Abstract
The last two decades have marked a growing understanding of the interaction occurring between bone and immune cells. The chronic inflammation and immune system dysfunction commonly observed to occur during the ageing process and as part of a range of other pathological conditions, commonly associated with osteoporosis has led to the recognition of these processes as important determinants of bone disease. This is further supported by the recognition that the immune and bone systems in fact share regulatory mechanisms and progenitor molecules. Research into this complex synergy has provided a better understanding of the immunopathogenesis underlying bone diseases such as osteoporosis. However, existing research has largely focussed on delineating the role played by inflammation in pathogenic bone destruction, despite increasing evidence implicating autoantibodies as important drivers of osteoporosis. This review shall attempt to provide a comprehensive overview of existing research examining the role played by autoantibodies in osteoporosis in order to determine the potential for further research in this area. Autoantibodies represent promising targets for the improved treatment and diagnosis of inflammatory bone loss.
Keywords: Osteoporosis, Autoantibodies, Bone remodelling, Bone mineral density, Fractures
Highlights
-
•
Immune and bone systems recognised to share regulatory mechanisms and progenitor molecules.
-
•
Osteoclasts and osteoblasts are continuously controlled by a variety of cells of the immune system.
-
•
Altered bone composition often observed to occur alongside diseases characterised by immune dysfunction.
-
•
Immune disturbance linked to pathogenic bone loss through the action of inflammatory markers and autoantibodies.
1. Introduction
Osteoporosis is a condition characterised by decreased bone strength that culminates in an increased risk of fractures in response to minimal or low velocity force (Lindsay and Cosman, 2014). Its prime fracture regions are around the vertebrae, hip and distal radius, though fractures can occur at almost any skeletal site (Dempster, 2011). In general, most fragility fractures occur at non vertebral sites where bone is composed mainly by compact or cortical tissue that accounts for 80% of the total bone mass of an adult skeleton, whilst trabecular tissue makes up the remaining 20% (Iolascon et al., 2013). Reduced bone strength results from a loss of bone tissue, a consequence of imbalances between bone formation and resorption, as well as a subsequent deterioration in skeletal microarchitecture (Kasper et al., 2016). As such, the presence of a fragility fracture or bone mineral density (BMD) measurements form the basis of diagnostic techniques that guide targeted intervention strategies (Duque et al., 2017). The working definition for osteoporosis is a BMD that falls 2.5 standard deviations (SD) below the mean for a healthy individual (i.e., a T score of <− 2.5), with a T score of < 1.0 considered indicative of an increased risk of developing osteoporosis (Kasper et al., 2016). For each standard deviation below peak bone mass (or 1 unit decrease in T-score), it is reported a woman's risk of fracture approximately doubles (Humes, 2011). Bone density results are usually reported using T scores rather than Z scores, particularly amongst older adults. It is however important to note that defining osteoporosis on the sole basis of T scores has failed to prove effective with more than 50% of all hip fractures occurring in individuals with T scores that are better than − 2.5 (Kasper et al., 2016). Moreover, the consequences of osteoporotic fracture include diminished quality of life, decreased functional independence, and increased morbidity and mortality (Cooper, 1997). There is therefore a need for research aimed at improving diagnostic strategies and subsequently optimizing both prevention and treatment of this condition.
Osteoporosis is classified into primary and secondary osteoporosis on the basis of the precipitating factors (Refer to Appendix A) (Kasper et al., 2016). The development of osteoporosis is traditionally attributed to result from the influence of a range of lifestyle and genetic factors as well as a range of other acquired and modifiable risk factors (Clarke, 2008). Recently, there has been a growing acknowledgement for the involvement of the immune system in the pathogenesis of osteoporosis precipitating the emergence of the field of osteoimmunology (Pietschmann et al., 2016). The immune system is postulated to play an important role in the aetiology of bone disease by disrupting the balance of activity between the effects of osteoblasts that form bone and those of osteoclasts that resorb bone (Caetano-Lopes et al., 2009). This review will seek to appraise existing literature in this field in an effort to better understand the interaction between the immune system and the skeletal system, with a particular focus on the role of autoantibodies in osteoporosis development.
2. The immune system and bone homeostasis
In a normal physiological state, the skeletal system carries out multiple functions including providing support, mobility, and protection for vital organs, as well as acting as a mineral reservoir for calcium and phosphate (Office of the Surgeon General (US), 2004). In order to effectively carry out these tasks, the skeleton exists in a dynamic equilibrium characterised by continuous osteoclast-mediated bone resorption and osteoblast-mediated bone deposition (Caetano-Lopes et al., 2009, Raggatt and Partridge, 2010, Rucci, 2008). The latter biological process, termed “bone remodelling”, occurs in a harmonious and simultaneous fashion, resulting in a negligible change in bone mass (Raggatt and Partridge, 2010, Rucci, 2008). After peak bone mass is reached at the end of the 3rd decade, the normal balance between bone formation and bone resorption changes with relative increases in bone resorption leading to net bone loss (Clarke and Khosla, 2010). Interestingly, according to recent research, the majority of bone loss after the age of 65 is cortical bone loss; however, bone loss after menopause is mainly trabecular bone loss (Hunter and Sambrook, 2000).
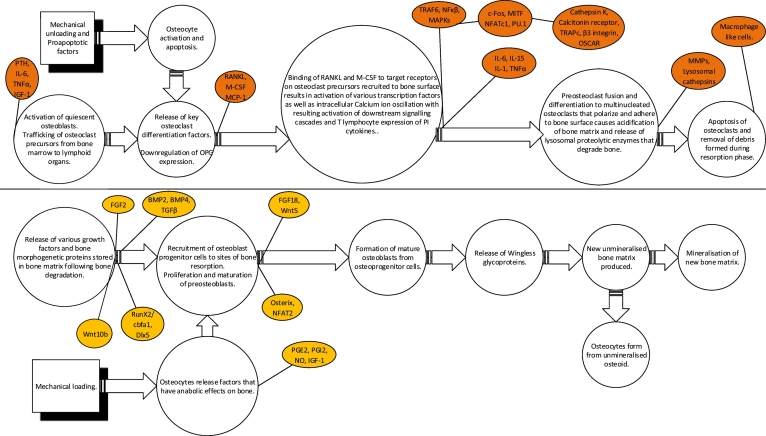
Briefly, bone remodelling follows the activation-resorption-formation (ARF) sequence (Baron R., 2000). The first step, called the activation phase, begins with stimulation of quiescent osteoblasts. In response to appropriate stimuli, the latter release key osteoclast differentiation factors triggering preosteoclast fusion and differentiation to multinucleated osteoclasts marking the end of the activation phase (Raggatt and Partridge, 2010, Baron, 2000). Once differentiated, osteoclasts polarize, adhere to the bone surface, and dissolve bone as part of the resorption phase. They then undergo apoptosis, as a means to prevent excessive bone resorption (Raggatt and Partridge, 2010, Clarke and Khosla, 2010). After this resorptive process, there is an intermediate phase preceding bone formation, called a reversal phase. At this time, some macrophage-like uncharacterized mononuclear cells are observed at the site of remodelling, whose function consists of removal of debris produced during matrix degradation (Teti and Rucci, 2010). The final phase termed bone formation is triggered by several growth factors stored in the bone matrix and released after its degradation, which are likely to be responsible for recruitment of osteoblasts in the resorbed area (Rucci, 2008, Teti and Rucci, 2010). Once recruited, osteoblasts produce new bone matrix, initially not mineralized (osteoid), and then they promote its mineralization, thus completing the bone remodelling process (Rucci, 2008). Both cortical and trabecular bone undergo a continuous process of structural remodelling as a means of maintiaing mineral homeostasis, adapting to mechanical changes and repairing damage to bone (Iolascon et al., 2013).
Research has successfully highlighted various shared molecules and characteristics between bone and immune systems. Firstly, osteoclasts have been identified to derive from the same myeloid precursor cells that give rise to macrophages and myeloid dendritic cells (Xiao et al., 2015, Schett and David, 2010). Moreover, osteoclasts are observed to exhibit the same lifecycles as dendritic cells, regulated by a variety of cytokines, transcription factors and inflammatory mediators (Xiao et al., 2015, Schett and David, 2010). On the other hand, bone forming osteoblasts are noted to derive from mesenchymal stem cells (Teitelbaum, 2007).
The coordinated stimulation of osteoclasts by osteoblasts and reciprocal activation of osteoblasts by osteoclasts is referred to as coupling (Rucci, 2008). It is widely acknowledged that the complementary regulatory activities of osteoclasts and osteoblasts are continuously controlled through direct cell to cell contact, via extracellular matrix interaction as well as by a variety of cells of the immune system (Caetano-Lopes et al., 2009, Chen et al., 2017). Osteoclasts represent the sole bone-resorbing cells in the body and are derived mainly following stimulation by two essential cytokines: the macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-kappa beta (RANK) ligand (RANKL), produced by osteoblasts (Pietschmann et al., 2016, Seeling and Nimmerjahn, 2015). In particular, M-CSF aided by transcription factor PU.1 is responsible for driving the commitment of osteoclast progenitor cells (Seeling and Nimmerjahn, 2015). Whilst M-CSF stimulates proliferation of osteoclast precursors and upregulates RANK expression, PU.1 positively regulates the transcription of M-CSF receptor Colony stimulating factor 1 receptor, namely, c – Fms (Seeling and Nimmerjahn, 2015). Osteoporotic hormones such as 1,25 dihydroxyvitamin D3 (1,25(OH)2 D3), parathyroid hormone (PTH) and prostaglandin E2 (PGE2) are responsible for upregulating expression of RANKL (Pietschmann et al., 2016, Seeling and Nimmerjahn, 2015).
Though the RANK/RANKL pathway represents the central process through which bone loss is regulated, there exist additional costimulatory pathways that are capable of modifying the net outcome resulting from bone remodelling. Osteoblast regulation of osteoclastogenesis additionally occurs through: interactions between immunoglobulin like receptors associated with immunoreceptor tyrosine based activation motif (ITAM) harbouring adaptor molecules (such as DNAX activating protein of 12KDa, known as DAP12 and Fc receptor common gamma subunit); via interactions between semaphorin 6D and its receptor plexin A1; and interactions between ephrin receptor B4 and ephrin B2 newly identified protein mediators of osteoblast – osteoclast interactions (Chen et al., 2017, Koga et al., 2004, Jones, 2015).
Additionally, a number of other cytokines are also reported to play a role in osteoclastogenesis. For example, tumor growth factor beta (TGFβ) is implicated in the enhanced recruitment of osteoblast progenitor cells to sits of bone resorption, whilst, tumor necrosis factor-α (TNF-α), is believed to facilitate the trafficking of osteoclast precursors from bone marrow to lymphoid organs (Pietschmann et al., 2016, Caetano-Lopes et al., 2009). RANKL expression which plays a central role in osteoclast biology, has also been observed on various subsets of proliferative T cells (CD8 and CD4, Th (helper) 1 and Th2 cells) as well as Forkhead box p3 (Foxp3) expressing regulatory T (Treg) cells (Pietschmann et al., 2016, Caetano-Lopes et al., 2009). Similarly, RANKL is also expressed on B lineage cells (i.e. B220 + cells that in the bone marrow represent multiple populations of early B cells precursors, immature B cells and mature B cells) (Pietschmann et al., 2016, Schett and David, 2010). The latter observations suggest a pro-osteoclastogenic and bone resorption role for these immune cells.
Additionally, osteoblast differentiation is achieved by the coordinated activity of runt related transcription factor 2/core binding factor alpha 1 (RunX2/cbfa1) and distal-less homeobox 5 (Dlx5) which carry out integral transcriptional regulation for the conversion of bone marrow mesenchymal stem cells to osteoprogenitors cells (Nakashima et al., 2002). The latter occurring following commitment of MSc towards osteo/chrondo progenitor outcome driven by the Wingless (Wnt) pathway and a number of bone morphogenetic proteins (BMP) (Nakashima et al., 2002). Subsequently, a zinc finger containing transcription factor operating downstream of RunX2 termed osterix that is predominantly expressed in bone and cartilage provides additional support for the transition of osteoprogenitors to osteoblasts (Nakashima et al., 2002). A range of cytokines (i.e., IL-1, IL-6, IL-4, and TNF-α, TGF-β) are also noted to aid in regulating the differentiation and function of osteoblasts (Mori et al., 2013). Osteoblasts are therefore also influenced by immune cells, although the physiological and pathological significance and the molecular mechanisms are less well understood than in the case of osteoclasts. The precise roles attributed to the range of immune cells implicated in bone remodelling are illustrated in Table 1 below.
Table 1.
Immune cells implicated in regulation of bone remodelling.
| Immune cell | Role in bone remodelling |
|---|---|
| Macrophage colony stimulating factor (M-CSF) | Homodimeric glycoprotein derived from osteoblasts as well as bone marrow stromal cells. Binds high affinity receptors expressed on cells of the monocyte/macrophage lineage acting as a cofactor for RANKL in the proliferation, differentiation and survival of osteoclast progenitor cells. |
| Receptor activator of nuclear factor-kappa B (RANK) | Receptor for RANKL expressed o osteoclast precursors. |
| Colony stimulating factor 1 receptor (c – Fms) |
Receptor for M-CSF expressed o osteoclast precursors. |
| Receptor activator of nuclear factor-kappa B ligand (RANKL) | Key factor for maturation, proliferation and fusion of pre-osteoclasts as well as osteoclast activation and survival. RANKL interacts with its receptor RANK activating signalling by recruting adaptor molecules belonging to the TNF receptor associated factors (TRAF) family. Binding of TRAF6 to RANK induces trimerization of TRAF6 leading to activation of nuclear factor kappa beta (NFκβ), and mitogen activated protein kinases (MAPKs). NFκβ upregulate transcription of several genes amongst them, the master regulator of osteoclastogenesis nuclear factor of activated T cells cytoplasmic 1 (NFATc1) as well as additional cofactors i.e. transcription factor complex AP-1 which is composed of c-Fos, critical for osteoclastogenesis. AP-1 activation along with calcium signalling further induce NFATc1 transcription, allowing its amplification. In cooperation with AP-1, PU.1, NFκβ and MITF, NFATc1 regulates the transcription of several target genes involved in osteoclast differentiation and function. RANKL additionally induces T lymphocyte expression of PI cytokines interleukin (IL)-6 and IL-1 and T lymphocyte growth and differentiation factors (IL-12 and IL-15). |
| Osteoprotegerin (OPG) also known as osteoclastogenesis inhibitory factor (OCIF) | Soluble glycoprotein that opposes RANK by acting as a competitive decoy receptor for RANKL. Balances RANKL activity protecting skeleton from excess bone resorption by binding to RANKL and preventing its binding to RANK. |
| Interleukin – 17 (IL-17) | Expressed on T helper cells (Th17). Induces osteoclast function supporting cells, i.e. synovial macrophages to release tumor necrosis factor alpha (TNFα) and IL-1 as well as upregulates fibroblast and osteoblast expression of RANKL, subsequently promoting bone resorption. |
| Interleukin – 7 (IL-7) | Promotes osteoclastogenesis by upregulating T and B cell derived RANKL. Lowers tolerance of T cells to weak antigenic responses, stimulating precursor expansion, thymic export and peripheral expansion of T cells. Additionally, increases T cell production of IL-1 and TNF-α. Also involved in B cell expansion. |
| Interleukin – 23 (IL-23) | Produced by activated dendritic cells and macrophages. Controversial role in bone erosion. Research suggests a role for this cytokine as a stimulus to IL-17 as well as acts as a direct stimulant of osteoclast formation. |
| Interleukin – 27 (IL-27) | Acts as an anti osteoclastogenic cytokine. Suppresses osteoclastogenesis both through a direct effect on osteoclasts decreasing their ability to differentiate into fully mature resorbing cells by abrogating RANKL mediated induction of NFATc1 and supressing proximal RANK signalling as well as indirectly through action on T helper cell subsets where it favours differentiation of Th1 and regulatory T cells whilst decreasing the differentiation of Th17 cells. |
| Interleukin – 6 (IL-6) | Classic bone resorbing pro-inflammatory cytokine. Suppresses osteoblast function whilst upregulating osteoclasts in the presence of RANKL. Stimulates RANK expression by osteoblasts. |
| Monocyte chemoattractant protein – 1 (MCP-1) | Recruits preosteoclasts to bone surface. Down regulates expression of OPG on osteoblasts. |
| Interferon gamma (IFN-γ) | Controversial effect on osteoclast activity and formation. Behaves as an anti osteoclastogenic cytokine in mouse models, however some human studies have suggested IFN-γ stimulates bone resorption. |
| Interleukin – 12 (IL-12) | Negative regulator of osteoclastogenesis. |
| Interleukin – 10 (IL-10) | Negative regulator of osteoclastogenesis. |
| Tumor necrosis factor alpha (TNFα) | Stimulate osteoclast development and function directly and indirectly. Facilitates trafficking of osteoclast precursors from bone marrow to lymphoid organs as well as rendering them more susceptible to further differentiation into osteoclasts by increasing production of M-CSF by bone marrow stromal cells and decreasing the release of OPG as well as stimulating IL-1β secretion and inducing production of IL-6. TNFα is able to directly stimulate osteoclastogenesis in the presence of M-CSF by stimulating the activation of NFκβ, mainly through TRAF2. |
| B cells | Active regulators of RANK/RANKL/OPG system. Key effector role in basal bone homeostasis, osteoclast formation and the regulation of bone resorption. B cells express both RANKL and OPG. |
| Transcription factors | The differentiation of osteoblasts from mesenchymal stem cells and osteoclasts from myeloid precursor cells that also originate a variety of other cells requires the activity of transcription factors expressed at specific time points during differentiation in turn defining various developmental stages of osteoblastogenesisand osteoclastogensis |
| Nuclear factor kappa beta (NFκβ) | Released by osteoclast precursors. Translocates to the nucleus where it upregulates cofactors that induce osteoclastogenesis and PI transcription factors. Required for NFATc1 induction. |
| The Wingless (Wnt) Family of Glycoproteins | Family of glycoproteins that upon engaging various membrane receptors activate numerous pathways that are either dependent on or independent of beta (β) catenin, both capable of promoting differentiation of osteoblast progenitors into mature osteoblasts as well as regulating osteoclastogenesis. In particular, Wnt5a binds to two different osteoclast precursor receptor complexes. Binding of one of those complexes, namely, frizzled and low density lipoprotein receptor related protein 5/6 (LRP 5/6) by Wnt5a acts through β cantenin to produce signalling for osteoblastogenesis. Binding of Wnt5a to the other complex, receptor tyrosine kinase like orphan receptor 2 (Ror 2) appears to increase RANK expression in osteoclasts, sensitising them to RANKL. Wnt10b shifts commitment of mesenchymal cells towards osteo/chondro progenitor outcome, as well as inhibits preadipocyte commitment by suppressing adipogenic transcription factors CCAAT enhancer binding protein alpha (C/EBPα) and peroxisome proliferator activated receptor (PPARγ) along with inducting osteoblastogenesis factors RunX2, Dlx5 and Osterix. High levels of Wnt signalling in the presence of RunX2 then promotes osteoblastogenesis at the expense of chondrocyte differentiation. |
| TRAIL | Expressed on osteoblasts. In normal conditions weakly sensitive to apoptotic effects, however in inflammatory conditions can affect osteoblast by stimulating their apoptosis in turn resulting in decreased bone formation and subsequently impairing bone remodelling. |
| Interleukin – 1 (IL-1) | Potent stimulator of bone resorption and inhibitor of bone formation. IL-1 acts by increasing RANKL and MCSF expression in turn upregulating the effect of TNF-α in turn promoting osteoclast differentiation. Additionally IL-1 decreases OPG mRNA expression mediated through PGE2 induction. In addition, it promotes fusion of osteoclast precursors as well as prolongs survival of mature osteoclasts. IL-1 synergises with RANKL to induce osteoclastogenesis and bone resorption, probably by stimulating TRAF6. |
| Interleukin - 11 (IL-11) | Bone resorption cue that stimulates osteoclastogenesis in a manner similar to IL-6. |
| T cells | Potent modulators of bone turnover and are important sources of osteoclastogenic cytokines. Express RANKL. |
| CD137 (Cluster of Differentiation 137) | Costimulatory member of the TNF receptor family that acts as an important regulator of immune responses. Expressed on dendritic cells and osteoclast precursors. CD137L ligation suppresses RANKL induced osteoclastogenesis by inhibiting the multinucleation process. |
| Sclerostin (SOST) | Produced mainly by osteocytes, mineralised hypertrophic chondrocytes and cementocytes. Inhibits the Wnt pathway in turn decreasing osteoblast formation and activity and consequently decreasing bone formation. SOST influences the Wnt signalling pathway, favouring bone resorption by binding to LRP 5/6. |
| Dickkopf 1 (DKK-1) | Glycoprotein produced by osteoblasts that negatively regulates osteoblastogenesis branch of Wnt5a signalling. Additionally activates osteoclast formation. |
| Interleukin – 4 (IL-4) | Negative regulator of osteoclastogenesis. IL-4 is an important inhibitor of RANKL induction on CD4 + and CD8 + T lymphocytes following T cell receptor/cluster of differentiation 3 (TCR/CD3) stimulation, (and CD28 mediated costimulation). |
| Interleukin – 3 (IL-3) | Negative regulator of osteoclastogenesis. |
| Interleukin – 15 (IL-15) | Master T cell growth factors. IL-15 signalling provides costimulatory functions for osteoclast development. |
| Interleukin - 8 (IL-8) | Recruits preosteoclasts to bone surface. Down regulates expression of OPG on osteoblasts. |
| Growth factors | Fibroblasts are a large family of proteins comprising 23 different ligands that transduce their signal through one of four fibroblast growth factor receptors. Fibroblast growth factors (FGF) initiate condensation of the mesenchyme and proliferation of progenitor cells. In particular, FGF2 is important for pre osteoblast proliferation and maturation while FGF18 is essential in mature osteoblast formation. FGFs regulate RunX2 by promoting its activation. Additionally, FGF23 secreted by osteoblasts and osteocytes is a key regulator of phosphate in balancing bone ion homeostasis and bone mineralisation. |
| Osteopontin | Cytokine produced by activated T lymphocytes. Promotes osteoclast attachment to bone matrix via the alpha beta 3 (αβ3) integrin and CD44 (hyaluronic acid receptor). Promotes chemotaxis of macrophages and dendritic cells to sites of inflammation. |
| Interferon beta (IFNβ) | Functions as a negative feedback regulator that inhibits the differentiation of osteoclasts by interfering with the RANKL induced expression of c-Fos. RANKL signalling induced IFNβ negative feedback regulation. |
| Immunoglobulin (Ig) like receptors associated with immunoreceptor tyrosine based activator motif harbouring adaptor molecules (DAP12 and Fcγ) | Osteoblasts can regulate osteoclast differentiation by interacting with Ig like receptors such as Osteoclast associated receptor (OSCAR). Phosphorylation of the ITAM sequence in DAP12 or FcγR resulting after RANK activation, allows recruitment of splenocyte tyrosine kinases (STK) and the consequent activation of the phospholipase C gamma (PLCγ) which in turn triggers calcium signalling. The latter promotes osteoclastogenesis by activating the calcium/calmodulin dependent protein kinase type IV (CAMKIV) that in accordance with c-Fos activation and calcinuem potentiates NFATc1 auto amplification. |
| Tumor growth factor beta (TGFβ) | Bone morphogenetic proteins (BMP) belonging to TGFβ superfamily, are expressed in bone (all except BMP1) and required for skeletal development and maintenance of adult bone homeostasis as well as fracture healing. BMP2 and BMP4 promote osteoblast differentiation and function. BMPs enhance expression of alkaline phosphatase (ALP), parathyroid hormone related peptide receptor type 1 (PTHrP), collagen 1 and osteocalcin as well as stimulates the formation of mineralised bone like nodules. ALP and PTHrP are early markers of osteoblast progenitors that increase as osteoblasts mature and deposit matrix. BMP3 inhibits the signal transduced by BMP2 or BMP4 working as a negative regulator of osteoblast differentiation. Overexpression of TGFβ also leads to decreased T cell proliferation and production of IFNγ as well as TNFα. TGFβ enhances recruitment of osteoblast progenitor cells to the site of bone resorption. BMPs and TGFβ regulate RunX2 by promoting its activation. |
Though the majority of studies aimed at understanding bone remodelling at the cellular level have mainly focussed on the role of mature osteoblasts and osteoclasts as well as their respective precursor cells in mediating bone remodelling, we must acknowledge the growing recognition of a role for the other two types of cells exhibited in bone, namely, osteocytes and bone lining cells (Bonewald, 2011). Osteocytes derive from osteoblasts that remain trapped in the matrix that they are actively synthesizing during the process of mineralization (Bonewald, 2011, Pajevic, 2009). Osteocytes are historically acknowledged to be mechanoreceptors, recognising mechanical forces applied on the bone and transforming this mechanical stimulus into biological signals (Bonewald, 2011, Pajevic, 2009). Although, our understanding of the precise manner in which this occurs remains inadequate, the importance of mechanical loading and osteocyte function in bone health continues to be unveiled (Pajevic, 2009). It is widely acknowledged that bone adjusts in shape and strength according to mechanical stress (Florencio-Silva et al., 2015). Mechanical loading is reported to prompt osteocytes to produce factors that exert anabolic function on bone such as prostaglandin E2 (PGE2), prostacyclin (PGI2), nitric oxide (NO) and insulin like growth factor 1 (IGF-1). Alternatively, mechanical unloading down regulates the latter anabolic factors and stimulates osteocytes to produce sclerostin and Dickkopf wingless signalling pathway inhibitor 1 (DKK-1), both inhibitors of osteoblast activity as well as specific factors that stimulate local osteoclastogenesis (osteocytes are key producers of RANKL) (Florencio-Silva et al., 2015). In fact, recent research has highlighted the observation of osteocytes surrounding fatigue microcracks in bone undergoing apoptosis and the precise co-localization of these regions containing apoptotic osteocytes with areas subsequently resorbed by osteoclasts (Pajevic, 2009). It has therefore been postulated, that the programmed cell death of osteocytes in fact represents the lay event that initiates bone remodelling (Pajevic, 2009). Subsequently, osteocyte activation and apoptosis are hypothesised to act as chemotactic signals for local osteoclast recruitment (Florencio-Silva et al., 2015). Osteocytes are additionally implicated in the control of bone mineral homeostasis through the synthesis and release of a range of proteins that are of critical importance for the regulation of phosphorous, namely, fibroblast growth factor 23 (FGF23), phosphatase regulating gene with homologies to endopeptidase on the X chromosome (PHEX), matrix extracellular phosphoglycoprotein (MEPE) and dentin matrix acidic phosphoprotein (DMP1) (Pajevic, 2009). The function of bone lining cells is a lot less clear, however, the latter appear to play a role in the coupling of bone resorption to bone formation (Florencio-Silva et al., 2015).
3. The immune system and bone disease
Perturbation of homeostatic bone remodelling resulting in reduced bone strength and subsequent fracture as a result of accelerated bone loss is closely linked to bone diseases such as osteoporosis (Takayanagi, 2007). The observation of altered bone composition occurring alongside diseases characterised by immune dysfunction depicted the earliest illustrations of an interaction between disturbances in the skeletal structure and the immune systems. Firstly, low BMD and osteoporosis are commonly observed amongst individuals living with the Human Immunodeficiency Virus (HIV), with a recent meta-analysis estimating HIV positive populations to incur an increased risk of low BMD and osteoporosis, 6.4 and 3.7 times that of the general population, respectively (Cotter and Mallon, 2014, Weitzmann, 2014). Whilst the use of anti retroviral therapy (ART) and low body weight have been implicated as causal factors, activated T cells as well as B cell dysfunction have also been implicated in HIV associated osteoporosis (Cotter and Mallon, 2014). Additionally, rapid bone loss and increased fracture risk are implicated in a range of autoimmune diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriatic arthritis (PSA), inflammatory bowel disease (IBD), and systemic lupus erythematosus (SLE) (Weitzmann, 2014). For example, patients with RA are reported to have a two-fold increased risk of developing osteoporosis, a two to six fold increased risk of incurring a vertebral fracture and a two to three fold increased risk of suffering a hip fracture when compared to the general population (Kamen and Alele, 2010). Similarly, epidemiological studies have shown individuals suffering from SLE to have an estimated prevalence of osteoporosis 20% greater than that observed amongst their healthy counterparts (Kamen and Alele, 2010). Early onset osteoporosis occurring amongst autoimmune disease cohorts is linked to a range of factors including the use of medications such as corticosteroids and cyclophosphamides as well as disease sequelae such as decreased functional capacity (Kamen and Alele, 2010). However, as research has clearly established a role for immune cells in regulating normal bone remodelling, it seems likely that the dysregulation of the immune system makes a pivotal contribution to the deleterious consequences on bone integrity and the subsequent observed increased osteoporotic risk occurring alongside this group of diseases (Goldring, 2015). In this regard, it is important to note that the profile of cytokines noted to be elevated in conditions such as RA and ankylosing spondylitis characterised by osteoporosis, are the same as those implicated in bone modulation, such as TNFα and IL-6 (Goldring, 2015). Moreover, animal studies have successfully illustrated that mice lacking B and T cells exhibit osteoporotic bones whilst bone loss is recognised as a typical presentation in transplant related inflammation (Li et al., 2007).
The immune mechanisms reported to target bone in autoimmune disease are said to be complex and diverse (Schett and David, 2010). At present, majority of existing research has focused on delineating the role of inflammation in the pathogenesis of skeletal manifestations observed in osteoporosis. Mediators of inflammation are major regulators of bone homeostasis as illustrated in Fig. 1 below, and therefore their involvement in the development of bone pathology is of importance.
Fig. 1.
Key inflammatory mediators implicated in the regulation of bone remodelling.
During chronic inflammation, the balance between bone formation and resorption is said to be skewed towards osteoclast-mediated bone resorption subsequently increasing the risk of fracture (Office of the Surgeon General (US), 2004). This occurs as various RANKL producing immune cells, i.e. neutrophils, monocytes, dendritic cells and T lymphocytes have the ability to induce osteoclast differentiation (Pietschmann et al., 2016, Caetano-Lopes et al., 2009, Mori et al., 2013). Moreover, these cells are also known for producing a variety of pro-inflammatory cytokines that also contribute to bone damage by potentiating the effects of RANKL signalling through its receptor RANK (Pietschmann et al., 2016, Caetano-Lopes et al., 2009, Mori et al., 2013).
The effects of inflammation on bone have been illustrated in various animal studies. In mouse RA models, inflammatory cytokines such as IL-1β, TNF-α, and IL-6 have been observed to activate the signal transducer and activator of transcription 3 (STAT3) either directly or indirectly in murine osteoblasts and fibroblasts (Mori et al., 2011). STAT3 activation has been shown to induce the expression of RANKL (Mori et al., 2011). This is in line with literature describing an osteoclast driven disturbance of bone homeostasis in RA (Kamen and Alele, 2010). The critical role of RANKL in osteoporosis pathogenesis is further supported by studies showing that the genetic deletion of RANKL or its receptor RANK in animal models of RA protects animals from both articular and systemic bone loss (Alves et al., 2016). In addition, researchers have illustrated that mice with osteoblast specific overexpression of TGFβ2 develop high turnover osteoporosis (Mori et al., 2011). Observations amongst animal studies supporting a role for inflammation in osteoporosis are further supported by clinical studies reporting a correlation between the production of IL-1, IL-6 and TNFα by peripheral blood monocytes and bone resorption as well as spinal bone loss amongst healthy pre-and post-menopausal women (Weitzman, 2013, Brincat et al., 2014, Teitelbaum, 2004, Ginaldi et al., 2005). In fact, experimental studies have indicated that the proinflammatory cytokines IL-1, IL-6 and TNFα are important immunoregulatory mediators of bone resorption in both age and oestrogen deficiency related bone loss at least in part (McLean, 2009, D'Amelio and Isaia, 2015).
It has become apparent that immune cells are capable of producing factors that both aid and suppress osteoclastogenesis (Refer to Table 1). The effect of these cells on bone homeostasis therefore depends on an altered balance between the expressions of these factors. The ultimate shift in the balance of bone remodelling towards resorption subsequently signifies the presence of an environment that strongly favours the activity of pro-osteoclastogenic factors. Therefore, the improvement of therapeutic and diagnostic regimens in osteoporosis depends largely on our improved understanding of the pathophysiology of this disease. In this regard, research aimed at uncovering the factors capable of modulating the risk of osteoporotic fracture is indeed crucial for optimal identification of those requiring treatment. This is particularly relevant in light of the growing prevalence of osteoporosis and the dangers associated with the resulting fragility fractures. Though research into the role of inflammation in osteoporosis has led to the development of novel therapeutic targets, there remains a need to expand our understanding of the interactions occurring between bone and immune cells in order to expand our treatment options. Moreover, it remains to be uncovered whether the same immune factors play the same role in bone loss in both men and women. Research into the role of autoantibodies in osteoporotic bone loss therefore presents an opportunity to identify major molecular pathways that can be effectively targeted to improve the treatment of and possibly reverse osteoporosis.
4. Autoimmunity and osteoporosis
Autoantibodies have been increasingly implicated in the pathological bone loss characteristic of conditions such as osteoporosis (Sokolove and Pisetsky, 2016, Harre et al., 2014). Clinical studies comprising autoimmune disease samples have illustrated the ability of autoantibodies to induce osteoclast differentiation and activation as well as alter bone mineral content. In fact, bone destruction in immunologically based conditions appears to result not only from inflammation, but autoimmunity as well. For example, autoantibodies such as rheumatoid factor (RF) and anti citrullinated protein (ACPA) are identified as independent risk factors for the development of bone erosions and osteoporosis in RA (Sokolove and Pisetsky, 2016, Harre et al., 2014, Kocijan et al., 2013, Orsolini et al., 2017, Bugatti et al., 2016). Rheumatoid factors are autoantibodies directed against the Fc portion of IgG and are frequently found in RA populations (50–90%) (Ingegnoli et al., 2013, Leslie et al., 2001). The latter are however not specific for RA and can also be observed in a number of other autoimmune conditions as well as in 5–10% of healthy populations (Leslie et al., 2001). The RF most commonly measured is an IgM RF, although others have been described (Ingegnoli et al., 2013). RF immune complexes are reported to bind to low affinity Fc gamma receptors (specifically, FcγRIIA) displayed on monocytes and macrophages triggering a cascade of pro-inflammatory events (Mori et al., 2013, Harre et al., 2014). In fact, Fc receptors make up pivotal elements expressed on the surface of bone marrow stem cells that control the activation or down regulation of immune responses (Takai et al., 2012, Takai, 2005). Animal studies have successfully illustrated that FcγR1, FcγRIIA, FcγRII and FcγRIV are activating receptors signalling through ITAM bearing FcγRs (Takai et al., 2012). On the other hand, inhibitory FcγRIIB mediates its downstream signalling through immunoreceptor tyrosine based inhibitory motif (ITIM) phosphorylation with subsequent counteraction of the activating FcγRs or other ITAM bearing receptors (Takai et al., 2012). Hematopoietic cells expressing Fcγ receptors include human osteoclasts and their myeloid precursors (Takai, 2005). FcγRs are reported to mediate intracellular signalling following crosslinking of their receptors through binding of immune complexes (Takai et al., 2012, Takai, 2005). Research has successfully illustrated the ability of activating Fcγ receptors to stimulate osteoclastogenesis following their crosslinking (Takai, 2005). Activation of FcγR leads to phosphorylation of phospholipase Cγ (PCγ) and calcium dependent activation of nuclear factor of activated T cells c1 (NFATc1), both costimulatory factors of osteoclastogenesis (Takai, 2005). It is therefore possible that RF mediates their effect by upregulating osteoporotic resorption through FcγR signalling.
Alternatively, ACPA autoantibodies are directed against modified proteins in which arginine residues have been transformed into citrulline by peptidilarginine deiminase and are highly specific for RA, detected in ~ 70% of sufferers (Leslie et al., 2001, Johansson et al., 2016). The main antigenic targets for ACPAs include citrullinated forms of fibrinogen, alpha-erolase and vimentin (Harre et al., 2014). Their role in pathological bone modification is supported by observations of ACPA autoantibody positive subjects incurring a decrease in cortical bone mass associated with the presence of the latter autoantibody prior to the onset of RA (Kleyer et al., 2014). In this regard, citrullinated fibrinogen and ACPA immune complex formation has been demonstrated to stimulate TNFα production (Harre et al., 2014). Alternatively, direct TNFα production is additionally initiated via binding of monomeric ACPA to citrullinated proteins on macrophages independent of immune complex formation (Harre et al., 2014). TNFα is a critical regulator of bone homeostasis through its ability to facilitate the trafficking of osteoclast precursors from the bone marrow to the lymphoid organs as well as render then more susceptible to further differentiation into osteoclasts (Harre et al., 2012). In RA, IgM RF has been shown to enhance the capacity of ACPA immune complexes (ACPA-ICs) to stimulate macrophage cytokine production, in turn enhancing the pathogenicity of ACPA-ICs in RA (Sokolove et al., 2014).
In addition, ACPA is reported to directly induce formation and activation of osteoclasts in animal models even in the absence of chronic inflammation (Harre et al., 2012, Schett and Gravallese, 2012). This independent role for autoantibodies in pathologic bone modification is supported by studies illustrating the ability of ACPAs with specificity for vimentin to stimulate systemic bone loss and osteoclast formation following adaptive transfer into mice in an experimental setting that excluded the interference of other RA specific factors (Harre et al., 2012). Osteoclasts as well as their precursors express citrullinated vimentin on the cell surface; making them a direct target for ACPA mediated activation in turn triggering osteoclastogenesis and bone resorptive activity (Harre et al., 2014, Harre et al., 2012). The association between ACPA and bone destruction is not confined to RA with ACPA linked to bone destruction in PSA and SLE as well (Budhram et al., 2014, Behrens et al., 2016, Kocijan et al., 2014). The prevalence of ACPA has been noted to vary across various autoimmune disease states with differing subsets of articular diseases. On the basis of existing literature, on average 7–13% of patients suffering from PSA are determined to be seropositive for ACPA (Vander Cruyssen et al., 2005, Alenius et al., 2006). Similarly, ACPA often distinguishes SLE patients with erosive disease as these autoantibodies are prevalent (20%) in this type of arthritis compared with other forms where they appear to be less prevalent (0–2%) (Taraborelli et al., 2012).
To date, ACPA has garnered majority of the attention for its association with reduced BMD, despite research also pointing to a potential role for RF in osteoporosis development. Though RF is often considered to act primarily as an enhancer of bone loss in ACPA positive patients, studies have highlighted the presence of more profound changes to trabecular bone architecture amongst individuals seropositive for RF autoantibodies when compared to their seronegative counterparts (Kocijan et al., 2014, Steenberg et al., 2014). In fact, research has successfully established that RF in ACPA negative patients was indeed associated with more severe erosive disease as well as confirmed its additive effect on bone erosion in ACPA positive patients (Steenberg et al., 2014). The latter observations are therefore strongly suggestive of a direct role for both ACPA and RF in bone destruction.
Another group of autoantibodies observed to occur in RA patients and implicated in osteoporosis are autoantibodies against osteoprotegerin (OPG). The latter autoantibodies are said to neutralise the effect of bone regulatory cytokine osteoprotegerin (Real et al., 2015). As highlighted in Table 1, OPG acts as a decoy receptor for RANKL leading to the inhibition of osteoclast differentiation and subsequently reducing bone resorption. The importance of this pathway in bone metabolism is demonstrated by studies illustrating the effective treatment of osteoporosis via the pharmacological blockade of RANKL coupled with observations of raised bone turnover and multiple fractures occurring amongst individuals with loss of function mutations of OPG (McClung, 2007). Similarly, anti OPG autoantibodies are associated with the development of high bone turnover osteoporosis (Hauser et al., 2015, Zhao et al., 2016). However, not all studies support a role for these autoantibodies in the pathogenesis of bone disease (Larussa et al., 2012).
Additionally, immunoglobulin (Ig) A anti endomysial autoantibodies (EMA) have been implicated in the reduced BMD levels characteristically present in celiac disease patients, with more than one quarter of celiac disease sufferers presenting with osteoporosis (Scott et al., 2000). Antibodies targeting endomysium, the perivascular connective tissue lining smooth muscle bundles, are serological markers of celiac disease and act as a measure of mucosal response and dietary compliance (James and Scott, 2000). It has recently been suggested that the target antigen in endomysium is tissue transglutaminase (TTG) (James and Scott, 2000, Brusco et al., 1999, Farrace et al., 2001). When it reacts with gliadin, neoepitopes are formed (Seissler et al., 2001). It is thought that the immunological response to these neoepitopes may initiate the mucosal damage in coeliac disease (Seissler et al., 2001). Though their role in celiac disease is widely recognised, contradictory observations exist surrounding the potential role played by these autoantibodies in pathological bone density (West et al., 2007). Nonetheless, we cannot disregard the possibility that these autoantibodies are an additional mechanism through which decreased bone mass occurs in celiac disease. This is further supported by observations of increased anti EMA seroprevalence amongst a premenopausal non-celiac female sample with low BMD (Heikkilä et al., 2015).
Moreover, research has also uncovered the presence of a significantly higher rate of anti bone autoantibodies, in a subgroup of celiac disease sufferers (Duerksen and Leslie, 2010). These autoantibodies were reported to recognise bone TTG as the autoantigen of interest and their titres subsided following the adoption of a gluten free diet (Sugai et al., 2002). Similarly, anti TTG autoantibodies are also highly implicated in bone disease. In a study comprising a large cohort of middle-aged or older women without previously known osteoporosis, anti TTG autoantibodies were observed to significantly associate with low BMD as well as be indicative of higher fracture frequency (Agardh et al., 2009). Moreover, a recent prospective study of a large adult population sample of celiac disease sufferers showed that study participants positive for anti TTG autoantibody had a higher risk of hip fracture compared to negative counterparts up to 3 decades later independent of age, sex, body mass index, vitamin D, gamma-glutamyl transferase, smoking, and self-rated health (Heikkilä et al., 2015). In fact, similar to ACPA, anti TTG autoantibodies are associated with osteoporosis not only in celiac disease, but in ankylosing spondylitis and psoriatic arthritis as well (Teichmann et al., 2010). More recently, a biological role for anti TTG autoantibodies in bone disease in the absence of celiac disease was illustrated in a population based prospective study that reported lower BMD and reduced growth trajectories in anti TTG autoantibody positive children not previously diagnosed with celiac disease (Jansen et al., 2015). Similarly, findings from other epidemiological studies comprising adult populations suggest that osteoporosis and low BMD are common in individuals with increased circulating concentrations of anti TTG or EMA, regardless of whether or not they had gastrointestinal symptoms of celiac disease, villous atrophy, or evidence of malabsorption (Dickey et al., 2005, Kurppa et al., 2010, Mustalahti et al., 1999).
TTGs are a widely distributed class of enzymes that stabilise protein assemblies by catalysing the calcium ions (Ca2 +) dependent formation of isopeptide bonds (Kaartinen et al., 2002). In bone TTG enzymes are reported to play an important role in the maturation of the extracellular matrix of bone into a biological substrate capable of mediating cell attachment, and inducing as well as regulating mineralisation (Kaartinen et al., 2002). Only tissue transglutaminase 2 (TTG2) and factor 13 a (FXIIIA) have been detected in the cartilage and bone expressed by osteoblasts (Cui et al., 2014). FXIIIA is the main active TTG orchestrating matrix crosslinking during matrix deposition by osteoblasts (Cui et al., 2014). In particular, FXIIIA secretion has been shown to facilitate collagen type 1 (COL 1) and fibronectin (FN) matrix assembly and stabilization, with inhibition of TTG activity from cell culture serum observed to significantly decrease FN matrix assembly as well as affect both the quantity as well as the quality of type 1 collagen, result in impaired lysyl oxidase and alkaline phosphatase activity and lead to decreased osteoblast mineralisation (Aeschlimann and Paulsson, 1994). In addition, to COL1 and FN, bone matrix also contains several other non-collagenous proteins that have been observed to act as substrates of TTG in vitro, i.e., osteopontin, osteonectin and fibrulin-1 (Kaartinen et al., 2002). Their structures are also modulated by TTG crosslinking activity and this may contribute to their function in bone matrix assembly and bone cell function (Kaartinen et al., 2002). Recently, a role for TTG2 as well as FXIIIA in resorption was highlighted in a study demonstrating compromised biomechanical properties and trabecular bone loss in the axial and appendicular skeleton of mice lacking TTG2 and FXIII-A transglutaminases, reported to result from increased osteoclastogenesis, a cellular phenotype that persists in vitro (Mousa et al., 2017). Moreover, increased potential of TTG2 and FXIII-A deficient monocytes to form osteoclasts was reversed by chemical inhibition of TTG activity, which revealed the presence of TTG in osteoclasts (Mousa et al., 2017). Interestingly, chemical inhibition of TTG activity significantly increased RANKL expression as well as upregulated TTG1 expression in osteoclasts (Mousa et al., 2017). The findings of this study presented a novel function for TTG in bone cells by identifying them as regulators of RANKL production and myeloid and MSC cell differentiation (Mousa et al., 2017). Collectively, the latter provide crucial evidence for TTG enzyme activity in bone remodelling. It is therefore possible that autoantibodies targeting this antigen contribute towards osteoporotic pathophysiology through the inactivation of TTG, which would have serious implications for bone homeostasis.
It is widely acknowledged that exposure to tobacco smoke is considered a risk factor for the development of autoimmune phenotypes (Costenbader and Karlson, 2006). In the same manner smoking associated lung diseases are also linked to many systemic abnormalities including osteoporosis (Cielen et al., 2014). Adaptive immune responses against a variety of autoantigens have been proposed as the means through which individuals exposed to cigarette smoke develop abnormal and often pathological bone demineralisation (Demoruelle et al., 2014). A recent study aimed at identifying antigen specific autoimmune responses relevant to both emphysema and osteoporosis amongst smoke exposed subjects reported that study participants exhibited autoantibodies to GRP78, an endoplasmic reticulum molecular chaperone highly expressed in lungs (Bon et al., 2014). The pathogenicity of anti GRP78 IgG autoantibodies in smokers is at least implied by the stringent, independent and overlapping associations of this specific autoantibody with concurrent emphysema, osteoporosis and increased bone turnover (Demoruelle et al., 2014). Moreover, abnormal autoantibody response to GRP78 has been associated with the pathogenesis of RA with serum anti GRP78 autoantibodies detected in up to 63% of RA patients (Park et al., 2014).
Glucose regulated protein 78 (GRP78) also referred to as immunoglobulin heavy chain binding protein (BiP) is a chaperone protein belonging to the Heat Shock Protein 70 (HSP70) family and residing primarily in the lumen of the endoplasmic reticulum (ER) (Park et al., 2014, Panayi and Corrigall, 2014). The ER is the site of biosynthesis for all proteins with the lumen hosting a unique environment critical for proper folding of proteins destined for secretion and display on the cell surface (Navid and Colbert, 2017). BiP requires an ER environment comprising high Ca2 + for its efficient functioning (Park et al., 2014, Panayi and Corrigall, 2014). The absence of the latter can lead to inappropriate secretion, aggregation and degradation of unassembled proteins (Park et al., 2014, Panayi and Corrigall, 2014). Subsequent increased concentrations of unfolded or misfolding proteins results in ER stress (Panayi and Corrigall, 2014). During ER stress, GRP78 cell surface expression is upregulated and triggers a coordinated adaptive program termed the unfolded protein response (UPR) responsible for maintaining ER homeostasis (Park et al., 2014, Panayi and Corrigall, 2014, Navid and Colbert, 2017). GRP78 propagates UPR signalling through three ER localised protein sensors, namely, inositol-requiring transmembrane kinase endoribonuclease 1 alpha (IREIα), double stranded ribonucleic acid dependent protein kinase like ER kinase (PERK) and activating transcription factor 6 (ATF6) (Bravo et al., 2013). In the resting state, GRP78 binds the N termini of IREIα, PERK and ATF6 preventing their activation, however upon activation, GRP78 binds to unfolded or misfolded proteins, and it releases IREIα, PERK and ATF6 in turn triggering UPR (Bravo et al., 2013). The intrinsic ribonuclease activity of IREIα results in the production of XBOX binding protein1 (XBP-1), a transcription factor that induces the expression of genes involved in restoring protein folding or in degrading unfolded proteins (Bravo et al., 2013, Tohmonda et al., 2015). The IREIα/XBP-1 pathway is the most evolutionarily conserved branch of the UPR, possessing additional functions not directly related to UPR, including the regulation of innate immunity, energy metabolism and cell differentiation (Tohmonda et al., 2015, Boot-Handford and Briggs, 2010). Research has highlighted that IREIα/XBP-1 pathway is transiently activated during osteoclastogenesis (Tohmonda et al., 2015). Notably, XBP-1 functions as a transcription factor for the NFATc1 gene, the master regulator of osteoclastogenesis (Tohmonda et al., 2015). Moreover, professional secretory cells such as antibody producing lymphocytes, chondrocytes, osteoblasts and fibroblasts are particularly susceptible to ER stress induction, which is a recognised component of the disease process in inflammatory conditions such as RA (Navid and Colbert, 2017). Nonetheless, the relevance of the generation of pathogenic antibodies to GRP78 as well as the direct contribution of anti GRP78 antibody - anti GRP78 complex to pathogenic bone loss characteristic of osteoporosis remains unclear.
Further evidence of the deleterious effects of anti GRP78 autoantibodies, however, lies in research illustrating the ability of patient derived anti GRP78 autoantibodies to activate monocyte lineage phagocytes and enhance their production of injurious mediators that are implicated in the development of osteoporosis (Kim et al., 2008, Lorenzo et al., 2008, Gosselink et al., 2010, Van Kempen and Coussens, 2002, Sundaram et al., 2007). In particular, anti GRP78 autoantibodies have been shown to increase circulating levels of IL-8 and monocyte chemoattractant protein – 1 (MCP-1), which are potent chemo-attractants of neutrophils and monocytes/macrophages and mediators of osteoclastogenesis (Kim et al., 2008, Lorenzo et al., 2008). Additionally, increased production of matrix metalloproteinase 9 (MMP-9) involved in the breakdown of extracellular matrix in normal physiological processes such as tissue remodelling has also been illustrated to occur in response to anti GRP78 autoantibodies (Gosselink et al., 2010, Van Kempen and Coussens, 2002, Sundaram et al., 2007). It is possible that the cumulative effect of this increased inflammatory environment is one proposed mechanism through which these autoantibodies lead to osteoporosis.
Other autoantibodies linked to bone disease and fragility fractures in existing literature, are anti-nuclear autoantibodies (ANA) and thyroid autoantibodies. Anti centromere and anti topoisomerase 1 (anti SCL70) autoantibodies have been observed to correlate with decreased BMD at various sites (Marot et al., 2015, Ibn Yacoub et al., 2012). Furthermore, a recent prospective study identified anti double stranded deoxyribonucleic acid (anti dsDNA) as an independent predictor of higher 10 year probability of hip fracture in SLE patients (Mak et al., 2013). Similarly, anti thyroglobulin (anti TG) and anti-thyroid peroxidase (anti TPO) autoantibodies have all been associated with an increased risk of fragility fractures in post-menopausal women independent of other known risk factors (age, menopausal age, BMI, thyroid hormones) (Lambrinoudaki et al., 2017). As the studies surrounding the role played by these autoantibodies in pathogenic bone loss are few, even less is known of their mechanisms of action in osteoporosis pathogenesis. Nonetheless, these studies provide further support for a role for autoantibodies as mediators of bone diseases such as osteoporosis.
5. Conclusion
It is important to note that majority of the studies discussed in this review consisted of clinically autoimmune samples. It is therefore apparent there exists a shortage of studies examining the potential role autoantibodies play in causing bone diseases such as osteoporosis in the absence of clinical autoimmune disease. Nonetheless, this review reinforces the notion that autoimmunity represents an important driver in pathogenic bone loss, in turn providing support for further research to enable definitive conclusions to be drawn.
Additionally, the mechanisms through which autoantibodies may mediate their effects remain poorly understood. Autoantibodies may play a role in inflammatory driven osteoporosis; however, research suggests an independent function for these immune markers in the deterioration of bone structure and resulting fragility fractures. The role played by autoantibodies in mediating immune processes driving osteoporosis pathogenesis carries significant implications for better skeletal preservation by reducing fracture rates and increasing BMD, particularly amongst older, postmenopausal females known to be at a significantly greater risk of developing this disabling condition. Furthermore, research has revealed the occurrence of preferential alteration of bone tissue when comparing the deterioration of microarchitecture between trabecular and cortical bone. However, the influence of the immune system of differing bone tissue remains poorly understood. There is therefore a clear need for prospective based population studies aimed at not only ascertaining the impact of autoantibodies on accelerated bone loss characteristic of osteoporosis, but to understand the differing effects of autoimmune processes on differing bone tissue between anatomic sites as well.
Appendix A. Classification of osteoporosis
| Type of osteoporosis | Characteristics |
|---|---|
| Primary osteoporosis (or idiopathic osteoporosis) |
Historically classified as postmenopausal or senile osteoporosis. Affects 80% of women and 60% of men with osteoporosis. Multifactorial, resulting from a combination of factors including nutrition, peak bone mass, genetics, level of physical activity, age of menopause (spontaneous vs. surgical), and age related decrease in bioavailable testosterone for men and oestrogen for both men and women.
|
| Secondary osteoporosis | Affects 20% of women and 40% of men with osteoporosis. Describes osteoporosis that exists as a common feature of another disease process, heritable disorder of connective tissue, or drug side effect. Secondary osteoporosis is reported to represent the central cause of majority (> 65%) of fractures occurring in men. |
N/B
In women, postmenopausal osteoporosis is often followed by a slower and sustained bone loss due at least in part to secondary hyperparathyroidism.
Men undergo a slow age related bone loss in primary osteoporosis that often begins by the 6th decade.
References
- Aeschlimann D., Paulsson M. Transglutaminases: protein cross-linking enzymes in tissues and body fluids. Thromb. Haemost. 1994;71:402e15. [PubMed] [Google Scholar]
- Agardh D., Bjorck S., Agardh C.-D., Lidfeldt J. Coeliac disease-specific tissue transglutaminase autoantibodies are associated with osteoporosis and related fractures in middle-aged women. Scand. J. Gastroenterol. 2009;44(5):571–578. doi: 10.1080/00365520902718929. [DOI] [PubMed] [Google Scholar]
- Alenius G.M., Berglin E., Dahlqvist S.R. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann. Rheum. Dis. 2006;65(3):398–400. doi: 10.1136/ard.2005.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves C.H., Farrell E., Vis M., Colin E.M., Lubberts E. Animal models of bone loss in inflammatory arthritis: from cytokines in the bench to novel treatments for bone loss in the bedside—a comprehensive review. Clin Rev Allergy Immunol. 2016;51:27–47. doi: 10.1007/s12016-015-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Anatomy and Ultrastructure of Bone – Histogenesis, Growth and Remodeling. In: De Groot L.J., Chrousos G., Dungan K., editors. Endotext [Internet] MDText.com, Inc.; South Dartmouth (MA): 2000. https://www.ncbi.nlm.nih.gov/books/NBK279149/ [Updated 2008 May 13] Available at. [Google Scholar]
- Behrens F., Koehm M., Thaçi D., Gnann H., Greger G. Anti-citrullinated protein antibodies are linked to erosive disease in an observational study of patients with psoriatic arthritis. Rheumatology (Oxford) 2016;55(10):1791–1795. doi: 10.1093/rheumatology/kew229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon J., Kahloon R., Zhang Y., Xue J., Fuhrman C.R. Autoreactivity to glucose regulated protein 78 links emphysema and osteoporosis in smokers. PLoS One. 2014;9(9):e105066. doi: 10.1371/journal.pone.0105066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L. The amazing osteocyte. J. Bone Miner. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot-Handford R.P., Briggs M.D. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res. 2010;339(1):197–211. doi: 10.1007/s00441-009-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Parra V., Gatica D., Rodriguez A.E., Torrealba N., Paredes F., Wang Z.V., Zorzano A., Hill J.A., Jaimovich E., Quest A.F., Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat S.D., Borg M., Camilleri G., Calleja-Agius J. The role of cytokines in postmenopausal osteoporosis. Minerva Ginecol. 2014;66(4):391–407. [PubMed] [Google Scholar]
- Brusco G., Muzi P., Ciccocioppo R., Biagi F., Cifone M.G. Transglutaminase and coeliac disease: endomysial reactivity and small bowel expression. Clin. Exp. Immunol. 1999;118(3):371–375. doi: 10.1046/j.1365-2249.1999.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhram A., Chu R., Rusta-Sallehy S., Ioannidis G., Denburg J.A., Adachi J.D., Haaland D.A. Anti-cyclic citrullinated peptide antibody as a marker of erosive arthritis in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. 2014;23(11):1156–1163. doi: 10.1177/0961203314540967. [DOI] [PubMed] [Google Scholar]
- Bugatti S., Bogliolo L., Vitolo B., Manzo A., Montecucco C. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res. Ther. 2016;18:226. doi: 10.1186/s13075-016-1116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Lopes J., Canhão H., Fonseca J.E. Osteoimmunology–the hidden immune regulation of bone. Autoimmun. Rev. 2009;8(3):250–255. doi: 10.1016/j.autrev.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast – osteoclast interactions. Connect. Tissue Res. 2017;8:1–9. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cielen N., Maes K., Gayan-Ramirez G. Musculoskeletal Disorders in Chronic Obstructive Pulmonary Disease. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/965764. (17 pp. doi: https://doi.org/10.1155/2014/965764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Suppl. 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B.L., Khosla S. Physiology of bone loss. Radiol. Clin. N. Am. 2010;48(3):483–495. doi: 10.1016/j.rcl.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. 103 (2); Suppl 1. 1997. The Crippling Consequences of Fractures and Their Impact on Quality of Life; pp. S12–S19. [DOI] [PubMed] [Google Scholar]
- Costenbader K.H., Karlson E.W. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- Cotter A.G., Mallon P.W. The effects of untreated and treated HIV infection on bone disease. Curr. Opin. HIV AIDS. 2014;9(1):17–26. doi: 10.1097/COH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Cui C., Wang S., Myneni V.D., Hitomi K., Kaartinen M.T. Transglutaminase activity arising from Factor XIIIA is required for stabilization and conversion of plasma fibronectin into matrix in osteoblast cultures. Bone. 2014;59:127–138. doi: 10.1016/j.bone.2013.11.006. 2014. [DOI] [PubMed] [Google Scholar]
- D'Amelio P., Isaia G.C. Male osteoporosis in the elderly. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/907689. 8 pp. doi:10.1155/2015/907689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoruelle M.K., Solomon J.J., Fischer A., Deane K.D. The lung may play a role in the pathogenesis of rheumatoid arthritis. Int. J. Clin. Rheumatol. 2014;9(3):295–309. doi: 10.2217/ijr.14.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster D.W. Osteoporosis and the burden of osteoporosis-related fractures. Am. J. Manag. Care. 2011;17(Suppl. 6):S164–9. https://www.ncbi.nlm.nih.gov/pubmed/21761955 [PubMed] [Google Scholar]
- Dickey W., Hughes D.F., McMillan S.A. Patients with serum IgA endomysial antibodies and intact duodenal villi: clinical characteristics and management options. Scand. J. Gastroenterol. 2005;40(10):1240–1243. doi: 10.1080/00365520510023747. [DOI] [PubMed] [Google Scholar]
- Duerksen D.R., Leslie W.D. Positive celiac disease serology and reduced bone mineral density in adult women. Can. J. Gastroenterol. 2010;24(2):103–107. doi: 10.1155/2010/285036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G., Demontiero O., Troen BR. Osteoporosis. In: Halter JB, Ouslander JG, Studenski S, High KP, Asthana S, Supiano MA, Ritchie C., editors. Hazzard's Geriatric Medicine and Gerontology 7e. McGraw-Hill; New York: NY: 2017. http://accessmedicine.mhmedical.com.ezproxy.newcastle.edu.au/content.aspx?bookid=1923§ionid=144562566 [Google Scholar]
- Farrace M.G., Picarelli A., Di Tola M., Sabbatella L., Marchione O.P. Presence of anti-"tissue" transglutaminase antibodies in inflammatory intestinal diseases: an apoptosis-associated event? Cell Death Differ. 2001;8:767–770. doi: 10.1038/sj.cdd.4400880. [DOI] [PubMed] [Google Scholar]
- Florencio-Silva R., Sasso G.R., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginaldi L., Di Benedetto M.C., De Martinis M. Osteoporosis, inflammation and ageing. Immun. Ageing: I & A. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S.R. Inflammatory signaling induced bone loss. Bone. 2015;80:143–149. doi: 10.1016/j.bone.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Gosselink J.V., Hayashi S., Elliot W.M., Xing L., Chan B. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U., Georgess D., Bang H., Bozec A., Axmann R. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 2012;122(5):1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U., Kittan N.A., Schett G. Autoantibody-mediated bone loss. Curr. Osteoporos. Rep. 2014;12(1):17–21. doi: 10.1007/s11914-013-0185-9. [DOI] [PubMed] [Google Scholar]
- Hauser B., Riches P.L., Gilchrist T., Visconti M.R., Wilson J.F. Autoantibodies to osteoprotegerin are associated with increased bone resorption in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74(8):1631–1632. doi: 10.1136/annrheumdis-2014-207219. [DOI] [PubMed] [Google Scholar]
- Heikkilä K., Heliövaara M., Impivaara O., Kröger H., Knekt P. Celiac disease autoimmunity and hip fracture risk: findings from a prospective cohort study. J. Bone Miner. Res. 2015;30(4):630–636. doi: 10.1002/jbmr.2380. [DOI] [PubMed] [Google Scholar]
- Humes D.H. Osteoporosis. In: Humes D.H., editor. Kelley's Essentials of Internal Medicine. 2ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. [Google Scholar]
- Hunter D.J., Sambrook P.N. Bone loss: epidemiology of bone loss. Arthritis Res. 2000;2(6):441–445. doi: 10.1186/ar125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibn Yacoub Y., Amine B., Laatiris A., Wafki F., Znat F. Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol. Int. 2012;32:3143–3148. doi: 10.1007/s00296-011-2150-1. 2011. [DOI] [PubMed] [Google Scholar]
- Ingegnoli F., Castelli R., Gualtierotti R. Rheumatoid Factors: Clinical Applications. Dis. Markers. 2013;35(6) doi: 10.1155/2013/726598. https://doi.org/10.1155/2013/726598 726598, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon G., Napolano R., Gioia M., Moretti A., Riccio I. The contribution of cortical and trabecular tissues to bone strength: insights from denosumab studies. Clin. Cases Miner. Bone Metabol. 2013;10(1):47–51. doi: 10.11138/ccmbm/2013.10.1.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M., Scott B. Endomysial antibody in the diagnosis and management of coeliac disease. Postgrad. Med. J. 2000;76(898):466–468. doi: 10.1136/pmj.76.898.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M.A., Kiefte-de Jong J.C., Gaillard R., Escher J.C., Hofman A. Growth trajectories and bone mineral density in anti-tissue transglutaminase antibody-positive children: the generation R study. Clin. Gastroenterol. Hepatol. 2015;13(5):913–920. doi: 10.1016/j.cgh.2014.09.032. (e5. doi: https://doi.org/10.1016/j.cgh.2014.09.032) [DOI] [PubMed] [Google Scholar]
- Johansson L., Pratesi F., Brink M., Ärlestig L., D'Amato C. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res. Ther. 2016;18:127. doi: 10.1186/s13075-016-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.R. A potential osteoporosis target in the FAS ligand/FAS pathway of osteoblasts to osteoclasts signalling. Ann. Transl. Med. 2015;3(14):189. doi: 10.3978/j.issn.2305-5839.2015.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M.T., El-Maadawy S., Räsänen N.H., McKee M.D. Tissue transglutaminase and its substrates in bone. J. Bone Miner. Res. 2002;17:2161–2173. doi: 10.1359/jbmr.2002.17.12.2161. [DOI] [PubMed] [Google Scholar]
- Kamen D.L., Alele J.D. Skeletal manifestations of systemic autoimmune diseases. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17:540–545. doi: 10.1097/MED.0b013e328340533d. [DOI] [PubMed] [Google Scholar]
- Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson J, Loscalzo J., editors. Harrison's Manual of Medicine, 19e. McGraw-Hill; New York, NY: 2016. Osteoporosis and Osteomalacia.http://accessmedicine.mhmedical.com.ezproxy.newcastle.edu.au/content.aspx?bookid=1820§ionid=127559876 [Google Scholar]
- Kim V., Rogers T.J., Criner G.J. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008;5:478–485. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyer A., Finzel S., Rech J., Manger B., Krieter M. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann. Rheum. Dis. 2014;73(5):854–860. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- Kocijan R., Harre U., Schett G. ACPA and bone loss in rheumatoid arthritis. Curr. Rheumatol. Rep. 2013;15(10):366. doi: 10.1007/s11926-013-0366-7. (doi: https://doi.org/10.1007/s11926-013-0366-7) [DOI] [PubMed] [Google Scholar]
- Kocijan R., Finzel S., Englbrecht M., Engelke K., Rech J. Differences in bone structure between rheumatoid arthritis and psoriatic arthritis patients relative to autoantibody positivity. Ann. Rheum. Dis. 2014;73(11):2022–2028. doi: 10.1136/annrheumdis-2013-203791. [DOI] [PubMed] [Google Scholar]
- Koga T., Inui M., Inoue K., Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- Kurppa K., Collin P., Sievanen H., Huhtala H., Maki M. Gastrointestinal symptoms, quality of life and bone mineral density in mild enteropathic coeliac disease: a prospective clinical trial. Scand. J. Gastroenterol. 2010;45(3):305–314. doi: 10.3109/00365520903555879. [DOI] [PubMed] [Google Scholar]
- Lambrinoudaki I., Armeni E., Pliatsika P., Rizos D., Kaparos G. Thyroid function and autoimmunity are associated with the risk of vertebral fractures in postmenopausal women. J. Bone Miner. Metab. 2017;35(2):227–233. doi: 10.1007/s00774-016-0752-0. [DOI] [PubMed] [Google Scholar]
- Larussa T., Suraci E., Nazionale I., Leone I., Montalcini T. No evidence of circulating autoantibodies against osteoprotegerin in patients with celiac disease. World J. Gastroenterol. 2012;18(14):1622–1627. doi: 10.3748/wjg.v18.i14.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie D., Lipsky P., Notkins A.L. Autoantibodies as predictors of disease. J. Clin. Invest. 2001;15; 108(10):1417–1422. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Toraldo G., Li A., Yang X., Zhang H. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay R., Cosman F. Osteoporosis. In: Kasper D., Fauci A., Hauser S., Longo D., Jameson J., Loscalzo J., editors. Harrison's Principles of Internal Medicine, 19e New York. McGraw-Hill; NY: 2014. http://accessmedicine.mhmedical.com.ezproxy.newcastle.edu.au/content.aspx?bookid=1130§ionid=79753850 [Google Scholar]
- Lorenzo J., Horowitz M., Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr. Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A., Lim J.Q., Liu Y., Cheak A.A., Ho R.C. Significantly higher estimated 10-year probability of fracture in lupus patients with bone mineral density comparable to that of healthy individuals. Rheumatol. Int. 2013;33(2):299–307. doi: 10.1007/s00296-012-2389-1. [DOI] [PubMed] [Google Scholar]
- Marot M., Valery A., Esteve E., Bens G., Muller A. Prevalence and predictive factors of osteoporosis in systemic sclerosis patients: a case control study. Oncotarget. 2015;6(17) doi: 10.18632/oncotarget.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung M. Role of RANKL inhibition in osteoporosis. Arthritis Research & Therapy. 2007;9(Suppl. 1):S3. doi: 10.1186/ar2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- Mori T., Miyamoto T., Yoshida H., Asakawa M., Kawasumi M. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 2011;23(11):701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- Mori G., D'Amelio P., Faccio R., Brunetti G. The interplay between the bone and the immune system. Clin. Dev. Immunol. 2013;2013:720504. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa A., Cui C., Song A., Myneni V.D., Sun H., Li J.J., Murshed M., Melino G., Kaartinen M.T. Transglutaminases factor XIII-A and TG2 regulate resorption, adipogenesis and plasma fibronectin homeostasis in bone and bone marrow. Cell Death Differ. 2017;24:844–854. doi: 10.1038/cdd.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustalahti K., Collin P., Sievanen H., Salmi J., Maki M. Osteopenia in patients with clinically silent celiac disease warrants screening. Lancet. 1999;354(9180):744–745. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B. 108(1) 2002. The Novel Zinc Finger-Containing Transcription Factor Osterix is Required for Osteoblast Differentiation and Bone Formation; pp. 17–29. [DOI] [PubMed] [Google Scholar]
- Navid F., Colbert R.A. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat. Rev. Rheumatol. 2017;13(1):25–40. doi: 10.1038/nrrheum.2016.192. [DOI] [PubMed] [Google Scholar]
- Office of the Surgeon General (US) The Basics of Bone in Health and Disease. Vol. 2. 2004. Bone health and osteoporosis: a report of the surgeon general. Rockville (MD): Office of the surgeon general (US) Available at: https://www.ncbi.nlm.nih.gov/books/NBK45504. [PubMed] [Google Scholar]
- Orsolini G., Caimmi C., Viapiana O., Idolazzi L., Fracassi E., Gatti D., Adami G., Rossini M. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif. Tissue Int. 2017 doi: 10.1007/s00223-017-0253-8. [DOI] [PubMed] [Google Scholar]
- Pajevic P.D. Regulation of bone resorption and mineral homeostasis by osteocytes. IBMS Bone Key. 2009;6:63–70. [Google Scholar]
- Panayi G.S., Corrigall V.M. Immunoglobulin heavy-chain-binding protein (BiP): a stress protein that has the potential to be a novel therapy for rheumatoid arthritis. Biochem. Soc. Trans. 2014;42(6):1752–1755. doi: 10.1042/BST20140230. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Yoo S.A., Kim W.U. Role of endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J. Korean Med. Sci. 2014;29(1):2–11. doi: 10.3346/jkms.2014.29.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann P., Mechtcheriakova D., Meshcheryakova A., Föger-Samwald U., Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62(2):128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggatt L.J., Partridge N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010;285(33):25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real A., Gilbert N., Hauser B., Kennedy N., Shand A. Characterisation of osteoprotegerin autoantibodies in coeliac disease. Calcif. Tissue Int. 2015;97(2):125–133. doi: 10.1007/s00223-015-0023-4. [DOI] [PubMed] [Google Scholar]
- Rucci N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metabol. 2008;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- Schett G., David J.P. The multiple faces of autoimmune-mediated bone loss. Nat. Rev. Endocrinol. 2010;6(12):698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- Schett G., Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012;8(11):656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E.M., Gaywood I., Scott B.B. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. Gut. 2000;46:I1–I8. doi: 10.1136/gut.46.suppl_1.I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeling M., Nimmerjahn F. Unlocking the bone: Fcγ-receptors and antibody glycosylation are keys to connecting bone homeostasis to humoral immunity. Ann. Translat. Med. 2015;3(12):163. doi: 10.3978/j.issn.2305-5839.2015.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seissler J., Wohlrab U., Wuensche C., Scherbaum W.A., Boehm B.O. Autoantibodies from patients with coeliac disease recognize distinct functional domains of the autoantigen tissue transglutaminase. Clin. Exp. Immunol. 2001;125(2):216–221. doi: 10.1046/j.1365-2249.2001.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove J., Pisetsky D. Bone loss, pain and inflammation: three faces of ACPA in RA pathogenesis. Ann. Rheum. Dis. Published Online First. 2016 doi: 10.1136/annrheumdis-2015-208308. [DOI] [PubMed] [Google Scholar]
- Sokolove J., Johnson D.S., Lahey L.J., Wagnar C.A., Cheng D., Thiele G.M., Michaud K., Sayles H., Reimold A.M., Caplan L., Cannon G.W., Kerr G., Mikuls T.R., Robinson W.H. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenberg H.W., Ajeganova S., Forsiland K., Svennson B., van der Helm-van Mil A.H.M. The effects of rheumatoid factor and anti citrullinated peptide antibodies on bone erosion in RA. Ann. Rheum. Dis. 2014;74 doi: 10.1136/annrheumdis-2014-206623. [DOI] [PubMed] [Google Scholar]
- Sugai E., Cherñavsky A., Pedreira S., Smecuol E., Vazquez H. Bone-specific antibodies in sera from patients with celiac disease: characterization and implications in osteoporosis. J. Clin. Immunol. 2002;22(6):353–362. doi: 10.1023/a:1020786315956. [DOI] [PubMed] [Google Scholar]
- Sundaram K., Nishimura R., Senn J., Youssef R.F., London S.D. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007;313:168–178. doi: 10.1016/j.yexcr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Takai T. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 2005;25(1):1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- Takai T., Nakamura A., Tobinai A., Endo S., Inui M. Fcγ receptor targeting in RA. Arthritis Res. Ther. 2012;14(Suppl. 1):017. [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Taraborelli M., Inverardi F., Fredi M., Ceribelli A., Cavazzana I., Tincani A., Franceschini F. Anti-cyclic citrullinated peptide antibodies in systemic lupus erythematosus patients with articular involvement: a predictive marker for erosive disease? Reumatismo. 2012;11;64(5):321–325. doi: 10.4081/reumatismo.2012.321. [DOI] [PubMed] [Google Scholar]
- Teichmann J., Voglau M.J., Lange U. Antibodies to human tissue transglutaminase and alterations of vitamin D metabolism in ankylosing spondylitis and psoriatic arthritis. Rheumatol. Int. 2010;30(12):1559–1563. doi: 10.1007/s00296-009-1186-y. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S.L. Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2004;101(48):16711–16712. doi: 10.1073/pnas.0407335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007;170(2):427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A., Rucci N. The unexpected links between bone and the immune system. Medicographia. 2010;32:341–348. [Google Scholar]
- Tohmonda T., Yoda M., Iwawaki T., Matsumoto M., Nakamura M., Mikoshiba K., Toyama Y., Horiuchi K. IRE1α/XBP1-mediated branch of the unfolded protein response regulates osteoclastogenesis. J. Clin. Invest. 2015;3; 125(8):3269–3279. doi: 10.1172/JCI76765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kempen L.C.L., Coussens L.M. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- Vander Cruyssen B., Hoffman I.E., Zmierczak H., Van den Berghe M., Kruithof E., De Rycke L., Mielants H., Veys E.M., Baeten D., De Keyser F. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann. Rheum. Dis. 2005;64(8):1145–1149. doi: 10.1136/ard.2004.032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M.N. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica. 2013;2013 doi: 10.1155/2013/125705. 29 pp. doi: https://doi.org/10.1155/2013/125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann M.N. T-cells and B-cells in osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(6):461–467. doi: 10.1097/MED.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Logan R.F., Hill P.G., Khaw K.T. The iceberg of celiac disease: what is below the waterline? Clin. Gastroenterol. Hepatol. 2007;5(1):59–62. doi: 10.1016/j.cgh.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Zijl S., Wang L., de Groot D.C., van Tol M.J. Identification of the common origins of osteoclasts, macrophages, and dendritic cells in human hematopoiesis. Stem Cell Rep. 2015;4(6):984–994. doi: 10.1016/j.stemcr.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Hauser B., Visconti M.R., Riches P.L., Ralston S.H. Autoantibodies to Osteoprotegerin and bone mineral density in axial Spondyloarthritis. Rheumatology (Oxford) 2016;55(Suppl_1):i143. [Google Scholar]