviewers reviewed the reference lists of relevant articles. The fixed-effects model and random-effects model were used to summarize relative estimates and 95% confidence intervals (CIs) according to the heterogeneity of the included studies.
Results
Three randomized controlled trials (RCTs) including 4050 patients were identified in this meta-analysis study. The pooled analysis showed that denosumab could significantly reduce SREs, series SREs [Odds Ratio (OR) = 0.84; 95% CI, 0.74–0.95, I2 = 0%, P = 0.008] in patients with bone metastases as compared with ZA. Similar results of spinal cord compression SRE and surgery to bone SRE were obtained with (OR = 0.84; 95% CI, 0.70–1.01, I2 = 0%, P = 0.07) and (OR = 0.92; 95% CI, 0.78–1.08, I2 = 0%, P = 0.28) separately, radiation to bone SRE (OR = 0.72; 95% CI, 0.46–1.10, I2 = 11%, P = 0.13) and pathological fracture SRE (OR = 0.78; 95% CI, 0.35–1.73, I2 = 25%, P = 0.54) showed similar results, there were no significant difference between denosumab and ZA in patients with bone metastases.
Conclusion
Denosumab was more effective than ZA in reducing the incidence of SRE in patients with bone metastases.
Keywords: Skeletal-related event, Denosumab, Zoledronic acid, Bone metastases, Meta- analysis
1. Introduction
Metastatic involvement of bone is one of the common complications in advanced cancer. Almost all of patients with myeloma, 65–75% of patients with breast or prostate cancer, and 30–40% of patients with lung cancer develop skeletal metastases [1]. Nearly 50% of patients with bone metastases develop one or more complications collectively termed skeletal-related events (SREs) (i.e., radiation to bone, pathological fracture, spinal cord compression, surgery to bone) [2], [3]. Incidence of SRE has been used as the composite primary endpoint in the trials conducted to reduce skeletal complications among patients with bony metastases since 2002 [4], [5]. SREs reduced performance status, quality of life (QOL) and reduced survival. SREs also increase patients' risk of hospitalization and the duration of hospital stays. They are estimated to cost 1.9 billion dollars every year in the United States, with the cost to treat a single SRE episode per patient varying from 6973 to 11979 USD [6], [7].
Bisphosphonates (BP) have played a central part in the prevention of skeletal complications. In particular, zoledronic acid (ZA) demonstrated the broadest range of clinical benefits. Evidence showed that ZA can significantly reduce the bone pain and the risk of SREs for patients with bone metastases secondary to a wide range of solid tumors [8], [9], [10]. Growing evidence indicates that denosumab is a promising new treatment option for patients with bone metastases. Denosumab is a fully humanized monoclonal antibody against receptor activator of nuclear factor-κB ligand (RANK-L) which plays a central role in osteoclast formation, differentiation, and survival [11], [12], [13]. Thus, denosumab can inhibit bone destruction through inhibiting the function of RANK-L. Two phase II studies showed that denosumab reduced levels of bone turnover markers consistently, regardless of tumor types or prior BP exposure [14], [15]. In addition, recent studies found that denosumab was non-inferior or even superior to ZA in delaying or preventing SREs in patients with bone metastasis [16], [17].
In the present study, we systematically reviewed and assessed the evidence to compare the efficacy of denosumab with that of ZA in reducing morbidity of SREs for patients with bone metastases.
2. Material and methods
2.1. Search strategies
We searched for both non-English and English articles in PUBMED, EMBASE, Cochrane Library, Web of Science with Conference Proceedings, Elsevier and China National Knowledge Infrastructure (CNKI) databases till August 2017. The following key words were searched as terms: skeletal-related event, SRE, denosumab, bisphosphonates, zoledronic acid, ZA, bone metastases, cancer. There was no restriction on time period, population, language. All eligible studies were retrieved, and their bibliographies were checked for other relevant publications. The computerized search was supplemented by a manual search of the bibliographies of all retrieved articles by two independent reviewers.
2.2. Inclusion/exclusion criteria of literature
The studies were included if they satisfied the following criteria: (1) RCTs and case series; (2) Information on the SRE results of denosumab versus ZA in patients with bone metastases; (3) The papers had to provide the size of the samples, distribution of the incidence of SRE or other information that can help us estimate an odds ratio (OR) and 95% confidence interval (95% CI).
The studies were excluded if one of the following existed: (1) The review articles and studies that contained overlapping data; (2) Not offering the source of cases and controls or other essential information; (3) If more than one study from the same group occurred, we only recruited the most recent or complete study.
2.3. Statistical analysis
The following information was extracted from each eligible study by two investigators independently with the standard protocol: the name of first author, year of publication, country of origin, study setting, study population, the sample size of denosumab group and ZA group, usage of denosumab and ZA, SREs (radiation to bone, pathological fracture, spinal cord compression, surgery to bone). To ensure the accuracy of the extracted information, two investigators checked the data extraction results and reached consensus on all of the data extracted. If different results were generated, they would check the data again and have a discussion to come to an agreement. The results were reviewed by a third investigator and disagreement was resolved by discussion.
OR and a 95% CI were used for presenting the statistical results for dichotomous outcomes. Weighted mean difference (WMD) and a 95% CI were employed for presenting the statistical results for continuous outcomes. Mantel-Haenszel analysis was utilized for dichotomous variables, and inverse variance method was used for continuous variables [18]. The statistical significance was set at P < 0.05.
The difference was considered to be statistically significant if a P value was less than 0.10 and was also quantitatively assessed by using the value of I-square (I2 < 25%, no heterogeneity; I2 between 25% and 50%, moderate heterogeneity; and I2 > 50%, high heterogeneity) [19]. If I2 < 50% or P > 0.10, it shows that the studies were homogeneous or slightly heterogeneous, and we will use the fixed-effects model to combine the effect size. If I2 > 50% or P < 0.10, indicating that the studies were moderately or highly heterogeneous, and we will employ the random-effects model to combine the effect size [20]. Statistical calculations were performed by using the computer program Review Manager (RevMan) Version 5.3 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
3. Results
3.1. Search results and features of studies included
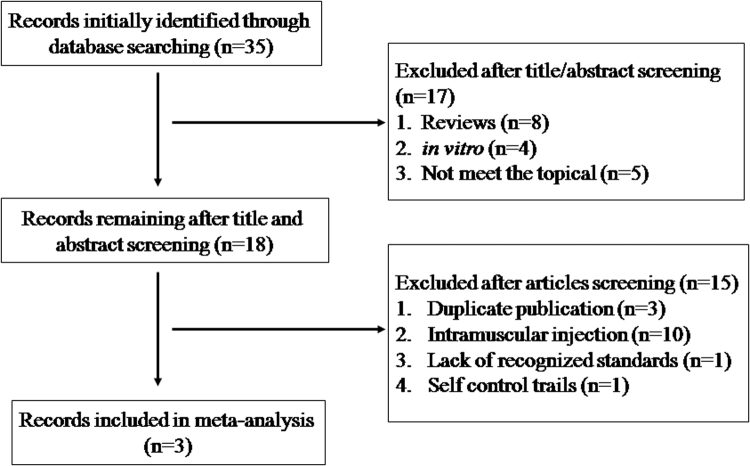
The initial search was independently executed by two reviewers, and 35 articles were preliminarily selected based on the research criteria. After screening by title and abstract in accordance with inclusion/exclusion criteria, 17 studies were excluded. After a thorough discussion between the 2 reviewers, three articles were found to be related to this study. The detailed steps of the literature search are shown in Fig. 1. This review covered 3 studies [21], [22], [23] from a total of 4050 participants. The name of first author, year of publication, country of origin, study setting, study population, the sample size of denosumab group and ZA group, usage of denosumab and ZA, and outcome parameter (SRE types) of each study were listed in Table 1. The information of SRE types and the sample size in denosumab group and ZA group of each study are given in Table 2.
Fig. 1.
Flow diagram of the literature search.
Table 1.
Characteristics of included studies in this meta-analysis.
| First author | Year | Country | Design | Study population | Sample (Denosumab/ZA) | Treatment | Assessment of outcomes (SRE types) |
|---|---|---|---|---|---|---|---|
| Size(Denosumab/ZA) | |||||||
| Outcome parameter | HWE | ||||||
| Population | HWE | ||||||
| Martin 21 | 2012 | Spain | RCT | Breast cancer | 2046 (1026/1020) | Denosumab 120 mg Ih | Radiation to bone, Pathological fracture, Spinal cord compression, Surgery to bone |
| Q4W; | |||||||
| Smith 22 | 2014 | America | RCT | Prostate cancer | 1901 (950/951) | ZA 4 mg IV Q4W | Radiation to bone, Pathological fracture, Spinal cord compression, Surgery to bone |
| Denosumab 120 mg Ih Q4W; | |||||||
| Hibiki 23 | 2017 | Japan | Case series | NSCLC | 103 (52/51) | Q4W; | Radiation to bone, Pathological fracture, Spinal cord compression, Surgery to bone |
| ZA 4 mg IV Q4W | |||||||
| NA |
Abbreviations: RCT, randomized controlled trial; ZA, Zoledronic Acid; IV, indicates intravenous infusion; Q4W, every 4 weeks; SER, skeletal-related event; NSCLC, non-small cell lung cancer; NA, not available.
Table 2.
Patients, SREs of the trials included in the meta-analysis.
| First author | Radiation to bone |
Pathological fracture |
Spinal cord compression |
Surgery to bone |
||||
|---|---|---|---|---|---|---|---|---|
| Denosumab (n/N) | ZA (n/N) | Denosumab (n/N) | ZA (n/N) | Denosumab (n/N) | ZA (n/N) | Denosumab (n/N) | ZA (n/N) | |
| Martin 21 | 82/1026 | 95/1020 | 215/1026 | 235/1020 | 10/1026 | 10/1020 | 10/1026 | 10/1020 |
| Smith 22 | 177/950 | 203/951 | 137/950 | 143/951 | 26/950 | 36/951 | 1/950 | 4/951 |
| Hibiki 23 | NA | NA | 2/52 | 1/51 | 0/52 | 4/51 | NA | NA |
| 26/950 | 36/951 | 1/950 | 4/951 | |||||
Abbreviations: SER, skeletal-related event; ZA, Zoledronic Acid; NA, not available.
3.2. Result of meta-analysis
The detailed steps of our literature search are shown in Fig. 1. In brief, we identified 4 RCTs that met the inclusion criteria for this meta-analysis.
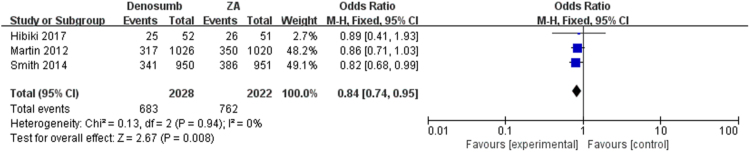
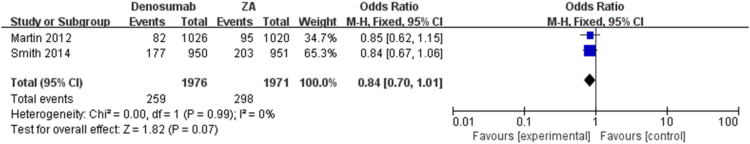
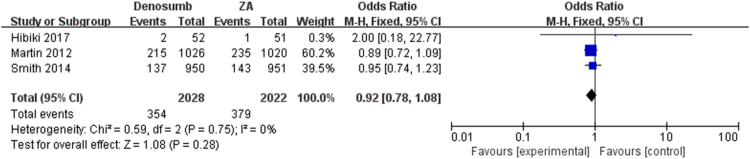
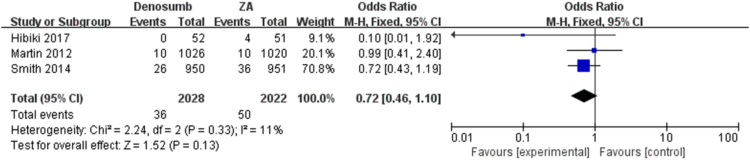
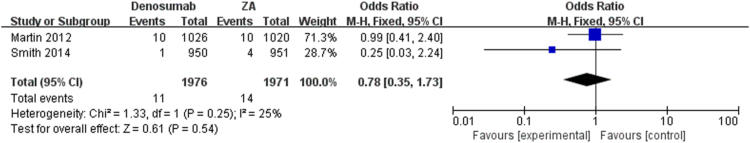
The pooled analysis showed that denosumab could significantly reduce SREs as compared with ZA, series SREs (OR = 0.84; 95% CI, 0.74–0.95, I2 = 0%, P = 0.008) (Fig. 2) in patients with bone metastases. The meta-analysis showed the similar results in spinal cord compression SRE (OR = 0.84; 95% CI, 0.70–1.01, I2 = 0%, P = 0.07), surgery to bone SRE (OR = 0.92; 95% CI, 0.78–1.08, I2 = 0%, P = 0.28), radiation to bone SRE (OR = 0.72; 95% CI, 0.46–1.10, I2 = 11%, P = 0.13) and pathological fracture SRE (OR = 0.78; 95% CI, 0.35–1.73, I2 = 25%, P = 0.54) separately (Fig. 3, Fig. 4, Fig. 5, Fig. 6), and there were no significant difference between denosumab and ZA in patients with bone metastases.
Fig. 2.
Forest plot: summary OR of denosumab compared with ZA for series SREs.
Fig. 3.
Forest plot: summary OR of denosumab compared with ZA for radiation to bone SRE.
Fig. 4.
Forest plot: summary OR of denosumab compared with ZA for pathological fracture.
Fig. 5.
Forest plot: summary OR of denosumab compared with ZA for spinal cord compression.
Fig. 6.
Forest plot: summary OR of denosumab compared with ZA for surgery to bone SRE.
3.3. Publication bias
We did not draw funnel plot to demonstrate publication bias, because the number of the included studies was comparatively small.
4. Discussion
This meta-analysis indicates that denosumab is associated with a decreased risk of SREs for patients with bone metastases when compared with ZA. However, the pooled analysis found no difference in clinical outcome of spinal cord compression SRE, surgery to bone SRE, radiation to bone SRE and pathological fracture SRE.
Although both denosumab and ZA inhibit bone resorption, the working mechanism is different. It is hypothesized that a vicious cycle of bone metastases is established between bone destruction and tumor growth [24], [25]. Tumor cells metastasized to the bone produce growth factors that induce osteoblasts to secrete RANK-L. Binding of RANK-L to its receptor RANK stimulates osteoclast formation, differentiation, and bone resorption. In turn, the increased bone resorption provides the tumor cells with growth factors from the bone matrix that stimulate tumor growth [26]. Denosumab disrupts the vicious cycle in the bone microenvironment by binding to RANK-L and inhibits its function, however, it is suggested that ZA interrupts this vicious cycle by preventing the prenylation of small GTPase proteins essential for osteoclast function and survival [27].
Denosumab is a cost-effective treatment option for the prevention of SREs in patients with advanced solid tumors and bone metastases compared to ZA. The overall value of denosumab is based on superior efficacy, favorable safety, and more efficient administration [28]. The safety profile for ZA and denosumab is similar but subcutaneous administration of denosumab offers advantages over intravenous administration with no need for renal monitoring. Denosumab is associated with fewer acute phase reactions, but has a higher incidence of hypocalcemia [29].
We performed a thorough search of the literature to capture all relevant information. In addition, the quality of studies included in our meta-analysis was satisfactory. They are all randomized, double-blind controlled trials. Nevertheless, our meta-analysis has several limitations. The main potential limitation is the very small number of clinical trials available for inclusion in the meta-analysis. Similarly, the evidence comparing the efficacy of denosumab and ZA on the incidence of SRE among the subgroups like lung cancers, colon cancer, and multiple myeloma could not be provided because of insufficient information. However, large, multicenter, and randomized trials are still needed to assess longer-term safety and efficacy of denosumab. Further research should also determine whether, in addition to patients naive to BP treatment, patients who did not respond well to ZA would benefit from denosumab therapy.
Conflict of interest
None of the authors have any conflicts of interest to declare.
Contributor Information
Zhicai Zhang, Email: zhicaizhang@126.com.
Feifei Pu, Email: pufeifeiemail@163.com.
Zengwu Shao, Email: szwjj@medmail.com.cn.
References
- 1.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Berenson J.R., Lichtenstein A., Porter L. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J. Clin. Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 3.Saad F., Gleason D.M., Murray R. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J.R., Williams G., Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J. Clin. Oncol. 2003;21:1404–1411. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 5.Major P.P., Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am. J. Clin. Oncol. 2002;25:S10–S18. doi: 10.1097/00000421-200212001-00003. [DOI] [PubMed] [Google Scholar]
- 6.Oefelein M.G., Ricchiuti V., Conrad W. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 2002;168:1005–1007. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 7.Saad F., Lipton A., Cook R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 8.Rosen L.S., Gordon D., Kaminski M. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, doubleblind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 9.Berenson J.R., Lichtenstein A., Porter L. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 10.Rosen L.S., Gordon D., Kaminski M. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 11.Lipton A., Goessl C. Clinical development of anti-RANKL therapies for treatment and prevention of bone metastasis. Bone. 2011;48:96–99. doi: 10.1016/j.bone.2010.10.161. [DOI] [PubMed] [Google Scholar]
- 12.Lacey D.L., Timms E., Tan H.L. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 13.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Fizazi K., Lipton A., Mariette X. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasm after intravenous bisphosphonates. J. Clin. Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 15.Lipton A., Steger G.G., Figueroa J. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin. Cancer Res. 2008;14:6690–6699. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 16.Henry D., Von Moos R., Vadhan-Raj S. A double-blind, randomized study of denosumab versus zoledronic acid for the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Eur. J. Cancer. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 17.Stopeck A.T., Lipton A., Body J.J. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Martin M., Bell R., Bourgeois H. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin. Cancer Res. 2012;18:4841–4849. doi: 10.1158/1078-0432.CCR-11-3310. [DOI] [PubMed] [Google Scholar]
- 22.Smith M.R., Coleman R.E., Klotz L. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 2015;26:368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udagawa H., Niho S., Kirita K. Impact of denosumab use on the survival of untreated non-squamous non-small cell lung cancer patients with bone metastases. J. Cancer Res. Clin. Oncol. 2017;143(6):1075–1082. doi: 10.1007/s00432-017-2350-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen F., Pu F. Safety of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. Oncol. Res. Treat. 2016;39(7–8):453–459. doi: 10.1159/000447372. [DOI] [PubMed] [Google Scholar]
- 25.Guise T.A., Mohammad K.S., Clines G. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006;12:6213–6216. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 26.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 27.Russell R.G., Watts N.B., Ebetino F.H. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 28.Stopeck A., Rader M., Henry D. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J. Med. Econ. 2012;15:712–723. doi: 10.3111/13696998.2012.675380. [DOI] [PubMed] [Google Scholar]
- 29.Vera Hirsh. Targeted treatments of bone metastases in patients with lung cancer. Front. Oncol. 2014;4:146. doi: 10.3389/fonc.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]