Abstract
Bacteria under stress conditions of excess of carbon (C) and limitations of nutrients divert its metabolism towards C storage as energy reservoir—polyhydroxyalkanoate (PHA). Different Bacillus species—B. cereus and B. thuringiensis, were monitored to produce PHA from different C sources—glucose, crude glycerol and their combination at 37 °C for period up to 192 h. PHA production and its composition was found to vary with feed and bacterial strains. PHA production on crude glycerol continued to increase up to 120 h, reaching a maximum of 2725 mg/L with an effective yield of 71% of the dry cell mass. Depolymerization of PHA was observe to initiate after 96 h of incubation up to 192 h. PHA degradation products have been envisaged to be applied in medical field: tissue engineering, drug carriers, memory enhancers, antiosteoporosis, biodegradable implants. The PHA production and degradation cycle for 192 h has not been reported previously in literature.
Keywords: Polyhydroxyalkanoate, Degradation, Bacillus, Glycerol, Co-polymers
Introduction
Bacterial metabolism is targeted to generate energy from substrates for their growth and development [1]. In general, the most energy efficient process is the tricarboxylic acid cycle (TCA). However, under stress conditions especially those arising due to nutritional imbalance i.e. excess of carbon and limitations of nitrogen, phosphorus, potassium, oxygen, magnesium, etc., bacteria are obliged to divert acetyl co-A towards polyhydroxyalkanoate (PHA) biosynthesis [1–6]. At high C:N ratio, the two enzymes of the TCA cycle, i.e. citrate synthase and isocitrate dehydrogenase are inhibited leading to its blockage. This in turn allows accumulation acetyl co-A and activation of β-ketothiolase, the acetoacetyl reductase and PHA synthase enzymes. It results in the production of PHA [7 ]. PHA production and its composition i.e. homopolymer and co-polymer is affected by the feed and bacterial strains [6]. Once favourable conditions are available, PHA gets depolymerized and utilize them as energy source [7–9]. This natural process of degradation can be exploited to recover intermediates, which can be modified and used. Hydroxyalkanoic acids (HAs) are the major products, which have been reported to be generated from PHA degradation. The composition of the byproducts of PHAs depend upon the type of depolymerases. Complete degradation of PHA occurs through two routes: (1) aerobically, monomers are metabolized to yield CO2 and water, and (2) where as under anaerobic conditions, methane, CO2 and water are generated [10–13].
PHA has been reported to have potential applications in diverse fields, such as medical (tissue engineering, drug carriers, memory enhancers, antiosteoporosis, biodegradable implants), energy (biofuels), probiotics, agriculture [6, 14–16]. However, it has been commercially uneconomical to produce PHA. The major issues are the cost of feed and that involved in polymer recovery process [17–20]. This has slowed down the pace of research on PHA. On the other hand, it has been shown that PHA degradation process leads to intermediates, which can be chemically modified to produce novel products with a wide range of applications [6, 21, 22].
For utilizing PHA and its degradation products, in the field of medicine, it is necessary to understand the cycle of PHA production and its degradation. Here, in this study, we have monitored the cycle of PHA production and its degradation pattern under three different physiological conditions, which affect the rate of the metabolic process and composition of PHA.
Materials and Methods
Organism and Growth Conditions
Bacillus thuringiensis strain EGU45 (Accession no. DQ508971), Bacillus cereus strains EGU43 (Accession no. DQ508969) and EGU520 (Accession no. DQ508978) were isolated previously in our laboratory [23]. These were grown on HiMedia nutrient broth (NB) (13 g/L) and incubated at 37 °C at 200 rpm for 16 h. The cultures of B. thuringiensis EGU45, B. cereus EGU43 and EGU520 were used as inoculum at the rate of 10 µg cellular protein/mL medium. All batch fermentations were carried out by inoculating 100 mL of the medium in 500 mL Erlenmeyer flasks. The incubations were done at 37 °C at 200 rpm for periods of 24, 48, 72, 96, 120, 144, 168, and 192 h [23].
Polyhydroxyalkanoate (PHA) Production
The ability of B. thuringiensis EGU45, B. cereus EGU43 and EGU520 to produce PHA were monitored on Yeast extract + Peptone (YE + PE) medium [(g/L): (1.5 YE; 5.0 PE)] supplemented with: (a) glucose (1%, w/v), (b) crude glycerol (CG) (1%, v/v), (c) glucose (0.1%, w/v) + CG (1%, v/v) + propionic acid (PA) (0.5%, v/v) + NH4Cl (0.5%, g/L). In combination (c), glucose (0.1%, w/v) and NH4Cl (0.5%, w/v) were added to YE + PE medium, at time zero, whereas CG (1% v/v) and PA (0.5% v/v) were added 3 h after initial incubation. Dry cell mass (DCM) and PHA production were monitored after 24, 48, 72, 96, 120, 144, 168, and 192 h of incubation.
Analytical Method
PHA analysis was done as described earlier [23]. In brief, For PHA analysis, 40 mg of DCM was dissolved in 2 mL chloroform and acidified propanol (2 mL) and benzoic acid (200 μL, as internal standard) were added. The mixture was heated at 120 °C for 2 h. It was cooled to room temperature, and 4 mL water was added. The mixture was vortexed for 5 min and allowed to stand for 24 h. The upper layer was discarded and lower layer (organic phase) was used for GLC analysis [23].
Results
Effect of Substrates on PHA Production
Glucose
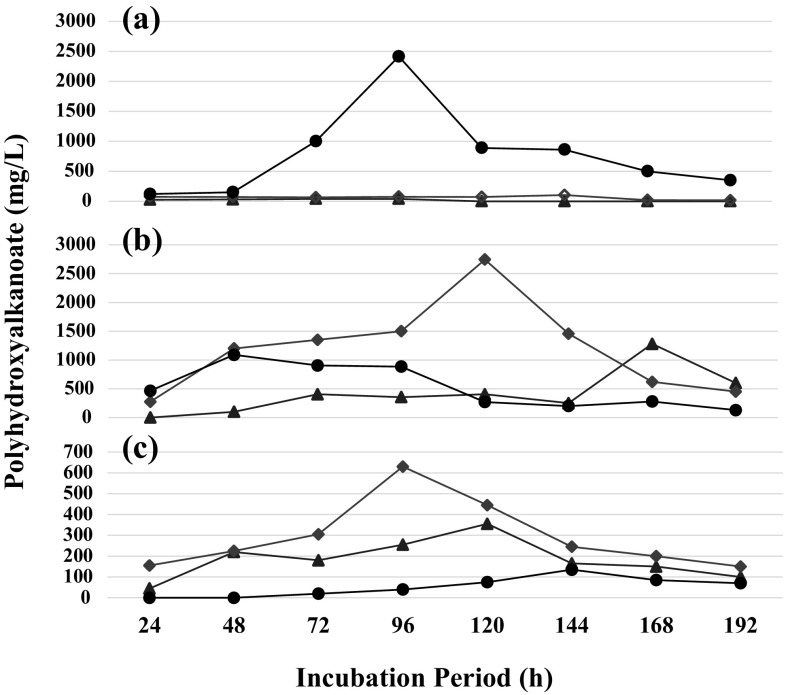
With glucose as C source, DCM of B. thuringiensis EGU45 continued to increase from 640 mg/L at 24 h after incubation (AI) to a maximum 910 mg/L at 144 h AI (Table 1). Thereafter, DCM was found to decline slowly to 450 mg/L by 192 h AI. PHB production by B. thuringiensis EGU45 on glucose as feed was found to reach a maximum value of 75 mg/L at 96 h AI. PHB yield varied from 11 to 14% up to 96 h AI. PHB production declined to 15 mg/L by 192 h AI, with a corresponding yield of 3%. The overall PHA yields were quite low i.e. 3–14%. With B. cereus EGU43, glucose proved to be an effective substrate for its growth (Fig. 1). The DCM was observed to achieve a DCM of 4530 mg/L by 144 h AI. However, PHA producing ability of B. cereus EGU43 were very low i.e. 25–40 mg/L. PHA production was recorded only up to 96 h AI, which completely disappeared thereafter. With B. cereus EGU520 grew very well on glucose, with a DCM of 3540 at 96 h AI. It declined thereafter to 1250 mg/L by 192 h AI (Table 1). This growth was accompanied by very high PHA production, with a maximum of 2415 mg/L at 96 h AI, which was equal to a yield of 68%. PHA production started declining thereafter and even by 192 h AI, it did not completely disappear. In all the three cases, no PHV production was recorded at any stage.
Table 1.
Polyhydroxyalkanoate production pattern in different Bacillus species
| Incubation period (h) | Feed | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose (1%, v/v) | Crude glycerol (1%, v/v) | Glucose (0.1%, w/v) + crude glycerol (1%, v/v) + propionic acid (0.5%, v/v) | |||||||||||||
| DCM | PHB | Yield | PHV | Yield | DCM | PHB | Yield | PHV | Yield | DCM | PHB | Yield | PHV | Yield | |
| (mg/L) | (%) | (mg/L) | (%) | (mg/L) | (%) | (mg/L) | (%) | (mg/L) | (%) | (mg/L) | (%) | ||||
| Bacillus thuringiensis EGU45 | |||||||||||||||
| 24 | 640 | 75 | 12 | 0 | NA | 690 | 275 | 40 | 0 | NA | 960 | 150 | 16 | 5 | 0.5 |
| 48 | 580 | 70 | 12 | 0 | NA | 1880 | 1200 | 64 | 0 | NA | 890 | 155 | 17 | 70 | 8 |
| 72 | 600 | 65 | 11 | 0 | NA | 2390 | 1350 | 56 | 0 | NA | 2000 | 305 | 15 | 20 | 10 |
| 96 | 550 | 75 | 14 | 0 | NA | 2480 | 1480 | 60 | 20 | 0.8 | 2830 | 625 | 22 | 5 | 0.2 |
| 120 | 620 | 70 | 11 | 0 | NA | 3820 | 2725 | 71 | 20 | 0.5 | 4410 | 440 | 10 | 5 | 0.1 |
| 144 | 910 | 65 | 7 | 0 | NA | 3290 | 1450 | 44 | 6 | 0.2 | 4590 | 240 | 05 | 5 | 0.1 |
| 168 | 500 | 20 | 4 | 0 | NA | 3960 | 620 | 16 | 0 | NA | 4750 | 200 | 4 | 0 | NA |
| 192 | 450 | 15 | 3 | 0 | NA | 1510 | 450 | 30 | 0 | NA | 4900 | 150 | 3 | 0 | NA |
| Bacillus cereus EGU43 | |||||||||||||||
| 24 | 490 | 25 | 5 | 0 | NA | 270 | 0 | 0 | 0 | NA | 1750 | 30 | 2.1 | 15 | 0.8 |
| 48 | 840 | 30 | 4 | 0 | NA | 360 | 100 | 28 | 0 | NA | 1460 | 50 | 3.4 | 170 | 12 |
| 72 | 2400 | 40 | 2 | 0 | NA | 830 | 405 | 49 | 0 | NA | 1800 | 80 | 4.4 | 100 | 6 |
| 96 | 3720 | 30 | 1 | 0 | NA | 940 | 355 | 38 | 0 | NA | 2220 | 130 | 5.8 | 125 | 6 |
| 120 | 3690 | 0 | 0 | 0 | NA | 1980 | 270 | 14 | 135 | 7 | 3130 | 65 | 2.1 | 290 | 9 |
| 144 | 4530 | 0 | 0 | 0 | NA | 3410 | 250 | 7 | 9 | 0.3 | 3190 | 165 | 5.2 | 0 | NA |
| 168 | 3500 | 0 | 0 | 0 | NA | 3430 | 1280 | 37 | 0 | NA | 3000 | 150 | 5 | 0 | NA |
| 192 | 3000 | 0 | 0 | 0 | NA | 2050 | 600 | 29 | 0 | NA | 2500 | 100 | 4 | 0 | NA |
| Bacillus cereus EGU520 | |||||||||||||||
| 24 | 710 | 120 | 17 | 0 | NA | 1380 | 465 | 34 | 0 | NA | 700 | 0 | 0 | 0 | NA |
| 48 | 970 | 150 | 15 | 0 | NA | 1680 | 1090 | 65 | 0 | NA | 540 | 0 | 0 | 0 | NA |
| 72 | 2500 | 1000 | 40 | 0 | NA | 1900 | 905 | 47 | 0 | NA | 2000 | 20 | 1 | 0 | NA |
| 96 | 3540 | 2415 | 68 | 0 | NA | 2160 | 885 | 41 | 0 | NA | 2620 | 40 | 1.5 | 0 | NA |
| 120 | 1930 | 890 | 46 | 0 | NA | 2860 | 270 | 9 | 0 | NA | 3240 | 75 | 2.3 | 0 | NA |
| 144 | 1450 | 860 | 59 | 0 | NA | 2170 | 200 | 9 | 0 | NA | 4250 | 135 | 3 | 0 | NA |
| 168 | 1200 | 500 | 41 | 0 | NA | 1500 | 280 | 18 | 0 | NA | 4000 | 85 | 2.1 | 0 | NA |
| 192 | 1550 | 350 | 22 | 0 | NA | 1000 | 130 | 13 | 0 | NA | 3500 | 70 | 2.0 | 0 | NA |
DCM Dry cell mass PHB Polyhydroxybutyrate PHV Polyhydroxyvalerate NA Not applicable
Fig. 1.
Evolution of polyhdroxyalkanoate production by B. cereus EGU43 (triangle), B. thuringiensis EGU45 (diamond), and B. cereus EGU520 (circle) grown on a glucose, (1%, w/v), b crude glycerol (1%, v/v), and c glucose (0.1% w/v) + CG (1%, v/v)
In summary, maximum PHA production was recorded at 72–96 h AI. Thereafter, PHA degradation was complete and rapid in the cases of B. thuringiensis EGU45, B. cereus EGU43. In the case of B. cereus EGU520, PHA production was high and the rate of degradation was also very slow. With glucose as feed, these three strains could produce only homo-polymers of PHB.
Crude Glycerol
With CG as feed, B. thuringiensis EGU45 showed a 4–5 times higher DCM as compared to that recorded on glucose (Table 1). Maximum PHA production of 2725 mg/L was recorded at 120 h AI, which was equivalent to 71% yield. PHA production declined to 450 mg/L by 192 h AI. Here, PHV production also recorded after 96 h AI, which degraded rapidly by 144 h AI. With B. cereus EGU43, DCM of 3410 mg/L was slightly lower than that recorded 4530 mg/L on glucose as feed by 144 h AI. PHV production was recorded from 48 to 120 h AI, which decreased after 144 h. B. cereus EGU520 showed slightly lower growth on CG in comparison to that recorded on glucose (Table 1, Fig. 1). Maximum DCM of 2860 mg/L was recorded at 120 h AI, whereas maximum PHA yield of 1090 mg/L was recorded 48 h AI. PHA yield in this case was 65%. Here, B. cereus EGU520 did not produce any PHV.
In conclusion, in comparison to glucose, CG proved to be a better good C substrate for PHA production, which was 20–40 fold higher in case of B. thuringiensis EGU45 and B. cereus EGU43. Thus, with CG as substrate, PHA production was highest with B. thuringiensis EGU45 at 2725 mg/L at 120 h AI, which declined to 450 mg/L at 192 h AI. In the case B. cereus EGU43, PHA production was slow and peaked at 168 h AI and started decreasing thereafter. The most rapid though low PHA production was recorded with B. cereus EGU520. Thus, CG could enhance PHA production and prolonged it metabolic period as well. In addition, PHA production and it degradation was greatly influenced by the bacterial strains.
Combination of CG and Glucose
With CG and glucose as substrates, supplemented with PA, PHA production was observed to be 625 mg/L at 96 h AI with B. thuringiensis EG45, 165 mg/L with B. cereus EGU43 and 135 mg/L with B. cereus EGU520 at 144 h AI (Table 1). These PHA production values are much lower than that recorded with CG alone. The PHA degradation process was also very slow. The only major difference in this combination of substrates, was the production of PHV in the range of 15–290 mg/L with B. cereus EGU43, whereas B. thuringiensis EGU45 produced only 70 mg/L of PHV at 48 h AI, and B. cereus EGU520 did not produce any PHV at all (Table 1, Fig. 1). In brief, the PHA production is greatly influenced by addition of PA, however it does permit PHA co-polymer production. The influence of bacterial strain on co-polymer production was also evident, as B. cereus EGU520 does not produce co-polymer under any physiological conditions.
Discussion
Most studies on PHA production abilities of different bacteria are targeted towards the most efficient period for its production. It has been reported quite repeatedly that maximum PHA production occurs between 24 and 96 h of incubation [19, 24–29]. Since our interest lies in using the PHA and its degradation products for biomedical applications, we have carried out the study for an extended period of 192 h. This strategy allowed us to analyse PHA production and its degradation pattern. Based on the previous works done with these Bacillus strains, we monitored the influence of the various combinations of substrates and strains on the PHA degradation [30]. We may conclude that the PHA production, its composition and degradation rates are dependent on strains and substrates. It thus allows one to decide on which combination is the most suitable for studying the PHA degradation pathway [31]. Another factor which will certainly have a strong bearing on the decision is the chemical composition of the degradation products [13]. This will pave way for potential chemical modifications of the intermediates and their application in various fields especially medical [32]. Modified PHAs have potential application in various industries: (1) Poly-3-hydroxy-6-acetylthiohexanoate-co-4-acetylthiobutanoate (PHACOS), a PHA modified form can be used for biomedical applications [15], (2) Short-chain fatty acids (SCFAs) produced on degradation of PHAs, can be used as bacteriostatic agents to control bacterial infections and other medical applications [33], (3) Poly-3-hydroxy-actylthioalkanoate-co-3-hydroxyalkanoate can be employed for antibacterial activity against methicillin resistant Staphylococcus aureus [9, 34], (4) Poly-3-hydroxybutyrate-hydoxyhexanoate has been exploited for osteoblast attachment, proliferation and differentiation [35, 36], (5) R-3HAs have used in pharmaceutical industry [37], (6) as oral drugs [38], (7) as antibiotic such as carbapenem or macrolides [39, 40], (8) Methyl esters of HAs have used as biofuels [16, 41], and (9) Monomers such as 3HB and 4HB can be used for preparing peptides, which are resistant to peptidases and can be employed to improve drug delivery [42].
Acknowledgements
The authors wish to thank the Director of CSIR-Institute of Genomics and Integrative Biology (CSIR-IGIB), CSIR-HRD project OLP1126 (ES Scheme No. 21 (1022)/16/EMR-2), Delhi, India, for providing the necessary funds, facilities and moral support. Authors are also thankful to Academy of Scientific and Innovative Research (AcSIR), New Delhi.
References
- 1.Kalia VC, Prakash J, Koul S. Biorefinery for glycerol rich biodiesel industry waste. Indian J Microbiol. 2016;56:113–125. doi: 10.1007/s12088-016-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy CSK, Ghai R, Kalia VC. Polyhydroxyalkanoates: an overview. Bioresour Technol. 2003;87:137–146. doi: 10.1016/S0960-8524(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 3.Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Kumar T, Singh M, Purohit HJ, Kalia VC. Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol. 2009;106:2017–2023. doi: 10.1111/j.1365-2672.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Patel SKS, Lee JK, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Ray S, Kalia VC. Co-metabolism of substrates by Bacillus thuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour Technol. 2017;224:743–747. doi: 10.1016/j.biortech.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Yuan MQ, Liu F, Jian J, Chen GQ. Enhanced production of medium-chain-length polyhydroxyalkanoates (PHA) by PHA depolymerase knockout mutant of Pseudomonas putida KT2442. Bioresour Technol. 2009;100:2265–2270. doi: 10.1016/j.biortech.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Patel SKS, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez V, Dinjaski N, De Eugenio LI, De la Pena F, Prieto MA. Cell system engineering to produce extracellular polyhydroxyalkanoate depolymerase with targeted applications. Int J Biol Macromol. 2014;71:28–33. doi: 10.1016/j.ijbiomac.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Jendrossek D, Handrick R. Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol. 2002;56:403–432. doi: 10.1146/annurev.micro.56.012302.160838. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama A, Kobayashi T, Shiraki M, Saito T. Roles of poly (3-hydroxybutyrate) depolymerase and 3HB-oligomer hydrolase in bacterial PHB metabolism. Curr Microbiol. 2004;48:424–427. doi: 10.1007/s00284-003-4227-x. [DOI] [PubMed] [Google Scholar]
- 12.Uchino K, Saito T, Gebauer B, Jendrossek D. Isolated poly (3-hydroxybutyrate)(PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J Bacteriol. 2007;189:8250–8256. doi: 10.1128/JB.00752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray S, Kalia VC. Biological significance of degradation of polyhydroxyalkanoates. In: Kalia VC, Kumar P, editors. Microbial applications: vol 1: bioremediation and boenergy. Cham: Springer; 2017. pp. 125–139. [Google Scholar]
- 14.Kalia VC, Raizada N, Sonakya V. Bioplastics. J Sci Ind Res. 2000;59:433–445. [Google Scholar]
- 15.Hazer DB, Kılıçay E, Hazer B. Poly(3-hydroxyalkanoate)s: diversification and biomedical applications: a state of the art review. Mater Sci Eng C. 2012;32:637–647. doi: 10.1016/j.msec.2012.01.021. [DOI] [Google Scholar]
- 16.Magdouli S, Brar SK, Blais JF, Tyagi RD. How to direct the fatty acid biosynthesis towards polyhydroxyalkanoates production? Biomass Bioenergy. 2015;74:268–279. doi: 10.1016/j.biombioe.2014.12.017. [DOI] [Google Scholar]
- 17.Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC. Biodiesel industry waste: a potential source of bioenergy and biopolymers. Indian J Microbiol. 2015;55:1–7. doi: 10.1007/s12088-014-0509-1. [DOI] [Google Scholar]
- 18.Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC. Biotechnology in aid of biodiesel industry effluent (glycerol): biofuels and bioplastics. In: Kalia VC, editor. Microbial factories: vol 1: biofuels, waste treatment. New Delhi: Springer; 2015. pp. 105–119. [Google Scholar]
- 19.Kumar P, Ray S, Patel SKS, Lee JK, Kalia VC. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol. 2015;78:9–16. doi: 10.1016/j.ijbiomac.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Kumar P, Ray S, Kalia VC. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J Microbiol. 2015;55:235–249. doi: 10.1007/s12088-015-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivakumar S, Jagadish SJ, Zatakia H, Dutta J. Purification, characterization and kinetic studies of a novel poly (β) hydroxybutyrate (PHB) depolymerase PhaZ from Penicillium citrinum S2. Appl Biochem Biotechnol. 2011;164:1225–1236. doi: 10.1007/s12010-011-9208-0. [DOI] [PubMed] [Google Scholar]
- 22.Ray S, Kalia VC. Biomedical applications of polyhydroxyalkanoates. Indian J Microbiol. 2017;57:261–269. doi: 10.1007/s12088-017-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Singh M, Mehariya S, Patel SKS, Lee JK, Kalia VC. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol. 2014;54:151–157. doi: 10.1007/s12088-014-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel SKS, Singh M, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of Bacillus spp. from glucose in two stage system. Indian J Microbiol. 2011;51:418–423. doi: 10.1007/s12088-011-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SKS, Singh M, Kumar P, Purohit HJ, Kalia VC. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 26.Patel SKS, Kumar P, Singh S, Lee JK, Kalia VC. Integrative approach for hydrogen and polyhydroxybutyrate production. In: Kalia VC, editor. Microbial factories: vol 1: biofuels, waste treatment. New Delhi: Springer; 2015. pp. 73–85. [Google Scholar]
- 27.Patel SKS, Lee JK, Kalia VC. Integrative approach for producing hydrogen and polyhydroxyalkanoate from mixed wastes of biological origin. Indian J Microbiol. 2016;56:293–300. doi: 10.1007/s12088-016-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P, Ray S, Kalia VC. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour Technol. 2016;200:413–419. doi: 10.1016/j.biortech.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Singh LK, Dhasmana N, Sajid A, Kumar P, Bhaduri A, Bharadwaj M, Gandotra S, Kalia VC, Das TK, Goel AK, Pomerantsev AP. clpC operon regulates cell architecture and sporulation in Bacillus anthracis. Environ Microbiol. 2015;17:855–865. doi: 10.1111/1462-2920.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray S, Kalia VC. Microbial co-metabolism polyhydroxyalkanoates co-polymers. Indian J Microbiol. 2017;57:39–47. doi: 10.1007/s12088-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh M, Kumar P, Patel SKS, Kalia VC. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J Microbiol. 2013;53:77–83. doi: 10.1007/s12088-012-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Yu H, Xia Y, Kang Z, Qi Q. Complete PHB mobilization in Escherichia coli enhances the stress tolerance: a potential biotechnological application. Microb Cell Fact. 2009;8:47. doi: 10.1186/1475-2859-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P. Short-chain fatty acids and poly-β-hydroxyalkanoates:(New) Biocontrol agents for a sustainable animal production. Biotechnol Adv. 2009;27:680–685. doi: 10.1016/j.biotechadv.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Dinjaski N, Fernandez-Gutierrez M, Selvam S, Parra-Ruiz FJ, Lehman SM, San Roman J, Garcia E, Garcia JL, Garcia AJ, Prieto MA. PHACOS, a functionalized bacterial polyester with bactericidal activity against methicillin-resistant Staphylococcus aureus. Biomaterials. 2014;35:14–24. doi: 10.1016/j.biomaterials.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YW, Wu Q, Chen J, Chen GQ. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials. 2005;26:899–904. doi: 10.1016/j.biomaterials.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Bian YZ, Wu Q, Chen GQ. Evaluation of three-dimensional scaffolds prepared from poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials. 2008;29:2858–2868. doi: 10.1016/j.biomaterials.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Babel W, Ackermann JU, Breuer U (2001) Physiology, regulation, and limits of the synthesis of poly (3HB). In: Babel W, Steinbuchel A (eds) Biopolyesters. Advances in biochemical engineering/biotechnology, vol 71. Springer, Berlin, Heidelberg, pp 125–157. doi:10.1007/3-540-40021-4_4 [DOI] [PubMed]
- 38.Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Chen GQ, Wu Q. Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol. 2005;67:592–599. doi: 10.1007/s00253-005-1917-2. [DOI] [PubMed] [Google Scholar]
- 40.Shivakumar S, Jagadish SJ, Zatakia H, Dutta J. Purification, Characterization and Kinetic Studies of a Novel Poly (β) Hydroxybutyrate (PHB) Depolymerase PhaZ from Penicillium citrinum S2. Appl Biochem Biotechnol. 2011;164:1225–1236. doi: 10.1007/s12010-011-9208-0. [DOI] [PubMed] [Google Scholar]
- 41.Gao X, Chen JC, Wu Q, Chen GQ. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol. 2011;22:768–774. doi: 10.1016/j.copbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Philip S, Keshavarz T, Roy I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol. 2007;82:233–247. doi: 10.1002/jctb.1667. [DOI] [Google Scholar]