Abstract
In this research work different shapes and sizes of gold nanoparticles (AuNPs) were synthesized through an intracellular biogenic approach, exploiting the chloroauric acid reducing and Au0 stabilizing potential of Laccaria fraterna EM-1083 mycelia. The intracellularly synthesized AuNPs exhibits anti-quorum sensing inhibitory potential against Pseudomonas aeruginosa. The synthesized AuNPs were characterized using UV–visible spectroscopy; transmission electron microscopy, X-ray diffraction, energy dispersive X-ray spectroscopy, and Fourier transform infrared spectroscopy. The characterization proved that the successful synthesis of highly stable crystalline AuNPs with various shapes. Here we tested inhibitory activity of AuNPs on QS-regulated biofilm development and pyocyanin production traits of P. aeruginosa. The qualitative and quantitative data demonstrated that AuNPs significantly inhibited the biofilm formation and pyocyanin production. In summary, our results signify the future use of intracellularly synthesized AuNPs in P. aeruginosa mediated diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0662-4) contains supplementary material, which is available to authorized users.
Keywords: Ectomycorrhiza, Intracellular, Gold nanoparticles, Anti-quorum, Biofilm, Pyocyanin
Introduction
A gram-negative bacterium Pseudomonas aeruginosa that causes ventilator related pneumonia, severe and chronic infections like nosocomial and cystic fibrosis (CF), urinary tract and catheter infections, etc. in suffering patients by producing quorum sensing regulated virulence factors (biofilm formation and pyocyanin secretion) [1, 2]. This pathogenic bacteria is accountable for ~10% of all hospital-related infections worldwide and has the knack to develop multi antimicrobial drug resistance (MDR) through phenotypic (biofilm production) and genetic (mutations, uptake of antibiotic resistance genes or plasmids) mechanisms [3, 4]. Subsequently, MDR strains of P. aeruginosa drastically reduced the efficacy of used antibiotics. The MDR against modern antibiotics in P. aeruginosa is currently considered as a worldwide health problem and has become an emerging problem in the twenty first century [5, 6]. Many biochemical features in the P. aeruginosa including expression and production of virulence factors (biofilm, pyocyanin, proteolytic and elastolytic enzymes, etc.) are regulated and produced by a well-coordinated cell-to-cell communication [7, 8]. Thus, there is an urgent need for the development of novel biotherapeutic agents to prevent infection of P. aeruginosa via inhibition the production of virulence traits rather than killing the bacteria. Past few years, a promising approach has been recognized as a target which is known as quorum sensing (QS) and in this one bacterial cell tries to communicate with the other cell with the help of different types of signals [9].
In recent years numerous studies have reported the organic and inorganic anti-QS agents, are able to interrupt QS-regulated characters of P. aeruginosa [10]. However, among these anti-QS agents (e.g. halogenated furanones) have been found toxic and unsuitable for therapeutics use [11]. Thus, exploring nanohybrid agents having anti-QS activity will be an effective alternative to antibiotics, taking this into account biosynthesized nanoparticles using plant and fungus have exhibited the property of disrupting bacterial QS systems [12–14]. Consequently, there is an increasing demand for the recognition of economically viable, novel nontoxic, biocompatible, stable anti-QS agents with dual mode of actions targeting both anti-QS and antibiofilm. Biofabrication of anti-QS agents like AuNPs from natural renewable resources of biological origin could open up into new ventures for the development of novel nanomaterials to battle MDR P. aregunosa strains infections. AuNPs have been utilized for centuries by vaidya (Sanskrit word meaning “physician” refers to a person who practices Ayurveda) and artists due to the medicinal and optical properties [15, 16]. More recently, these unique medicinal and optoelectronic potentials have been explored and utilized in high technology applications such as development of therapeutic agents and sensors. These properties of AuNPs are tunable by changing the size, crystal structure and surface chemistry state [17–19]. Thus, AuNPs have been widely applied in biomedicine based on their unique properties and numerous surface functionalities. In past few decades various methods like physiochemical and biological have been developed for the synthesis and to control the size, crystal structure and surface chemistry of AuNPs. The property, sensitivity and specificity of AuNPs can be tuned and tailored by functionalizing the NPs surface with different biological macromolecules through biological synthesis approach. This complex change of the AuNPs surface has also offered opportunities for the development novel nanomaterials of medicinal importance [20–22].
The culturable fungi are now preferred for producing surface functionalized AuNPs. The biomass (wet as well dry in some cases) and the secreted intra and extracellular metabolites of fungi have been utilized for the reduction of Au+3 ions to AuNPs via redox active biological macromolecules. Of course, the filamentous fungi have some distinguishing advantages over bacteria, including inherent properties like metal tolerance/resistance nature and biomass production. The mycologically synthesized AuNPs are already well known for its medicinal activities, but in this study, we have attempted to explore bioprospection of the transition and post transition metals tolerant Laccaria fraterna, EM-1083 isolated from Eucalyptus globulus for intracellular synthesis of AuNPs [23]. The synthesized AuNPs has been utilized as a novel nanoanti-quorum sensing inhibitor against P. aeruginosa QS regulated traits e.g. biofilm and pyocyanin production inhibition. Thus, main aims of the present research were to study the (1) intracellular biosynthesis of AuNPs using mycelia biomass of metals tolerant strain EM-1083 of L. fraternal, (2) characterization of AuNPs for their optical, electrical, structural, morphological, elemental and functional properties (3) investigation of the anti-QS property of AuNPs against P. aeruginosa. Overall, data suggest a key role of L. fraterna, EM-1083 as a renewable bioresource for eco-friendly production of AuNPs that can have extensive application in development of a novel nontoxic anti-QS agent.
Materials and Methods
Materials
All chemicals used were of analytical grade and purchased from Sigma Aldrich (India) or Merck (India) unless otherwise stated.
Collection of Ectomycorrhizal Fungal Strain EM-1083
The ectomycorhizal strain EM-1083 used in this study was obtained from the Centre for Mycorrhizal Culture Collection (CMCC), The Energy and Resources Institute (TERI), New Delhi, India. This strain was recovered from root sample of Eucalyptus globulus, Flyash pond of Korba Super Thermal Power Station, Jamanipali, Korba, Chattisgarh, India (22.3858°N; 82.6816°E) by Ray et al. [24]. The ectomycorrhizal strain EM-1083 has been characterized as Lacaria fraterna and studied for its inherent broad spectrum transition and post transition metals tolerance and adsorption characteristic under In-vitro condition [23–25]. The strain was maintained and subcultured regularly in modified Melin-Norkrans (MMN) agar during the study period.
Mycelia Biomass Production of Strain EM-1083 for Intracellular Synthesis of AuNPs
In order to synthesize AuNPs intracellularly by using ectomycorrhizal strain EM-1083 biomass, the sterilized MMN broth medium was inoculated with pure culture of strain EM-1083 and incubated under shaking condition at 28 ± 1 °C for 10 days. After that biomass was separated through standard 300 µm mess sieve and obtained biomass was three times washed thoroughly by sterile MilliQ water through the centrifugation at 10,000 rpm (Velocity 18 R, Bench top centrifuge Dynamica, United Kingdom) for 10 min. The obtained medium free biomass was used for the intracellular synthesis of AuNPs. About ~20 g of washed wet biomass was suspended in 250 ml Erlenmeyer flasks containing 100 mL sterile Milli Q water and treated with 1 mM (v/v) chloroauric acid solution. The biomass mediated intracellular bioreduction of the Au+3 to Au0 was performed on shaker at 140 rpm for 24 h [26]. A gradual color change of biomass to red-pink, confirms the intracellular synthesis of AuNPs. Bioreduction of the chloroauric acid to AuNPs was examined by using a Series 3000 double beam UV–Vis spectrophotometer (UV-2450, Shimadzu, Japan) [27]. The media free biomass was allowed to react with the chloroauric acid solution in the working concentration of 1 mM for different time intervals (0, 4, 12, and 24 h). After mentioned time intervals (0, 4, 12 and 24 h), each time biomass was separated from the MilliQ containing metal salts and sonicated to break the cells (200 Hz for 20 min with 5 min pulse on) (Vibra-Cell VC 750, Sonics, USA). Sonicated content was centrifuge to collect the pellets (contains nanoparticles). Pellet was resuspended into equal amount of MilliQ and absorbance spectra were recorded from 200 to 800 nm.
Characterization of Intracellularly Synthesized AuNPs
The intracellular synthesis of AuNPs was examined by measuring UV–Vis absorption spectra (UV2450, Shimadzu, Japan) between the wavelength ranges of 200–800 nm. Further Intracellular AuNPs synthesis was confirmed through the TEM analysis. Briefly, purple color biomass containing the AuNPs was fixed in 2% glutaraldehyde in 25 mM phosphate buffer (pH-7.4) at 25 °C for 1 h. Rapid washing of the fixed biomass sample was done with 25 mM phosphate buffer (pH-7.4) and then stained with 0.5% osmium tetroxide and incubated at 4 °C for 45 min. After the staining, fixative was completely washed using 50 mM phosphate buffer at (pH-7.4). Then the prepared biomass after fixing and staining was sequentially dehydrated with a series of ethanol solutions (10, 20, 30, 50, 70 and 90%), prepared in sterile MilliQ water. The processed sample was analyzed for intracellular synthesis of AuNPs under a TEM instrument with 80 kV an accelerating voltage. The size, shape and elemental composition of AuNPs was analyzed using transmission electron microscope (TEM) [28] (TECNAI G2 T20 TWIN, The Netherlands) fitted with energy dispersive X-Ray spectrometer (EDS) (EDX Inc. The Netherlands). For the shape and size analysis ~10 mg AuNPs was dissolved in 1 mL MQ water and sonicated for 5 min for disintegration of the large particles clumps. The sonicated sample (~10 μl) was drop casted under air dried and dark conditions on a carbon-coated copper grid. Then the grid was analyzed at an accelerated voltage of 200 kV and the TEM micrographs and EDX spectrum images were obtained. The powder X-ray diffraction helped to analyze crystalline nature of the AuNPs. The X-ray diffraction (XRD) patterns of powder sample of AuNPs was recorded on MiniFlex™ II benchtop XRD system (Rigaku Corporation, Tokyo, Japan) which operates at 40 kV and using a current of 30 mA with Cu Ka radiation (λ = 1.54 A0). The diffracted intensities were recorded at two angles, from 20° to 80°. The Fourier transform infrared spectroscopy (FTIR) spectrum was recorded between 4000 and 500 cm−1 with 1 cm−1 resolution (Nicolet-6700, Thermo-fisher, USA).
Assessment of Anti-quorum Sensing Potential of Intracellularly Synthesized AuNPs
Quantification of Minimum Inhibitory Concentration and Growth Curve of AuNPs
Broth microdilution protocol explained by Clinical and Laboratory Standards Institute standards was used to quantify the minimum inhibitory concentration (MIC) of intracellularly synthesized AuNPs against P. aeruginosa [29]. Briefly, an active bactrim inoculum of 1 × 106 CFU/mL was added in Müller–Hinton medium in which 10–200 µg/mL AuNPs was pre supplemented. The MIC here in this scenario was defined as the minimum concentration of AuNPs that prohibited visible growth of the P. aeruginosa. For measurement of growth curve, sub-MIC concentration of the AuNPs (100 µg/mL) was selected; overnight grown active cultures of P. aeruginosa was diluted in fresh LB, to obtain optical cell density of ~0.06 at 600 nm (OD600). Then, the bacterial active cultures were supplemented with increasing concentrations of AuNPs and incubated at 37 °C under continuous agitation conditions. After specifically mentioned time point, ~2 mL of the active bacterial culture was taken out for measurement of its growth at OD600 using an UV–Vis spectrophotometer (UV-2450, Shimadzu, Japan) and data was accounted to generate a growth curve (Fig. S1).
Structural Analysis of Biofilm Formation
Fluorescence, Light microscope and TEM, were used to monitor the changes in the structure of biofilm treated with AuNPs. After 48 h of biofilm formation, the grown culture was collected and gently washed with sterile MQ water using centrifugation 5000 rpm, for 5 min. The pellet of the biofilm formed cells dissolve in 500 μl sterile MQ water, and this suspension was used for the biofilm structural analysis. Fluorescence and light microscope structural analysis of biofilm was done on borosilicate glass slide, after mild heat fixation of 50 μl above prepared suspension. For TEM above suspension was diluted 50 fold in sterile MQ water and drop casted on copper coated carbon grid. The analysis was done following the method described in “Characterization of intracellularly synthesized AuNPs” section.
Quantification of Biofilm Development
The development of biofilm in P. aeruginosa was analyzed in the 96-well microtitre plate, after dye staining by using crystal violet (CV) to the different treatment to various concentrations of AuNPs [30]. Resulting biofilm was fixed with 200 μl of 99% concentrated methanol (Sigma–Aldrich). After 15 min, methanol was removed by decanting it, the plate was dried at room temperature. 200 μl of CV dye (1%, v/v) (Sigma–Aldrich) were added to the each well of microtitre plate containing treated biofilm, followed by a 10 min incubation at room temperature. The plate was washed once with 200 μl of deionized water after the removal of CV dye. The washed plate was incubated for 30 min at room temperature followed by addition 200 μl of acetic acid (33%, v/v) (Sigma–Aldrich) to solubilize the adhered CV dye. The absorbance of the plate was recoded at 570 nm using a microtiter plate reader (Synergy H1 Multi Mode Reader, BioTek, USA) and obtained data was analyzed to calculate the % inhibition of biofilm formation by AuNPs.
Quantification Pyocyanin Production
The production of pyocyanin pigment was measured by quantitative chemical assay, given by Essar et al. [31]. Briefly, 1 mL of active bacterial culture was pelleted down by centrifugation. The supernatant obtained after centrifugation contains the pyocyanin, which was then extracted with 0.6 mL of chloroform, followed by sequential extraction with 1 mL of 0.2 M HCl and absorbance was monitored at 520 nm.
Results
Intracellular Synthesis of AuNPs Using Mycelia Biomass of Strain EM-1083
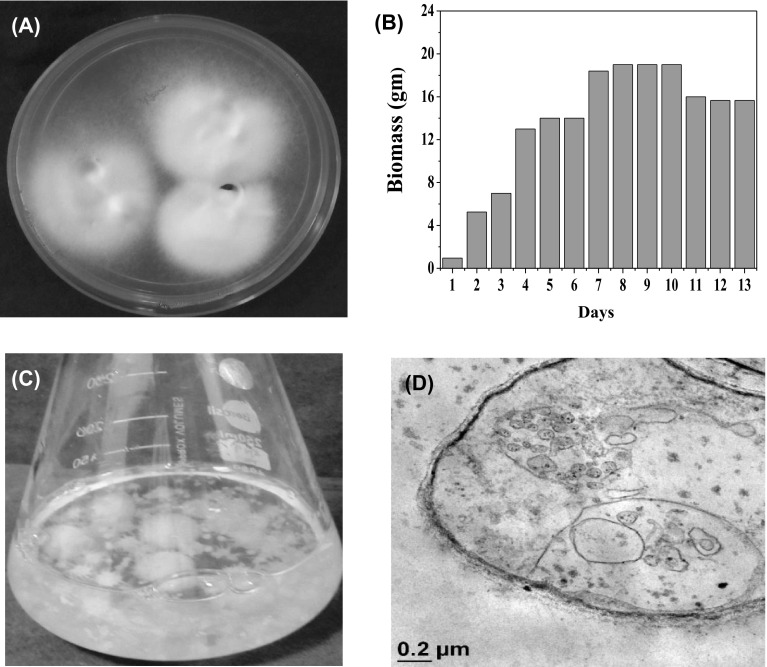
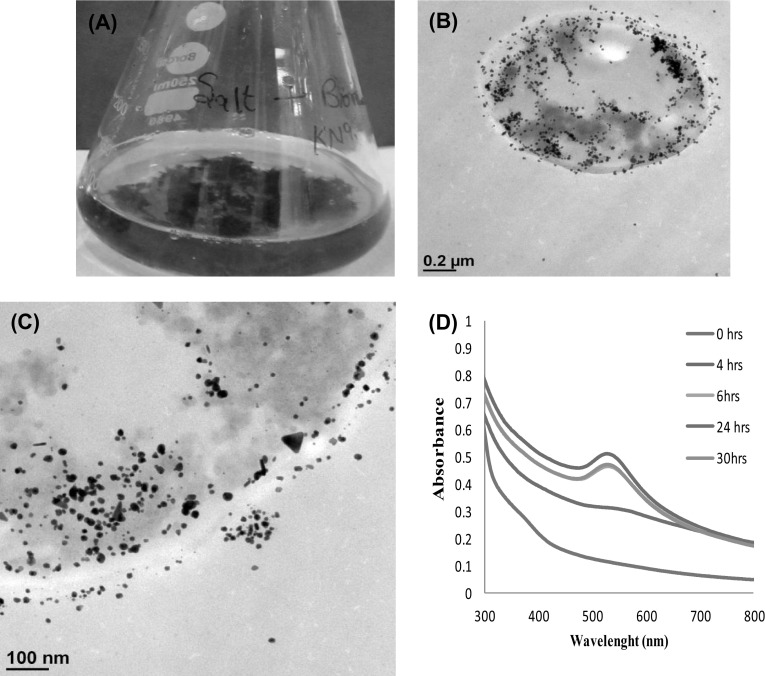
The intracellularly synthesized AuNPs was preliminary screened by visually observing the time dependent color change of biomass of strain EM-1083 in presence of AuCl4. At the initial stage, the color of the biomass was creamish yellow (Fig. 1a) and gradually turns to baby pink color after incubation of 4 h at 28 °C temperature on shaker at 140 rpm for 24 h. Unreacted colorless biomass was harvested and washed. Thin sections were done by using ultramicrotome and taken as control during TEM analysis where absence of AuNPs on the inner and outer surfaces of the mycelia was observed (Fig. 1c and d). The light pink color of the biomass changed to vivid purple color after incubation of 24 h and then the color of biomass remained unchanged. The visible pink color of the biomass indicated the intracellular bioreduction of Au+3 to Au0 and aqueous solution became colorless indicating no extracellular synthesis of AuNPs (Fig. 2a). The SEM analysis of the control and reacted mycelia of strain EM-1083 were performed to understand the interaction of Au ions and the macromolecules throughout the synthesis process. The comparative analysis of obtained SEM images at 10 and 2 µm scales confirms deposition of AuNPs on the mycelial surface, which suggested that mycelial surface acts as a biotemplate for the nucleation, growth and AuNPs synthesis initiation (Fig. S2a–d). The reacted mycelia surface showed bright dots due to the electron dense metallic character of intracellularly synthesized AuNPs (Fig. S2c),which were more prominent in the higher magnification image (Fig. S2d). The negatively charge mycelia surface functional groups enables the electrostatic biosorption of negatively charged AuCl4 and biosynthesis take place by the cell surface bound reducing biological macromolecules. Further, to investigate the intracellular site of the bioreduction of Au+3 to Au0 by the biological macromolecules of mycelia, TEM analysis of thin sections of the pink purple color biomass sample was done. The obtained micrographs showed the distribution of crystalline AuNPs within the outer and inner surfaces of the mycelia of analyzed biomass (Fig. 2b, c). It is well established that the bioreduction of Au+3 to Au0 mainly occurred on the cell wall of the mycelia as well as on the cytoplasmic membrane [32]. The distinctive pink-purple coloration of the biomass was due to the surface plasmon resonance (SPR) of intracellular AuNPs, where bioreduction of Au+3 to Au0 was confirmed by using the UV–Vis spectrophotometry. Absorption spectrum of AuNPs was recorded as a function of time of the harvested AuNPs in aqueous solution from biomass. The harvested AuNPs showed a signature absorbance spectrum of SPR band at about 540 nm after 4 h of incubation which then gradually exhibited a blue shift (~500 nm) and increases in intensity with time of incubation up to 24 h incubation (Fig. 1d). It is reported that the various types of AuNPs absorb light in the visible region of the electromagnetic spectrum upon the excitation of AuNPs SPR [33]. The absorbance data under UV range (200–300 nm) was critically analyzed to ascertain the role of strain EM-1083 mycelia cell wall and cytoplasmic membrane proteins in the AuNPs intracellular synthesis (Fig. S3). In the very beginning of the synthesis reaction, the absorption spectrum showed a prominent peak under UV region at ~280 nm due to the π–π* transition of the tryptophan and tyrosine amino acids of the proteins. The analysis revealed the gradual reduction in the absorbance at ~280 nm during the AuNPs synthesis indicating that these proteins have important role to play in the intracellular synthesis of AuNPs (Fig. S3). The AuCl4 bind to these proteins via free amine groups and negatively charged carboxylate groups and therefore, Au+3 nucleation, AuNPs synthesis and stabilization were performed.
Fig. 1.
Description of the Laccaria fraternal strain EM-1083. a Growth behavior of strain EM-1083 on modified Melin-Norkrans (MMN) agar medium. b Optimization of the higher mycelia biomass production. c Harvested and washed mycelia biomass in sterile MQ water (1:10 w/v). d TEM image of the mycelia internal structures
Fig. 2.
Intracellular synthesis of AuNPs using L. fraternal strain EM-1083 mycelia biomass. a Harvested and washed mycelia biomass of strain EM-1083 was challenged with 1 mM auric chloride up to 24 h under aqueous condition and gradually development of ruby color revealed the bioreduction of Au+3 to AuNPs, due to the excitation of surface plasmon resonance (SPR). b, c TEM micrographs showing the intracellular synthesis of AuNPs at 200 and 100 nm scales, respectively. d Time dependent UV–visible absorption spectrum of intracellularly synthesized AuNPs, after extraction from the mycelia biomass
Characterization of AuNPs
Particle size analysis was performed to measure the hydrodynamic diameter of AuNPs. The mean value of hydrodynamic diameter size of AuNPs was recorded as 79.69 nm. To investigate the morphology of AuNPs TEM study was performed. Obtained images of the intracellularly synthesized AuNPs after 24 h of reaction, showed the presence of different shapes of AuNPs. Additionally, Fig. 3b–d shows the HR-TEM images of the various shapes of AuNPs include spherical, pentagons, hexagons, and triangle, respectively. The (SAED) pattern i.e. selected-area electron diffraction from one of the AuNP of triangular shape in Fig. 3d clearly exhibits its single crystalline properties [34]. The hexagonal diffraction spots is a clear indication that the Au triangle nanoplate are highly oriented to [111] crystal face and presence of the 1/3 [422] face reflections (denoted by circled spot) suggests that the surface is atomically flat. However, a general mechanism for the formation of these AuNPs anisotropic shapes has not been fully understood yet. This heterogeneous behavior of AuNPs synthesis was attributed due to the following reasons (1) lower rate of synthesis of nanoparticle seed (2) kinetically controlled growth, specific or preferential direction due to the adsorption of capping biological macromolecules to specific facets of crystal can hinder or enhance the growth in a particular direction (3) Specific physico-chemical conditions like pH, temperature, final concentration of precursor salt and reducing agents [35–37].
Fig. 3.
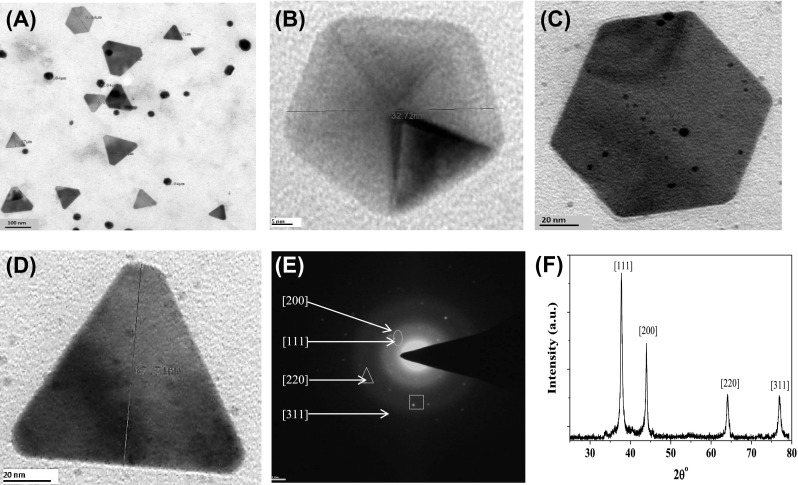
Structural characterization of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass. a TEM image showing various size and morphology of polydispersed AuNPs. b–d HRTEM of pentagon, hexagon, and triangle crystalline AuNPs. e SAED pattern of a triangle AuNPs. f X-ray diffraction (XRD) pattern of intracellularly synthesized AuNPs, showing its crystalline nature
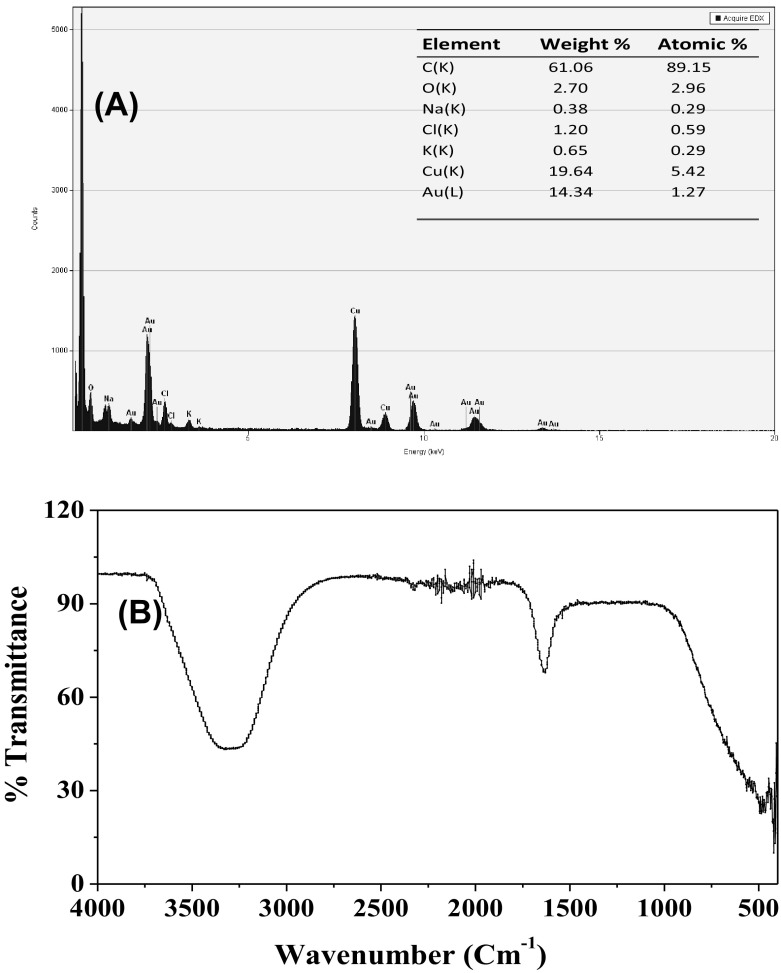
The EDX analysis was done for of the harvested AuNPs for the elemental composition of the intracellular synthesized AuNPs. The data indicated strong signals of gold atoms at various range of energy (2.40, 8.15 and 11.3 keV) (Fig. 3f). This data obtained implies that the nanostructures were made of solely gold element. However, presence of other EDX peaks for O, C, Cl, K, Na and Al, suggesting that they were came from the cell filtrate of EM-1083 [38]. The XRD analysis was performed to elucidate the crystallinity and lattice parameters of AuNPs, The obtained and analyzed XRD data AuNPs revealed the presence of intense peaks at values of 37.92, 44.04, 64.19, and 76.90, which correspond to Bragg’s reflections at [111], [200], [220] and [311] of Au nanocrystals, respectively (Fig. 3e) [27]. The Debye–Scherrer’s formula was used for the determination of the mean crystalline size of AuNPs from the calculated FWHM of the [111] Bragg reflection. The average crystalline size of the AuNPs was approximated about ~32 nm [35].
The enzymes/proteins involved in intracellular synthesis of AuNPs were demonstrated by the Fourier transform infrared spectroscopy (FTIR) technique. The spectrum obtained from unreacted mycelial biomass showed strong and individual stretching vibration bands at 1650 and 1542 cm−1, which corresponds to the amide I and II bonds of the enzymes/proteins (Fig. 4a). The peak observed at 1374 cm−1 was an assigned peak to the COO– symmetric stretch of enzymes/proteins [13]. The absorption bands in the region of 1155–1038 cm−1 were corresponding to P–OH stretching vibrations, which indicated the presence of phosphorylated enzymes/proteins. After the intracellular synthesis of AuNPs, the amide I and II bands were shifted to 1654 and 1541 cm−1, respectively, (Fig. 4b). Das et al. [39] also reported that shifting of amide I and II bands during the biosynthesis of AuNPs using the Rhizopus oryzae protein extract. In addition, the corresponding absorption band of the carboxyl and phosphate groups shifted towards at the 1366 and 1155–1038 cm−1 on completion of the AuNPs intracellular synthesis, The FTIR data evidently specifies that amine and carboxyl groups of phosphorylated proteins are mainly accountable for the intracellular synthesis and stabilization of AuNPs [39].
Fig. 4.
Elemental and functional characterizations of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass. a EDX spectrum of AuNPs and inset of the figure showing the its elemental composition. b FTIR spectrum showing possible interaction between AuNPs and biological macromolecules of mycelia biomass of strain EM-1083
Anti-QS Activity of AuNPs
Anti-biofilm Formation
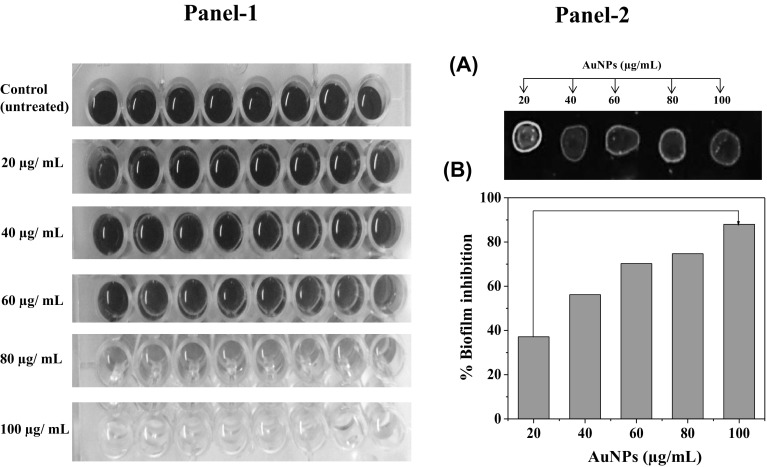
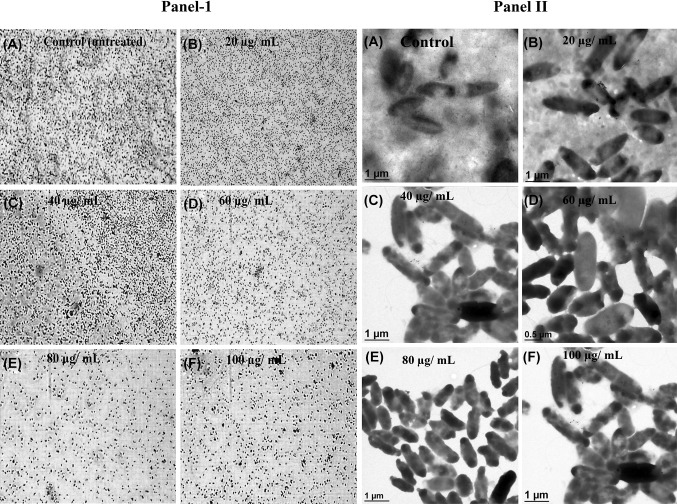
Light microscopy was used to study the preliminary indication of anti-biofilm activity of AuNPs. Therefore crystal violet (CV) staining assay was done for P. aeruginosa biofilm formation in the presence of synthesized AuNPs (Fig. 5, Panel I). A dense biofilm (microcolonies) was in untreated control. The effect of AuNPs on P. aeruginosa biofilm development was also measured using a fixed microtitre plate biofilm and chemiluminescence assays (Fig. 5, Panel I and II). AuNPs in a dose dependent manner inhibited biofilm formation by 7–93% as shown in Fig. 5, Panel II-b. However, AuNPs mediated, a dose-dependent decrease in quantity of microcolonies was detected in P. aeruginosa biofilm formation ability (Fig. 6, Panel I-a–e). Furthermore, AuNPs also interrupted the native structure of biofilm, as it was more marked by TEM analysis (Fig. 6, Panel II-a–e). Furthermore, we examined the interaction between AuNPs with P. aeruginosa biofilm or cells, the samples were analyzed by TEM-EDAX at the higher magnification. A clear interaction of the AuNPs on the cells and biofilm was observed and it was finally proved by elemental analysis (Fig. S4a, b).
Fig. 5.
Inhibitory effects of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass on quorum sensing regulated biofilm formation of P. areuginosa after 48 h exposure. (Panel-I) Crystal violet microtiter plate assay showing the dose dependent inhibitory effects of AuNPs on the biofilm formation. (Panel-II a) Fluorescence based assay showing the inhibitory effects of AuNPs on the biofilm formation. (Panel-II b) Quantification of biofilm formation inhibition by the AuNPs (color figure online)
Fig. 6.
Structural analysis quorum sensing regulated biofilm formation of P. areuginosa after 48 h exposure of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass. (Panel-I a–f) Light microscopic images showing the dose dependent inhibitory effects of AuNPs on biofilm structure. (Panel-II a–f) TEM images showing the dose dependent inhibitory effects of AuNPs on biofilm structure
Anti-pyocyanian Production
The probability of QS reduction by AuNPs was firstly examined by microtitre plate anti-QS assay. A bio-indicator strain P. aeruginosa, which develops an AHL-regulated green-colored ‘pyocyanin’ pigment was used for this assay. In this assay, the increase of pyocyanin signifies AHL-based QS signaling, while the inhibition of pyocyanin indicates the anti-QS property of AuNPs via decrease of AHL formation [40]. A concentration based inhibitory effect of the AuNPs on pyocyanin production was detected (Fig. 7 Panel I). The maximum inhibition was verified at 80 μg/mL while no activity was inspected at 10 μg/mL (Fig. 7, Panel II-a, b). However, 100 μg/mL of AuNPs showed the some extent growth inhibitory effect P. aeruginosa. Similar effects were noticed in spectrophotometric measurement of pyocyanin production as 100% inhibition was detected by 40 μg/mL of AuNPs (Fig. 7, Panel I and II). Next, we wanted to examine the effect of AuNPs (80 μg/mL) on growth of P. aeruginosa. The recorded data showed that the cell concentrations/densities of bacteria did not considerably vary between the control and treatment, exposed to 80 μg/mL of AuNPs (Data not shown). The results achieved confirmed that AuNPs could have inhibitory effects on QS without any lethal effect via reduction of biochemical pathways of AHL production.
Fig. 7.
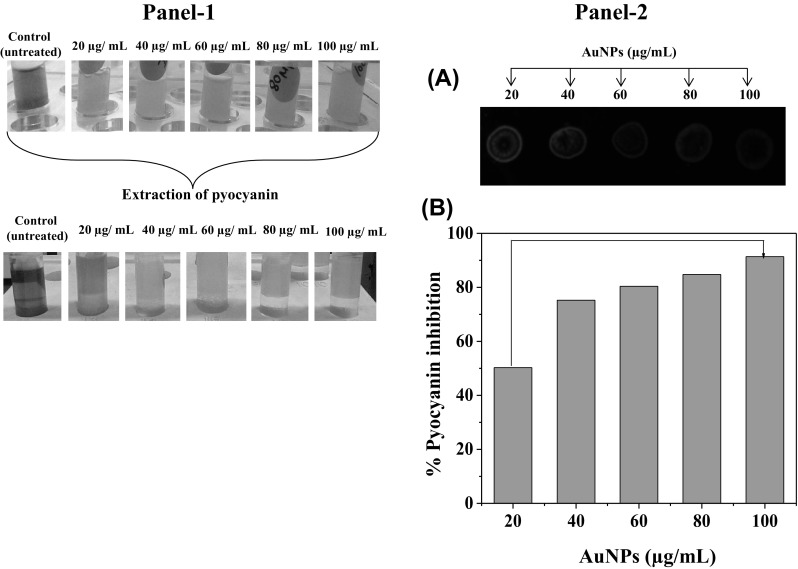
Inhibitory effects of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass on quorum sensing regulated pyocyanin production of P. areuginosa after 48 h exposure. (Panel-I) Microcentrifuge tubes showing the dose dependent inhibitory effects of AuNPs on the pyocyanin production (a) and extracted pyocyanin (b). (Panel-II a) Fluorescence based assay showing the inhibitory effects of AuNPs on the pyocyanin production. (Panel-II b) Quantification of pyocyanin production inhibition by the AuNPs
Stability of AuNPs
We evaluated the stability of AuNPs up to 3 years at 4 °C by observing the change in UV–Vis absorbance patterns and particle size analysis. Significant change in aggregation and SPR of AuNPs during storage was not observed, suggesting that the storage does not significantly affect AuNPs’ SPR stability, polydispersity index (PDI) and hydrodynamic size (Fig. 8a–d). It was also found that the colloidal AuNPs remained stable for 3 years and no significant change in the absorbance was measured.
Fig. 8.
Stability of intracellularly synthesized AuNPs using L. fraternal strain EM-1083 mycelia biomass, after 24 h synthesis and >3 years storage at 4 °C. a Glass vials showing the physical characteristics (SPR and colloidal nature) of synthesized AuNPs. b Absorbance spectrum showing the SPR of the AuNPs. c Polydispersity index (PDI) of AuNPs. d Hydrodynamic size of AuNPs
Discussion
Ectomycorrhizal fungi occur all over the world and have been recognized as a promising bioresources for agriculture and cost effective production of industrially important primary and secondary metabolites [41]. Interest in nanotechnology has increased recently because of their inherent metal reduction potential use in the mycosynthesis of metallic NPs [26]. In this paper we have utilized the inherent metal reducing capability of strain EM-1083 for the intracellular synthesis of AuNPs. The synthesis was confirmed by visual assessment, followed by standard techniques. The recorded spectrum clearly shows that the absorbance of SPR band intensity increased up to 24 h. The absorbance data clearly shows blue shift (From 540 nm to 500 nm) of the signature SPR absorption band of intracellularly synthesized AuNPs, due to the difference in the aspect ratio of the different crystalline shapes of particles. It has been reported that the SPR of AuNPs affected by various factors include (1) reaction medium dielectric constant, pH and temperature (2) method of biosynthesis (3) biological macromolecules involved in nucleation and growth of the particles and (4) nanoparticles shape, size and functionalization [26, 34]. These reasons explain the L. fraterna, EM-1083 intracellularly synthesized AuNPs variation in the shape and size. Moreover, during intracellular AuNPs synthesis, it is expected that the biological macromolecules act as a reducing as well as a capping agent, which direct the nucleation and Au crystalline nanostructured growth. Hence, the involvement of the concoction of biological macromolecules with in the L. fraterna, EM-1083 mycelia as a reducing and capping agent can effectively alter the shape and size of intracellularly synthesized AuNPs. The elemental composition of the harvested AuNPs from mycelia was determined through EDX. AuNPs area specific analysis shows strong peaks of Au along with the C, O, and K elements. The C, O, and K elemental signals in the EDX spectrum were come from the biological macromolecules bound to the AuNPs surface [42]. The higher stability of intracellularly synthesized AuNPs validates that the interactions between AuNPs and the negatively charged biological macromolecules in the form of casing, which were sufficient enough to prevent NPs aggregation and oxidation under aqueous condition [26, 34, 42]. Here in our study, we demonstrated that the L. fraterna, EM-1083 possessed inherent ability to intracellularly synthesized different shapes of AuNPs. Further, identification of crystalline shape specific (AuNPs) biological macromolecules may lead us to understand the role of individual molecules in nucleation growth of shape specific AuNPs. Thus, it is indicating that considerable future importance of such a renewable ectomycorrhizal bioresource for the specific bio- production of AuNPs [40].
We proved that the intracellularly synthesized AuNPs has the potential for the attenuation P. aeruginosa QS system, without a significant effect on its growth. Production of pyocyanin pigment and biofilm formation by P. aeruginosa is regulated by AHL-mediated QS system. Inhibition of pyocyanin production and biofilm formation by AuNPs clearly demonstrated their concentration dependent anti-QS activity. This is in accordance with the previous reports on the inhibition of pyocyanin production and biofilm formation by anti-QS nanostructured materials (NMs) [43, 44]. In addition, AuNPs inhibited the P. aeruginosa biofilm formation. Anti-QS and anti-biofilm activities were initiated may be with the down-regulation of LasIR–RhlIR transcriptional activity by AuNPs, which was evident by the pyocyanin and biofilm formation assays. LasIR and RhlIR are two principle QS systems that have been reported in P. aeruginosa which is reported to chronic infections of the respiratory tract. Recently, our group reported the anti-QS potential of NMs through inhibition of transcriptional activities of LasIR and RhlIR in P. aeruginosa PAO1. LasI and RhlI synthases are prerequisite for the production of C12-AHL and C4-AHL, respectively [13, 14]. The C4-AHL governed the expression of several virulence factors encoding genes (e.g. elastase, exotoxin, protease, and alkaline phosphatase), while C12-AHL controls the production of these virulence factors [45]. In our study, pyocyanin production inhibition by AuNPs might have interfered with the normal secretion of P. aeruginosa other tissue destructive virulence factors. A similar anti-QS effect has been examined with mycofabricated silver nanoparticles [14]. The probability of AuNPs to inhibit the production of AHLs has to be needed at this stage by the direct and indirect assays like biochemical quantification and indicator strain bioassay (C. violaceum 12472-based anti-QS spectrophotometric assay), respectively. Likewise, NMs mediated inhibition of AHLs production in P. aeruginosa PAO1 and Vibrio fischeri have been reported recently [14, 43]. The key evidence that QS regulates P. aeruginosa biofilm formation, which has been recognized an important factor in the pathogenesis thereof. Therefore, targeting QS inhibition P. aeruginosa could be a good therapeutic approach for biofilm formation pathogenesis [46, 47]. Our results of structural biofilm analysis demonstrated that the synthesized AuNPs not only inhibited biofilm formation in P. aeruginosa, but also reduced the microcolonies development, and changed biofilm architecture too. As reported by the previous studies describing the effect of QS inhibitors, our AuNPs also inhibited biofilm formation [44]. The present study clearly demonstrated that AuNPs attenuated P. aeruginosa QS systems without a significant effect on its growth and possible mechanism is depicted in Fig. 9.
Fig. 9.
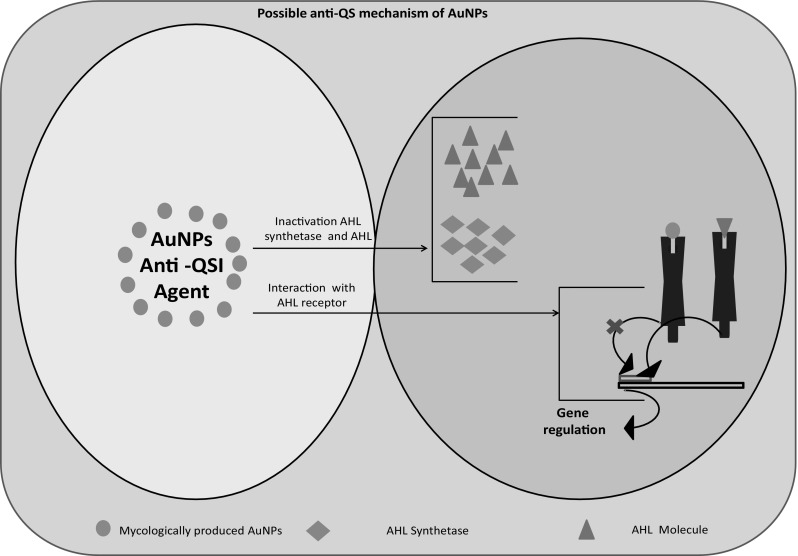
Possible anti-QS mechanism of mycologically produced AuNPs using L. fraterna strain EM-1083
Conclusion
Overall, the results elucidated a rapid, ecofriendly, renewable, economic, and facile method for AuNPs synthesis, and substantial inhibitory effects of AuNPs on biofilm formation and pyocyanin production, unequivocally suggested the anti-QS potential. Thus, synthesized AuNPs could be useful as a nanoanti-quorum sensing inhibitor against P. aeruginosa mediated acute and chronic diseases management.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the research funding supported by the TERI-Deakin Nanobiotechnology Centre, New Delhi India. We would like to thank Dr. Sunil Kumar Deshmukh for his valuable editorial inputs, throughout the preparation of this research article. Special thanks to Mr. Aditya Gaur, Miss. Priyanka Gupta and Mr. Chandrakant Tripathi for helping in TEM sample preparations, imaging and EDX analysis.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0662-4) contains supplementary material, which is available to authorized users.
References
- 1.Costerton JW, Stewart PS, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Behnia M, Logan SC, Fallen L, Catalano P. Nosocomial and ventilator-associated pneumonia in a community hospital intensive care unit: a retrospective review and analysis. BMC Res Notes. 2014;7:232. doi: 10.1186/1756-0500-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrino FLPC, et al. Occurrence of a multidrug-resistant Pseudomonas aeruginosa clone in different hospitals in Rio de Janeiro, Brazil. J Clin Microbiol. 2002;40:2420–2424. doi: 10.1128/JCM.40.7.2420-2424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43:S43–S48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M, Repetto E, Trecarichi EM, Bernardini C, et al. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect. 2011;139:1740–1749. doi: 10.1017/S0950268810003055. [DOI] [PubMed] [Google Scholar]
- 7.Juhas M, Eberl L, Tümmler B. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol. 2005;7:459–471. doi: 10.1111/j.1462-2920.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 8.Dong YH, Xu JL, Li XZ, Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci. 2000;97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiaen SE, Matthijs N, Zhang XH, Nelis HJ, Bossier P, Coenye T. Bacteria that inhibit quorum sensing decrease biofilm formation and virulence in Pseudomonas aeruginosa PAO1. Pathog Dis. 2014;70:271–279. doi: 10.1111/2049-632X.12124. [DOI] [PubMed] [Google Scholar]
- 10.Chong YM, Yin WF, Ho CY, Mustafa MR, Hadi AHA, et al. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod. 2011;74:2261–2264. doi: 10.1021/np100872k. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M, Høiby N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother. 2004;53:1054–1061. doi: 10.1093/jac/dkh223. [DOI] [PubMed] [Google Scholar]
- 12.Chen CW, Hsu CY, Lai SM, Syu WJ, Wang TY, Lai PS. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv Drug Deliv Rev. 2014;78:88–104. doi: 10.1016/j.addr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Singh BN, Rawat AKS, Khan W, Naqvi AH, Singh BR. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipids. PLoS ONE. 2014;9:e106937. doi: 10.1371/journal.pone.0106937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh BR, Singh BN, Singh A, Khan W, Naqvi AH, Singh HB. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci Rep. 2015;5:13719. doi: 10.1038/srep13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul W, Sharma CP. Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2:14–22. doi: 10.4103/0974-7788.83183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, Prajapati PK. Nanotechnology in medicine: leads from Ayurveda. J Pharm Bioallied Sci. 2016;8:80. doi: 10.4103/0975-7406.171730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Darabdhara G, Reddy TM, Borah A, Bezboruah P, et al. Synthesis, characterization and catalytic application of Au NPs-reduced graphene oxide composites material: an eco-friendly approach. Catal Commun. 2013;40:139–144. doi: 10.1016/j.catcom.2013.06.021. [DOI] [Google Scholar]
- 18.Kar PK, Murmu S, Saha S, Tandon V, Acharya K. Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PloS one. 2014;9:e84693. doi: 10.1371/journal.pone.0084693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Moyano DM, Parnsubsakul A, Papadopoulos A, Wang LS, Landis RF, Das R, Rotello VM. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl Mater Interfaces. 2016;8:14096–14101.20. doi: 10.1021/acsami.6b02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew Chem Int Ed. 2010;49(19):3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, El Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. doi: 10.1016/j.jare.2010.02.002. [DOI] [Google Scholar]
- 22.Tomić S, Đokić J, Vasilijić S, Ogrinc N, Rudolf R, et al. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE. 2014 doi: 10.1371/journal.pone.0096584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray P, Tiwari R, Reddy UG, Adholeya A. Detecting the heavy metal tolerance level in ectomycorrhizal fungi in vitro. World J Microbiol Biotechnol. 2005;21:309–315. doi: 10.1007/s11274-004-3572-7. [DOI] [Google Scholar]
- 24.Ray P, Reddy UG, Lapeyrie F, Adholeya A. Effect of coal ash on growth and metal uptake by some selected ectomycorrhizal fungi in vitro. Int J Phytorem. 2005;7:199–216. doi: 10.1080/16226510500214673. [DOI] [PubMed] [Google Scholar]
- 25.Ray P, Adholeya A. Development of molecular markers of ectomycorrhizal fungi based on ITS region. Curr Microbiol. 2008;57:23–26. doi: 10.1007/s00284-008-9146-4. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J Biomed Nanotechnol. 2005;1:47–53. doi: 10.1166/jbn.2005.012. [DOI] [Google Scholar]
- 27.Liu X, Atwater M, Wang J, Huo Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf B. 2007;58:3–7. doi: 10.1016/j.colsurfb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Glauert AM, Lewis PR. Biological specimen preparation for transmission electron microscopy. Princeton: Princeton University Press; 2014. [Google Scholar]
- 29.Jorgensen J, Turnidge J. Susceptibility test methods: dilution and disk diffusion methods. In: Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, Richter S, Warnock D, editors. Manual of clinical microbiology. Washington, DC: ASM Press; 2015. pp. 1253–1273. [Google Scholar]
- 30.O’Toole GA. Microtiter dish biofilm formation assay. JoVE (J Vis Exp) 2011;47:e2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, et al. Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed. 2001;40:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::AID-ANIE3585>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 33.Haiss W, Thanh NT, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV–Vis spectra. Anal Chem. 2007;79:4215–4221. doi: 10.1021/ac0702084. [DOI] [PubMed] [Google Scholar]
- 34.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 35.Fazal S, Jayasree A, Sasidharan S, Koyakutty M, Nair SV, Menon D. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl Mater Interfaces. 2014;6:8080–8089. doi: 10.1021/am500302t. [DOI] [PubMed] [Google Scholar]
- 36.Tofanello A, Miranda EG, Dias IW, Lanfredi AJ, Arantes JT, Juliano MA, Nantes IL. pH-dependent synthesis of anisotropic gold nanostructures by bioinspired cysteine-containing peptides. ACS Omega. 2016;1:424–434. doi: 10.1021/acsomega.6b00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plascencia-Villa G, Torrente D, Marucho M, José-Yacamán M. Biodirected synthesis and nanostructural characterization of anisotropic gold nanoparticles. Langmuir. 2015;31:3527–3536. doi: 10.1021/acs.langmuir.5b00084. [DOI] [PubMed] [Google Scholar]
- 38.Bradley AJ, Jay AH. A method for deducing accurate values of the lattice spacing from X-ray powder photographs taken by the Debye–Scherrer method. Proc Phys Soc. 1932;44:563. doi: 10.1088/0959-5309/44/5/305. [DOI] [Google Scholar]
- 39.Das SK, Dickinson C, Lafir F, Brougham DF, Marsili E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012;14:1322–1334. doi: 10.1039/c2gc16676c. [DOI] [Google Scholar]
- 40.Borovička J, Dunn CE, Gryndler M, Mihaljevič M, et al. Bioaccumulation of gold in macrofungi and ectomycorrhizae from the vicinity of the Mokrsko gold deposit, Czech Republic. Soil Biol Biochem. 2010;42:83–91. doi: 10.1016/j.soilbio.2009.10.003. [DOI] [Google Scholar]
- 41.Sawrnakar MK, Channashettar V, Sarma S, Adholeya A. Ectomycorrhizas: extending the capabilities of chromium-nanoparticles biosynthesis. Mycorrhiza News. 2009;21:34–39. [Google Scholar]
- 42.Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M. Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;3:482–488. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- 43.Naik K, Kowshik M. Anti-quorum sensing activity of AgCl–TiO2 nanoparticles with potential use as active food packaging material. J Appl Microbiol. 2014;117:972–983. doi: 10.1111/jam.12589. [DOI] [PubMed] [Google Scholar]
- 44.Singh BN, Pandey G, Jadaun V, Singh S, Bajpai R, Nayaka S, Naqvi AH, Rawat AKS, Upreti DK, Singh BR. Development and characterization of a novel Swarna-based herbo-metallic colloidal nano-formulation–inhibitor of Streptococcus mutans quorum sensing. RSC Adv. 2015;5:5809–5822. doi: 10.1039/C4RA11939H. [DOI] [Google Scholar]
- 45.Singh BN, Singh HB, Singh A, Singh BR, et al. Lagerstroemia speciosa fruit extract modulates quorum sensing-controlled virulence factor production and biofilm formation in Pseudomonas aeruginosa. Microbiology. 2012;158:529–538. doi: 10.1099/mic.0.052985-0. [DOI] [PubMed] [Google Scholar]
- 46.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacteria drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 47.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.