Abstract
Durian is one important tropical fruit with high nutritional value, but its shell is usually useless and considered as waste. To explore the efficient and high-value utilization of this agricultural and food waste, in this study, durian shell was simply hydrolyzed by dilute sulfuric acid, and the durian shell hydrolysate after detoxification was used for bacterial cellulose (BC) production by Gluconacetobacter xylinus for the first time. BC was synthesized in static culture for 10 days and the highest BC yield (2.67 g/L) was obtained at the 8th day. The typical carbon sources in the substrate including glucose, xylose, formic acid, acetic acid, etc. can be utilized by G. xylinus. The highest chemical oxygen demand (COD) removal (16.40%) was obtained at the 8th day. The highest BC yield on COD consumption and the highest BC yield on sugar consumption were 93.51% and 22.98% (w/w), respectively, suggesting this is one efficient bioconversion for BC production. Durian shell hydrolysate showed small influence on the BC structure by comparison with the structure of BC generated in traditional Hestrin–Schramm medium detected by FE-SEM, FTIR, and XRD. Overall, this technology can both solve the issue of waste durian shell and produce valuable bio-polymer (BC).
Keywords: Durian shell, Bacterial cellulose, Gluconacetobacter xylinus, Acid hydrolysate, Metabolism and structure
Introduction
Durian is a tropic fruit and it is popular by people because of its high nutritional value. Although durian pulp is delicious, a large number of durian shells will be generated after taking off of durian pulp [1]. For example, according to the Malaysia Ministry of Agriculture and Agro-Based Industry statistical crop data, the production of durian is about 320,164 MT (metric ton) in 2013. For each durian, only 50–65% flesh is consumed as food, and the rest is usually discarded [2]. In addition to Malaysia, many countries in tropic such as Thailand, Indonesia, Philippines, etc. also generate durian [3]. Besides, many countries [3] import durian from these tropical countries to satisfy the need of food. Obviously, a lot of arbitrarily discarded durian shell is hard to dispose, and this not only causes the problem of environmental pollution, but also wastes resources. Therefore, it is wise and necessary to explore the application of durian shell. Nowadays, most researches mainly focus on the effective utilization of durian shell component [4–6], because these active ingredients can be used for many aspects [7–10]. Besides, durian shell can also be used for adsorption or medical application [11–14]. However, above application of durian shell does not reduce the volume of durian shell greatly that most durian shell after treatment is still solid waste. Therefore, it is wise and necessary to explore new method to treat durian shell which can significantly reduce its volume. Recently, hydrolysis of lignocellulosic biomass is proven to be one promising technology that can convert various solid agricultural wastes into liquid substrate for fermentation [15–17]. Similarly, durian shell can also be turned into liquid substrate by hydrolysis. However, little study focuses on this point.
Bacterial cellulose (BC) has excellent properties such as tensile strength, water-binding capability, adaptability to the living body, and biodegradability, thus it has great potential in application in many fields including food, biomedical material, papermaking and transducer diaphragms [18, 19]. Gluconacetobacter xylinus is the bacterium usually used for BC production [20, 21]. Though the technology of BC production has become more mature, the high cost of substrate is still one barrier for the large scale production and application of BC. Nowadays, there are a lot of substrates have been used for BC production by G. xylinus, including fruit juice, agricultural and forestry waste, wheat straw, wastewater and so on [22–26]. So far, there is no report on the use of durian shell hydrolysate as culture medium for BC production by G. xylinus.
In this study, durian shell was hydrolyzed by dilute sulphuric acid, and the hydrolysate was used as the substrate for the BC production by G. xylinus for the first time. Then, the BC produced in durian shell hydrolysate was compared with that produced in traditional HS medium by Scanning electron microscope (SEM), Fourier transformed infrared (FT-IR), and X-ray diffraction (XRD) analysis. By this study, the potential and possibility of using waste durian shell as one alternative substrate for BC production can be proven.
Methods and Materials
Preparation of Durian Shell Dilute Acid Hydrolysate
Durian shell collected from local fruit market was used as feedstock, the durian shell was treated by 2% (w/v) dilute sulphuric acid with a solid/liquid ratio of 1/10 (w/v) at 120 °C for 60 min. After that the hydrolysate was recovered by vacuum filtration. The pH value of this hydrolysate was adjusted to 5.0 with CaO, and the hydrolysate was recovered by vacuum filtration. Then, the activated carbon was added into the hydrolysate with a solid/liquid ratio of 1/100 (w/v) at 50 °C and 170 rpm for 60 min. Finally, the pH of hydrolysate was adjusted to 6.0 with CaCO3 and the hydrolysate was recovered by vacuum filtration.
Fermentation Strain, Culture Medium and Fermentation Condition
In this study, G. xylinus CH001 (Laboratory of Energy and Biochemical Engineering, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences) was used for BC production. The pre-culture was performed on pre-cultivation medium (g/L, mannitol 25, yeast extract 5, peptone 3, initial pH 6.0) at 28 °C and 150 rpm for 48 h. The durian shell hydrolysate was used as the medium for BC production by G. xylinus. Seed culture [8% (v/v)] was translated into the durian shell dilute acid hydrolysate and the fermentation was performed in a 250 mL conical flask containing 30 mL medium in static culture at 28 °C for 10 days. The fermentation was also carried out in the classical Hestrin–Schramm (HS) medium (g/L, glucose 20, yeast extract 5, peptone 5, disodium hydrogen phosphate 2.7, citric acid 1.15, initial pH 6.0) [27], and the BC products obtained from the durian shell acid hydrolysate were compared with that obtained from HS medium. The fermentation was performed in duplicate and an average value of BC yield was given.
The Treatment of Bacterial Cellulose
After cultivation, the BC was separated from the fermentation broth. Then, the BC was treated by 1.5% (w/v) NaOH at 80 °C for 1.5 h to remove the remnant cells and then was washed by deionized water until the pH was neutral. In order to get dry product, the BC was lyophilized by freeze dryer. Subsequently, the dry mass of BC was weighed and the BC yield was calculated.
Analytical Methods
The concentration of various components in the durian shell hydrolysate was analyzed by HPLC (Waters 2685 systems, Waters Corp., USA), with a RI detector (Waters 2414), and on Aminex HPX-87H column (300 mm × 7.8 mm, Bio Rad Corp., USA) using 5 mM H2SO4 solution as mobile phase at 0.5 mL/min, and the HPLC was operated at 55 °C. COD in the durian shell hydrolysate was detected by Water Quality Analyzer (Beijing Shuanghui Jingcheng Electronic Product Ltd., China). The structure and composition of BC products were evaluated by FE-SEM, FTIR, and XRD according to the previous researches [28, 29].
Results and Discussion
Bacterial Cellulose Production in Durian Shell Hydrolysate by G. xylinus
After durian pulp was consumed, durian shell is usually discarded, thus its cost is very low and its availability is great in many countries consume durian. Base on this, durian shell can be one promising bio-resource for further application. In this study, the potential and possibility of using durian shell as one alternative feedstock for the high value carbohydrate polymer (BC) production by G. xylinus was evaluated. In order to create a suitable fermentation environment, the durian shell hydrolysate was detoxified by neutralization and adsorption, which were used most frequently in detoxification of lignocellulosic hydrolysates [30–34]. After detoxification, the durian shell hydrolysate can be used for fermentation by G. xylinus directly without adding other nutrients including nitrogen sources and trace elements. During the whole fermentation process, the BC accumulation, substrates utilization, and the change of BC structure were evaluated systematically.
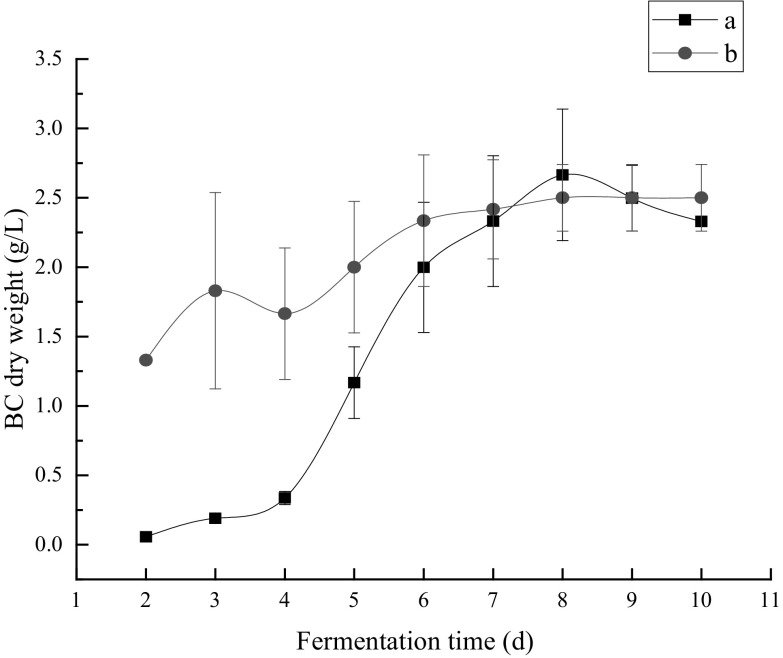
The result of BC accumulation is shown in Fig. 1. As seen from the figure, the accumulation of BC from durian shell acid hydrolysate was not apparent in the first 4 days, and this was similar with other researches [29, 35]. The lag phase was possibly caused by the inhibitors existing in the lignocellulosic hydrolysate that these inhibitory compounds are usually toxic to different microorganisms and their products formation. It is worth noting that besides hemicellulose and cellulose, lignin can also be degraded during acid hydrolysis and generate various aromatic compounds, and these compounds usually have high toxicity to microorganisms, the effect of inhibitors generated from lignin on BC production has been reported in other research [36] and therefore the lag phase might be caused by the existence of these compounds. In contrast, no inhibitory compound exist in the HS medium and therefore BC production was also carried out in this medium to evaluate the effect of durian shell hydrolysate environment containing inhibitory compounds on BC production.
Fig. 1.
BC production in durian shell acid hydrolysate and HS substrate by G. xylinus. a Durian shell acid hydrolysate, b HS medium
After the 4th day when G. xylinus has adapted to the environment of durian shell hydrolysate, the BC yield increased rapidly, and the maximum BC dry weight (2.67 g/L) was obtained at the 8th day of fermentation. After that, the BC dry weight decreased. This interesting phenomenon is possibly due to the exhaustion of fermentable carbon sources in the durian shell hydrolysate and the BC might be degraded to generate additional nutrients to maintain the growth of G. xylinus. By comparison, the maximum BC dry weight (2.50 g/L) was also obtained at the 8th day in the HS medium, and the BC yield remained unchanged later. Although there existed lag phase during the BC fermentation in durian shell acid hydrolysate, there was little difference between the maximum BC yield from the two media (the maximum BC yield in the durian shell acid hydrolysate was approximate 6.4% lower than that in the HS medium), indicating that the lag phase had little effect on the conversion rate from substrate to product.
Metabolism of Substrates in Durian Shell Hydrolysate During Fermentation Process
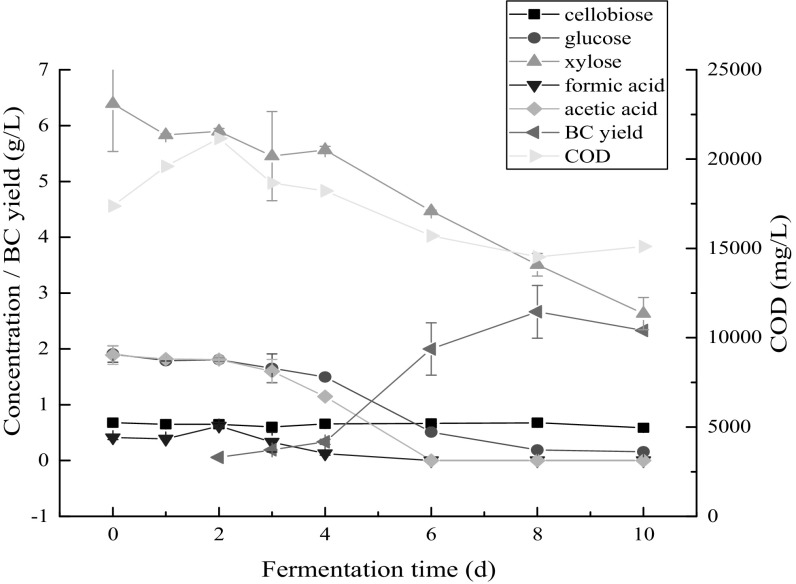
In this work, durian shell acid hydrolysate was used as substrate for BC production, and the metabolism of substances in the hydrolysate during the fermentation process was analyzed by HPLC. The components in the durian shell acid hydrolysate mainly included cellobiose, glucose, xylose, formic acid, acetic acid and so on, which was similar to other lignocellulosic hydrolysates [32, 37–39]. The metabolism of these substrates in the hydrolysate during fermentation is shown in Fig. 2.
Fig. 2.
Evolution of substrates concentration and COD during fermentation in durian shell acid hydrolysate
A lot of lignocellulosic biomass exists in the durian shell, and lignocellulosic biomass usually contains three components, namely cellulose, hemicellulose and lignin. Compared with cellulose, hemicellulose can be hydrolyzed easily by diluted acid, and xylose is the main product after hydrolysis of hemicellulose [40]. It can be seen that xylose was the main sugar in the durian shell acid hydrolysate, and its concentration was as high as 6.39 g/L in the early stage of the fermentation, suggesting that hemicellulose was degraded efficiently after diluted acid hydrolysis. During the first 4 days of fermentation, the xylose concentration showed small change, and the same phenomenon also happened in the metabolism process of the glucose. After the 4th day of fermentation, both xylose and glucose were consumed rapidly and their concentration decreased obviously, showing that the strain has adapted the fermentation environment and began to utilize the carbon sources in the hydrolysate for its growth and BC production. Interestingly, both xylose and glucose can be utilized simultaneously by G. xylinus as the fermentation went on. In addition to the above two sugars, there existed other substances in the hydrolysate, including cellobiose, formic acid, acetic acid and so on. The initial concentration of acetic acid was almost the same as that of glucose. The existence of acetic acid and formic acid maybe caused by the degradation of sugars and the fracture of acetyl [41]. After the 3rd day of fermentation, acetic acid was consumed rapidly, and it was consumed completely after the 4th day of fermentation. Similarly, formic acid was also consumed completely after the 4th day of fermentation. The change of cellobiose was not obviously, it showed a little increase in the process of fermentation.
COD is one important index to evaluate the concentration of organic materials in liquid medium and it reflects the total organic materials with high or low fermentability. The evolution of COD is also showed in Fig. 2 and this can partly reflect the metabolism of G. xylinus. The initial COD of durian shell hydrolysate was 17,373 mg/L, and in the early stage of fermentation, the COD continued to rise till the 2nd day, then it decreased to 14,523 mg/L after 8 days’ fermentation, and the COD removal ratio was 16.40%. The highest BC yield on COD consumption was 93.51% (w/w), and the highest BC yield on sugar consumption was 22.98% (w/w). Thus, the BC biosynthesis in durian shell acid hydrolysate is one efficient bioconversion process that it has great potential for BC production. It is worth noting that the durian shell was hydrolyzed by merely 2% sulfuric acid in this study and most cellulose component in durian shell was still not degraded (glucose is main sugar after degradation of cellulose) in spite that the hemicellulose of durian has been converted into xylose. Therefore, the remaining cellulose after acid hydrolysis of durian shell could further degraded by enzymatic hydrolysis [42] in order to fulfill comprehensive utilization of durian shell resource for BC production. In many situations, glucose is much easier carbon source than xylose for utilization by microorganisms [43]. Therefore, G. xylinus has great potential for comprehensive utilization of durian shell resource for BC production because both xylose and glucose are its promising carbon sources.
Development of BC Structure During Fermentation
In this research, the influence of durian shell acid hydrolysate environment on the micro-morphologies, functional groups, and crystallinity of BC samples were measured by SEM, FT-IR and XRD at different fermentation period and compared with those of the BC samples obtained in classical HS medium.
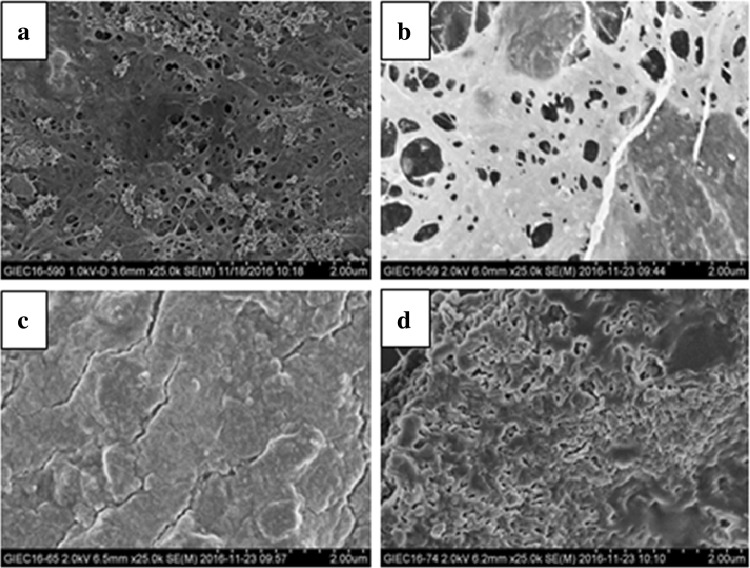
The change of BC micro-morphologies was detected by SEM (Fig. 3). On the 4th day and 8th day of fermentation, the network structure of cellulose fiber could be observed in the BC sample from durian shell hydrolysate, and there existed various obvious pores in the network. But in the later stage of fermentation, the network and the pores disappeared, and were replaced by the more compact structure. By contrast, the BC samples generated in HS medium (Fig. 3) showed more loose structure. Some reports illuminated that the narrow pores among the cellulose may contribute to the anisotropic mechanical strength [44].
Fig. 3.
SEM pictures of the BC samples. a BC sample obtained in durian shell acid hydrolysate after 4 days of fermentation, b BC sample obtained in durian shell acid hydrolysate after 8 days of fermentation, c BC sample obtained in durian shell acid hydrolysate after 10 days of fermentation, d BC sample obtained in HS medium after 10 days of fermentation
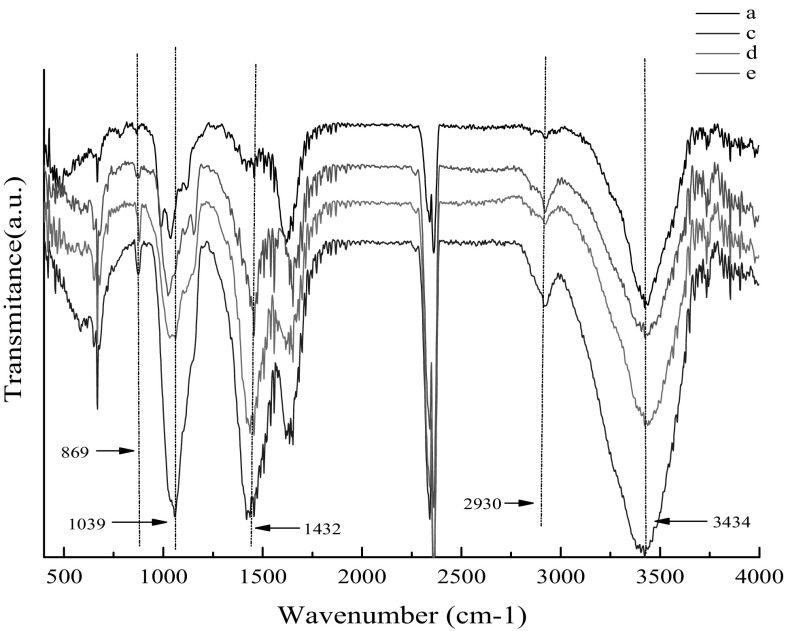
The functional group of BC samples obtained in durian shell acid hydrolysate was measured by FT-IR and compared with that obtained from traditional HS medium. The product synthesized in durian shell acid hydrolysate appeared at and 3434 cm−1 for the stretching vibration of hydroxyl groups (–OH), at 2930 cm−1 for the asymmetric stretching vibration of methylene (–CH2–), at 1432 cm−1 for the asymmetric deformation vibration of methyl and methylene, at 1039 cm−1 for C–O stretching vibration of sugar ring, at 869 cm−1 for (COC) in plane, symmetric stretching. For functional groups, there was no obvious difference between BC samples obtained from durian shell acid hydrolysate and that generated from HS medium, showing that the environment of durian shell acid hydrolysate had little influence on the functional groups composition of BC (Fig. 4).
Table 1.
Crystallinity, index, and size of crystals of BC samples produced from different fermentation medium at different fermentation time
| Sample | Crystallinity (%) | Size of crystals | Crystallinity index Segal equation (%) | ||
|---|---|---|---|---|---|
| (1Ī0) plane | (110) plane | (200) plane | |||
| BCdurian-4th | 80.08 | 74.12 | 367.68 | 52.75 | 56.02 |
| BCdurian-8th | 58.76 | 109.45 | 193.25 | 32.41 | 27.14 |
| BCdurian-10th | 51.83 | 88.00 | 163.09 | 24.15 | 26.13 |
| BCHS-10th | 84.56 | 87.93 | 163.12 | 53.25 | 50.07 |
Fig. 4.
FT-IR spectra of the BC samples. a BC sample obtained in durian shell acid hydrolysate after 4 days of fermentation, b BC sample obtained in durian shell acid hydrolysate after 8 days of fermentation, c BC sample obtained in durian shell acid hydrolysate after 10 days of fermentation, d BC sample obtained in HS medium after 10 days of fermentation
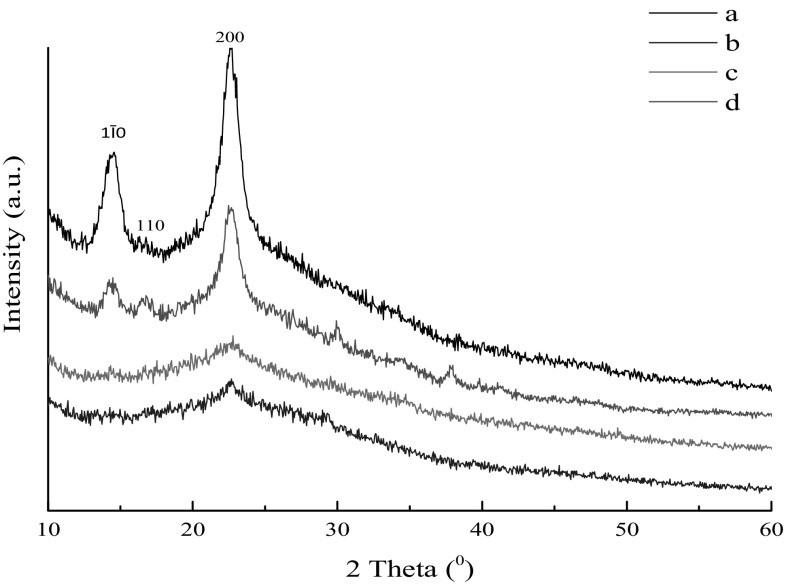
The change of crystal structure of BC samples obtained from durian shell acid hydrolysate was detected by XRD (Fig. 5). The (1Ī0), (110), and (200) crystal planes corresponded to peaks at 2θ angles of 14.5°, 16.8°, and 22.7°. Crystallinity index (CI) was calculated from the related peak intensity data by the Segal method, and the results indicated that the crystallinity and CI of BC from durian shell acid hydrolysate showed a trend to decrease with the increasing days of fermentation, and in the late stage of fermentation, the value of crystallinity and CI were both too low. This phenomenon was corresponding to the low signal of the characteristic peak. Some researches indicated that the low crystallinity and CI leads to smaller particles of BC [45], and the small particles of BC were more efficient in retaining filler granules [46].
Fig. 5.
XRD patterns of the BC samples. a BC sample obtained in durian shell acid hydrolysate after 4 days of fermentation, b BC sample obtained in durian shell acid hydrolysate after 8 days of fermentation, c BC sample obtained in durian shell acid hydrolysate after 10 days of fermentation, d BC sample obtained in HS medium after 10 days of fermentation
Conclusion
Durian shell is often discarded after the pulp has been eaten, and in this research, it is the first time to use the waste durian shell as feedstock for BC production by G. xylinus. This study fulfilled the effective application of waste durian shell, and it is an efficient way to convert the waste into valuable product. Further research should focus more on the comprehensive utilization of cellulose and hemicellulose in durian shell for BC production to make this technology more attractive for industrial application.
Acknowledgements
The authors acknowledge the financial support of the Science and Technology Project of Guangdong Province (2016A010105016), National Natural Science Foundation of China (21606229, 51378486, 31600475), project of Guangzhou Science and Technology (201610010014), Project of Huai-An Science and Technology (HAS2015035), Youth Innovation Promotion Association CAS (2015290), and Foundation of Director of Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (y407r41001), Key Laboratory of Renewable Energy, Chinese Academy of Sciences (Y707j41001).
References
- 1.Chandra TC, Mirna M, Sudaryanto Y, Ismadji S. Adsorption of basic dye onto activated carbon prepared from durian shell: studies of adsorption equilibrium and kinetics. Chem Eng J. 2007;127:121–129. doi: 10.1016/j.cej.2006.09.011. [DOI] [Google Scholar]
- 2.Manshor MR, Anuar H, Nur Aimi MN, Ahmad Fitrie MI, Wan Nazri WB, Sapuan SM, EI-shekeil YA, Wahit MU. Mechanical, thermal and morphological properties of durian skin fibre reinforced PLA biocomposites. Mater Des. 2014;59:279–286. doi: 10.1016/j.matdes.2014.02.062. [DOI] [Google Scholar]
- 3.Zhao C, Cui XY, Liu Y, Zhang RH, He YF, Wang W, Chen C, Liu GQ. Maximization of the methane production from durian shell during anaerobic digestion. Bioresour Technol. 2017;238:433–438. doi: 10.1016/j.biortech.2017.03.184. [DOI] [PubMed] [Google Scholar]
- 4.Aimi NN, Anuar H, Manshor M, Nazri WW, Sapuan S. Optimizing the parameters in durian skin fiber reinforced polypropylene composites by response surface methodology. Ind Crop Prod. 2014;54:291–295. doi: 10.1016/j.indcrop.2014.01.016. [DOI] [Google Scholar]
- 5.Lazim ZM, Hadibarata T, Puteh MH, Yusop Z. Adsorption characteristics of bisphenol a onto low-cost modified phyto-waste material in aqueous solution. Water Air Soil Pollut. 2015;226:1–11. doi: 10.1007/s11270-015-2318-5. [DOI] [Google Scholar]
- 6.Tham Y, Latif PA, Abdullah AM, Shamala-Devi A, Taufiq-Yap Y. Performances of toluene removal by activated carbon derived from durian shell. Bioresour Technol. 2011;102:724–728. doi: 10.1016/j.biortech.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 7.Amin AM, Ahmad AS, Yin YY, Yahya N, Ibrahim N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocoll. 2007;21:273–279. doi: 10.1016/j.foodhyd.2006.04.004. [DOI] [Google Scholar]
- 8.Hameed B, Hakimi H. Utilization of durian (Durio zibethinus Murray) peel as low cost sorbent for the removal of acid dye from aqueous solutions. Biochem Eng J. 2008;39:338–343. doi: 10.1016/j.bej.2007.10.005. [DOI] [Google Scholar]
- 9.Hokputsa S, Gerddit W, Pongsamart S, Inngjerdingen K, Heinze T, Koschella A, Harding SE, Paulsen BS. Water-soluble polysaccharides with pharmaceutical importance from Durian rinds (Durio zibethinus Murr.): isolation, fractionation, characterisation and bioactivity. Carbohydr Polym. 2004;56:471–481. doi: 10.1016/j.carbpol.2004.03.018. [DOI] [Google Scholar]
- 10.Leontowicz H, Leontowicz M, Haruenkit R, Poovarodom S, Jastrzebski Z, Drzewiecki J, Ayala ALM, Jesion I, Trakhtenberg S, Gorinstein S. Durian (Durio zibethinus Murr.) cultivars as nutritional supplementation to rat’s diets. Food Chem Toxicol. 2008;46:581–589. doi: 10.1016/j.fct.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Chandra TC, Mirna MM, Sunarso J, Sudaryanto Y, Ismadji S. Activated carbon from durian shell: preparation and characterization. J Taiwan Inst Chem Eng. 2009;40:457–462. doi: 10.1016/j.jtice.2008.10.002. [DOI] [Google Scholar]
- 12.Chansiripornchai N, Chansiripornchai P, Pongsamart S (2007) A preliminary study of polysaccharide gel extracted from the fruit hulls of durian (Durio zibethinus) on immune responses and cholesterol reduction in chicken. In: International workshop on medicinal and aromatic plants, pp 57–61. doi:10.17660/ActaHortic.2008.786.4
- 13.Ho LH, Bhat R. Exploring the potential nutraceutical values of durian (Durio zibethinus L.)—an exotic tropical fruit. Food Chem. 2015;168:80–89. doi: 10.1016/j.foodchem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Kurniawan A, Sisnandy VOA, Trilestari K, Sunarso J, Indraswati N, Ismadji S. Performance of durian shell waste as high capacity biosorbent for Cr(VI) removal from synthetic wastewater. Ecol Eng. 2011;37:940–947. doi: 10.1016/j.ecoleng.2011.01.019. [DOI] [Google Scholar]
- 15.Anwar Z, Gulfraz M, Irshad M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci. 2014;7:163–173. doi: 10.1016/j.jrras.2014.02.003. [DOI] [Google Scholar]
- 16.Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- 17.Sarkar N, Ghosh SK, Bannerjee S, Aikat K. Bioethanol production from agricultural wastes: an overview. Renew Energy. 2012;37:19–27. doi: 10.1016/j.renene.2011.06.045. [DOI] [Google Scholar]
- 18.Czaja W, Krystynowicz A, Bielecki S, Brownjr R. Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Hu W, Chen S, Yang J, Li Z, Wang H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr Polym. 2014;101:1043–1060. doi: 10.1016/j.carbpol.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen D, Flanagan B, Dykes G, Gidley M. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol. 2009;107:576–583. doi: 10.1111/j.1365-2672.2009.04226.x. [DOI] [PubMed] [Google Scholar]
- 21.Keshk S. Vitamin C enhances bacterial cellulose production in Gluconacetobacter xylinus. Carbohydr Polym. 2014;99:98–100. doi: 10.1016/j.carbpol.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 22.Bae S, Shoda M. Bacterial cellulose production by fed-batch fermentation in molasses medium. Biotechnol Prog. 2004;20:1366–1371. doi: 10.1021/bp0498490. [DOI] [PubMed] [Google Scholar]
- 23.Kongruang S. Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. Appl Biochem Biotechnol. 2008;148:245–256. doi: 10.1007/s12010-007-8119-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C-H, Lin P-J, Lee C-K. Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J Chem Technol Biotehnol. 2010;85:1346–1352. doi: 10.1002/jctb.2439. [DOI] [Google Scholar]
- 25.Moon S-H, Park J-M, Chun H-Y, Kim S-J. Comparisons of physical properties of bacterial celluloses produced in different culture conditions using saccharified food wastes. Biotechnol Bioprocess Eng. 2006;11:26. doi: 10.1007/BF02931864. [DOI] [Google Scholar]
- 26.Wang B, Qi G-X, Huang C, Yang X-Y, Zhang H-R, Luo J, Chen X-F, Xiong L, Chen X-D. Preparation of bacterial cellulose/inorganic gel of bentonite composite by in situ modification. Indian J Microbiol. 2015;56:72–79. doi: 10.1007/s12088-015-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hestrin S, Schramm M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J. 1954;58:345–352. doi: 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Yang D, Zhang H-R, Huang C, Xiong L, Luo J, Chen X-D. Preparation of esterified bacterial cellulose for improved mechanical properties and the microstructure of isotactic polypropylene/bacterial cellulose composites. Polymers. 2016;8:129. doi: 10.3390/polym8040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XY, Huang C, Guo HJ, Xiong L, Li YY, Zhang HR, Chen XD. Bioconversion of elephant grass (Pennisetum purpureum) acid hydrolysate to bacterial cellulose by Gluconacetobacter xylinus. J Appl Microbiol. 2013;115:995–1002. doi: 10.1111/jam.12255. [DOI] [PubMed] [Google Scholar]
- 30.Frazer F, McCaskey T. Wood hydrolyzate treatments for improved fermentation of wood sugars to 2, 3-butanediol. Biomass. 1989;18:31–42. doi: 10.1016/0144-4565(89)90079-6. [DOI] [Google Scholar]
- 31.Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO. Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Prog. 2001;17:287–293. doi: 10.1021/bp0001720. [DOI] [PubMed] [Google Scholar]
- 32.Mussatto SI, Roberto IC. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol. 2004;93:1–10. doi: 10.1016/j.biortech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Parajó J, Domínguez H, Domínguez J. Charcoal adsorption of wood hydrolysates for improving their fermentability: influence of the operational conditions. Bioresour Technol. 1996;57:179–185. doi: 10.1016/0960-8524(96)00066-1. [DOI] [Google Scholar]
- 34.Zhang W, Liu Z, Liu Z, Li F. Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett Appl Microbiol. 2012;55:240–246. doi: 10.1111/j.1472-765X.2012.03283.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Guo H-J, Xiong L, Wang B, Shi S-L, Chen X-F, Lin X-Q, Wang C, Luo J, Chen X-D. Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr Polym. 2016;136:198–202. doi: 10.1016/j.carbpol.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Winestrand S, Guo X, Chen L, Hong F, Jönsson LJ. Effects of aromatic compounds on the production of bacterial nanocellulose by Gluconacetobacter xylinus. Microb Cell Fact. 2014;13:1–11. doi: 10.1186/1475-2859-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H-J, Ramaswamy S, Tschirner U, Ramarao B. A review of separation technologies in current and future biorefineries. Sep Purif Technol. 2008;62:1–21. doi: 10.1016/j.seppur.2007.12.011. [DOI] [Google Scholar]
- 38.Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol. 2000;74:17–24. doi: 10.1016/S0960-8524(99)00160-1. [DOI] [Google Scholar]
- 39.Rubin EM. Genomics of cellulosic biofuels. Nature. 2008;454:841–845. doi: 10.1038/nature07190. [DOI] [PubMed] [Google Scholar]
- 40.Mamman AS, Lee JM, Kim YC, Hwang IT, Park NJ, Hwang YK, Chang JS, Hwang JS. Furfural: hemicellulose/xylosederived biochemical. Biofuel Bioprod Bioresour. 2008;2:438–454. doi: 10.1002/bbb.95. [DOI] [Google Scholar]
- 41.Jönsson LJ, Alriksson B, Nilvebrant N-O. Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels. 2013;6:16. doi: 10.1186/1754-6834-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dyk JS, Pletschke BI. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv. 2012;30:1458–1480. doi: 10.1016/j.biotechadv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Liu YT, Wang YP, Liu HJ, Zhang JA. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour Technol. 2015;180:32–39. doi: 10.1016/j.biortech.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, Kawano S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv Funct Mater. 2004;14:1124–1128. doi: 10.1002/adfm.200305197. [DOI] [Google Scholar]
- 45.Watanabe K, Tabuchi M, Morinaga Y, Yoshinaga F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose. 1998;5:187–200. doi: 10.1023/A:1009272904582. [DOI] [Google Scholar]
- 46.Hioki N, Hori Y, Watanabe K, Morinaga Y, Yoshinaga F, Hibino Y, Ogura T. Bacterial cellulose; as a new material for papermaking. Jpn TAPPI J. 1995;49:718–723. doi: 10.2524/jtappij.49.718. [DOI] [Google Scholar]