Abstract
The present study was aimed to develop and evaluate dot–blot assays for rapid detection of staphylococcal enterotoxin-A (SEA) in food. Dot blots were developed in two formats, indirect and sandwich utilizing mouse monoclonal anti-SEA and rabbit polyclonal anti-SEA antibodies. In indirect dot–blot format, recombinant SEA was directly coated on NCM dot–blot strip and detection was carried out by anti-SEA antibodies. In sandwich dot–blot format, SEA was trapped between anti-SEA capture and detection antibodies. Both the dot–blot assays exhibited a sensitivity of ~48 ng ml−1 when tested in different food matrices. The developed assays were highly specific as no cross-reactivity was detected with other classical staphylococcal enterotoxins, toxigenic bacteria and foodborne pathogens. Sensitivity and specificity of developed indirect and sandwich dot–blot assays with respect to PCR was found to be 100 and 99%, respectively. The results shows that the developed dot–blot assays can be used as rapid preliminary screening tests for detection of SEA in food or determining the toxigenic potential of staphylococci, especially in resource-limited settings.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0671-3) contains supplementary material, which is available to authorized users.
Keywords: Staphylococci, Enterotoxins, Detection, Dot blots
Staphylococcal food poisoning (SFP) is a common foodborne intoxication caused by consumption of preformed staphylococcal enterotoxins (SEs) produced from enterotoxigenic staphylococci [1]. It has been estimated that about 20–100 ng of SE is sufficient to cause human illness [2]. Till date, 23 different types of SEs have been reported (SEA to SElV except SEF). SEF is renamed as TSST-1 [3]. Classical SEs (SEA-SEE) are responsible for >95% of food poisoning outbreaks associated with staphylococci, of which SEA is reported to be the most common [4]. SEs are highly resistant to heat, freezing, drying, proteolytic enzymes and low pH and therefore persist after processing and treatment of foods even when the bacteria which produced them may have been eliminated. In such conditions, attempts to isolate staphylococci from such foods by conventional culture-based methods may give a misinterpretation. Additionally, conventional culture based methods used for isolation and identification of pathogens are laborious, time-consuming, and bear low sensitivity, therefore rapid detection methods are important pre-requisite for effective prevention and control of foodborne infections. To overcome the difficulties of conventional methods, nucleic acid amplification methods have been developed. PCR is one of the most common nucleic acid based tests used for screening of enterotoxigenic staphylococci [5]. Loop mediated isothermal amplification (LAMP) assays based on different targets have been developed and found to sensitive and specific as well as less time consuming than PCR [6]. A real time LAMP has been reported for identifying S. aureus, with the detection limit of 10 ng DNA template per reaction [7]. However, mere presence of an enterotoxin gene in isolated Staphylococcus from food sample does not ensure the production of biologically active toxin in that food. Reverse transcription PCR was attempted to determine the level of expression of the enterotoxin by staphylococci, however, this again is an indirect indication by quantifying level of production of mRNA. Immunological methods are the most common methods used for detection of microbial pathogens or their toxins. Commonly used immunological techniques for microbial diagnosis include latex agglutination test (LAT), enzyme linked immunosorbent assay (ELISA), Dot–blot and lateral flow assays. Although, ELISA is highly sensitive, specific and quantitative, the method bears limitations being technically demanding, time-consuming and requiring specific equipment for data acquisition in addition to high cost of commercial ELISA kits. In comparison, latex agglutination, dot–blot, and lateral flow assays are simple, rapid, sensitive and specific qualitative or semi-quantitative tests which do not require any specific equipment or trained manpower. Recently, mass spectrometric methods [8] and biosensors [9] have also been developed and shown to have good sensitivity and specificity but these methods require costly equipment and sophisticated laboratory setup and therefore are challenging for routine sample analysis. Several reports have been published in the recent past on the development of ELISA for classical enterotoxins [10, 11], however, none of the studies has been carried out on the development of any simple test for detection of SEA in food that may be utilized for preliminary screening purpose in resource limited settings under field conditions. Therefore, the current study was undertaken to develop and evaluate dot–blot assays for rapid identification of SEA in food.
Antigen preparation involved expression and purification of recombinant SEA using clone (SEA-Tru15-pProEXHT/1) available at Food Microbiology Laboratory, Division of Livestock Products Technology, Indian Veterinary Research Institute, Izatnagar (Bareilly) UP, India. For standardizing the indirect dot–blot, 1.0 μl of fivefold serially diluted recombinant SEA antigen (1:25, 1:125, 1:625, 1:3125) and 1.0 μl of 10 mM TBS (Tris buffed saline) (negative control) were coated on nitrocellulose membrane (NCM) dot–blot strip. Blocking was carried out for 60 min. NCM strips were washed three times for 5 min each with Tris buffed saline with Tween 20 (TBS-T) to remove blocking reagent. NCM dot–blot strips were dipped in twofold serially diluted (1:1000, 1:2000, 1:4000, 1:8000) rabbit anti-SEA-polyclonal antibody or mouse anti-SEA-monoclonal antibody prepared in diluent for 45 min. Washing step was repeated as described earlier followed by dipping of strips in goat anti-rabbit HRP (Horseradish peroxidase) or goat anti-mouse HRP conjugate (1:1000 in diluent) for 45 min. The strips were then washed three times and dipped in DAB solution for 5 min followed by dipping of strips in stop solution (water) for stopping the reaction.
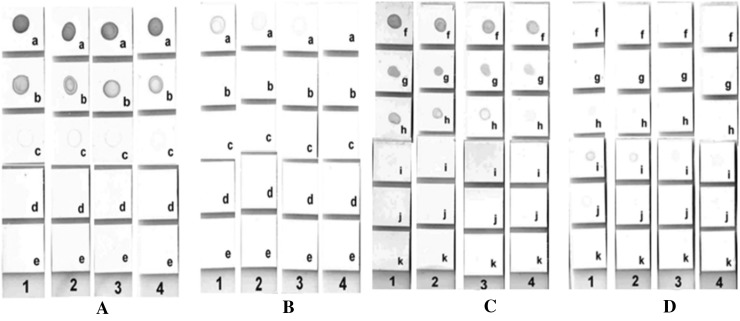
In sandwich dot–blot format, 1.0 μl each of un-diluted and fivefold serially diluted (1:5, 1:25, 1:125, 1:625) mouse anti-SEA-monoclonal or rabbit anti-SEA-polyclonal antibodies were coated on NCM dot–blot strips. After coating, NCM dot–blot strips were dipped in blocking reagent for 60 min. NCM strips were washed thrice for 5 min each with TBS-T to remove blocking reagent. Strips were dipped in 1:25 diluted (1.2 µg) recombinant staphylococcal enterotoxin-A antigen for 90 min, whereas, negative control strips were dipped in TBS-T. After washing thrice with TBS-T, NCM dot–blot strips were dipped in twofold serially diluted (1:1000, 1:2000, 1:4000, 1: 8000) rabbit anti-SEA-polyclonal or mouse anti-SEA-monoclonal antibody prepared in diluent for 45 min. Rest of the steps were similar to indirect dot–blot as described earlier. Results of the study indicated that mouse anti-SEA-monoclonal was superior as capture and rabbit polyclonal anti-SEA as detector antibody in sandwich dot–blot while in indirect dot–blot, rabbit anti-SEA-polyclonal was superior to mouse anti-SEA-monoclonal for detection of SEA (Fig. 1). This may be due to the fact that polyclonal antibodies carry multiple paratopes which might be binding strongly to many epitopes present on complex antigen like SEA. Similar findings have been reported earlier where polyclonal antibodies were found to be more sensitive and producing faster and stronger visual signal than monoclonal antibodies [12, 13]. Additionally, use of polyclonal antibodies may be advantageous over monoclonal as polyclonal antibodies are easy to produce and relatively less expensive. Pair-wise interaction analysis confirmed that the highest sensitivity (48 ng ml−1) was observed when mouse anti-SEA-monoclonal (1.0 µg) was chosen as the capture antibody and rabbit anti-SEA-polyclonal (1:1000 diluted) as detector antibody, in sandwich dot–blot. The developed assays were found to be highly specific as no cross-reaction was observed with other classical staphylococcal enterotoxins (SEB and SEC), toxigenic bacteria or foodborne pathogens tested (Table 1). Spiking studies in food matrices indicated that both indirect and sandwich dot–blot had the sensitivity of 48 ng ml−1. Although, the sensitivity of the developed dot–blots for detection of SEA in food appears to be lower, but it falls well below the 100 ng ml−1 limit, which is reported to be the amount of toxin required for causing human illness. Since, there is no commercially available dot–blot assay for detection of SEA, PCR was taken as the reference method for comparative evaluation. Out of 86 isolates of Staphylococcus spp. tested under the study, six were found to be positive for enterotoxin-A by both indirect and sandwich dot–blot assays while only five were positive by enterotoxin-A gene specific PCR (Table 1). Sensitivity and specificity of developed indirect and sandwich dot–blot assay with respect to PCR was calculated to be 100 and 99%, respectively, and based on kappa statistics the level of agreement between the two tests (PCR and dot–blot) was 0.9 (perfect agreement).
Fig. 1.
Optimization of Indirect and Sandwich dot–blot assay. a Indirect dot–blot: Rabbit anti-SEA-polyclonal as detector antibody. b Indirect dot–blot: Mouse anti-SEA-monoclonal as detector antibody. c Sandwich dot–blot: Mouse anti-SEA-monoclonal as capture antibody and rabbit anti-SEA-polyclonal as detector antibody. d Sandwich dot–blot: Rabbit anti-SEA-polyclonal as capture antibody and mouse anti-SEA-monoclonal as detector antibody. (1) 1:1000 diluted detector antibody, (2) 1:2000 diluted detector antibody, (3) 1:4000 diluted detector antibody, (4) 1:8000 diluted detector antibody. (a) 1:25 diluted capture SEA antigen, (b) 1:125 diluted capture SEA antigen, (c) 1:625 diluted capture SEA antigen, (d) 1:3125 diluted capture SEA antigen, (e) negative control, (f) undiluted capture Antibody, (g) 1:5 diluted capture antibody, (h) 1:25 diluted capture antibody, (i) 1:125 diluted capture antibody, (j) 1:625 diluted capture antibody, (k) negative control
Table 1.
Microorganisms used in the study
| Bacterial genera | Strain number | Characteristics | SEA PCR | Dot blots |
|---|---|---|---|---|
| S. aureus | ATCC 700699 | SEA positive | Positive | Positive |
| S. aureus | ATCC 29213 | SEA positive | Positive | Positive |
| S. aureus | ATCC 43300 | SEA positive | Positive | Positive |
| S. aureus | FRI 722 | SEB positive | Negative | Negative |
| S. aureus | MTCC 96 | SEA negative | Negative | Negative |
| Staphylococcus spp. (81 isolates) | MS 1- MS 81 (food and clinical isolates) | Relative sensitivity and specificity with PCR | 2 Positive | 3 Positive |
| 79 Negative | 78 Negative | |||
| B. cereus | ATCC 10876 | Specificity | Negative | Negative |
| C. perfringens | ATCC 13124 | Specificity | Negative | Negative |
| V. parahaemolyticus | ATCC 17802 | Specificity | Negative | Negative |
| E. coli O157:H7 | ATCC 43888 | Specificity | Negative | Negative |
| Salmonella spp. | 09/epi clinical isolate | Specificity | Negative | Negative |
| Y. enterocolitica | ATCC 23715 | Specificity | Negative | Negative |
| L. monocytogenes | ATCC 19115 | Specificity | Negative | Negative |
Although, numerous reports have been published in the recent past for detection and quantification of SEs in food, most of them are using methodologies which require costly equipment and sophisticated laboratory set up. Several assays reported in recent past include, a double-antibody sandwich ELISA [10] with sensitivity of 32 and 64 pg ml−1 SEA in buffer and milk respectively; a monoclonal antibody-based sandwich ELISA [11] with sensitivity of 0.0282 ng ml−1 SEA; hydrogel-based microarray biochips [14] with detection limit of 0.5 ng ml−1 SEA in milk; a sandwich ELISA [15] for staphylococcal enterotoxin B (SEB) with the sensitivity of 0.25–0.45 ng ml−1 in different foods. Several kits for detection and quantification of classical SEs are also available commercially viz., RIDASCREEN (R-Biopharm GmbH, Germany), Transia (Transia-Diffchamb S.A. Lyon, France), VIDAS Staph enterotoxin 2 (SET2) and TECRA (3M Science, USA). The RIDASCREEN kit is able to discriminate different classical SEs while Transia kit cannot differentiate SEs. Both the kits are monoclonal antibody-based and reported to have sensitivities of 0.25 ng to 0.375 ng toxin ml−1 and 0.2 ng ml−1, respectively. VIDAS Staph enterotoxin 2 (SET2) kit (Biomerieux Inc., USA) works on the VIDAS automated immuno-analyzer having sensitivity of 1.0 ng ml−1 in food matrices. Sandwich ELISAs by TECRA (3M Science, USA) as SETVIA96 (polyvalent configuration) and as SIDVIA72 (monovalent configuration) are available for detection of classical staphylococcal enterotoxins (A–E). The dot–blots developed in the present study were found to be sensitive, specific, repeatable and user-friendly. Developed dot–blot assays were almost equal to PCR in terms of sensitivity and specificity, which is considered to be highly sensitive technique. The assays were faster than the ELISA and may facilitate rapid detection of SEA. The developed assays can be used as an alternative tool to PCR for determining the enterotoxigenic potential of the staphylococcal isolates. In the published literature, a simple and rapid test for detection of SEs is not available. This is the first report of the development of simple and user-friendly tests for detection of SEA without involving any pre-treatment or extraction procedures. The developed dot–blots are sensitive and specific. However, more work needs to be carried out to further enhance the sensitivity of these developed dot–blot assays.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely acknowledge the Director and Joint Director (Research), IVRI, Izatnagar 243122 Bareilly (UP) India for providing funds and necessary infrastructural facilities to carry out the present study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0671-3) contains supplementary material, which is available to authorized users.
Contributor Information
Mamta Singh, Email: mamt.si23@yahoo.in.
Ravi Kant Agrawal, Email: ravikantvet@gmail.com.
Bhoj Raj Singh, Email: brs1762@gmail.com.
Sanjod Kumar Mendiratta, Email: mendiratta_65@yahoo.co.in.
Rajesh Kumar Agarwal, Email: grace_bly@yahoo.com.
Mithilesh Kumar Singh, Email: drmithileshsingh@yahoo.com.
Deepak Kumar, Email: deep_biotek@yahoo.com.
References
- 1.Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal foodborne disease: an ongoing challenge in public health. Biomed Res Int. 2014;2014:1–9. doi: 10.1155/2014/827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal enterotoxins. Toxins. 2010;2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Duan N, Gu H, Hao L, Ye H, Gong W, Wang Z. A review of the methods for detection of Staphylococcus aureus enterotoxins. Toxins. 2016;8:176–195. doi: 10.3390/toxins8070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas D, Chou S, Dauwalder O, Lina G. Diversity in Staphylococcus aureus enterotoxins. Chem Immunol Allergy. 2007;93:24–41. doi: 10.1159/000100856. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Lin CW, Wang J, Oh DH. Advances in rapid detection methods for food-borne pathogens. J Microbiol Biotechnol. 2014;24:297–312. doi: 10.4014/jmb.1310.10013. [DOI] [PubMed] [Google Scholar]
- 6.Lim KT, Teh CSJ, Thong KL. Loop-mediated Isothermal amplification assay for the rapid detection of Staphylococcus aureus. Biomed Res Int. 2013;2013:1–5. doi: 10.1155/2013/895816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D. Novel primers for increased specificity and sensitivity for the detection of Staphylococcus aureus by real-time LAMP. CyTA-J Food. 2016;14:88–91. doi: 10.1080/19476337.2015.1048530. [DOI] [Google Scholar]
- 8.Sospedra I, Marin R, Manes J, Soriano JM. Rapid whole protein quantification of staphylococcal enterotoxin B by liquid chromatography. Food Chem. 2012;133:163–166. doi: 10.1016/j.foodchem.2011.12.083. [DOI] [Google Scholar]
- 9.Salmain M, Ghasemia M, Boujday S, Spadavecchia J, Técher C, Val F, Le Moignee V, Gautier M, Briandet R, Pradier CM. Piezoelectric immunosensor for direct and rapid detection of staphylococcal enterotoxin A (SEA) at the ng level. Biosens Bioelectron. 2011;29:140–144. doi: 10.1016/j.bios.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Clarisse T, Michele S, Olivier T, Valérie E, Vincent LM, Jacques-Antoine H, Michel G, Florence V. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. Food Control. 2013;32:255–261. doi: 10.1016/j.foodcont.2012.11.021. [DOI] [Google Scholar]
- 11.Kuang H, Wang W, Xu L, Ma W, Liu L, Wang L, Xu C. Monoclonal antibody-based sandwich ELISA for the detection of staphylococcal enterotoxin A. Int J Environ Res Public Health. 2013;10:1598–1608. doi: 10.3390/ijerph10041598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer A, Von Eiff C, Kuczius T, Katsuhiko O, Peters G, Becker K. A quantitative real-time immuno-PCR approach for detection of staphylococcal enterotoxins. J Mol Med. 2007;85:461–469. doi: 10.1007/s00109-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Choi BK, Vijayachandran LS, Ayyappan V, Chong CK, Lee KS, Kim SC, Choi CW. Immuno detection of canine parvovirus (CPV) in clinical samples by polyclonal antisera against CPV-VP2 protein expressed in Escherichia coli as an antigen. J Virol Methods. 2007;146:281–287. doi: 10.1016/j.jviromet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Rubina AY, Filippova MA, Feizkhanova GU, Shepeliakovskaya AO, Sidina EI, Boziev KM, Laman AG, Brovko FA, Vertiev YV, Zasedatelev AS, Grishin EV. Simultaneous detection of seven staphylococcal enterotoxins: development of hydrogel biochips for analytical and practical application. Anal Chem. 2010;82:8881–8889. doi: 10.1021/ac1016634. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal R, Singh PK, Sharma SK, Kamboj DV, Goel AK, Singh L. Highly expressed recombinant SEB for antibody production and development of immunodetection system. Indian J Microbiol. 2012;52:191–196. doi: 10.1007/s12088-011-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.