Abstract
The present study was aimed to investigate the health of vegetative and reproductive parts of sesame plants during Bacillus methylotrophicus KE2 interaction by their pigments, sugars, organic acid, amino acids, hormones and antioxidant production analysis. In a green-house study, B. methylotrophicus KE2 was sprayed to sesame plants at late flowering stage. The bacterial treatment enhanced photosynthetic pigments of plants including pods than their controls. The shoots of plants had higher amount of sucrose, glucose, galactose, xylitol and malic acid, and while the pods of plants showed the more accumulation of sucrose, glucose, inulin and xylitol in bacterium treated plants. However, alanine, cysteine, valine, isoleucine, leucine, tyrosine, phenylalanine, arginine and proline content in shoots and cysteine in pods were increased by the effect of KE2 inoculation. Salicylic acid production was declined in shoots and increased in pods during bacterial exposure. In addition, abscisic acid concentration was lower in pods due to the effect of B. methylotrophicus KE2 in pods over controls. The total polyphenol synthesis was increased in shoots and pods of sesame plants by bacterial interaction. The results of this study revealed that foliar spray of B. methylotrophicus KE2 on sesame plants triggered the plant growth promoting and defense metabolites in vegetative and reproductive organs to improve the health status of sesame.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0666-0) contains supplementary material, which is available to authorized users.
Keywords: Amino acids, Antioxidant, Bacillus, Pigments, Sesame, Sugars
The climatic changes on earth and continues cropping of sesame in same agricultural field decline the crop productivity and soil health [1]. The growth and yield of sesame are affected by environmental factors and various diseases, and specifically their low genetic yield potential causes a major reduction of plant yield [2, 3]. Although, chemical fertilizers increase the plant growth and yield in agricultural field, it pollutes the soil, water and air. Alternatively, the application of plant beneficial microorganisms to crop field can enhance the soil fertility and improve the plant growth [4, 5]. The utilization of plant growth promoting bacteria (PGPR) is considered as an environmental friendly biotechnological method to enhance the plant growth and yield. Several studies revealed that soil and endophytic bacterial isolates have the capacity to produce plant growth regulators such as auxins, cytokinins and gibberellins, and to solubilize the phosphate, and then fix the atmospheric nitrogen, which are used by plants for their growth and development [6, 7]. The bacteria belonging to Bacillus are identified from soil and other sources, and well-reported on plant growth promotion. The spore formation character of Bacillus species is giving more attention than other PGPR to commercialize the viable bacterial fertilizers [8]. A commercial product of Bacillus species, Alinit application influenced the plant growth to increase the yield (40%) of crop plants [9]. Bacillus species are ubiquitous in nature, and enhance the plant growth directly by producing indole acetic acid, gibberellins and phosphate solubilization or indirectly induce systemic resistance of plants against phytopathogens through the secretion of lytic enzymes, antimicrobial peptides and competition for nutrient and space [10]. Very few Bacillus species were successfully sprayed on plants to promote the plant growth and to protect them from disease causing agents or pests. The foliar application of Bacillus thuringiensis to crop plants controls pest infection is widely reported [11]. Besides some of the studies were conducted with foliar spray of Bacillus species to control diseases in crop plants, no work has been carried out to study the effect of foliar spray of Bacillus species on plant functional chemicals to health aspect. The sign of plant health has been proved by enhancing photosynthetic rate, carbohydrates, amino acids, antioxidants and reducing stress related components. In current study was aimed to elucidate the effect of foliar spray of B. methylotrophicus KE2 on sesame plants by analyzing photosynthesis pigments, carbohydrates, organic acid, amino acids, hormones and antioxidant content.
The bacterial isolate, B. methylotrophicus KE2 was cultured [12] and applied to sterilized sesame seeds containing petri-dish and inoculated 25 °C in a dark incubator. The rate of seed germination was measured at 3 days. In a green-house study, the sterilized sesame seeds were sown in a tray containing autoclaved horticulture soil mixture and inoculated at 30 ± 2 °C and irrigated at periodic intervals. At the time of flowering, plants were sprayed with 50% diluted B. methylotrophicus KE2 culture to eleven-week-old sesame plants. The plants and pods were harvested after one-month of treatment. The chlorophyll and carotenoid were quantified according to the method of Arnon [13] and Lichtenthaler [14]. The carbohydrate (sucrose, glucose, inulin, galactose and xylitol) and malic acid content in pods and shoots of plants sprayed with or without B. methylotrophicus KE2 were quantified by the method of Hinesley et al. [15]. The individual amino acids concentrations were quantified by respective amino acids standard peak values [5]. However, plant hormones such as abscisic acid and salicylic acids content were determined by the method of Kang et al. [7]. Total polyphenol extraction and quantification were done according to the procedure followed by Kumazawa et al. [16]. The results were presented as mean values ± standard error, and analysis of variance (ANOVA) to compare the statistical difference based on Duncan’s multiple range test (DMRT) by using SPSS software, at significance level of p ≤ 0.05.
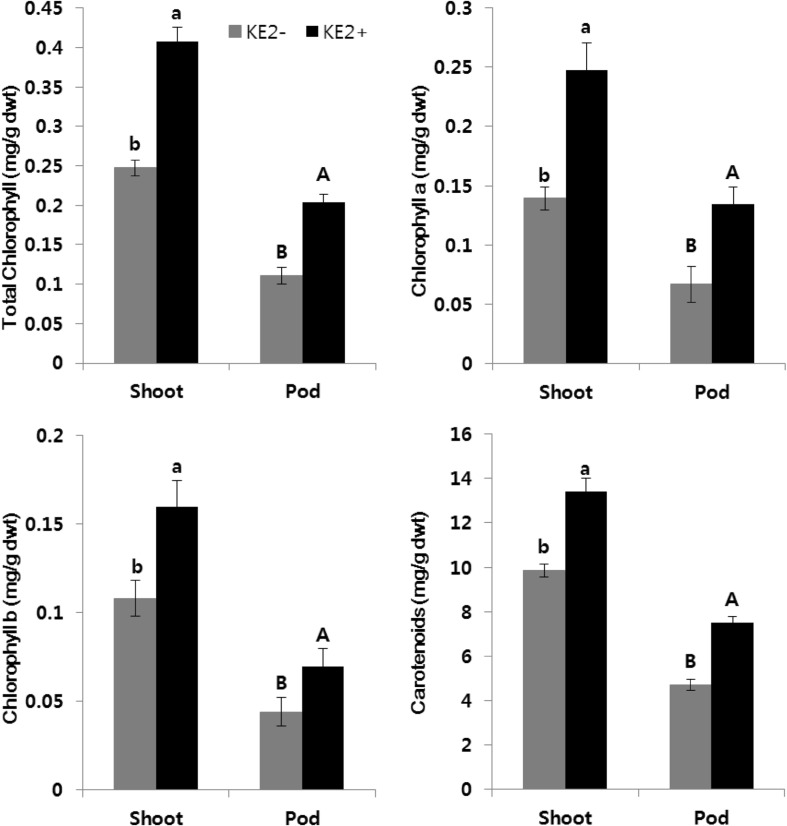
The secretion of secondary metabolites including plant growth promoting substances from bacteria plays major role on plant growth [4]. We found the beneficial effect of B. methylotrophicus KE2 to increase the rate of seed germination (11%) than their controls (Supplementary Fig. 1). Previously, we detected the varying concentration of diverse gibberellins, indole-acetic acid in B. methylotrophicus KE2 culture triggered the rate of seed germination of various crop plants [12]. The availability of those plant growth promoting substances in B. methylotrophicus KE2 bacterial culture was recommended to apply the culture as foliar spray in green-house-grown sesame plants at post-flowering stage to know the vegetative and reproductive health of plants. There were no disease symptoms in sesame after the bacterial foliar treatment. However, B. methylotrophicus KE2-associated plants had higher amount of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in shoots and pods than bacterium-free plants (Fig. 1). The status of chlorophyll concentration in plants is one of the important factors to determine the photosynthetic efficiency of plants. The higher synthesis of photosynthetic pigments can harvest more light energy and converts it into chemical energy in cells, which is used for growth and development of plants. On the other hand, carotenoids act as precursor for vitamin A and antioxidants to scavenge the reactive oxygen species in plant cells [17]. The presence of GAs and IAA in B. methylotrophicus KE2 might be supported the sesame plant health by increasing photosynthetic pigments.
Fig. 1.
Changes on photosynthetic pigments in sesame during B. methylotrophicus KE2 interaction. Means (n = 3) followed by the same letter were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
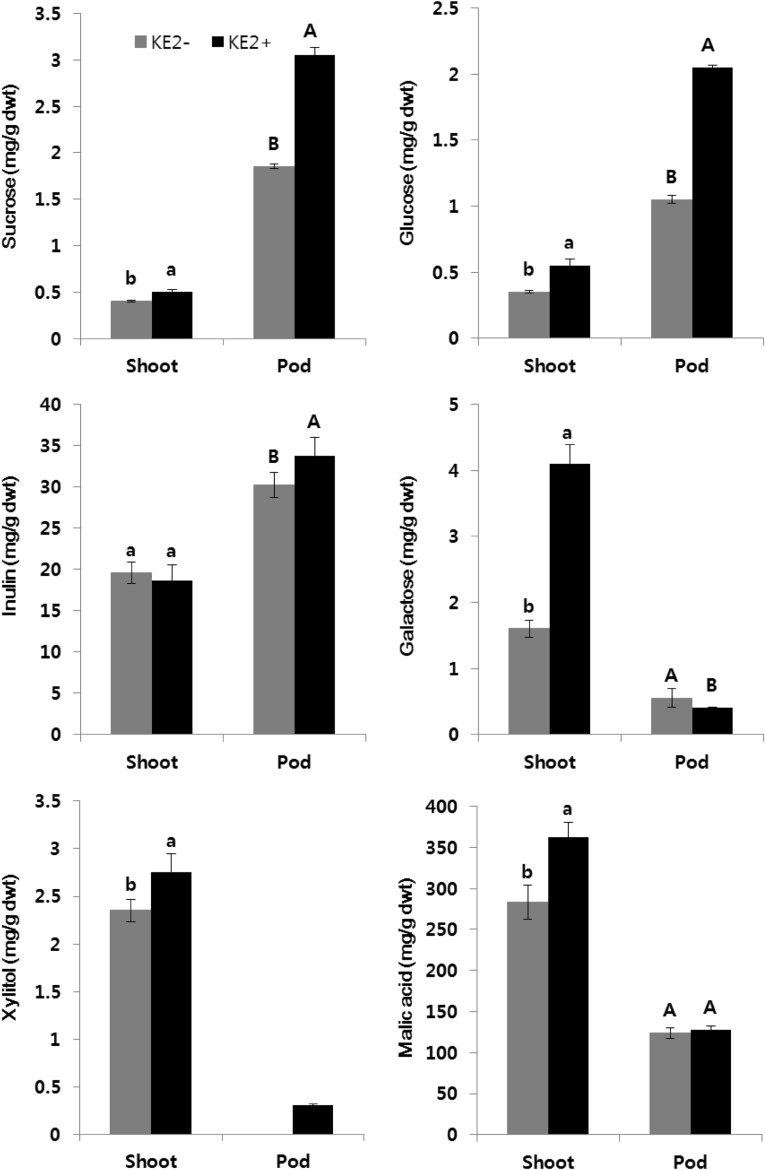
In current study, foliar spray of B. methylotrophicus KE2 influenced the photosynthetic machinery in sesame and increased the accumulation of sucrose, glucose, galactose and xylitol in shoots (Fig. 2). The leaf-associated bacteria secrete biosurfactants and contribute to increase the diffusion of sugars in plants through cuticles and stomatal pores [18]. Although, carobhydrates play a vital role in plant immunity, their actual mechanism in plant–microbe interactions still remains unknown. In plants, sugars and hormones coordinate several metabolisms for growth and development of plants and response to biotic and abiotic stresses [19]. Wang et al. [20] reported that accumulation of sugars and amino acids enhances abiotic stress tolerance in plants. In addition, the sucrose and glucose induce pathogen resistant proteins through salicylic acid dependent or independent pathway [21]. B. methylotrophicus KE2 sprayed sesame plants in this experiment showed higher concentration of sucrose, glucose and inulin, lower amount of galactose in pods over the controls. Xylitol was found only in pods at bacterial treatment (Fig. 2). The galactose is a monosaccharide and one of the major neutral sugars in pectin. The hydrolytic cleavage of galactose-rich pectin side chains is caused to decrease of galactose. Stolle-Smits et al. [22] and Louvet et al. [23] suggested that during fruit development stage, galactose content slowly reduces in pods. Gonzalez-Rodriguez et al. [24] reported that sucrose and glucose content were increased in pineapple plants sprayed with Azotobacter chroococcum. Sucrose is an osmotic substance in plants prevents oxidative stress induced from biotic and abiotic factors [25] and their perception is increased by malic acids [26]. In current study, we found B. methylotrophicus KE2 treatment increased the malic acids concentration in sesame plants (Fig. 2). Plant growth promoting bacteria secrete malic acids to promote the plant growth is widely reported [4]. Malic acid plays a vital role in Krebs’ cycle for producing energy [27]. The subsequent process of higher photosynthetic rate and sugars production by the influence of B. methylotrophicus KE2 on sesame plants led to regulates the amino acids synthesis. In general, number of Bacillus species has the ability to fix the atmospheric nitrogen and supply the nutrients to plants. The amino acids, a source of nitrogen can be possibly uptake by plant roots through amino acids transporters [28]. The micro-organisms impact the amino acids uptake by plants from soil [3]. Recently, we reported that B. megaterium inoculation regulates endogenous plant carbohydrates and amino acids synthesis to promote the mustard plant growth [4]. However, the IAA secreting Enterbacter sp. SE992 induced amino acids, sugars and hormonal regulation in cucumber helped to enhance the salt tolerance of plants [29]. Very few studies were reported the PGPR-induced changes on amino acids in crop. In current study showed that B. methylotrophicus KE2 foliar inoculation favored to sesame vegetative (shoot) growth by enhancing nine amino acids (Ala, Cys, Val, Ile, Leu, Tyr, Phe, Arg and Pro) content and suppressed the amino acids production in pods except Cys synthesis (Table 1). Although, the amount of individual amino acids was comparatively higher in pods than sesame shoots at controlled environments, bacterial treatment inhibited the production of several amino acids in pods. Best of our knowledge, there is no report on amino acids metabolic regulation in pods during bacterial foliar spray. We made the first attempt to elucidate the bacterium induced metabolic alteration in amino acids synthesis in sesame plants. The beneficial bacterial inoculation in soil significantly influenced the amino acids metabolism in plants as documented in our recent studies. For instant, B. megaterium, Rhodobacter sphaeroides, Lactobacillus plantarum, and Saccharomyces cerevisiae enhanced the several individual amino acids to promote the plant growth [4, 5]. The foliar application of B. methylotrophicus KE2 at flowering period might be impacted amino acids synthesis during pod development, which led to reduce the accumulation of amino acids in pods.
Fig. 2.
Influence of B. methylotrophicus KE2 association on sugars and organic acid production in sesame plants. Means (n = 3) followed by the same letter were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
Table 1.
B. methylotrophicus KE2 induced changes on amino acids synthesis in sesame plants. Means (n = 3) followed by the same letter in the column were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
| Amino acids | Bacterial Treatment | Shoot (mg/g dwt) | Pod (mg/g dwt) |
|---|---|---|---|
| Aspartic acid | KE− | 4.846 ± 0.20a | 6.855 ± 0.32a |
| KE+ | 4.748 ± 0.21a | 6.549 ± 0.34a | |
| Threonine | KE- | 2.095 ± 0.13a | 3.334 ± 0.10a |
| KE+ | 1.796 ± 0.02b | 2.863 ± 0.03b | |
| Serine | KE− | 1.954 ± 0.07a | 3.003 ± 0.12a |
| KE+ | 1.472 ± 0.01b | 2.540 ± 0.07b | |
| Glutamic acid | KE− | 4.983 ± 0.10a | 12.433 ± 1.3a |
| KE+ | 4.149 ± 0.02b | 10.099 ± 0.4b | |
| Glycine | KE− | 0.871 ± 0.01a | 2.563 ± 0.11a |
| KE+ | 0.307 ± 0.01b | 1.658 ± 0.05b | |
| Alanine | KE− | 2.760 ± 0.13b | 5.031 ± 0.21a |
| KE+ | 3.538 ± 0.10a | 4.649 ± 0.16b | |
| Cystine | KE− | 1.292 ± 0.04b | 0.942 ± 0.04b |
| KE+ | 1.710 ± 0.03a | 2.341 ± 0.13a | |
| Valine | KE− | 2.283 ± 0.13b | 4.849 ± 0.14a |
| KE+ | 3.062 ± 0.11a | 4.251 ± 0.10b | |
| Methionine | KE− | 0.292 ± 0.01a | 1.152 ± 0.02a |
| KE+ | 0.093 ± 0.00b | 0.746 ± 0.01b | |
| Isoleucine | KE - | 1.763 ± 0.04b | 3.898 ± 0.16a |
| KE+ | 2.467 ± 0.11a | 3.466 ± 0.13b | |
| Leucine | KE− | 2.602 ± 0.10b | 7.286 ± 0.42a |
| KE+ | 3.150 ± 0.16a | 6.452 ± 0.29b | |
| Tyrosine | KE− | 1.255 ± 0.05b | 3.164 ± 0.12a |
| KE+ | 1.986 ± 0.07a | 2.569 ± 0.12b | |
| Phenylalanine | KE− | 1.673 ± 0.02b | 5.533 ± 0.28a |
| KE+ | 2.027 ± 0.11a | 4.645 ± 0.17b | |
| Histidine | KE− | 1.128 ± 0.02a | 2.443 ± 0.10a |
| KE+ | 1.093 ± 0.03a | 2.024 ± 0.09b | |
| Arginine | KE− | 1.820 ± 0.06b | 9.091 ± 0.42a |
| KE+ | 2.254 ± 0.12a | 6.867 ± 0.27b | |
| Proline | KE− | 2.746 ± 0.13b | 3.895 ± 0.15a |
| KE+ | 3.264 ± 0.16a | 0.156 ± 0.04b |
The understanding of hormonal interaction during plant growth is still major challenge for plant researchers. B. methylotrophicus KE2 suppressed the synthesis of salicylic acid in sesame shoots and abscisic acid in pods represents, this bacterial spray can useful to avoid the stress condition in plants (Table 2). Previously, we documented that various soil bacterium stimulated gibberellins production and reduced the abscisic acid for promoting cucumber plant growth [5]. Physiological role of salicylic acid and abscisic acid is unkown on plant growth and development, but their contribution on stress condition was studied well and suggested that salicylic acid is most responsive in pathogen attach and abscisic acid prevent the abiotic environmental stresses [30]. During oxidative stress, plants synthesis more amount of abscisic acid to prevent the water loss by closing stomata [31]. In addition, B. methylotrophicus KE2 increased the antioxidant such as total polyphenol in shoots and pods of sesame plants (Table 2), which might be scavenged the photooxidative reactive oxygen species and protected the plants against environmental stresses [32].
Table 2.
Effect of B. methylotrophicus KE2 on endogenous level of abscisic acid, salicylic acid and total polyphenol content in sesame plants
| Phytohormones and polyphenol | Bacterial Treatment | Shoot | Pod |
|---|---|---|---|
| Salicylic acid (µg/g dwt) | KE− | 6656.005 ± 384a | 619.8561 ± 28.6b |
| KE+ | 3372.298 ± 167b | 722.6387 ± 32.7a | |
| Abscisic acid (ng/g dwt) | KE− | 822.9244 ± 35.6a | 220.8092 ± 10.4a |
| KE+ | 852.5388 ± 34.4a | 23.61727 ± 1.1b | |
| Total polyphenol (mg/g dwt) | KE− | 21.35948 ± 0.6b | 18.56354 ± 0.5b |
| KE+ | 22.64816 ± 0.2a | 21.16954 ± 0.3a |
Means (n = 3) followed by the same letter in the column were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test
In conclusion, the foliar spray of B. methylotrophicus KE2 successfully promoted the health of sesame plants was evidenced from the higher synthesis of physiological components such as photosynthetic pigments, sugars, malic acid, amino acids, total polyphenol and, declined level of stress hormones, salicylic acid and abscisic acid in shoots and pods of sesame plants. In this study suggest that either soil drench or spray of B. methylotrophicus KE2 can able to increase the vegetative and reproductive phage of plant health during their interaction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Effect of B. methylotrophicus KE2 on seed germination of sesame. Means (n = 30) followed by the same letter were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test. (DOC 48 kb)
Acknowledgement
Authors thank Rural Development Administration, Republic of Korea for providing financial support through Agenda Program (Project No. PJ01228603).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0666-0) contains supplementary material, which is available to authorized users.
Contributor Information
Ramalingam Radhakrishnan, Email: glyrrkri@gmail.com.
In-Jung Lee, Phone: + 82-53-950-5708, Email: ijlee@knu.ac.kr.
References
- 1.Hua JL, Liu GR, Huang JS. Effect of continuous cropping of sesame on rhizospheric microbial communities. Acta Ecol Sin. 2012;32:2936–2942. doi: 10.5846/stxb201104010422. [DOI] [Google Scholar]
- 2.Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY. IAA producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.) J Microbiol Biotechnol. 2013;23:856–863. doi: 10.4014/jmb.1209.09045. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan R, Khag SM, Baek IY, Lee IJ. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J Plant Interact. 2014;9:754–762. doi: 10.1080/17429145.2014.930524. [DOI] [Google Scholar]
- 4.Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Ind J Microbiol. 2014;54:427–433. doi: 10.1007/s12088-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang SM, Radhakrishnan R, Youb YH, Khan AL, Park JM, Lee SM, Lee IJ. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric Scand Sec B Soil Plant Sci. 2015;65:36–44. [Google Scholar]
- 6.Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- 7.Kang SM, Radhakrishnan R, Khan AL, Kim MJ, Park JM, Kim BR, Shin DH, Lee IJ. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent Pseudomonas. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 9.Kilian M, Steiner U, Krebs B, Junge H, Schmiedeknecht G, Hain R. FZB24®Bacillus subtilis-mode of action of a microbial agent enhancing plant vitality. Pflanzenschutz Nachr Bayer. 2000;1:72–93. [Google Scholar]
- 10.Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control. 2009;50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 11.Devi VS, Rao PA, Sharma SP, Sharma HC. Interaction of acid exudates in chickpea with biological activity of Bacillus thuringiensis towards Helicoverpa armigera. J Appl Entomol. 2014;138:289–296. doi: 10.1111/jen.12056. [DOI] [Google Scholar]
- 12.Radhakrishnan R, Lee IJ. Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol Biochem. 2016;109:181–189. doi: 10.1016/j.plaphy.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Arnon DI. Copper enzyme in isolated chloroplasts and polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenthaler HK. Chlorophylls and carotenoids: pigment of photosynthetic biomembranes. Med Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- 15.Hinesley LE, Pharr DM, Snelling LK, Funderburk SR. Foliar raffinose and sucrose in four conifer species relationship to seasonal temperature. J Am Soc Hortic Sci. 1992;117:852–855. [Google Scholar]
- 16.Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339. doi: 10.1016/S0308-8146(03)00216-4. [DOI] [Google Scholar]
- 17.Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 2002;155:349–361. doi: 10.1046/j.1469-8137.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 18.Eichert T, Goldbach HE. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces - further evidence for astomatal pathway. Physiol Plant. 2008;132:491–502. doi: 10.1111/j.1399-3054.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 19.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Chang L, Wang B, Wang D, Li P, Wang L, Yi X, Huang Q, Peng M, Guo A. Comparative proteomics of Thellungiella halophila leaves under different salinity revealed chloroplast starch and soluble sugar accumulation played important roles in halophyte salt tolerance. Mol Cell Proteomics. 2013;12:2174–2195. doi: 10.1074/mcp.M112.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibaud MC, Gineste S, Nussaume L, Robaglia C. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol Biochem. 2004;42:81–88. doi: 10.1016/j.plaphy.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Stolle-Smits T, Beekhuizen JG, Kok MTC, Pijnenburg M, Recourt K, Derksen J, Voragen AGJ. Changes in cell wall polysaccharides of green bean pods during development. Plant Physiol. 1999;121:363–372. doi: 10.1104/pp.121.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louvet R, Rayon C, Domon JM, Rusterucci C, Fournet F, Leaustic A, Crepeau MJ, Ralet MC, Rihouey C, Bardor M, Lerouge P, Gillet F, Pelloux J. Major changes in the cell wall during silique development in Arabidopsis thaliana. Phytochem. 2011;72:59–67. doi: 10.1016/j.phytochem.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rodriguez RM, Serrato R, Molina J, Aragon CE, Olalde V, Pulido LE, Dibut B, Lorenzo JC. Biochemical and physiological changes produced by Azotobacter chroococcum (INIFAT5 strain) on pineapple in vitro-plantlets during acclimatization. Acta Physiol Plant. 2013;35:3483–3487. doi: 10.1007/s11738-013-1373-z. [DOI] [Google Scholar]
- 25.Radhakrishnan R, Lee IJ. Effect of low dose of spermidine on physiological changes in salt stressed cucumber plants. Russian J Plant Physiol. 2014;61:90–96. doi: 10.1134/S1021443714010129. [DOI] [Google Scholar]
- 26.Fabian F, Blum H. Relative taste potency of some basic food constituents and their competitive and compensatory action. Food Sci. 1943;8:179–193. doi: 10.1111/j.1365-2621.1943.tb16560.x. [DOI] [Google Scholar]
- 27.Werbach MR. Nutritional strategies for treating chronic fatigue syndrome. Altern Med Rev. 2000;5:93–108. [PubMed] [Google Scholar]
- 28.Jones DL, Healey JR, Willett VB, Farrar JF, Hodge A. Dissolved organic nitrogen uptake by plants an important N uptake pathway? Soil Biol Biochem. 2005;37:413–423. doi: 10.1016/j.soilbio.2004.08.008. [DOI] [Google Scholar]
- 29.Kang S, Radhakrishnan R, Lee SM, Park YG, Kim AY, Seo CW, Lee IJ. Enterobacter sp. SE992-induced regulation of amino acids, sugars, and hormones in cucumber plants improves salt tolerance. Acta Physiol Plant. 2015;37:149. doi: 10.1007/s11738-015-1895-7. [DOI] [Google Scholar]
- 30.Radhakrishnan R, Lee IJ. Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol Plant. 2013;35:263–269. doi: 10.1007/s11738-012-1072-1. [DOI] [Google Scholar]
- 31.Zhu SQ, Chen MW, Ji BH, Jiao DM, Liang JS. Roles of xanthophylls and exogenous ABA in protection against NaCl induced photo-damage in rice (Oryza sativa L) and cabbage (Brassica campestris) J Exp Bot. 2011;62:4617–4625. doi: 10.1093/jxb/err170. [DOI] [PubMed] [Google Scholar]
- 32.Sreenivasulu N, Grimm B, Wobus U, Weschke W. Differential response of antioxidant compounds to salinity stress in salt tolerant and salt sensitive seedlings of foxtail millet (Setaria italica) Physiol Plant. 2000;109:435–442. doi: 10.1034/j.1399-3054.2000.100410.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Effect of B. methylotrophicus KE2 on seed germination of sesame. Means (n = 30) followed by the same letter were not significantly different (p ≤ 0.05) according to Duncan’s multiple range test. (DOC 48 kb)