Abstract
Background
In a previous case-control study, we demonstrated that children with PKU and non-PKU controls preferred sweet foods. Additionally, children with PKU exhibited food neophobia, with no preference for bitter tasting foods associated with the taste of phenylalanine (Phe)-free L-amino acid supplements.
Objective
In an observational extension study, we evaluated the influence of parental food choice and neophobia on their children's taste preferences and food neophobia.
Methods
Male and female parents/caregivers of 35 children with PKU and 35 control parents, completed a neophobia and food frequency questionnaire for comparison using the same questionnaires that they completed for their children.
Results
Both groups of children (PKU and non PKU control) were rated as more food neophobic and exhibited more neophobic behaviour than parents, although children with PKU more so than non-PKU controls (PKU food neophobia p < 0.0001vs control 0.001; PKU general neophobia p = 0.003 vs control p = 0.04). Both groups of children ate significantly more sweets, sweetened drinks and potato fries than their parents but differences were greater for children with PKU who also consumed more high carbohydrate (low protein) staple foods such as bread and pasta, and more sweet snacks such as biscuits than their parents. Non-PKU control children's food choices were closer to their parent's choices.
Conclusions
In PKU, parental food choices and their food neophobia have limited influence on their children's eating habits. Food neophobia in children with PKU may be associated with fear of eating unfamiliar foods potentially containing a source of protein or aspartame. Their preference for sweet foods may be influenced by limited food choices and habitual consumption of artificially sweetened L-amino acid supplements.
Keywords: Metabolic disorders, Phenylketonuria (PKU), Neophobia, Taste preferences, Food frequency questionnaire, L-amino acid supplements
1. Introduction
In phenylketonuria (PKU), an essential component of management is a low phenylalanine (Phe) diet, and in newborn screened infants is usually commenced within the first 2 weeks of life. Dietary management involves a severe restriction of high protein foods such as meat, fish, eggs, cheese, nuts, seeds and pulses; in addition to supplementation with bitter-tasting Phe-free L-amino acids. Due to severe restriction of high protein foods, the diet by necessity is high in carbohydrate, particularly plant and cereal starches to meet energy requirements. In addition, the artificial sweetener aspartame, that contains phenylalanine, must be avoided thereby limiting intake of some sugar-free products. It is well established that low Phe diets are high in carbohydrate (59–67% of energy) but lower in fat (23–26%) [1], [2], [3], [4], [5] than the general populations diet (50–52% carbohydrate and 33% fat) [6]. Eating behaviours evolve in the early years of development, and in PKU, a high carbohydrate diet may influence taste preferences and eating behaviour, potentially influencing the development of obesity in later life [1], [7], [8], [9]. Parents help mould children's early experiences with food, providing both the genes and the environment for children [10]. It is important to understand the influence of parents on food preferences in PKU.
Although children with PKU might be expected to have a preference for bitter tasting foods associated with frequent exposure to bitter tasting Phe-free L-amino acid supplements from early infancy, a recent study in children with PKU aged 4–13 years [11], concluded that food neophobia (avoidance of unfamiliar foods) played a more significant role in food refusal than taste preferences. Children with and without PKU were given 10-blinded puree foods to taste and rank in order of preference; parents also completed questionnaires about their children's neophobia. Both groups demonstrated a strong preference for sweet foods, although children with PKU did show a stronger preference for savoury foods (i.e. vegetables) than control children. Fruits and vegetables were consumed in similar quantities in both groups of children.
Studies have identified that food neophobia (avoidance of unfamiliar food) in children is associated with less healthy food choices, [12] reduced variety [13] and fewer new foods being served in the family home thereby influencing everyday food choices [14], [15]. Neophobia and food choice particularly in mothers, has been shown to influence the development of food neophobia in children [15], [16], [17], [18], [19], [20]. In non-PKU studies, it is observed that food neophobia is familial i.e. children whose caregivers or siblings are food neophobic, are more likely to exhibit food neophobia [17], [18], [20], [21]. The impact of parental food choice and general neophobia (fear of anything new or unfamiliar) is unreported in PKU.
The aim of this study was to evaluate the influence of parental food choice on food preferences and neophobia in children with PKU and control children.
2. Methods
2.1. Subjects
Male and female parents/caregivers of thirty-five children with PKU aged 4–13 years, and thirty-five age and gender matched control children previously studied and described [11] were recruited. PKU children were all following a low phenylalanine diet providing ≤ 10 g/day of natural protein allocated from 50 mg phenylalanine exchanges (equivalent to 1 g natural protein) from diagnosis (< 14 days of life).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and the local research ethics committee gave a favourable opinion. Written informed consent was obtained from all parents/caregivers.
2.2. Study design
This was a single-centre, observational, prospective, follow-on, case-control study. Male and female parents/caregivers from PKU and control groups completed a food and general neophobia questionnaire using a 7-item scale (ranging from “always” to “never”). This was modified from an existing questionnaire [22] and was the same questionnaire used in the previous study for the children [11]. This included 9 questions specifically related to food and 5 questions on general neophobia. A lower score indicated more neophobia. Parents/caregivers from the PKU and control groups also completed a food frequency questionnaire to assess the number of times 60 commonly eaten foods from all the major food groups (milk and dairy products; fruit and vegetables; cereals, pasta and bread; meat and meat alternatives; ready meals; snack foods; fats and oils; and drinks) were consumed each day/week.
2.3. Statistical methods for data analysis
Neophobia and food frequency were compared between parent (male and female) and child. Descriptive statistics were used for quantitative outcome measures and more detailed analysis of all parameters was assessed using Kruskal Wallis and Mann Whitney nonparametric tests.
3. Results
3.1. Subjects (Table 1)
Table 1.
Subject demographics – parental age, ethnicity and highest educational level achieved.
| PKU |
Control |
|||
|---|---|---|---|---|
| Mother n = 35 | Father n = 31 | Mother n = 34 | Father n = 31 | |
| Median age range (years) | 36–40 | 41–45 | 41–45 | 41–45 |
| Ethnicity | 80% white 9% Asian 11% Mixed/other |
84% white 6% Asian 10% Mixed/other |
85% white 9% Asian 6% Mixed/other |
94% white 6% Asian 0% Mixed/other |
| Highest educational level | % (n) | % (n) | % (n) | % (n) |
| None | 14% (5) | 10% (3) | 6% (2) | 3% (1) |
| GCSE | 46% (16) | 26% (8) | 9% (3) | 10% (3) |
| A-level | 9% (3) | 13% (4) | 3% (1) | 6% (2) |
| NVQ | 3% (1) | 16% (5) | 21% (7) | 0 |
| Diploma | 17% (6) | 26% (8) | 6% (2) | 19% (6) |
| Degree | 9% (3) | 6% (2) | 6% (2) | 35% (11) |
| Post Grad | 3% (1) | 3% (1) | 21% (7) | 0 |
| Masters | 0 | 0 | 18% (6) | 13% (4) |
| PhD | 0 | 0 | 9% (3) | 13% (4) |
| Unknown | 0 | 0 | 3% (1) | 0 |
The control parents of one child were lost to follow-up and only 31 of 35 fathers were available in both groups. Therefore, 35 mothers and 31 fathers of children with PKU and 34 mothers and 31 control fathers were studied. Median ‘age range’ for mothers of children with PKU was 36–40 years, and for fathers of children with PKU and both control parents median age was 41–45 years. Eighty two percent of parents of children with PKU and 89% of parents of control children were white Caucasian, 8% in both groups were of Asian origin and the remainder were of other descent. Parents in the control group generally achieved a higher level of education (p < 0.0001).
3.2. Neophobia
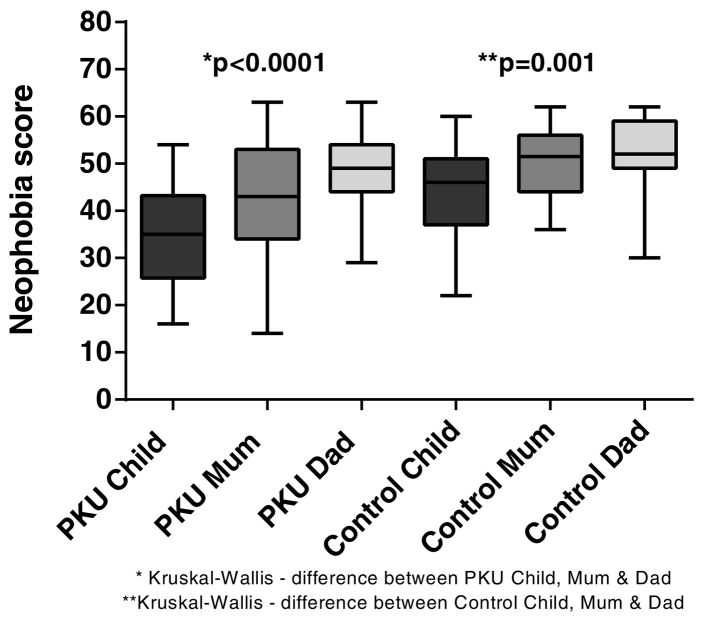
3.2.1. Food neophobia (Fig. 1)
Fig. 1.
Median total scores for food neophobia for children with PKU and Control children and their parents (lower score = more neophobic).
When total scores were calculated, children with PKU were significantly more food neophobic than both their mother (p = 0.004) and father (p < 0.0001). Control children were also more food neophobic than their parents (mother p < 0.0001; father p = 0.0003) but overall, less significantly than children with PKU. Children with PKU were more food neophobic than control children (p = 0.0001). There was no difference between mothers and fathers of children with PKU or between control mothers and fathers, but the parents of children with PKU were more food neophobic than control parents (mothers p = 0.004; fathers p = 0.05).
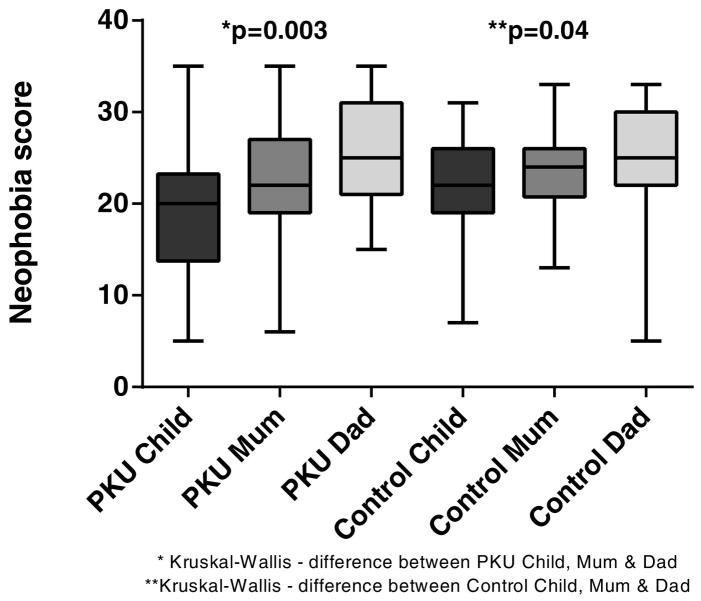
3.2.2. General neophobia (Fig. 2)
Fig. 2.
Median total scores for general neophobia for children with PKU and Control children and their parents (lower score = more neophobic).
Children with PKU were significantly more neophobic than both their parents (mothers = 0.05; fathers p = 0.0006). Control children exhibited similar neophobia to their mothers but were more neophobic than their fathers (p = 0.01). There was no significant difference between children with PKU and control children or between mothers and fathers from either group with respect to neophobia.
3.3. Food frequency questionnaire (Table 2)
Table 2.
Median frequency (per week) of consumption of certain food/food groups consumed by parents and children from both PKU and Control groups.
| PKU |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Child | Mum | Dad | P value⁎ | Child | Mum | Dad | P value⁎ | |
| Fruit | 18 | 16 | 11 | 0.06 | 17 | 21 | 15 | 0.26 |
| Vegetables | 21 | 19 | 18 | 0.79 | 20 | 26 | 21 | 0.04 |
| Potato fries | 3 | 1 | 2 | 0.007 | 1 | 1 | 1 | 0.02 |
| Bread (including LP) | 12 | 7 | 8 | 0.0007 | 9 | 9 | 9 | 0.31 |
| Pasta (including LP) | 3 | 2 | 2 | < 0.0001 | 2 | 2 | 2 | 0.31 |
| Rice (including LP) | 1 | 1 | 2 | 0.61 | 2 | 2 | 2 | 0.34 |
| Butter/Margarine | 10 | 7 | 9 | 0.45 | 7 | 7 | 5 | 0.55 |
| Sweet biscuits (including LP) | 4 | 1 | 2 | 0.0001 | 3 | 3 | 3 | 0.49 |
| Cake (including LP) | 2 | 0 | 1 | 0.03 | 2 | 1 | 2 | 0.41 |
| Sweets | 5 | 0 | 1 | < 0.0001 | 2 | 0 | 2 | < 0.0001 |
| Ice-cream (including LP) | 1 | 0 | 0 | 0.006 | 1 | 1 | 1 | 0.001 |
| Crisps | 5 | 3 | 2 | 0.12 | 2 | 2 | 3 | 0.35 |
| Chocolate (including LP) | 1 | 2 | 2 | 0.29 | 3 | 3 | 3 | 0.93 |
| Sugary drinks | 5 | 0 | 1 | < 0.0001 | 1 | 0 | 0 | 0.02 |
| Natural fruit juice | 5 | 3 | 2 | 0.61 | 4 | 4 | 5 | 0.69 |
| Water | 21 | 21 | 15 | 0.45 | 14 | 14 | 15 | 0.54 |
| Sugar-free drinks | 0 | 0 | 0 | 0.34 | 2 | 0 | 0 | 0.24 |
| Median number of different foods/week (range) |
33 (12–45) | 40 (19–61) | 44 (14–62) | < 0.0001 | 54 (25–80) | 51 (28–69) | 52 (26–74) | 0.09 |
Kruskall Wallis LP = low protein.
3.3.1. Parent vs child
Unsurprisingly, the control children's food intake was more similar to parental food intake than children with PKU. Children with PKU ate significantly more high carbohydrate staple foods such as bread, pasta and snack foods such as sweet biscuits and cake (low protein varieties) and there was a non-significant trend for children with PKU to consume more fruit and water and less chocolate than their parents. Both groups of children ate significantly more sweets, sweetened drinks, ice-cream and potato fries than parents. However, except for ice-cream, children with PKU ate significantly more of these foods (although less than daily) compared with control children. The differences between parent and child were greater for children with PKU.
3.3.2. Mothers vs fathers
Control group fathers ate fewer vegetables (p = 0.04) and more potato fries (p = 0.02) and sweets (p = 0.03) than control mothers but there were no significant differences between parents of children with PKU.
3.3.3. Parents of children with PKU vs control parents
Parents of children with PKU ate significantly less chocolate (p = 0.04) than control parents (possibly due to avoidance of eating this in front of their child), and control mothers had more vegetables (p = 0.04) and biscuits (p = 0.008) than mothers of children with PKU. Fathers of children with PKU had significantly more sweetened drinks (p = 0.02) than control fathers and mothers of PKU children (along with their children) consumed more water than control parents (although not statistically significant).
3.3.4. Number of foods consumed per week
The variety of foods consumed by children with PKU was significantly less than control children (p < 0.0001) and both their parents (mothers p < 0.0001; father p = 0.0002) (Table 2). Control children were not significantly different from either parent. Both groups of parents were not significantly different from each other but mothers of PKU children had significantly less variety than control mothers (p = 0.001).
3.4. Influence of mother's age and educational level on food neophobia and food variety
There was a significant difference (p < 0.0001) between PKU and control groups for mother's highest level of education. Mother's (PKU and control groups) with a higher level of education (Diploma or higher), had less food neophobia (median score 55 vs 45; p = 0.006) and ate a wider variety of foods (median number of different foods per week 51 vs 40; p = 0.001) than those with a lower educational level, as did their children (47 vs 37; p = 0.005). However, when divided into PKU and control groups, there was no significant difference between PKU group mothers, or their children, irrespective of educational level (Table 3). Conversely, control mothers with a lower level of education (below Diploma level), and their children, were significantly more food neophobic and consumed less variety in their diet. The age of the mother did not significantly influence the level of neophobia or food variety in either the mother or child.
Table 3.
Comparison of food neophobia and food variety with mother's educational level.
| PKU mothers |
Control mothers |
|||||
|---|---|---|---|---|---|---|
| Lower education | Higher education | P value⁎ | Lower education | Higher education | P value⁎ | |
| Mother's median food neophobia score | 43 | 44 | 0.51 | 46 | 55 | 0.03 |
| Child's median food neophobia score | 31 | 36 | 0.52 | 39 | 48 | 0.003 |
| Mother's median food variety no./week | 39 | 41 | 0.73 | 40 | 58 | 0.0009 |
| Child's median food variety no./week | 33 | 33 | 0.78 | 47 | 63 | < 0.0001 |
Kruskal Wallis.
4. Discussion
This is the first study in PKU to compare parental and child neophobia, food preferences and food intake in comparison with age matched control children and their parents. Both groups of children had more general and food neophobia than their parents, although children with PKU were more food neophobic than control children. In addition, both parents of children with PKU (particularly mothers) were significantly more food neophobic than control parents and this in turn may influence the type and variety of foods offered. Children with PKU ate more staple foods such as bread and pasta than their parents or than control children; these are low in phenylalanine, satiating, and more accessible than some other low protein foods. Furthermore, both groups of children, but particularly children with PKU consumed more sweet foods than their parents. The apparent preference for sweet containing food may be influenced by restricted food choices, lack of ‘quick’ alternative snack foods, avoidance of sugar-free drinks due to their aspartame content or by the ‘sweetened’ Phe-free L-amino acid supplements which are prescribed and consumed at least three times daily.
Although neophobia may be influenced by genetics [21], several studies show that neophobia declines with age associated with increasing exposure to new and different foods [15], [23], [24], [25], peaking at around 2–6 years of age [23]. Our children with PKU had a minimal age of 4 years but still had significant neophobia. It is likely many other factors may have influenced their neophobia including innate fear of eating ‘unsafe’ food, limited food choice, food palatability, and poor appetite associated with regular daytime intake of Phe-free L-amino acids [26]. The mealtime environment is undoubtedly important, including the strategies employed by parents to persuade their children to eat, frequency of exposure to foods, and the effects of parental modelling [23]. When children eat in the presence of others, their eating behaviour is socially facilitated [24] generally leading to an increase in the amount of food consumed relative to the number of people present [23]. Previous studies have demonstrated that children with PKU may eat separately to the rest of the family and receive limited positive encouragement during mealtimes [26], [27], exacerbating food neophobia. There is also evidence that parental style and controlling behaviour increases neophobia in children [28]. This type of behaviour may well be reflective of the pressure experienced by parents of PKU children to ensure their children choose appropriate foods in their diet.
The influence of caregivers in food choice and taste preferences cannot be underestimated. Overall, parents of children with PKU lacked variety in their diet compared with control parents who ate fruit and vegetables more frequently. In addition, mothers of children with PKU were more food neophobic than control mothers and this may in turn have influenced the foods offered to children. Food neophobia and food preferences in children are affected by socio-economic status and parents educational level [23] and the quality of children's dietary intake has been shown to be associated with maternal educational level, namely in the form of less variety and less healthy choices [29], [30], [31]. In our study cohort, mothers of children with PKU generally achieved a lower educational level than control mothers. However, no difference was found in children with PKU or their mothers with respect to food neophobia and variety of foods consumed irrespective of the mother's education level. Conversely, educational level did influence food neophobia and food variety in control children and their mothers. This suggests that there are other more significant factors influencing neophobia and food variety in PKU. Parents of children with PKU may adjust some of their own eating patterns because of their child with PKU. In addition, food choices may be limited in PKU associated with the complexities of low protein cooking, with parents opting for pre-measured manufactured foods for dietary phenylalanine allocation instead of preparing foods from fresh ingredients.
There were some study limitations. The study population had been previously selected for another study with different objectives; therefore, parents were not matched for age and educational level. Moreover, statistical comparisons of mothers' educational level were limited due to the small subject numbers studied. Parents of PKU children generally had lower education levels than control parents and mothers of PKU children were also on average younger than control mothers and these are factors known to influence food choice. However, when controlled for, these factors did not appear to influence results in the PKU group. In addition, the questionnaires for the children in the study were completed predominantly by the children's mothers rather than children themselves. It may be that they perceived their children to be more neophobic than they were and it is possible that their own neophobia or food rejections may have limited exposure to different foods in their children. Furthermore, the BMI of parents was not recorded and it is acknowledged that this may have influenced eating habits. However, previous study in this group of PKU children has indicated that the incidence of obesity is no different than in the general population [8].
In conclusion, this study suggests that food neophobia in the parents/caregivers of children with PKU, has limited influence on their children's food neophobia. However, parental food choice may constrain the exposure to food variety. In early childhood years, although practical guidance is offered on improving children's exposure to different low phenylalanine foods (principles of ‘food modelling’, eating together as a family, and maintaining positive messages about food choices), this advice adds to the pressure surrounding the central message that blood phenylalanine control should always be maintained within target range. The convenience of offering children low phenylalanine foods that are pre-measured, easy to prepare and readily accepted by their children in a diet that is difficult to maintain is probably an important factor but it remains unstudied. Overall, in PKU, further longitudinal cohort studies are required about dietary patterning, food portion sizes and food choice availability to see how these influence longer-term eating patterns and nutritional status.
Funding
Funding for this research was obtained through a research grant from Birmingham Children's Hospital Research Foundation (37-3-707) who also provided peer review for the study design.
Conflict of interest
Anne Daly – research funding from Vitaflo; financial support from Nutricia and Vitaflo to attend study days and conferences.
Sharon Evans - research funding from Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences.
Anita MacDonald - research funding and honoraria from Nutricia, Vitaflo International and Merck Serono, Member of European Nutrition Expert Panel (Biomarin), Member of Sapropterin Advisory Board (Biomarin), Member of the Advisory Board for Element (Danone-Nutricia) and Arla.
Acknowledgments
Acknowledgements
The authors would also like to thank all the parents/carers and their families for participating in the study and the Birmingham Children's Hospital Research Foundation for funding.
References
- 1.Rocha J.C., MacDonald A., Trefz F. Is overweight an issue in phenylketonuria? Mol. Genet. Metab. 2013;110:S18–S24. doi: 10.1016/j.ymgme.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Rocha J.C., van Spronsen F.J., Almeida M.F., Soares G., Quelhas D., Ramos E. Dietary treatment in phenylketonuria does not lead to increased risk of obesity or metabolic syndrome. Mol. Genet. Metab. 2012;107(4):659–663. doi: 10.1016/j.ymgme.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Schulz B., Bremer H.J. Nutrient intake and food consumption of adolescents and young adults with phenylketonuria. Acta Paediatrica (Oslo, Norway: 1992) 1995;84(7):743–748. doi: 10.1111/j.1651-2227.1995.tb13748.x. [DOI] [PubMed] [Google Scholar]
- 4.Rose H.J., White F., MacDonald A., Rutherford P.J., Favre E. Fat intakes of children with PKU on low phenylalanine diets. J. Hum. Nutr. Diet. 2005;18(5):395–400. doi: 10.1111/j.1365-277X.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald A. University of Birmingham; 1999. The Dietary Management of Phenylketonuria. [Google Scholar]
- 6.NatCen Social Research . MRC Human Nutrition Research University College London, Medical School; 2015. National Diet and Nutrition Survey Years 1-4, 2008/09-2011/12. (10 November 2016) [Google Scholar]
- 7.Doulgeraki A., Skarpalezou A., Theodosiadou A., Monopolis I., Schulpis K. Body composition profile of young patients with phenylketonuria and mild hyperphenylalaninemia. Int. J. Endocrinol. Metab. 2014;12(3) doi: 10.5812/ijem.16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokmen Ozel H., Ahring K., Belanger-Quintana A., Dokoupil K., Lammardo A.M., Robert M. Overweight and obesity in PKU: the results from 8 centres in Europe and Turkey. Mol. Genet. Metab. Rep. 2014;1:483–486. doi: 10.1016/j.ymgmr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson L.V., McStravick N., Ripley S., Weetch E., Donald S., Adam S. Body mass index in adult patients with diet-treated phenylketonuria. J. Hum. Nutr. Diet. 2013;26(Suppl. 1):1–6. doi: 10.1111/jhn.12054. [DOI] [PubMed] [Google Scholar]
- 10.Savage J.S., Fisher J.O., Birch L.L. Parental influence on eating behavior: conception to adolescence. J. Law Med. Ethics. 2007;35(1):22–34. doi: 10.1111/j.1748-720X.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans S., Daly A., Chahal S., MacDonald J., MacDonald A. Food acceptance and neophobia in children with phenylketonuria: a prospective controlled study. J. Hum. Nutr. Diet. 2016;29(4):427–433. doi: 10.1111/jhn.12346. [DOI] [PubMed] [Google Scholar]
- 12.Cooke L., Carnell S., Wardle J. Food neophobia and mealtime food consumption in 4–5 year old children. Int. J. Behav. Nutr. Phys. Act. 2006;3(1):14. doi: 10.1186/1479-5868-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falciglia G.A., Couch S.C., Gribble L.S., Pabst S.M., Frank R. Food neophobia in childhood affects dietary variety. J. Am. Diet. Assoc. 2000;100(12):1474–1481. doi: 10.1016/S0002-8223(00)00412-0. [DOI] [PubMed] [Google Scholar]
- 14.Koivisto U.K., Sjoden P.O. Food and general neophobia in Swedish families: parent-child comparisons and relationships with serving specific foods. Appetite. 1996;26 doi: 10.1006/appe.1996.0009. [DOI] [PubMed] [Google Scholar]
- 15.Koivisto Hursti U.-K., Sjoden P.O. Food and general neophobia and their relationship with self-reported food choice: familial resemblance in Swedish families with children of ages 7–17 years. Appetite. 1997;29 doi: 10.1006/appe.1997.0108. [DOI] [PubMed] [Google Scholar]
- 16.Galloway A.T., Lee Y., Birch L.L. Predictors and consequences of food neophobia and pickiness in young girls. J. Am. Diet. Assoc. 2003;103(6):692–698. doi: 10.1053/jada.2003.50134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pliner P., Loewen E.R. Temperament and food neophobia in children and their mothers. Appetite. 1997;28(3):239–254. doi: 10.1006/appe.1996.0078. [DOI] [PubMed] [Google Scholar]
- 18.Falciglia G., Pabst S., Couch S., Goody C. Impact of parental food choices on child food neophobia. Child. Health Care. 2004;33(3):217–225. [Google Scholar]
- 19.Coulthard H., Sahota S. Food neophobia and enjoyment of tactile play: associations between preschool children and their parents. Appetite. 2016;97:155–159. doi: 10.1016/j.appet.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Cooke L.J., Haworth C.M., Wardle J. Genetic and environmental influences on children's food neophobia. Am. J. Clin. Nutr. 2007;86(2):428–433. doi: 10.1093/ajcn/86.2.428. [DOI] [PubMed] [Google Scholar]
- 21.Knaapila A., Tuorila H., Silventoinen K., Keskitalo K., Kallela M., Wessman M. Food neophobia shows heritable variation in humans. Physiol. Behav. 2007;91(5):573–578. doi: 10.1016/j.physbeh.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Pliner P., Hobden K. Development of a scale to measure the trait of food neophobia in humans. Appetite. 1992;19(2):105–120. doi: 10.1016/0195-6663(92)90014-w. [DOI] [PubMed] [Google Scholar]
- 23.Dovey T.M., Staples P.A., Gibson E.L., Halford J.C. Food neophobia and ‘picky/fussy’ eating in children: a review. Appetite. 2008;50(2–3):181–193. doi: 10.1016/j.appet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Addessi E., Galloway A.T., Visalberghi E., Birch L.L. Specific social influences on the acceptance of novel foods in 2–5-year-old children. Appetite. 2005;45(3):264–271. doi: 10.1016/j.appet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 25.McFarlane T., Pliner P. Increasing willingness to taste novel foods: effects of nutrition and taste information. Appetite. 1997;28(3):227–238. doi: 10.1006/appe.1996.0075. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald A., Rylance G.W., Asplin D.A., Harris G., Booth I.W. Abnormal feeding behaviours in phenylketonuria. J. Hum. Nutr. Diet. 1997;10:163–170. [Google Scholar]
- 27.MacDonald A., Rylance G.W., Asplin D.A., Hall K., Harris G., Booth I.W. Feeding problems in young PKU children. Acta Paediatr. Suppl. 1994;407:73–74. doi: 10.1111/j.1651-2227.1994.tb13457.x. [DOI] [PubMed] [Google Scholar]
- 28.Wardle J., Carnell S., Cooke L. Parental control over feeding and children's fruit and vegetable intake: how are they related? J. Am. Diet. Assoc. 2005;105(2):227–232. doi: 10.1016/j.jada.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Cribb V.L., Jones L.R., Rogers I.S., Ness A.R., Emmett P.M. Is maternal education level associated with diet in 10-year-old children? Public Health Nutr. 2011;14(11):2037–2048. doi: 10.1017/S136898001100036X. [DOI] [PubMed] [Google Scholar]
- 30.Rogers I., Emmett P. The effect of maternal smoking status, educational level and age on food and nutrient intakes in preschool children: results from the Avon longitudinal study of parents and children. Eur. J. Clin. Nutr. 2003;57(7):854–864. doi: 10.1038/sj.ejcn.1601619. [DOI] [PubMed] [Google Scholar]
- 31.Vereecken C.A., Keukelier E., Maes L. Influence of mother's educational level on food parenting practices and food habits of young children. Appetite. 2004;43(1):93–103. doi: 10.1016/j.appet.2004.04.002. [DOI] [PubMed] [Google Scholar]