Abstract
Endometrial cancer (EC) is the most common familiar gynecologic malignant tumor identified in the female reproductive system and has been increasing yearly. In this study, we will identify the surface markers and stem cell markers related with cancer stem cells (CSCs) of EC. Tissue samples were obtained from endometrial cancer patients during surgical procedures. Single cells were isolated from the tissues for culturing, transfection into nude mice, and histopathology analysis. RT-PCR demonstrated that the cultured cells strongly expressed stemness-related genes, such as c-Myc, Sox-2, Nanog, Oct 4A, ABCG2, BMI-1, CK-18, Nestin and β-actin. The expression of surface markers CD24, CD133, CD47, CD29, CD44, CXCR4, SSEA3 and SSEA4, CD24, and CD133 and chemokine markers such as CXCR4 were measured by flow cytometry. Then the double percentage of CD133+CXCR4+ cells constituted 7.2% and 9.3% in EC cells originated from two different patients, respectively. The CD133+CXCR4+ primary endometrial cancer cells grew faster, exhibited high expression of mRNA of stemness-related genes, produced more spheres, and had higher clonogenic ability than other subpopulations. They are also more resistant to anti-cancer drugs than other subpopulations. These findings indicate that CD133+CXCR4+ cells may possess some characteristics of CSCs in primary endometrial cancer. These cell surface markers may be useful for the development of drugs against CSC molecular targets or as a predictive marker for poor prognosis in primary endometrial cancer.
Introduction
Endometrial cancer (EC) is one of the most common malignancies in the world. American cancer statistics indicate that the incidence of endometrial cancer is increasing yearly, with 49,560 new cases diagnosed in 2013 and 54,870 new cases in 2015, respectively [1], [2]. There are several pathological types of EC, which include endometrioid carcinoma, mucinous adenocarcinoma, papillary serous adenocarcinoma, clear cell adenocarcinoma, undifferentiated carcinoma, and mixed carcinoma. Although conventional treatments, such as operation and chemotherapy, have been established, its recurrence is common because cancer stem cells (CSCs) have certain abilities which include self-renewal, invasion, anti-tumor drug resistance, and tumor recurrence [3]. The CSC theory emerged as a prominent instance for explaining tumor heterogeneity. According to the CSC hypothesis, tumors are organized in a hierarchy of heterogeneous cell populations and only a small subpopulation of the cells within a cancer constitute as CSCs; CSCs have the ability to maintain formation and growth [4]. The CSC hypothesis not only provides a mechanism for therapeutic methods but also explains failures in the fight against cancer. The validity of the CSC theory has been demonstrated in leukemic cells; CSCs have been shown to account for <1% of total tumor cells [5], [6], [7]. According to the CSC hypothesis, CSCs express surface markers or other specific markers that can differentiate them from the bulk of cancer cells; these markers can be used to identify a population of CSCs from total tumor cells. The theory has had a significant influence on our understanding of cancer metastasis, biology, and progression and has provided a molecular target for anti-cancer therapeutic methods.
It is widely accepted that CSCs play an important role in cancer development and progression. A number of studies have shown that CSCs are associated with cancer metastasis [8]. CSCs are similar to common stem cells in their capacity to preserve themselves via self-renewal and generate huge differentiated cell populations [9], [10]. Cells express various surface markers that can be used to isolate CSCs from tumor cells. In order to identify CSCs, patient-derived cancer cells are stained with labeled antibodies against multiple cell surface markers, including single or double markers, and the labeled and unlabeled cells can then be separated.
CSCs have been discovered in a wide range of tumors including breast [11], prostate [12], pancreas [13], and melanoma tumors [14]. A number of cell surface markers, including CD133, CD24, ALDH1, and side population (SP) fraction, are common to several types of cancer [15]. The precise function of these markers have already been established, and they may be associated with stem cell functions such as self-renewal or differentiation.
Previous studies have found it challenging to identify and isolate CSCs from solid tumors because most tumors are unlike the hematopoietic, the normal tissue developmental gradation has not been identified and therefore, it is more difficult to select candidate markers [16]. However, the techniques developed to date have enabled the isolation of CSCs from solid breast, brain, lung, liver, mouth, ovary, prostate, and colon tumors. The first minority subpopulation was isolated from a solid human breast cancer tumor [17]. This cell population was identified by the cell surface marker CD44+CD24−/low Lineage-; these cells are tumorigenic; when low numbers of CD44+CD24− cells were injected into immunodeficient mice, tumors formed at very high frequency, while alternate phenotypes failed to form tumors. Gargett et al. identified the epithelial stem/progenitor cells in the human endometrium [18]. The human endometrium is a highly dynamic tissue that undergoes cycles of growth, differentiation, shedding, and regeneration throughout the reproductive life of women and contains rare populations of epithelial and stromal colony-forming cells [19]. Several studies have reported the existence of CSCs in EC. Friel et al. showed that SP cells isolated from the EC cell lines AN3CA and Ishikawa were chemoresistant and had high proliferative activity and tumorigenicity [20]; while Hubbard et al. demonstrated that the small population of clonogenic cells isolated from EC patient tissues possessed self-renewal, differentiation, and tumorigenic abilities.
CD133 (human prominin-1) is a membrane glycoprotein with a putative function in plasma membrane organization. It is the first marker used to identify and isolate CSCs from the EC. Rutella et al. [21] and Nakamura et al. [22] analyzed tumor samples for CD133 expression. They isolated CD133+ cells and assessed their phenotypic characteristics, self-renewal capacity, ability to maintain CD133 expression and form sphere-like structures in long-term cultures, sensitivity to chemotherapeutic agents, gene expression profiles, and the ability to initiate tumors in NOD/SCID mice [22], [23].
CXC motif chemokine receptor 4 (CXCR4) is a stromal cell-derived factor-1 receptor secreted by bone marrow, liver, lung, and neural cells [24]. A previous study detected CXCR4 expression in malignant tumor cells and showed its activation causes signaling through numerous pathways, leading to enhanced survival, increased proliferation, drug resistance, degradation of extracellular matrix, and angiogenesis [25].
Based on these previous studies, we hypothesized that the cell surface markers CD133 and CXCR4 could constitute as potential EC markers [26], [27]. Thus, in this study we isolated the CD133+ and CXCR4+ subpopulation and examined its functional characteristics in vitro and in vivo. Our findings could be used to identify CSCs in EC and develop new molecular target treatments.
Materials and Methods
Human Primary Endometrial Cancer Tissues
All primary EC tissues were collected in accordance with the guidelines of the Research Ethics Committee of the University of Toyama. The tumor samples were obtained during surgical resection after obtaining informed consent from the EC patients (Table 1). The sampled tumor tissue was divided in three. One section was fixed with 4% paraformaldehyde for pathology, one was washed and dissociated into 1-2 mm3 fragments, then transplanted subcutaneously into nude mouse (6–8 weeks), and one was used to isolate cancer cells.
Table 1.
Clinical Characteristics of Patients
| Diagnosis/Pathology | ID | Previous Clinical Background |
|||||
|---|---|---|---|---|---|---|---|
| At Surgery Age |
Diagnosis |
Comments |
|||||
| FIGO 2008 |
Grade in Adenocarcinoma | Therapy | Isolatedcells | ||||
| Endometrioid carcinoma | 1 | 69 | G1 | death | |||
| 2 | 77 | IVb | G3 | TC | |||
| 3 | 52 | IVb | G2–3 | TC, RT, AP,TC | relapse | ||
| 4 | 85 | IVb | G2 | TC | ◯ | ||
| 5 | 68 | IIIc | G2 | TC | |||
| 6 | 62 | IIIc | G3 | TC | ◯ | ||
| 7 | 63 | IIc | G1 | TC | |||
| 8 | 70 | IIb | G2 | TC | |||
| 9 | 91 | IIb | G1 | TC | |||
| 10 | 63 | Ib | G1 | ||||
| 11 | 70 | Ib | TC | ||||
| 12 | 55 | Ib | G2 | ||||
| 13 | 59 | Ib | G1 | TC | |||
| 14 | 62 | Ia | G3 | TC | |||
| 15 | 48 | Ia | G2 | TC | |||
| 16 | 59 | Ia | G1 | ||||
| 17 | 46 | G1 | |||||
| Serous adenocarcinoma | S1 | 67 | IIIc | TC, AP,TC | death | ||
| S2 | 72 | IIIa | TC, AP, TC | death | |||
| S3 | 68 | Ia | TC | ||||
FFIGO: International Federation of Gynecology and Obstetrics surgical staging system.
 : growth well.
: growth well.
◯: growth slowly.
Human Primary Endometrial Cancer Isolation
The small tissue fragments were washed with phosphate-buffered saline (PBS), digested twice with 0.4% collagenase for 15 min in a 37 °C water bath, and then filtered with gauze. Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Sigma-Aldrich, USA) supplemented with 1% penicillin–streptomycin (Nacalai Tesque, Japan), 1% L-glutamine (Nacalai Tesque), and 20% fetal bovine serum (Biosera, Australia) in a cell culture incubator at 37 °C under 5% CO2.
Flow Cytometric Analysis
Cells at 70%–85% confluence were dissociated with trypsin (0.25%,Sigma-Aldrich), incubated in PBS, then blocked with 3% bovine serum albumin (BSA) for 30 minutes. Cells (1 × 106) were stained with antibodies against CD24 (anti CD24-FITC; BD Pharmingen, Franklin Lakes, NJ, USA), CD133 (anti CD133-FITC; Miltenyi Biotec, Germany), CD47 (anti CD47-FITC; Santa Cruz Biotechnology, USA), CD29 (anti CD29-PE; BD Pharmingen), CD44 (anti CD44-PE; BD Pharmingen), CXCR4 (anti CXCR4-PE; Beckman Coulter, USA), SSEA3 (anti SSEA3-FITC; BD Pharmingen), and SSEA4 (anti SSEA4-PE; BD Pharmingen), then washed twice with PBS and analyzed using the FACS Canto II system (BD Biosciences, USA).
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer's protocol. Isolated RNA (1 μg) was reverse-transcribed with the ReverTra Ace qPCR RT Kit Master Mix (TOYOBO, Japan) according to the manufacturer's protocol. Complementary DNA was amplified using the Taq DNA polymerase kit (Qiagen) with specific primers for c-Myc, Sox-2, Nanog, Oct4A, ABCG2, BMI-1, Nestin, and β-actin (Table 2) under the following conditions: initial denaturation (95 °C, 4 min); 35 cycles of denaturation (94 °C, 1 min), annealing (60 °C, 56 °C, 56 °C, respectively, for 1 min), extension (72 °C, 1 min); and a final extension (72 °C, 10 min). Primers and PCR conditions are detailed in Table 1. PCR products were separated by electrophoresis on 2% agarose gels (Wako, Japan), visualized with ethidium bromide (Wako, Japan), and analyzed using the Image Reader LAS-3000 software.
Table 2.
PCR Primer and PCR Condition Details
| Genes | Primer | Size (bp) |
Annealing Temperature(°C) |
Cycle Number |
|---|---|---|---|---|
| c-Myc | F:GATTCTCTGCTCTCCTCGACGGAG | 273 | 56 | 35 |
| R:GCGCTGCGTAGTTGTGCTGATGTG | ||||
| SOX-2 | F:AGTCTCCAAGCGACGAAAAA | 410 | 56 | 35 |
| R:GGAAAGTTGGGATCGAACAA | ||||
| Nanog | F:CAGAAGGCCTCAGCACCTAC | 216 | 56 | 35 |
| R:CTGTTCCAGGCCTGATTGTT | ||||
| Oct A | F:GAAGCTGGAGAAGGAGAAGCTG | 224 | 60 | 35 |
| R:CAAGGGCCGCAGCTTACACATGTTC | ||||
| ABCG2 | F:TCAGGTAGGGCAATTGTGAGG | 210 | 56 | 35 |
| R:CTTCAGCATTCCACGATATGG | ||||
| Bmi-1 | F:AATCAAGGAGGAGGTGA | 370 | 50 | 35 |
| R:CAAACAAGAAGAGGTGGA | ||||
| CK-18 | F:TGGTCACCACACAGTCTGCT | 357 | 56 | 35 |
| R:CCAAGGCATCACCAAGATTA | ||||
| Nastin | F:ACAGCGGAATTCCTGGAG | 410 | 56 | 35 |
| R:CTGAGGACCACGACTCTCTA | ||||
| β-actin | F:CGGGACCTGACTGACTAC | 252 | 56 | 35 |
| R:GAAGGAAGGCTGGAAGAG |
Magnetic-Activated Cell Sorting (MACS)
CD133+CXCR4+ EC cells were separated by Magnetic-Activated Cell Sorting technology (MACS; Miltenyi Biotec). Cultured EC cells at 70%–85% confluence were trypsinized by 0.25% trypsin and resuspended in cold (2–8 °C) MACS buffer (PBS [pH 7.2], 0.5% BSA, and 2 mM EDTA). Cells were stained with the CD133 FITC-conjugated primary antibody (Miltenyi Biotec) according to the manufacturer's recommendations. CD133 FITC-conjugated antibody was added per 107 total cells. The cells were then resuspended and 20 μL anti-FITC Multisort microbeads and 80 μL buffer were added per 107 total cells, mixed well, and incubated 15 min at 2–8 °C. The cells were then washed, applied to a column placed in the magnetic field of the MACS Separator (Miltenyi Biotec). For the second magnetic labeling and separation, 20 μL of MultiSort Release Reagent was added per 1 mL of cell suspension, Next, the MACS Micro Beads were added to magnetically label the cells for the second marker, the CXCR4 PE conjugated antibody (Beckman coulter, USA). The reaction was mixed well and incubated according to the manufacturer's recommendations.
Immunohistochemistry Analyses for Patients
EC patient tissue samples were obtained, fixed in 4% paraformaldehyde, and embedded into paraffin for sectioning with a microtome. The specimens were incubated with primary antibodies against CXCR4 (1:500, Abcam, UK), CD133 (1:100, Novus Biologicals USA) over night. The sections were treated with biotinylated anti-rabbit IgG antibody (Nichirei biosciences, Japan). Color developing agent was obtained by treatment with DAB kit (Nichirei biosciences, Japan). The samples were examined using a Leica microscope (Leica DMRBE, Germany).
Immunofluorescence
Cells (25,000) were suspended in 50 μL PBS, dripped onto glass slides, and then centrifuged at 800 rpm for 7 min using a cytospin (Cytospin 4, Thermoscientific, USA). Slides were air-dried overnight, fixed in acetone for 15 min at −20 °C, and blocked in Block Ace for 15 min. The cells were then incubated with primary antibodies (Santa Cruz Biotechnology, USA) against c-Myc (1:200), Klf-4 (1:200), Oct3/4 (1:200), Nanog (1:200), and Sox-2 (1:50) overnight at 4 °C. The cells were washed three times with PBS, then incubated with an Alexa Flour-conjugated secondary antibody in the dark at 37 °C for 1 h. The cells were counterstained with 4,6-diamidino-2-phenylidole (DAPI) to demonstrate the presence of nuclei. DAPI-stained negative groups were stained without a primary antibody. Stained cells were examined using a Leica fluorescent microscope with DP Controller software and images were obtained with a digital camera (DP70; OLYMPUS, Tokyo, Japan).
Cell Proliferation Assay
Parental, CD133+CXCR4+, and CD133−CXCR4− cells were seeded into 24-well plates at a concentration of 5 × 103 per well. Cells were cultured in DMEM/F-12 supplemented with 1% penicillin–streptomycin, 1% L-glutamine, and 20% fetal bovine serum in a cell culture incubator at 37 °C under 5% CO2. Medium was changed every 24 h and the cells of each subgroups were counted from day 2 to day 12.
Sphere Formation Assay
Following cell sorting, parental, CD133+CXCR4+, and CD133−CXCR4− cells were seeded at a density of 1000 cells in 24-well ultralow attachment dishes (Corning, USA) containing serum-free sphere medium, then cultured at 37 °C under 5% CO2 for 7 days.
Soft Agar Colony Formation Assay
Cells (2 × 104) from each group were added to 3 mL 0.4% Noble Agar (BD, USA), mixed well, then seeded into 60 mm dishes containing 5 mL 0.5% Bacto Agar (BD, USA). Cells were cultured at 37 °C under 5% CO2 in an incubator for 14 days then examined using a microscope (Nikon, Japan). Colonies with a diameter greater than 500 μm were counted and analyzed.
Chemosensitivity Assay
Chemosensitivity was evaluated using the Cell Counting Kit-8 (Dojindo, Japan) assay according to the manufacturer's protocol; 5 × 103 cells per well were seeded in 96-well plates, pre-incubated for 24 h, then treated with different concentrations of cisplatin (Sigma-Aldrich) and paclitaxel (Sigma-Aldrich). Following 24 h incubation, 10 μL of CCK-8 solution was added to each well and the cells were further incubated for 2 h at 37 °C under 5% CO2 in an incubator. Cell viability was determined by measuring absorbance at 450 nm with a Multi-Mode Microplate Reader (Filter Max F5, Molecular Devices, USA).
Xenograft Tumor Formation Assay
Male, 6- to 8- week-old nude mice were purchased from Japan SLC (Tokyo, Japan) and housed under pathogen-free conditions. Tumorigenicity experiments were performed according to the guidelines provided by the Experimental Animal Center, Toyama University. To determine tumorigenicity, cells were sorted and various amounts (1 × 104, 1 × 103, and 1 × 102) of CD133+CXCR4+, and CD133−CXCR4− cells were diluted in PBS. Next, the CD133+CXCR4+ cells were subcutaneously injected into the left flanks of nude mice and CD133−CXCR4− cells were subcutaneously injected into the right flanks. Tumor formation was evaluated 12 weeks post injection.
Statistical Analysis
Data are expressed as the mean ± standard deviation. Statistical analysis was carried out using the statistical software Statistical Product and Service Solutions. Student's t-test was used to compare the means between the different groups. P < .05 was considered significant.
Results
-
1.
Expression of stem cell markers and chemokines in primary EC cells
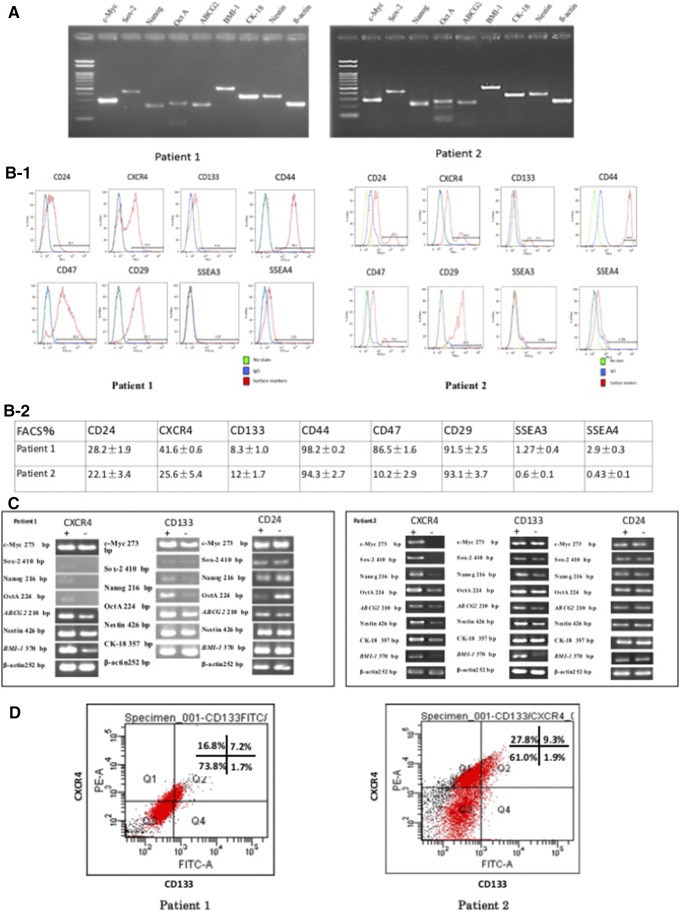
The stemness of primary cells isolated from endometrial cancer patient tissue samples was determined by measuring mRNA expression using semi-quantitative RT-PCR. Several stemness genes, including c-Myc, Sox-2, Nanog, Oct 4A, ABCG2, BMI-1, CK-18, Nestin and β-actin were expressed in these isolated cancer cells (Figure 1A).
Figure 1.
The expression of stemness genes and surface markers in primary endometrial cancer cells. (A) RT-PCR showed both two patients expressed stemness related genes including c-Myc, Sox-2, Nanog, Oct4A, ABCG2, BMI-1, CK-18, Nestin and β-actin, β-actin is the negative control. (B-1 and B-2) The expression levels of CD24, CD133, CD47, CD29, CD44, CXCR4, SSEA3, and SSEA4 by flow cytometry. (C) The mRNA expression of comparison and analysis between CD24, CD133, and CXCR4 positive and negative subpopulation in the two patients by RT-PCR. (D) The double CD133+CXCR4+ cells ration is 7.2% and 9.3%, respectively.
Next, the presence of CD24, CXCR4, CD133, CD44, CD49, CD29, SSEA-3, and SSEA-4 in the isolated primary cells was examined by flow cytometry; CD24, CXCR4, and CD133 were expressed 28.2%, 41.6%, and 8.3% (patient1), and 22.1%, 25.6%, and 12% (patient2), respectively. CD44, CD47, and CD29 were expressed 98.2%, 86.5%, and 91.5% (patient1) and 94.3%, 10.2%, and 93.1% (patient2), respectively. However, SSEA-3 and SSEA-4 were expressed only 1.27% and 0.6% (patient1) and 2.9% and0.43% (patient2), respectively (Figure 1, B-1 and B-2).
The mRNA levels of the stemness genes were higher in CD133+ and CXCR4+ cells than in CD133- and CXCR4− cells. However, there was no difference in the presentation of CD24 between these cells (Figure 1C).
Next, the rate CXCR4 and/or CD133-positive cells was analyzed. The proportion of CD133+CXCR4+ cells was 7.2% and 9.3% in patient1 and patient2, respectively (Figure 1D).
-
2.
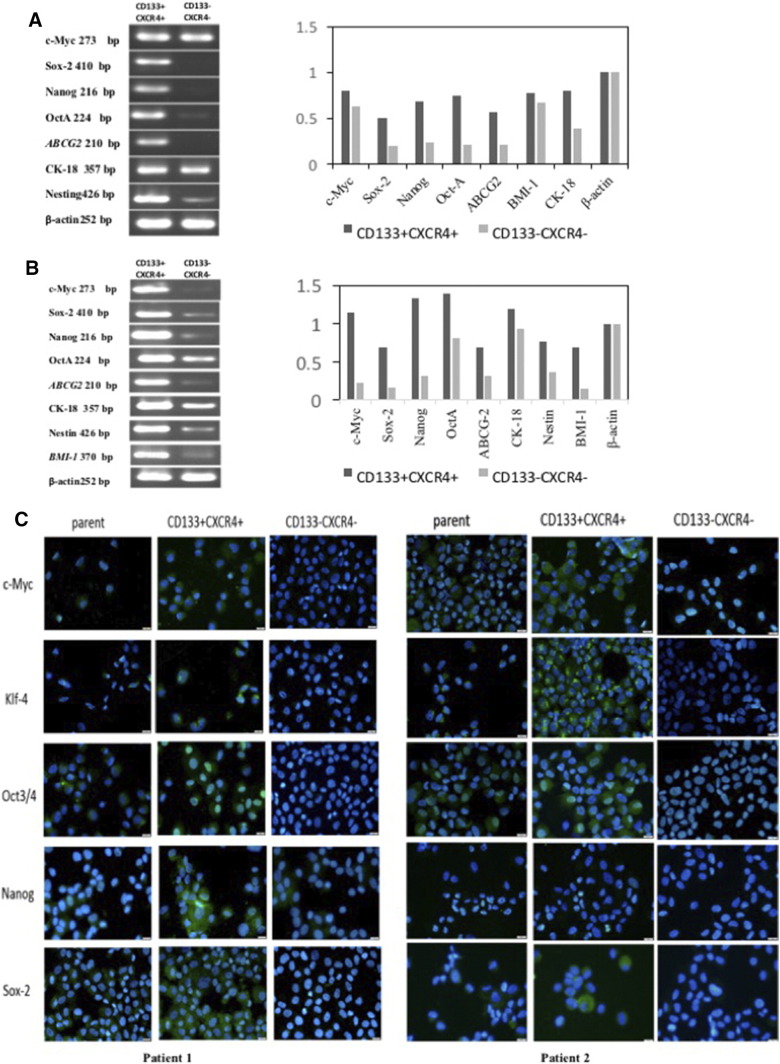
Expression of stemness genes in CD133+CXCR4+ and CD133−CXCR4− cells
We next examined the expression of genes thought to play key roles in stem cell biology, such as c-myc, sox-2, nanog, Oct4A, abcg-2, bmi-1, ck-18, and nestin, in CD133+CXCR4+ cells. The RT-PCR results showed that the expression of c-myc, sox-2, nanog, Oct4A, abcg-2, bmi-1, and nestin was increased in the CD133+CXCR4+ population and lower in the CD133−CXCR4− population. Similarly, a mild but not significant increase in the expression of CK-18 was observed in CD133+CXCR4+ cells (Figure 2, A and B). Immunocytochemistry staining further demonstrated that c-Myc, KLF4, OCT3/4, NANOG, and SOX2 levels were higher in CD133+CXCR4+ cells compared to unsorted and CD133−CXCR4− cells (Figure 2C).
-
3.
CD133+CXCR4+ cells have increased proliferative and clonogenic capacity
Figure 2.
Reverse transcriptase PCR analysis between double positive and double negative EC cell. (A) The cells from patient's tissue expressed higher stemness genes such as c-Myc, Oct4A, Sox2, Nanog, ABCG2, and Nestin in CD133+CXCR4+ cells than CD133−CXCR4− cells. β-actin was used as parameter. (B) The other patient showed the similar result, include BMI-1. (C) The immunocytochemistry stain of the each subgroups. Nuclei were stain with DAPI.
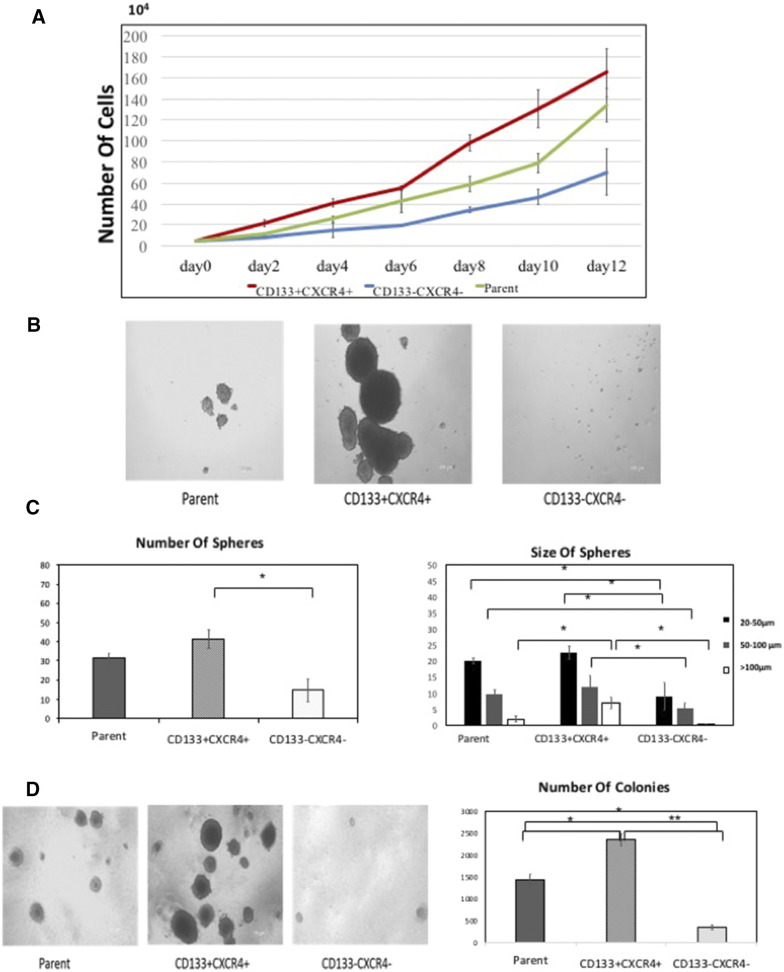
The proliferative capacity of the groups was determined in vitro. Single positive and negative cells were first selected for sphere formation assays. As shown in Figure 3A, the number of spheres formed by CD133+ and CXCR4+ was higher than the CD133- and CXCR4− groups. There was no apparent difference between the CD24+ and CD24− groups. Sorted CD133+CXCR4+ cells were cultured in normal medium for 12 days. The growth curve demonstrates that CD133+CXCR4+ cells grow faster than the parental and CD133−CXCR4− cells (Figure 3B). In addition, sphere formation was examined (Figure 3C); CD133+CXCR4+ cells formed the highest number of spheres compared with the other subpopulations (P < 0.05). The formed spheres were then divided into three groups, large (L, >100 μm), medium (M, 50–100 μm), and small (S, 20–50 μm). This analysis revealed that CD133+CXCR4+ cells formed more spheres than the parental and CD133−CXCR4− cells and that these spheres were the largest in size (Figure 3D).
Figure 3.
Characteristics of unsorted cells, positive and negative cells. (A) The growth curve of EC cells that counted day 2 to day 12 after sorting cell. (B) The photograph of spheres after sorting, cultured for 7 days. (C) The number and size of spheres. Spheres were observed in all subpopulations, but whether the numbers or the size of the spheres, the CD133+CXCR4+ cells were the most and largest (* P < .05). (D) Colony-forming assay demonstrated the difference in the each subgroups (* P < 0.05, ** P < 0.01).
We next examined the colony-formation of the cells on soft agar. As shown in Figure 3E, CD133+CXCR4+ cells formed an average of 2345 colonies with larger sizes, while the CD133−CXCR4− cells and parental cells showed an average of 354 and 1432 colonies, respectively, with smaller sizes. This difference in colony-forming ability was significant (P < 0.05).
-
4.
CD133+CXCR4+ cells possess tumorigenic potential
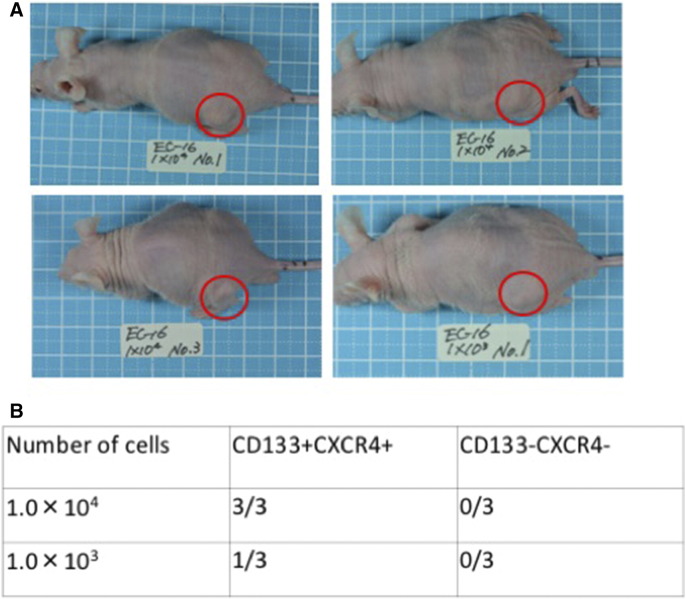
The tumorigenic potential of CD133+CXCR4+ cells and CD133−CXCR4− cells was evaluated in vivo using nude mice. Subcutaneous tumors were initiated in nude mice by 1 × 104 CD133+CXCR4+ cells (3/3) and 1 × 103 cells CD133+CXCR4+ (1/3). In contrast, CD133−CXCR4− cells completely failed to form tumors in nude mice (Figure 4, A and B).
-
5.
CD133+CXCR4+ cells show enhanced anti-drug resistance
Figure 4.
Tumorigenicity ability of sorted cells in vivo. (A) Tumor formation in nude mice after subcutaneous injection (1.0 × 104). (B) Tumor formation groups were compared between the double positive and negative cells.
The chemoresistance of CD133+CXCR4+ cells was tested, using CD133+CXCR4+, CD133−CXCR4-, and parental cells treated with different concentrations of cisplatin and paclitaxel. As shown in Figure 5, A and B, CD133+CXCR4+ cells were significantly more resistant to cisplatin and paclitaxel compared to the other subpopulations at different concentrations.
-
6.
Immunohistochemistry of tumor tissue
Figure 5.
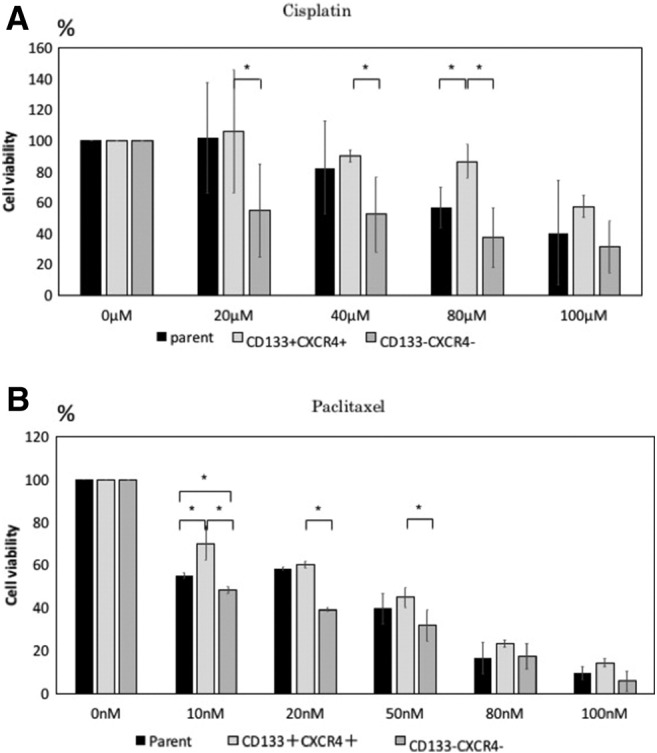
Drug resistance of the each subpopulation. (A and B) Cisplatin and Paclitaxel were added into the sorted cells with different concentration. Then evaluated the cell viability by CCK-8 kit (*P < .05).
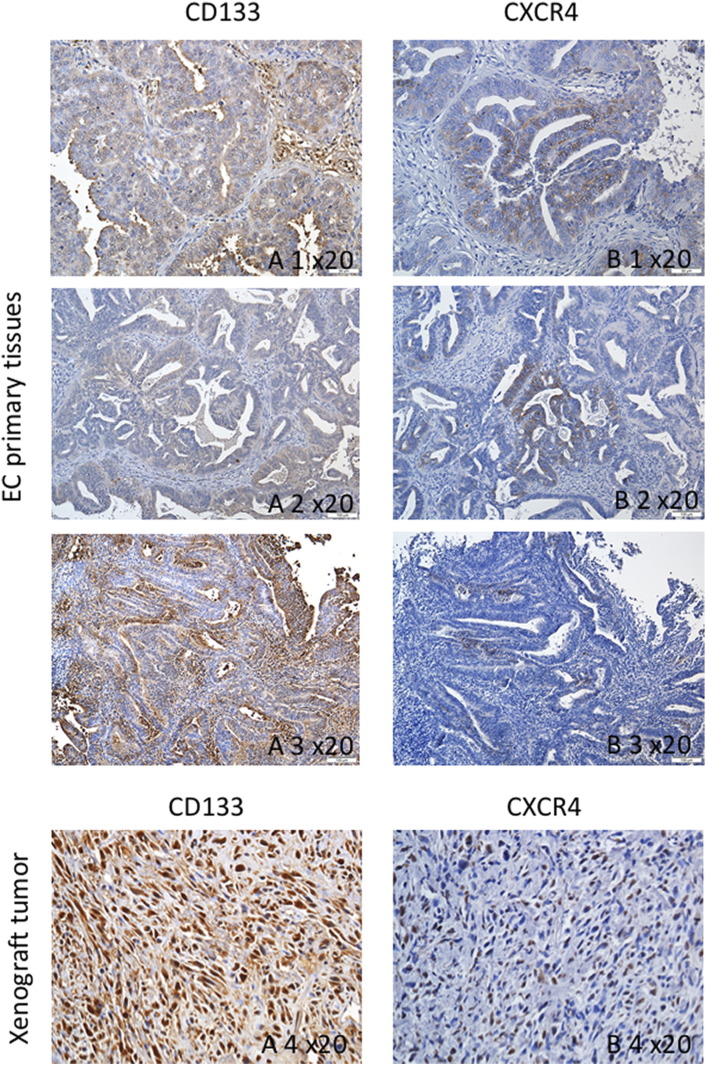
The clinical relevance of the markers was tested in patients with endometrial cancer. The expression of CD133, and CXCR4 was analyzed with patients and transfected tumors. Both the patient samples and xenograft tumors expressed CD133, and CXCR4 (Figure 6).
Figure 6.
Immunohistochemistry of CD133, CXCR4 in tumor tissue which from the endometrial cancer patient. The expression of CD133 in the patients (A 1-3) and the tissue of xenograft tumor (A 4). The expression of CXCR4 in the patients (B 1-3) and the tissue of xenograft tumor (B 4).
Discussion
Cancer stem cells express a variety of markers on their surface. The expression or deficiency of these markers has been used to identify and isolate subpopulations of cancer cells for CSCs. CD133 and CXCR4 have been shown to be expressed in many types of human cancers including endometrial cancer. Several cell surface markers, such as CD24, CD44, and CD133, are common in certain solid tumors including breast, brain, colorectal, and endometrial cancers [28]. Thus in this study, we examined marker expression by flow cytometry to determine which marker was adaptive for endometrial cancer cell lines. Our initial results indicated that CD133+ and CXCR4+ EC cells exhibited stronger expression of stem-related genes, such as c-Myc, Sox-2, Nanog, Oct4A, ABCG2, BMI-1, CK-18, and Nestin, while there was hardly any difference between CD24+ and CD24− cells. Therefore, we focused on the CD133 and CXCR4 markers and hypothesized that both markers might be involved in tumor progression.
CD133 is an 866-amino-acid single-chain transmembrane glycoprotein with a molecular weight of 120 kDa. Previous studies have indicated that CD133 expression is risk factor for EC [29]. In our study, we detected CD133 expression in 8.3% and 12%, respectively, of EC primary cells. Nakamura et al. [23] reported that CD133+ EC cells possess increased proliferative and tumorigenic potentials and are resistant to cisplatin- and paclitaxel- induced cytotoxicity. Vincent et al. [30] also identified CD133+ as a reliable marker for CSC characterization in the Colo205 colon adenocarcinoma cell line. Moreover, CD133+ EC cells have been shown to exhibit higher expression of MT1-MMP, through which their increased invasive ability is mediated [31].
CXCR4 has been shown to be expressed in all types of human tumors including EC; CXCR4 is significantly up-regulated in EC compared to atypical, simple hyperplasia, and normal endometrium tissue [27]. Beverly et al. [32] showed that the CXCL12/CXCR4 axis is involved in tumor progression, angiogenesis, metastasis, and survival; CXCR4 is thought to generate key signaling pathways thus promoting chemotaxis, survival proliferation, and gene transcription expression.
Our results show that CD133+CXCR4+ EC cells represent less than 10% of the total population, which is consistent with previous findings that CSCs constitute a small population of cancer cells in malignant tumors. CD133+ cells account for 10.1% and 20.1% in the Ishikawa and MFE280 EC cell lines, respectively [23]. Over the past 2 decades a number of researchers have sought to identify appropriate markers for CSCs, most of which were single markers, however, much of the research has demonstrated the importance of combination markers. Hermann et al. [33] have demonstrated that CD133+ and CXCR4+ constitute two distinct subpopulations in pancreatic cancer with a migratory and invasive phenotype. Other studies have shown that patients with high ratios of CD133+CXCR4+ exhibit a significantly reduced two-year survival rate compared with patients with low CD133+CXCR4+ cell ratios [34]. A recent study has reported that the use of a novel class of CXCR4 antagonists [35], alone or in combination with chemotherapeutic agents and/or CD133 targeting agents, might reduce anti-drug ability and the development of tumor formation [36].
Importantly, although many reports have shown that CSCs isolated from cell lines could be successfully used for in vitro and in vivo experiments, these cannot be considered as accurate surrogates for clinical cancers. Thus, to overcome the deficiencies of cell lines, we chose to use primary cancer cells from EC patients. Our results indicate that CD133 and CXCR4 expression is closely associated with cell proliferation in vitro; CD133+CXCR4+ cells grew faster than CD133−CXCR4− cells under normal culture conditions. In addition, we found that CD133+CXCR4+ cells could form more spheres and colonies than the CD133−CXCR4− and parental cells. Sphere formation has been observed in stem cells from various normal and cancer tissues, indicating that sphere formation might constitute an ordinary characteristic of stemness [37], [38], [39], [40]. Spheres have also been shown to have higher tumorigenic ability than parental cancer cells in xenograft experiment [41].
Boyer et al. [42] have suggested that Oct4, Sox-2, and Nanog contribute to pluripotency and self-renewal by activating their own genes, which encode components of key signaling pathways. BMI-1 is a polycomb gene associated with maintenance of self-renewal ability, which has been implicated in various cancers [43], [44], [45].
In addition, it has been reported that down-regulation of ABCG-2 genes expression inhibits the self-renewal capacity of cells and significantly enhances the efficacy of chemotherapy-induced apoptosis in colon adenocarcinoma cells and CD133-positive colorectal carcinoma cells [46]. Nestin, an intermediate filament protein and a stem cell marker, is expressed in several tumors. Bokhari et al. found that of the EC cancer lines, AN3CA and KLE cells exhibited a significantly higher number of CD133+ cells and higher Nestin expression levels than Ishikawa cells [47], while CK18 expression varied in different cancer types.
Zhang et al. [48] demonstrated that CK18 expression is correlated with clinical stage, lymph node metastasis, number of positive lymph nodes, and recurrence and metastasis in non-small cell lung cancer. They also found that patients with high CK18 expression have poorer overall survival and disease-free survival than patients with low CK18 expression. In the present study, we found that CD133+CXCR4+ cells exhibited higher expression of the stemness genes compared to CD133−CXCR4− cells. Moreover, immunofluorescence staining also showed that the levels of c-Myc, KLF-4, OCT3/4, NANOG, and SOX-2 were increased in CD133+CXCR4+ cells compared to the parental and CD133−CXCR4− cells. We found that CD133+CXCR4+ cells formed tumors when inoculated into nude mice, while CD133−CXCR4− cells did not establish tumor formation by injecting 1 × 103 cells.
Studies performed with several cancer lines have revealed that CD133+ cells are more resistant to anti-tumor drugs and radiotherapy. The CD133+ human fibrosarcoma cell line exhibits significant resistance to both cisplatin and doxorubicin, drugs currently used in the clinical setting for the treatment of fibrosarcoma [49]. Cioffi et al. [36] evaluated the sensitivity of sorted CD133+CXCR4+ ovarian cells to cisplatin, which is a drug commonly used for the treatment of ovarian cancer, and found that CD133+CXCR4+ ovarian cells expressed the highest level of ABCG2, a surface marker transporter involved in resistance to chemotherapy. Consistent with those findings, our results show that sorted CD133+CXCR4+ EC cells were more resistant to cisplatin and paclitaxel, drugs routinely used for the treatment of endometrial cancer. It is very difficult to isolate the primary cells from the tumor tissue, so we collected 21 patients' specimens, several of them successful. Most of the cell isolation failed, or the cells were weak. The cells which were able to passage several times and grow well have expressed CD133 and CXCR4 strongly with immunocytochemistry. The immunohistochemical study and tumor classification in accordance with high CD133 and CXCR4 expression were associated with poorer overall survival of patients in the esophageal squamous cell carcinoma colon cancer cells [34].
All these data indicate that CD133+CXCR4+ EC cells possess greater proliferation, clonogenic, tumorigenic, and chemoresistance abilities like as CSCs. Although further studies will be required to resolve the mechanism and/or pathways relationship with these molecule and drug resistance and proliferation, our results suggest that the surface marker of CD133 and CXCR4 constitute excellent novel molecular targets for endometrial cancer therapy.
Acknowledgments
Acknowledgements
I would like to thank Ms. Furuichi for providing expert technical assistance, and Otsuka Toshimi Scholarship Foundation for supporting my life. This work was supported in part by Grant-in-Aid for JSPS KAKENHI GRANT Number JP16713604, JP25460264.
Contributor Information
Yi Sun, Email: sunyimao123@hotmail.com.
Toshiko Yoshida, Email: toshikoyoshida9@gmail.com.
Motonori Okabe, Email: okabe@med.u-toyama.ac.jp.
Kaixuan Zhou, Email: joe_nicky@hotmail.com.
Chika Soko, Email: koike@med.u-toyama.ac.jp.
Sigeru Saito, Email: s30saito@med.u-toyama.ac.jp.
Toshio Nikaido, Email: tnikaido@med.u-toyama.ac.jp.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2015. Cancer Facts & Figures 2015. [Google Scholar]
- 3.Clarke MF, Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H., Jones D.L., Visvader J., Weissman I.L., Wahl G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol. 2016;9(1):74. doi: 10.1186/s13045-016-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapidot T, Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M, Paterson B., Caligiuri M.A. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324(5935):1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Li Q. Cancer stem cells and tumor metastasis (Review) Int J Oncol. 2014;44(6):1806–1812. doi: 10.3892/ijo.2014.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 10.Morrison BJ, Morris JC, Steel JC. Lung cancer-initiating cells: a novel target for cancer therapy. Target Oncol. 2013;8(3):159–172. doi: 10.1007/s11523-012-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins AT, Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 13.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V., Wicha M., Clarke M.F., Simeone D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 14.Monzani E, Facchetti F., Galmozzi E., Corsini E., Benetti A., Cavazzin C., Gritti A., Piccinini A., Porro D., Santinami M. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43(5):935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 16.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18(5):460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Gargett CE, Schwab K.E., Zillwood R.M., Nguyen H.P., Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 19.Friel AM, Sergent P.A., Patnaude C., Szotek P.P., Oliva E., Scadden D.T., Seiden M.V., Foster R., Rueda B.R. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle. 2008;7(2):242–249. doi: 10.4161/cc.7.2.5207. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard SA, Friel A.M., Kumar B., Zhang L., Rueda B.R., Gargett C.E. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69(21):8241–8248. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 21.Rutella S, Bonanno G., Procoli A., Mariotti A., Corallo M., Prisco M.G., Eramo A., Napoletano C., Gallo D., Perillo A. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15(13):4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Kyo S., Zhang B., Zhang X., Mizumoto Y., Takakura M., Maida Y., Mori N., Hashimoto M., Ohno S. Prognostic impact of CD133 expression as a tumor-initiating cell marker in endometrial cancer. Hum Pathol. 2010;41(11):1516–1529. doi: 10.1016/j.humpath.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Gleichmann M, Gillen C., Czardybon M., Bosse F., Greiner-Petter R., Auer J., Müller H.W. Cloning and characterization of SDF-1gamma, a novel SDF-1 chemokine transcript with developmentally regulated expression in the nervous system. Eur J Neurosci. 2000;12(6):1857–1866. doi: 10.1046/j.1460-9568.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 24.Sison EA, Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev Hematol. 2011;4(3):271–283. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy MS, Qamar L., Behbakht K., Post M.D., Sheeder J., Sartorius C.A., Spillman M.A. Progestin treatment decreases CD133+ cancer stem cell populations in endometrial cancer. Gynecol Oncol. 2016;140(3):518–526. doi: 10.1016/j.ygyno.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Liu P., Long P., Huang Y., Sun F., Wang Z. CXCL12/CXCR4 axis induces proliferation and invasion in human endometrial cancer. Am J Transl Res. 2016;8(4):1719–1729. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P., Long P., Huang Y., Sun F., Wang Z. Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol Res Pract. 2016;212(1):10–16. doi: 10.1016/j.prp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Vincent Z, Urakami K., Maruyama K., Yamaguchi K., Kusuhara M. CD133-positive cancer stem cells from Colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile. Genes Cancer. 2014;5(7–8):250–260. doi: 10.18632/genesandcancer.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura M, Zhang X., Mizumoto Y., Maida Y., Bono Y., Takakura M., Kyo S. Molecular characterization of CD133+ cancer stem-like cells in endometrial cancer. Int J Oncol. 2014;44(3):669–677. doi: 10.3892/ijo.2013.2230. [DOI] [PubMed] [Google Scholar]
- 30.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 31.Hermann PC, Huber S.L., Herrler T., Aicher A., Ellwart J.W., Guba M., Bruns C.J., Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SS, Han Z.P., Jing Y.Y., Tao S.F., Li T.J., Wang H., Wang Y., Li R., Yang Y., Zhao X. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012;10:85. doi: 10.1186/1741-7015-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portella L, Vitale R., De Luca S., Alterio C.D.’, Ieranò C., Napolitano M., Riccio A., Polimeno M.N., Monfregola L., Barbieri A. Preclinical development of a novel class of CXCR4 antagonist impairing solid tumors growth and metastases. PLoS One. 2013;8(9):e74548. doi: 10.1371/journal.pone.0074548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cioffi M, Alterio CD’C, Camerlingo R., Tirino V., Consales C., Riccio A., Ieranò C., Cecere S.C., Losito N.S., Greggi S. Identification of a distinct population of CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci Rep. 2015;5:10357. doi: 10.1038/srep10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Balch C., Chan M.W., Lai H.C., Matei D., Schilder J.M., Yan P.S., Huang T.H., Nephew K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dontu G, Abdallah W.M., Foley J.M., Jackson K.W., Clarke M.F., Kawamura M.J., Wicha M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang D, Nguyen T.K., Leishear K., Finko R., Kulp A.N., Hotz S., Van Belle P.A., Xu X., Elder D.E., Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 38.Toma JG, Akhavan M., Fernandes K.J., Barnabé-Heider F., Sadikot A., Kaplan D.R., Miller F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 39.Grange C, Lanzardo S., Cavallo F., Camussi G., Bussolati B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia. 2008;10(12):1433–1443. doi: 10.1593/neo.08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyer LA, Leer T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park IK, Qian D., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 42.Abdouh M, Facchino S., Chatoo W., Balasingam V., Ferreira J., Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29(28):8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Dontu G., Mantle I.D., Patel S, Ahn N.S., Jackson K.W., Suri P., Wicha M.S. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma L, Liu T., Jin Y., Wei J., Yang Y., Zhang H. ABCG2 is required for self-renewal and chemoresistance of CD133-positive human colorectal cancer cells. Tumour Biol. 2016;37(9):12889–12896. doi: 10.1007/s13277-016-5209-5. [DOI] [PubMed] [Google Scholar]
- 45.Bokhari AA, Baker T.M., Dorjbal B., Waheed S., Zahn C.M., Hamiltoni C.A., Maxwell G.L., Syed V. Nestin suppression attenuates invasive potential of endometrial cancer cells by downregulating TGF-beta signaling pathway. Oncotarget. 2016;7(43):69733–69748. doi: 10.18632/oncotarget.11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Wang J., Liu W., Yin Y., Qian D., Zhang H, Shi B., Li C., Zhu J., Zhang L. Cytokeratin 18 knockdown decreases cell migration and increases chemosensitivity in non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142(12):2479–2487. doi: 10.1007/s00432-016-2253-x. [DOI] [PubMed] [Google Scholar]
- 47.Feng BH, Liu A.G., Gu W.G., Deng L., Cheng X.G., Tong T.J., Zhang H.Z. CD133+ subpopulation of the HT1080 human fibrosarcoma cell line exhibits cancer stem-like characteristics. Oncol Rep. 2013;30(2):815–823. doi: 10.3892/or.2013.2486. [DOI] [PubMed] [Google Scholar]
- 48.Lu C, Xu F., Gu J., Yuan Y., Zhao G., Yu X., Ge D. Clinical and biological significance of stem-like CD133(+)CXCR4(+) cells in esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2015;150(2):386–395. doi: 10.1016/j.jtcvs.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 49.Silinsky J, Grimes C., Driscoll T., Green H., Cordova J., Davis N.K., Li L., Margolin D.A. CD 133+ and CXCR4+ colon cancer cells as a marker for lymph node metastasis. J Surg Res. 2013;185(1):113–118. doi: 10.1016/j.jss.2013.05.049. [DOI] [PubMed] [Google Scholar]