Abstract
A natural red dye which is produced by the tiny insects Kerria lacca while feeding on host trees is popularly known as lac dye. Lac dye is a mixture of at least five closely related pure compounds all being anthraquinone derivatives designated as laccaic acid A, B, C, D and E. Anthraquinones isolated from different natural sources and reported to have potent antimicrobial activity. The lac dye, which is also a mixture of anthraquinone derivatives, is expected to exhibit antifungal and antibacterial activity. Lac dye cannot be used as antibacterial and antifungal agent due to its low water solubility and high polarity. Therefore, it is modified into its methyl derivative to enhance its bio-efficacy. Methylated lac dye is characterized with the help of TLC, UV–Vis spectroscopy and FT-IR, NMR analysis. An in vitro spore germination assay was carried out to evaluate the antifungal efficacy of methylated lac dye against some phytopathogenic fungi which commonly caused a various foliar diseases in crop plants viz., Alternaria solani, Curvularia lunata, Erysiphe pisi, Helminthosporium oryzae and Verticillium sp. Among the tested fungi, Verticillum sp. showed highest sensitivity, which showed 100% inhibition at 750 and 1000 µg/ml as compared to control. However, E. pisi an obligate parasite also showed varied sensitivity but at 1000 µg/ml showed 100% spore germination as compared to control. Methylated lac dye also showed strong antibacterial properties against Ralstonia solanacearum at very low concentration (40 and 50 µg/ml). Hence, lac dye may serve as potent antifungal and antibacterial agent in plant disease management.
Keywords: Lac dye, Anthraquinone, Methylation, Antimicrobial efficacy
Introduction
Lac dye is one of the most ancient of natural dyes which is also synonymously known as lac color, lac, lake and lacca etc. It is red colored natural dye obtained during washing stick lac. It is secreted by the tiny insects feeding on the sap of specific host tree, allegedly to protect themselves from harmful UV radiations of the sun. The insects belong to Kerria spp. (Family-Tachardiidae, order-homoptera) feeding on resiniferous trees and bushes cultivated mostly in India, Thailand and China [1]. Lac dye is obtained during washing of stick lac, lac dye goes off from the lac resin and solubilized in the wash-water. This water is then processed to get lac dye powder, which has multiple applications in food, pharmaceutical and cosmetics industries.
Pure dye is sparingly soluble in water, is orange red in acidic pH and reddish violet in alkaline pH. In alkaline solutions, it decomposes rapidly [2]. Its principal color imparting component is laccaic acid, a hydroxy-anthraquinone carboxylic acid. Lac dye is a mixture of at least five closely related pure compounds all being anthraquinone derivatives designated as laccaic acid A, B, C, D and E [3–8], the structure is given in Fig. 1.
Fig. 1.
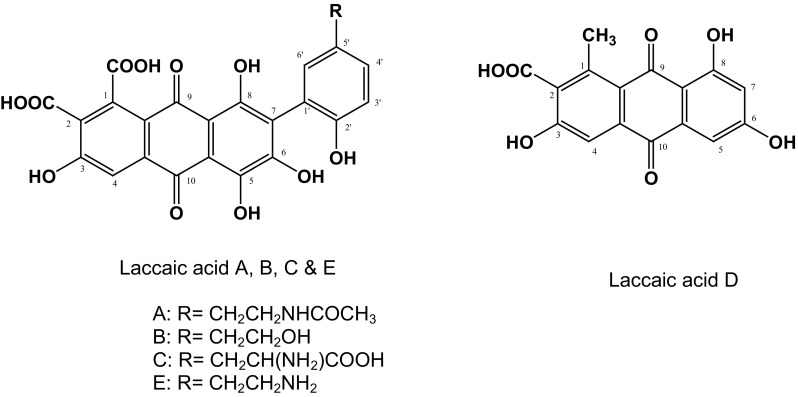
Structure of laccaic acid A, B, C, D and E
Indiscriminate use of synthetic fungicides has created several emerging environmental and toxicological issues which cause deleterious effect on flora, fauna, human and animals. Tackling these issues, thousands of researches are going on for the development of ecofriendly, potent alternative disease controlling formulations to reduce dependency on synthetic/chemical fungicides [9, 10]. The utilization of natural ecofriendly plant products to control fungal and bacterial pathogens has received added attention over synthetic formulations. Among the ecofriendly formulations, several formulations were developed by the researcher of the origin of plants, microbes and insects [11]. Currently, several ecofriendly bio-origin products have been formulated by the manufacturers at a large-scale for eco-friendly management of plant pests.
Anthraquinones and their derivatives isolated from natural sources are reported to have potent antibacterial and antifungal activities [12, 13]. The lac dye which is also a mixture of anthraquinone derivatives may have the potential to exhibit the antifungal and antibacterial activity. Lac dye cannot be used as antibacterial and antifungal agent due to its low water solubility and high polarity. Modification of lac dye was attempted to increase its permeability in the in the pathogen which is essentially require to cause the mortality of the microbes.
The present communication deals with a naturally occurring anthraqunone dye isolated from lac resin and its modification into its methyl derivatives which is further evaluated against some phytopathogenic fungi and a phytobacterium.
Materials and Methods
All the chemicals, solvents and reagents used in isolation of pure lac dye, synthesis of reagent for preparing methyl derivative of lac dye, physico-chemical characterization and bioassay for antifungal activity was procured from Sigma-Aldrich, Bengaluru, India and Merck India Ltd., Mumbai, India. Lac dye was isolated from stick lac samples through the pilot plant installed at Processing and Demonstration Unit, ICAR-IINRG.
Purification of Lac Dye
Technical grade lac dye prepared in the pilot plant was subjected to purification through precipitation of Calcium salt of lac dye with mineral acid and crystallization under cold condition to give pure lac dye.
Preparation of Methylated Lac Dye
Lac dye was methylated using Arndt–Einstert reaction in which nitrosomethylurea was prepared reacting methylamine and urea. Diazomethane was prepared using standard procedure [14] which ultimately used for methylation of lac dye. The mixture of 50 per cent aqueous solution of potassium hydroxide and ether is cooled to around 5 °C in an ice bath. The ethereal solution of nitrosomethylurea is slowly added in the above reaction mixture with gentle shaking. The ether layer became pale yellow due to the formation of diazomethane. The ether layer was separated using a separating funnel and ethereal solution of diazomethane was added slowly to ice-cooled solution of lac dye (Fig. 2). The reaction mixture was kept overnight under refrigerated condition for completion of methylation reaction. The methylated lac dye was extracted with methanol and are dried under vacuum and stored in desiccator.
Fig. 2.
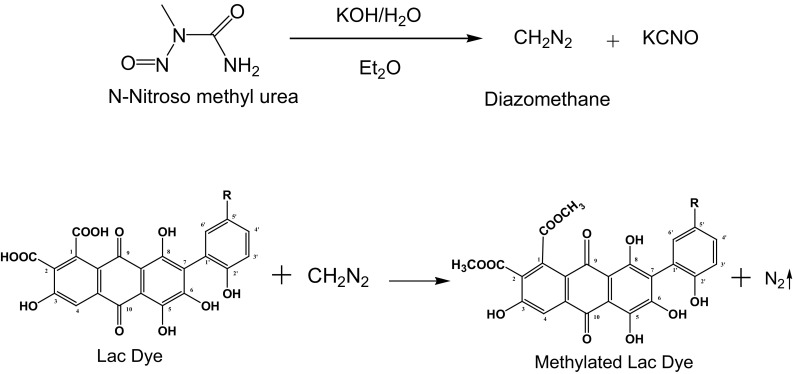
Scheme of preparation of methylated lac dye
Characterization of Methylated Lac Dye
Thin Layer Chromatography (TLC) Study
TLC of methylated lac dye was carried out keeping the pure lac dye as standard. Methylated lac dye was dissolved in methanol and loaded on the activated silica gel TLC plate with capillary tube. The plate was eluted with standardized solvent system (Toluene:Acetone in the ratio of 60:40) and developed TLC plate was visualized under UV light and Rf value of the different spots on TLC plate were recorded.
UV–Vis Spectrophotometric Studies of Lac Dye
Samples of lac dye and methylated lac dye were diluted with methanol and UV–Vis spectra from 200 to 900 nm wavelength range was recorded using CECIL CE 7200, UK UV–Vis spectrophotometer.
FT-IR Spectroscopy of Lac Dye
FT-IR spectra of dried lac dye and methylated lac dye samples was recorded using KBr pellet for identification of functional groups using Shimadzu IR prestige 21, Japan FT-IR spectrophotometer.
NMR Spectroscopy
1H NMR spectra lac dye and methylated lac dye samples was recorded using in 400 MHz NMR Spectrometer, Bruker, UK. Both the samples were dissolved in deuterated dimethyl sulfoxide (DMSO-d6) and trimethylsilane (TMS) was added as an internal standard.
Antibacterial Bioassay of Methylated Lac Dye Using Agar Well Diffusion Techniques
Ralstonia solanacearum, a bacterial wilt causing Gram negative, rod shaped phyto-bacterial pathogen is a widespread in tropics, subtropics and warm temperate region a major limiting factor in the production of Solanaceaous crop plant around the world. It has an extremely wide host range, rapid in growth and potential to adapt any environments. It is a dreaded soil–borne phytopathgenic bacteria which causes bacterial wilt in Solanaceous crops. The methylated Lac dye are allowed to diffuse out into the medium and interact in a plate freshly seeded with the test organism R. solanacearum. The stock solution (1000 μg/ml) of methylated lac dye was prepared by dissolving calculated amount of methylated lac dye in methanol. For further experimentation 10, 20, 30, 40 and 50 μl of the stock solution were used for testing their antibacterial activity against R. solanacearum. First of all, 30 ml of nutrient agar medium were poured into Petri dishes. After solidification 0.25 ml of test strain R. solanacearum was inoculated in the medium separately and spreading in a smear with the help of cotton swab. The experiment was conducted under strict aseptic conditions to avoid contamination. After inoculation of bacteria in the medium, a well was made in the plates with sterile borer (5 mm). The 10–50 μl prepared solution was introduced into the well and plates were incubated at 32 ± 2 °C for 24–36 h. All test concentrations were tested in triplicates along methanol (50 μl) was kept as control. Microbial growth was determined by measuring the diameter of zone of inhibition.
Spore Germination Bioassay for Assessment of Antifungal Activity of Methylated Lac Dye
An in vitro experiment was designed to see the antifungal efficacy of methylated lac dye against some test fungi which commonly caused various foliar diseases in crop plants viz., Alternaria solani, Curvularia lunata, Erysiphe pisi, Helminthosporium oryzae and Verticillium sp. The test fungi were isolated on PDA (Peeled potato-250 g, dextrose-20 g, agar–agar-15–18 g, distilled water 1000 ml) from their respective hosts and culture were maintained on PDA slants for further experimentation. Spores of obligate phytopathogenic fungi were directly used from the diseased plants.
Seven to 10-day-old cultures were used in the experimentation. The spore of obligate parasite, i.e., E. pisi of pea was directly picked up from their respective host. Stock solution of (1000 µg/ml) of methylated lac dye was prepared in aqueous methanol and then after further different test concentrations (250, 500, 750 and 1000 µg/ml) were prepared by serial dilution. A drop of 30–40 µl of the test solution was placed on a grease free glass slide. Then a loop full spore (200–300 approx.) was mixed in the solution with the help of sterile inoculation needle. Then slide was placed in a moist chamber made by placing two sterile moist filter papers on the lid and base of the Petri plates. The moist Petri plates were incubated in the BOD incubator at 27 ± 2 °C. The experiment was conducted maintaining three replications for each treatment along with one set of control. In control sample only aqueous methanol was added.
Results and Discussion
Preparation of Methylated Lac Dye
The methylation of lac dye using diazomethane is considered to be advantageous over other methods as the reaction was carried out at low temperature (0–5 °C) and it leaves no residues or by-products after methylation process is completed. The yield of methylated lac dye obtained from this process is higher than in comparison with other methods of methylation. Once the methylation process is completed minimum work up of the reaction is required which lead to the minimum loss of methylated dye.
Characterization of Methylated Lac Dye
TLC of lac dye (LD) and methylated lac dye (MLD) revealed that on methylation the polarity of the lac dye was reduced and MLD was separated on the TLC plate. Five spots out of which three major fractions (Rf value of 0.38, 0.75 and 0.91) were observed as reported in Yamada et al. [15]. Due to the high polarity lac dye is strongly adsorbed on the TLC plate and become inseparable.
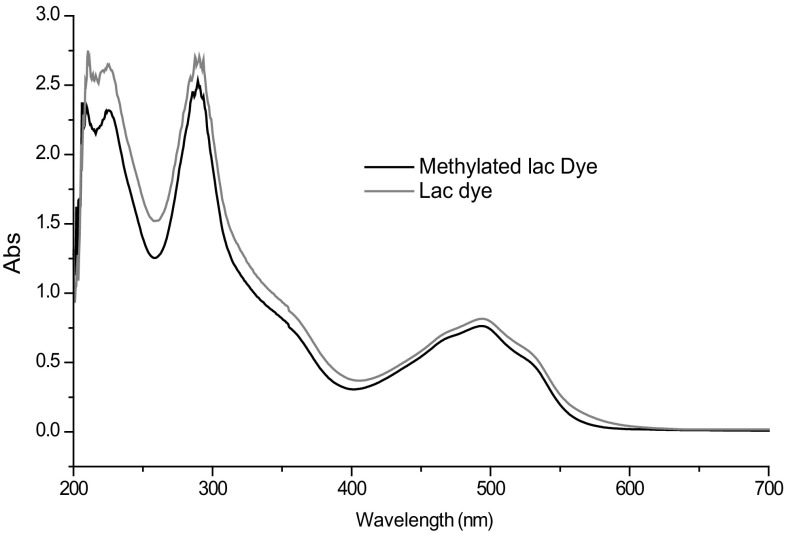
Samples of lac dye (LD) and methylated lac dye (MLD) were scanned for 200–900 nm wavelength range. Both lac dye as well as methylated derivative absorb similarly in visible region (493.5–497.5) of spectra. Absorption of lac dye in UV range is somewhat reduced due to methylation in which hydroxyl groups get replaced with methyl group (Fig. 3).
Fig. 3.
UV–Vis spectra of lac dye (LD) and its methylated derivative (MLD)
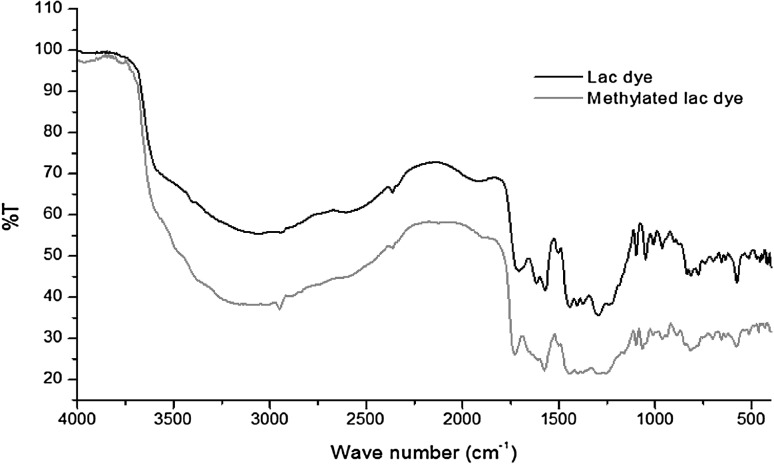
In the FT-IR spectra of lac dye, medium peaks were observed at the region 1100–950 cm−1 mainly due to O–H rocking in hydroxyl and carboxylic groups whereas strong peaks were recorded in the region 1750–1625 cm−1 due to C=O stretching for carboxyl groups in conjugation with the C=C bonds in aromatic ring, C–H stretching (3000–2850 cm−1) and vibration band of OH groups (around 3300 cm−1) were also observed. The results obtained here are in full conformity with the finding reported earlier by Nacowong and Saikrasun [16]. On comparing the FT-IR spectra of methylated lac dye, it clearly shows that peak of carboxylic group at 1625 cm−1 of lac dye get disappeared due to the formation of methyl ester in methylated lac dye (Fig. 4).
Fig. 4.
FT-IR spectra of lac dye (LD) and methylated lac dye (MLD)
The 1H –NMR spectrum of pure lac dye shows showed four aromatic protons at δH at 6.814, 6.835, 6.884 and 7.670, along with three phenolic protons at δH at 3.215–3.559 as broad peak. Peak due to carboxyl (–COOH) protons appeared at δH 13.197 and 13.638 respectively as reported by Nishizaki et al. [17]. Solvent peak of DMSO-d6 was observed at δH 2.5 ppm (Table 1). The methylated lac dye shows aromatic proton at δH ranging from 6.255 to 7.884 and phenolic protons appeared at δH 3.517–3.958. The chemical shifts of protons due to carboxylic group disappeared on methylation and new peak for methyl ester protons (–COOCH3) at δH 4.095 and 4.121 was observed (Table 1). The 1H NMR shift for methyl protons confirms the formation of methylated lac dye.
Table 1.
1H NMR chemical shifts of lac dye (LD) and methylated lac dye (MLD)
| No. | Lac dye (LD) | Methylated lac dye (MLD) |
|---|---|---|
| 1 | 13.197 (1H, s)Ar-COOH | 4.121 (1H, s)Ar-COOCH3 |
| 2 | 13.638 (1H, s)Ar-COOH | 4.095 (1H, s)Ar-COOCH3 |
| 3 | 3.559 (1H, br s)Ar-OH | 3. 691 (1H, br s)Ar-OH |
| 4 | 7.670 (1H, s)Ar-H | 7.884 (1H, s)Ar-H |
| 5 | 3.421 (1H, br s)Ar-OH | 3. 691 (1H, br s)Ar-OH |
| 6 | 3.540 (1H, br s)Ar-OH | 3.958 (1H, br s)Ar-OH |
| 7 | – | – |
| 8 | 3.215 (1H, br s)Ar-OH | 3.517 (1H, br s)Ar-OH |
| 9 | – | – |
| 10 | – | – |
| 1′ | – | – |
| 2′ | 3.540 (1H, br s)Ar-OH | 3.958 (1H, br s)Ar-OH |
| 3′ | 6.884 (1H, q J = 1.9 Hz)Ar-H | 6.995 (1H, q J = 1.9 Hz)Ar-H |
| 4′ | 6.814 (1H, d J = 1.4 Hz)Ar-H | 7.163 (1H, d J = 1.4 Hz)Ar-H |
| 5′ | – | – |
| 6′ | 6.835 (1H, s)Ar-H | 7.280 (1H, s)Ar-H |
Solvent DMSO-d6, s singlet, br s broad singlet, d doublet, q quartet
Antifungal and Antibacterial Activity of Methylated Lac Dye
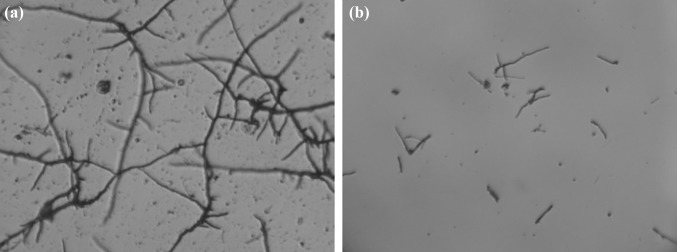
Antifungal activity of Lac dye by spore germination bioassay was performed against five test fungi viz., A. solani, C. lunata, E. pisi, H. oryzae and Verticillium sp. The effect of methylated Lac dye on spore germination of some phytopathogens showed varied sensitivity. Among the tested fungi, Verticillum sp. showed highest sensitivity, which showed 100% inhibition at 750 and 1000 µg/ml but the LC50 concentration was 500 µg/ml which inhibited 73.68% spore germination against Verticillium sp. Moreover, methylated Lac dye also effective against Erysiphe pisi, 100% spore germination inhibition was recorded at 1000 µg/ml while LC 50 concentration was recorded 750 µg/ml which inhibited 50% spore germination of E. pisi (Fig. 5). However, A. solani, C. lunata and H. oryzae showed least sensitive against methylated Lac dye and no LC50 concentration of methylated Lac dye were recorded against A. solani, C. lunata and H. oryzae (Table 2). Antibacterial efficacy of different concentration of methylated Lac dye (10, 20, 30, 40 and 50 µl of stock solution of 1000 µg/ml) was tested against a dreaded soilborne phytobacterial pathogen R. solanacearum. Among the tested concentration, 40 and 50 µl showed strong antibacterial efficacy which showed prominent zone of inhibition (12 and 15 mm) as compared to other test concentration and control (Fig. 6).
Fig. 5.
Effect of methylated lac dye on spore germination of Verticillium sp. a Control and b 1000 µg/ml conc
Table 2.
Spore germination assay of methylated lac dye against selected phytopathogenic fungi
| Tested fungi | Host | % Inhibition of spore germination | |||
|---|---|---|---|---|---|
| Concentration (µg/ml) | |||||
| 250 | 500 | 750 | 1000 | ||
| Alternaria solani | Solanum lycopercium | 7.29 | 12.50 | 18.75 | 22.91 |
| Curvularia lunata | Sweet potato | 6.12 | 10.20 | 14.28 | 18.36 |
| Helminthosporium oryzae | Oryza sativa | 4.34 | 9.78 | 16.30 | 21.74 |
| Verticillium sp. | Insect | 34.21 | 73.68 | 100 | 100 |
| Erysiphe pisi | Pisum sativum | 21.42 | 35.71 | 50.00 | 100 |
Fig. 6.
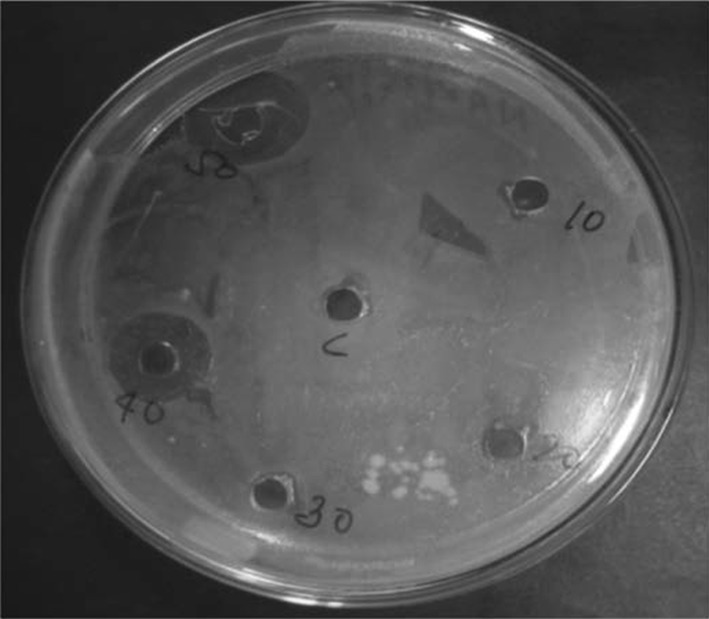
Antibacterial efficacy of methylated lac dye against Ralstonia solanacearum (central well-control and 10, 20, 30, 40 and 50 µl clockwise)
Several reports indicated that the as per results indicated that methylated lac dye have strong antifungal efficacy against Verticillium sp. and E. pisi as well as it has also antibacterial efficacy against a test bacterium, R. solanacearum a dreaded soilborne phytobacterium causing bacterial wilt in Solanaceous crop plants. As per results and their antifungal and antibacterial efficacy can be exploited in development of fungicidal and bactericidal formulations for wide area management of the target pathogens. Singh et al. [18] reported that the sensitivity of the E. pisi a powdery mildew of pea causing pathogen has very sensitive against garlic extracts and their constituents. They also reported that a compound isolated from garlic ajoene (1000 µg/l) showed strong disease inhibitory potential as compared to Sulphur (0.2%) and Carbendazim 50 WP (0.05%) against pea powdery mildew. However, the test formulation originally derived from the Lac resin and potentiated in the form of methylated Lac dye and tested their antifungal and antibacterial efficacy against the test pathogen prove their biological properties as a antifungal and antibacterial efficacy and can be exploited in the management of foliar diseases as well as bacterial wilt of solanaceaous crop plants.
Conclusions
The lac dye was methylated using diazomethane which subsequently purified and characterized through TLC, UV–Vis, FT-IR and NMR analysis. The spectroscopic analysis clearly indicated the insertion of methyl group in the carboxylic end of the lac dye. The process of methylation help to reduce the polarity of lac dye which find its application as antimicrobial agent. The antimicrobial action of methylated lac dye is speculated as enhanced permeability of pathogen cell wall through dissolving its lipid layer. Besides, lac dye have anthraquinonoid ring in its structure which also exhibit antimicrobial activity by inhibition of nucleic acid synthesis.
The spore germination assay was designed to see the antifungal efficacy of methylated lac dye against some test fungi which commonly caused various foliar diseases in crop plants viz., A. solani, C. lunata, H. oryze and Verticillium sp. The effect of methylated Lac dye on spore germination of some phytopathogens showed varied sensitivity. Among the tested fungi, Verticillum sp. showed highest sensitivity, which showed 100% inhibition at 750 and 1000 µg/ml as compared to control. However, E. pisi, an obligate parasite also showed varied sensitivity but at 1000 µg/ml showed 100% inhibition of spore germination as compared to control in which only aqueous methanol was used and more than 90% spore germination were recorded. On scrutinizing the data on spore germination assay it could safely be concluded that lac dye can be used as potent antifungal agent.
Acknowledgements
The authors express their sincere gratitude to Indian Council of Agricultural Research, for providing financial assistance to carry out this study. The authors also thankfully acknowledge the Director, ICAR-IINRG, Ranchi for his kind help and timely facilitating this study for its successful completion.
References
- 1.Baboo B, Goswami DN. Lac: an introduction. In: Baboo B, Goswami DN, editors. Processing, chemistry and applications of lac. New Delhi: Directorate of Information and Publications of Agriculture, Indian Council of Agricultural Research; 2010. pp. 1–6. [Google Scholar]
- 2.Srivastava S, Ray DP, Pandey SK, Prasad KM, Prasad M and Baboo B (2013) Pure lac dye: a potential natural food additive. Int J Emerg Technol Adv Eng 3:589–594
- 3.Chairat M. Thermodynamics study of lac dyeing of silk yarn coated with chitosan. Walailak J Sci Technol. 2009;6:93–107. [Google Scholar]
- 4.Pandhare ED, Rama Rao AV, Srinivasan R, Venkataraman K. Lac pigments. Tetrahedron. 1966;8:229–239. doi: 10.1016/S0040-4020(01)82186-4. [DOI] [Google Scholar]
- 5.Pandhare ED, Rama Rao AV, Shaikh IN, Venkataraman K. The constitution of laccaic acid B. Tetrahedron Lett. 1967;26:2437–2440. doi: 10.1016/S0040-4039(00)90827-X. [DOI] [Google Scholar]
- 6.Pandhare ED, Rama Rao AV, Shaikh IN. Lac pigments: part III—isolation of laccaic acids A and B and the constitution of laccaic acid A. Indian J Chem. 1969;7:977–986. [Google Scholar]
- 7.Rama Rao AV, Shaikh IN, Venkataraman K. Laccaic acid C, the first natural anthraquinone with an amino acid side chain. Indian J Chem. 1968;7:188–189. [Google Scholar]
- 8.Bhide NS, Pandhare ED, Rama Rao AV, Shaikh IN, Srinivasan R. Lac pigments: part IV—constitution of laccaic acid B. Indian J Chem. 1969;7:987–995. [Google Scholar]
- 9.Maurya S, Srivastava JS, Jha RN, Pandey VB, Singh UP. Efficacy of alkaloid (−)-corypalmine against spore germination of some fungi. Folia Microbiol. 2002;47:287–290. doi: 10.1007/BF02817654. [DOI] [PubMed] [Google Scholar]
- 10.Singh, UP, Maurya S, Singh DP (2003) Antifungal activity and induced resistance in pea by aqueous extract of vermicompost and for control of powdery mildew of pea and balsam. J Plant Dis Prot 110:544–553. https://www.jstor.org/stable/43215548?seq=1#page_scan_tab_contents
- 11.Verma R, Choudhary AK, Datta A and Maurya S (2015) Antimicrobial potential of an animal peptide and some antibiotics against a dreaded soil borne phytopathogen Ralstonia solanacearum. International Journal of Current Research 7:23015-23022. http://www.journalcra.com/sites/default/files/11347.pdf
- 12.Agarwal SK, Sudhir SS, Verma S, Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol. 2000;72:43–46. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 13.Khan SA, Ahmad A, Khan MI, Yusuf M, Shahid M, Manzoor N, Mohammad F. Antimicrobial activity of wool yarn dyed with Rheum emodi L. (Indian Rhubarb) Dyes Pigments. 2012;95:206–214. doi: 10.1016/j.dyepig.2012.04.010. [DOI] [Google Scholar]
- 14.Arndt F. Diazomethane. Org Synth. 1935;15:3. doi: 10.15227/orgsyn.015.0003. [DOI] [Google Scholar]
- 15.Yamada S, Noda N, Mikami E, Hayakawa J, Yamada M. Analysis of natural coloring matters in food. III. Application of methylation with diazomethane for the detection of lac color. J Assoc Off Anal Chem. 1989;72:48–51. [PubMed] [Google Scholar]
- 16.Nacowong P, Saikrasun S. Thermo-oxidative and weathering degradation affecting coloration performance of lac dye. Fash Text. 2016;3:18. doi: 10.1186/s40691-016-0070-0. [DOI] [Google Scholar]
- 17.Nishizaki Y, IshizukiK Akiyama H, Tada A, Sugimoto N, Sato K. Preparation of a ammonia-treated lac dye and structure elucidation of its main component. Food Hyg Saf. 2016;57:193–200. doi: 10.3358/shokueishi.57.193. [DOI] [PubMed] [Google Scholar]
- 18.Singh UP, Prithiviraj B, Wagner KG and Plank-Schumacher K (1995) Effect of ajoene, a constituent of garlic (Allium sativum), on powdery mildew (Erysiphe pisi) of pea (Pisum sativum). J Plant Dis Prot 102:399–406. https://www.jstor.org/stable/43386857?seq=1#page_scan_tab_contents