Abstract
Plants are confronted with a variety of environmenmtal stresses resulting in enhanced production of ROS. Plants require a threshold level of ROS for vital functions and any change in their concentration alters the entire physiology of plant. Delicate balance of ROS is maintained by an efficient functioning of intriguing indigenous defence system called antioxidant system comprising enzymatic and non enzymatic components. Down regulation of antioxidant system leads to ROS induced oxidative stress causing damage to important cellular structures and hence anomalies in metabolism. Proper mineral nutrition, in addition to other agricultural practices, forms an important part for growth and hence the yield. Potassium (K) is a key macro-element regulating growth and development through alterations in physiological and biochemical attributes. K has been reported to result into accumulation of osmolytes and augmentation of antioxidant components in the plants exposed to water and salt stress. In the present review an effort has been made to revisit the old findings and the current advances in research regarding the role of optimal, suboptimal and deficient K soil status on growth under normal and stressful conditions. Effect of K deficiency and sufficiency is discussed and the information about the K mediated antioxidant regulation and plant response is highlighted.
Keywords: ROS, Antioxidants, Osmolytes, Polyphenols, Potassium, Stress amelioration
Introduction
Abiotic stresses affect the growth and productivity of crop plants (Tiwari et al. 1998; Agarwal et al. 1999; Jatav et al. 2014; Ramegowda and Senthil-Kumar 2015). Salinity, water deficit, high temperature, cold and freezing stresses are detrimental to the plants reducing their yield. Hence, for sustaining food security, due consideration should be given to the evaluation and amelioration strategies to be adopted (Fracasso et al. 2016).
Stress induced production of ROS results in anomalies in several important cellular biochemical pathways/reactions which operate in cellular organelles like chloroplast and mitochondria (Miyake 2010) activating calcium (Ca2+) and potassium (K+) permeable cation channels at the plasma membrane thereby mediating Ca2+ based signaling events, K+ ion leakage and PCD. Potassium efflux channels activated by ROS include GORK, SKOR and annexins resulting in considerable K+ efflux through these channels thereby causing dramatic K+ loss from plant cells. This ROS induced loss of K+ ions stimulate activity of proteases and endonucleases promoting PCD (see Demidchik et al. 2014).
Plant cells are equipped with well evolved antioxidant defence system to avoid ROS induced damage maintaining the redox state of cell which help in scavenging ROS, minimizing the adverse effects of indigenously generated ROS. Cell have one or more antioxidants in every organelle acting on a particular ROS to detoxify it (Ahmad et al. 2010). Enhanced synthesis and accumulation of low molecular weight compounds is another important strategy adapted by plants exposed to water and salinity stresses. Plants tend to accumulate the free amino acids, free proline, non-structural carbohydrates and quaternary ammonium compounds (betaines) to cope up with stressful conditions (Jatav et al. 2014; Tomar et al. 2015). These compounds are usually called as osmoprotectants and their accumulation reduces osmotic potential and therefore help in restoration and maintenance of the potential gradient between the plant cell and external soil solution. Puniran-Hartley et al. (2014) have observed a strong correlation between enhanced accumulation of compatible osmolytes and oxidative damage tolerance in salt stressed wheat and barley seedlings.
Potassium mitigates the negative effects of water stress by enhancing the translocation and bringing water balance maintenance. K is actively implicated in several basic biochemical and physiological functions such as stomatal movement, enzyme activation, protein synthesis, photosynthesis, osmoregulation, phloem loading and in reduction of excess uptake of ions like Na (Zorb et al. 2014; Erel et al. 2015). In cells, K helps to maintain the transmembrane voltage gradients, cytoplasmic pH and the transport of metabolites and inorganic anions (Zorb et al. 2014). Plants growing on K deficient agricultural soils employ several strategies to maintain optimal required quantity of K which include enhanced potential for high-affinity K+ uptake from the soil, redistribution of potassium ions between the cytosolic and vacuolar pools, cytosolic homeostasis and modifications in the development and architecture of root system (Cherel et al. 2014).
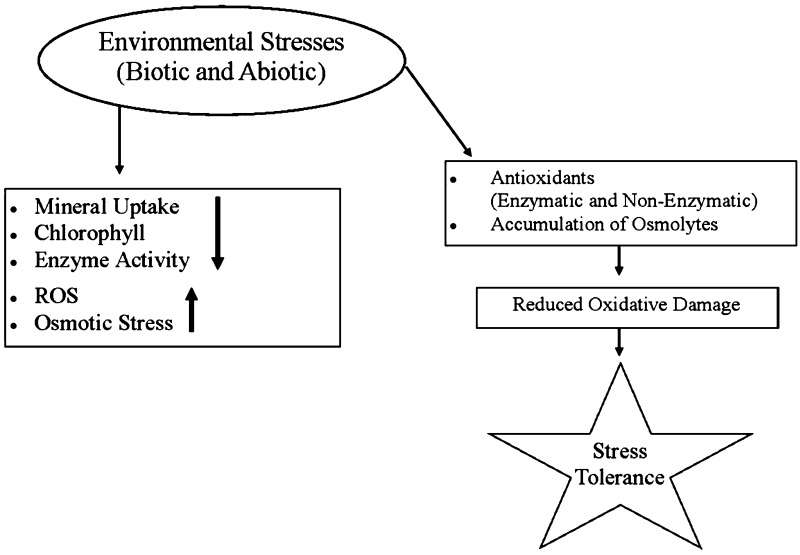
Plant growth under salinity and water stress is influenced by the ability of plant to control K+/Na+ ratio in the tissues (Jatav et al. 2014). Severity of deleterious effects of sodium are greater under low potassium concentrations and plants having efficient activity of potassium transporters maintain optimal levels of potassium required for normal growth under such conditions (Bacha et al. 2015). Present review encompasses stress (mainly abiotic) (Fig. 1) and potassium induced changes and possible alleviation of ill effects of stress by added potassium.
Fig. 1.
Fow chart indicating stress-induced changes and associated tolerance mechanisms in plants
Plant growth under abiotic stresses and the underlying tolerance mechanisms
Prevailing environmental conditions affect the growth, development and distribution of plant species. Extreme environmental conditions initiate numerous responses leading to changed gene expression pattern and cellular metabolism resulting in hampered growth rate and yield. Tolerance of individual plant species is a cumulative effect of several attributes which are under direct control of expression of genes leading to either stress acclimatization or hardening. Plants experience water stress when water availability to roots is low and transpiration rate exceeds absorption. Reduced water availability affects sink and source, reducing the yield potential which varies with the timing and the severity of stress with respect to plant phenology (Blum 1996). Different responses are provoked in plants to counter water deficiency during different phases of growth with the generative phase and initiation of flowering the most sensitive ones. In order to circumvent the adverse conditions, plants can employ strategies like escape, avoidance and tolerance (Touchette et al. 2009).
Water stress and plant growth
Water deficit conditions can be defined as a situation in which water potential of the plant is reduced to such levels that hinder the normal cellular functions. Water stress is often characterized by reduction in cell water content, turgor loss leading to wilting, stomatal closure and decreased cell enlargement. Exposure of plants to severe water stress may result in the arrest of photosynthetic and drastic disturbances in normal metabolism resulting in cessation of growth or death (Khan et al. 2015). Extreme stress conditions such as water deficit initiates a wide variety of morphological, physiological and developmental changes which probably enable them to survive and perform better. Physiological and biochemical responses that are elicited due to deficient irrigation have direct impact on the yield attributes (Agarwal et al. 1999; Jatav et al. 2014; Ahanger and Agarwal 2017a).
Water stress induced reduction in nutrient uptake in plants is ascribed to reduced root hydraulic conductance, root length, root surface area and impeded root hair growth because of increased production of ABA in dehydrating roots leading to ABA induced stomatal closure and consequent reduced transpiration rate. For improving water use efficiency at critical stages, both efficient root system and soil have important roles to play (Vadez 2014). Reduced root and shoot hydraulic conductivity under water stress conditions can be due to the interruptions in the xylem water column. Under high vapor deficit conditions plants control water use through controlled leaf area development and stomatal movements which are in turn controlled by hydraulic processes. Availability of adequate quantity of soil moisture content at different important growth stages of plant optimizes the efficiency of important metabolic processes and also increases the effectiveness of the supplemented mineral salts on crop plants (Rahil and Qanadillo 2015). Water deficit reduces chlorophyll content by affecting the chloroplast membranes, promoting swelling and distortion of the lamellae vesiculation and the formation of lipid droplets (Kaiser 1982). Reduction in cholorphyll content may be responsible for causing decline in other photosynthetic attributes in water stressed Gossypium herbaceum L. (Deeba et al. 2012). Water deficit disturbs the basic physiological processes such as photosynthesis which is attributed to the limited access to external CO2 due to decreased permeability of stomata and altered activity of the enzymes participating in photosynthesis (Karimi et al. 2015).
Pandey et al. (2004) found water stress to reduce the activity of aspartate aminotransferase and alanine aminotransferase and an increase in protease activity concomitant with increased free proline in Oryza sativa L. Proteomic studies have revealed differential regulation of drought stress responsive proteins, transcription factors, signalling associated kinases, antioxidant enzymes and ion transport channels (Deeba et al. 2012). Drought reduces the seed germination, alters seed anatomy and the productivity (Chamorro et al. 2016).
Salt stress and plant growth
Increasing salinity continuously leads to conversion of arable agricultural land into unproductive waste lands. Significant proportions of irrigated land all over the world are salt affected reflecting on average yields of major food crops (Sairam and Tygai 2004). Salts at lower concentration suppress the growth whereas higher concentration of salts can lead to death of the plant. Salinity induced alterations in growth and development of plants are due to its cumulative effect on several physiological as well as biochemical attributes like mineral ion homeostasis, water balance, osmolyte accumulation, antioxidant metabolism, nitrogen fixation and photosynthetic capacity of plants (Iqbal et al. 2015). Saline soils are characterized by high pH, sodium adsorption ratio, exchangeable sodium percentage and electrical conductivity. Synergistic influence of edaphic as well as prevailing environmental factors like temperature, existing soil profile, rainfall and water table aggravate the problem. Excess accumulation of Na ions or increase in soil salinity causes reduction in soil stability resulting in dispersion of soil particles (Yadav et al. 2011). Normally saline stress conditions arise when salt concentrations in soils or plants surpass the threshold level. Na and Cl ions taken up by plants in large amount impair the normal physiology of plants by altering the metabolic processes (Iqbal et al. 2015; Ahanger and Agarwal 2017b).
Increasing tolerance to osmotic stress and efficient Na+ and Cl− exclusion or tolerance to the excess Na+ and Cl− (that gets accumulated) can help withstand such stresses (see Munns and Tester 2008). Besides, it affects the uptake of many fundamental nutrients thereby affecting the photosynthetic electron transport system to a large extent having direct bearing on photosynthesis. Salinity stress induced reduction is evident in photosynthetic attributes like net photosynthesis, intracellular CO2 concentration, quantum efficiency of PSII and activity of Rubisco (Khan et al. 2009; Iqbal et al. 2015). D1 and associated proteins form important constituents of photodamage repair cycle (Mittal et al. 2012).
Salt tolerance is the consequence of molecular cascades mediating stress sensing, transduction and expression of stress responsive genes resulting in the production of several protective metabolites (Turkan and Demiral 2009). Compartmentalization preferably into the vacuole by the active participation of ion transporters, proton pumps and osmotic constituents like proline, glycine betaine etc. contribute towards tolerance mechanisms against excess Na and Cl in the soil. Tolerance to salinity induced osmotic stress is achieved by the long distance signals, may be due to ROS induced signal regulation (Mittler et al. 2011) and ion exclusion mechanism predominantly operating in roots inhibiting accumulation of Na+ and Cl− ions in the leaves thereby maintaining their levels below the toxic concentrations. Ion exclusion requires active participation of transporters at plasma membrane and tonoplast level (see Roy et al. 2014). HKT and SOS (SOS1, SOS2, SOS3) pathway have a pivotal role in maintaining the Na+ transport and homeostasis within the plant. Efficient functioning of HKT mediates the maintainence of high K concentration in cytosol whereas SOS1 acts as Na+/H+ antiporter. Tonoplast Na+/H+ exchanger helps plants improve tolerance by sequestering Na ions into vacuoles. Maintainence of Na and Cl concentrations below toxic levels through the exclusion can also be achieved by retrieving excess toxic ions from xylem, compartmentalizing into the vacuole of root cortex and effluxing into the soil (Zhu 2003; Roy et al. 2014). Transgenic finger millet plants overexpressing the vacuolar H+ pyrophosphatase exhibited enhanced salt tolerance by maintaining the higher levels of K as compared to Na, showing direct impact on the antioxidant enzyme system thus preventing the oxidative damage to membranes (Anjaneyulu et al. 2014). Fan et al. (2015) have demonstrated salt stressed potato plants to maintain the increased expression of vacuolar Na/H antiporter thereby maintaining the pace of compartmentalizing Na and preventing the possible cellular damage. Increased Na/H antiporter has positive associations with the tissue K concentration resulting in increased K/Na ratio. Himabindu et al. (2016) have suggested halophyte salt tolerant genes encoding SOS, NHX, Na/K, energy related pumps like plasma membrane and vacuolar H+-ATPase, vacuolar H+-PPase and potassium transporters like HKT to be the potential targets worth exploring for understanding and improving the mechanism of tolerance in glycophytes. Expression of these transport proteins is modulated by the external salt concentrations and internally regulated by several genes. Transgenic rice overexpressing vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from halophyte grass Spartina alterniflora exhibited greater salt tolerance by maintaining higher K/Na ratio reflecting in growth enhancement by affecting photosynthesis, the cellular expansion, modulations in ABA-mediated signalling and the expression of ion transporters like NHX (Baisakh et al. 2012).
Halophytes present an ideal model for understanding the complex tolerance mechanisms at physiological and genetic levels. In future halophyte and forage species may serve as alternative for farming on salinized soils and their restoration to productive soils (Panta et al. 2014).
ROS: production and significance
Molecular oxygen receives electrons from high energy level to produce ROS which are deleterious to plant cells at high concentration. Under severe stress conditions, rate of ROS generation exceeds the scavenging potential of cellular defence system resulting in oxidative stress. Oxidative stress damages cellular components resulting in their dysfunction and ultimately cell death. ROS includes H2O2, O2 − as well as free radicals i.e., 1O2, O2 2−, O2H, OH−, RO− etc., which are toxic to plant metabolism (Gill and Tuteja 2010; del-Rio 2015) affecting every cellular macromolecule including DNA (Tuteja et al. 2009). Another class of ROS includes RNS, which include NO, NO2, peroxinitrite etc. which have been shown to be involved in plant stress reactions (Qiao et al. 2014; del-Rio 2015). Nitric oxide shows important cross-talk association with other radicals including H2O2 eliciting stress signal in response to several abiotic stresses including water and salinity which subsequently result in induction of tolerance mechanisms (Qiao et al. 2014).
Under restricted water supply conditions, internal CO2 level is reduced due to early stomatal closure and continuous exposure to sunlight causes transfer of electrons to molecular oxygen resulting in generation of superoxide ions at PSI by the process called as Mehler reaction (Asada 2006). ROS induced damage to DNA leads to genotoxic stress (Tuteja et al. 2009). In addition, stresses provoke damage to membrane PUFA either enzymatically by activating lipoxygenases and cyclooxygenases or non-enzymatically by ROS. Enzyme mediated oxidation of PUFA gives HPODEs. Inactivation of lipoxygenases paves path for radical mediated peroxidation (Wiesner et al. 2003). ROS attack the membrane PUFA resulting in their breakdown into small hydrocarbon fragments like ketones or MDA or other related product (Garg and Manchanda 2009). Lemna gibba L. subjected to salt stress exihibit strong correlation between enhanced ROS and inhibition in the activities of PSI, PSII and the photosynthetic electron transport chain (Oukarroum et al. 2015). Besides being toxic to plant metabolism, ROS controls different processes in plants when present below threshold concentrations. Explicit evidence and reports are available suggesting that ROS integrate signalling pathways involved in maintaining plant growth, development, phytohormone action (see Mittler 2002; Mittler et al. 2011; Baxter et al. 2014) and many other physiologically important phenomena like pathogen defence, PCD, stomatal behavior, responses to biotic and abiotic stresses (del-Rio 2015).
Antioxidant system
Excess production of ROS causes oxidative stress leading to cellular damage and ultimately cell death. To prevent or alleviate the ROS induced damage allowing the beneficial functions of ROS to continue, plants have evolved an intriguing antioxidant defence system which functions to keep levels of reactive or active oxygen species under control. Antioxidant defence systems comprises both enzymatic as well as non-enzymatic components.
Enzymatic components
Toxic radicals produced at the site of PSI are scavenged by a series of sequential and parallel reactions called as water–water cycle (WWC) or Mehler-ascorbate peroxidase (MAP) pathway. Several physiologically important functions of the WWC contributing to the regulation of photosynthesis as well as mitigation of photoinhibition, mediating acclimatization to environmental stresses include: (1) ROS scavenging in chloroplasts, (2) electron sinks, (3) dissipation of excess excitation energy, and (4) ATP supply for CO2 assimilation in photosynthesis (Ort and Baker 2002; Miyake 2010).
SOD catalyses dismutation of O2 − into H2O2 and is frontline defence against the oxidative stress reducing the chances of formation of OH radicals through metal catalysed Haber-Weis reaction. Water stress increases expression of Mn-SOD, Fe-SOD and Cu/Zn-SOD isozymes in Flaveria (Uzilday et al. 2014). Catalases (CATs) are mainly found in the peroxisomes and are tetrameric heme containing enzymes mediating conversion of H2O2 to O2 and H2O. Broadly speaking plant catalases have been classified into three major classes i.e., catalases which are most prominently found in the photosynthetic tissues and mediate dismutation of H2O2 generated during photorespiration, the other class of catalases are often found in the vascular tissues having a probable role in lignification whereas the third class of catalases are abundant in seeds and young plants and are involved in the removal of excess H2O2 generated in the glyoxylate cycle, during the fatty acid degradation, in glyoxisomes (Willekens et al. 1994). Exposure to stressed conditions causes proliferation of peroxisomes resulting in diffusion of H2O2 from cytosol into peroxisomes (Lopez-Huertas et al. 2000).
Ascorbate–glutathione cycle operating in chloroplasts, mitochondria, apoplast, cytosol and peroxisomes is another important ROS scavenging pathway i.e., Foyer–Halliwell–Asada pathway. This cycle has a crucial role in maintaining the balance of ROS within harmless levels helping thereby in averting the stress induced oxidative damage. Like WWC, ascorbate–glutathione cycle comprises both enzymatic as well as non enzymatic components. APX, GR, MDHAR and DHAR are the key enzymes linking to the non-enzymatic antioxidant metabolites, ascorbic acid (AsA) and glutathione (GSH). This cycle also operates for scavenging of H2O2 where net electron flow is from NADPH to H2O2 (Noctor and Foyer 1998; Mittler 2002). In the process of detoxification through ascorbate–glutathione pathway, APX reduces H2O2 to water using two molecules of ascorbate with the concomitant generation of equal number of MDHA. MDHA can also directly be reduced to ascorbate by enzyme MDHAR and either reduced ferredoxin or NAD(P)H may serve as electron donor (Mittler 2002). DHA is reduced back into ascorbate by the enzyme DHAR using GSH as reductant. During this reaction, GSSG is generated which is again re-reduced to GSH by GR using NADPH electron donor. Hence, ascorbate and glutathione only participate in the cyclic transfer of reducing equivalents thereby contributing to the reduction of H2O2 to H2O using electrons from NADPH.
Antioxidant enzymes including SOD, CAT and APX are important for maintaining the steady state level of superoxide and hydrogen peroxide radicals thereby preventing the formation of more toxic hydroxyl radical via the Fenton or metal-dependent Haber–Weiss reactions. As compared to CAT, APX shows greater affinity for H2O2. In plants five isozyme forms of APX exist, which include thylakoidal and microsomal membrane bound forms, chloroplastic stromal soluble form and cytosolic and apoplastic form (Caverzan et al. 2012). GR is an important enzyme of ascorbate–glutathione cycle contributing for the maintainence of GSH pool by catalyzing the NADPH-dependent reduction of oxidised glutathione (GSSG). GR predominantly exists in chloroplast stroma and rarely in mitochondria, peroxisomes and cytoplasm. In addition to mediating H2O2 scavenging, GR confers stress tolerance through maintaining the GSH levels which itself is not only an important component of ascorbate–glutathione cycle but also serves as substrate for other enzymes like GST. The enhanced activity of GR increases ratio of NADP/NADPH ensuring availability of NADP- for accepting electrons from the photosynthetic electron transport chain and minimizing the generation of O2 − by bringing down the electron flow to O2 (Ahmad et al. 2010). GST, the glutathione transferases, are another important large and important group of non photosynthetic enzymes catalysing the conjugation of electrophilic substrates (mostly xenobiotics) with the glutathione. Mostly found in cytosol, GST enzymes have the ability to bind non-catalytically to a range of endogenous and exogenous ligands. GST mediates several functions like detoxification of herbicides and H2O2, tyrosine metabolism, regulation of apoptosis, hormonal homeostasis and responses to stresses as well (Dixon et al. 2010).
Non enzymatic components
Ascorbic acid (AsA)
AsA, a water soluble vitamin commonly called vitamin C, is most essential metabolite for plants. Plants maintain relatively good levels of ascorbate with concentrations in leaves ranging from 1 to 5 mM and going up to 25 mM in chloroplasts (Wheeler et al. 1998). In plants, AsA serves as a cofactor for enzymes involved in photosynthesis, biosynthesis of phytohormones, anthocyanins, secondary metabolites and the regeneration of antioxidants like α-tocopherol (Gallie 2013). In chloroplasts, high ascorbate levels are required to maintain the photosynthetic rate and transmembrane electron transport (Wheeler et al. 1998) and overcome photoinhibition caused by strong light (Miyaji et al. 2015). AsA is synthesized in mitochondria and subsequently transported to other cellular components either through proton-electrochemical gradient or facilitated diffusion (Horemans et al. 2000).
AsA protects metabolic processes against the deleterious impact of H2O2 and other toxic radicals derived from oxygen. During free radical scavenging, AsA acts essentially as reductant e.g., two molecules of AsA are utilized by APX in ascorbate–glutathione cycle to reduce H2O2 to water. AsA protects membranes either by directly scavenging the toxic radicals like 1O2, O2 − and OH− or indirectly by regenerating α-tocopherol from tocopheroxyl radical and synthesizing zeaxanthin in the xanthophyll cycle (Eskling et al. 1997). Plants exposed to drought (Alam et al. 2014; Shafiq et al. 2015) and salinity (El-Sayed et al. 2014; Shan and Zhao 2014) had increased AsA content. Rasool et al. (2012) have demonstrated that tolerant cultivars maintain higher AsA concentrations. Maintainence of cellular redox homeostasis prevent excessive reduction and oxidation (see Foyer and Noctor 2011). Rice seedlings defective in AsA biosynthetic pathway exhibited increased oxidative damage to membranes (Liu et al. 2013). Potassium supplementation resulted in increased AsA contents possibly strengthening the antioxidant system and reflecting on yield (Shrivastava et al. 2016).
Reduced glutathione
GSH, a tripeptide (γ-glutamyl-cysteinyl-glycine; γ-Glu-Cys-Gly) is an abundant, ubiquitous thiol and has been observed in all cellular locations including cytoplasm, chloroplasts, mitochondria, endoplasmic reticulum and vacuoles. It is believed to play a key role in triggering the expression of stress-responsive genes (Mullineaux and Rausch 2005). It also has an important role in adaptations to different environmental stresses which result in its altered cellular concentration. Glutathione and other thiols are involved in integrative control of the photorespiratory and respiratory metabolism and also in bringing about modulation in the phytohormone mediated signalling by triggering the proper modifications of sensitive protein cysteine residues (Noctor et al. 2012). GSH plays a significant role in the event of growth and development such as; cell differentiation, senescence, cell death, pathogen resistance and enzymatic regulation. Under metal stressed conditions, GSH serves as a precursor for the synthesis of important phytochelatins (Flores-Caceres et al. 2015).
Besides scavenging H2O2, GSH reacts non-enzymatically with ROS including O2 −, 1O2 and OH− radicals (see Noctor and Foyer 1998). Active participation of glutathione in protection of photosynthetic system from deleterious impact of salt stress with amelioration of oxidative damage has been observed in Brassica juncea L. (Fatma et al. 2014). Increase in GSH under water stress conditions helps maintain the optimal activities of ascorbate–glutathione cycle enzymes (Uzilday et al. 2014). Interaction of GSH with important growth hormones like salicylic acid and ethylene has also been reported (Ghanta et al. 2014). In rice, Cao et al. (2015) have noticed amelioration of cadmium stress induced deleterious changes in photosynthetic and yield attributes due to exogenous application of GSH. GSH treated seedlings showed reduction in the accumulation of cadmium in edible parts. Ahanger and Agarwal (2017a) have reported potassium induced improvement in reduced glutathione under water deficit conditions.
Plant secondary metabolites
Phenolic or polyphenolic compounds constitute a large and chemically diverse group of plant secondary metabolites such as; phenylpropanoids and their polymers including lignins, tannins, flavonoids, isoflavonoids, coumarins and anthocyanins. Prime pathway operating in plants leading to biosynthesis of these polyphenols is shikimic acid pathway in addition to mevalonic acid pathway. Accumulation of phenolic compounds is regulated by a variety of environmental factors like water availability (Tomar and Agarwal 2013), atmospheric CO2 levels (Goufo et al. 2014), wounding, herbicides, insect herbivory (Gols 2014), nutrient deficiencies (Giorgi et al. 2009) as well as nutrient availability (Ahanger et al. 2015), exposure to UV radiations (Yao et al. 2015), plant developmental stage and the prevailing environmental conditions (Tomar et al. 2015).
Phenols can chelate toxic metals to restrict formation of toxic radicals through Fenton reaction (Rice-Evans et al. 1997). Interestingly, another important mechanism underlying the antioxidant property of polyphenolics, especially flavonoids, is their ability to alter peroxidation kinetics by bringing modification in the order of lipid packing arrangement thereby decreasing the fluidity of the membranes (Arora et al. 2000). Such changes can bring steric restriction in free radical diffusion resulting in lesser chances of peroxidative reactions. Binding and chelation to polyphenols has been observed in water lily rhizomes treated with chromium, lead and mercury (Lavid et al. 2001). In plants, phenolics donate electrons to guaiacol-type peroxidases for the detoxification of H2O2 conferring on them antioxidant property (Sakihama et al. 2002). During the phenolics mediated scavenging of toxic radicals, phenoxyl radical intermediates are formed. These phenoxy radical intermediates are relatively stable and do not propagate radical reactions further and can either terminate the radical chain reaction by interacting with other free radicals or are recycled back to parent phenolics either enzymatically or non enzymatically (Kagan and Tyurina 1998). Polyphenols including glycosides, derivatives of hydroxycinnamic acid and flavonoids are involved in secondary cell wall thickening therefore increasing the mechanical strength of tissues which is one of the key anatomical features for improving water deficit tolerance. Polyphenol induced strengthening of cell wall coupled with their related functions may induce enhanced resistance to biotic as well as abiotic stress induced oxidative damage (Agati et al. 2012). Potassium supplementation and application of salicylic acid to barley improved the drought and salinity tolerance by enhancing the accumulation of phenolics (Fayaz and Bazaid 2014). Potassium enhanced the synthesis of polyphenols including tannins and improved growth under water stress (Tomar and Agarwal 2013; Ahanger and Agarwal 2017a).
Phenolics lead to the maximal K+ retention within guard cells for the maintenance of major functions of stomata and such functions vary with type of phenol leading to reverse the ABA-induced stomatal closure which is more often initiated by K+ efflux (Purohit et al. 1992). Synthesis of secondary metabolites is controlled by transcription factors including MYB, HLH, ERF, WRKY, zinc finger, NAC etc. leading to integration of external and internal cues by acting on the cis-binding elements and modulating enzymes involved in metabolite synthesis (Yang et al. 2012). Environmental stresses like drought, high salinity, temperature and water logging significantly affect the synthesis and accumulation of important phenolic compounds. Altered contents of polyphenols can be beneficial for plant adaptations to stressful conditions. Water stress increased the expression of PAL genes in lettuce (Oh et al. 2009). Fagopyrum esculentum plants exposed to increasing salt concentration showed concentration dependent increase in accumulation of phenols imparting higher DPPH free radical scavenging potential (Lim et al. 2012). Exogenous application of coumarins enhances the production and accumulation of important phenolic compounds as well as the antioxidant potential concomitant with increased PAL activity in wheat growing under salt stressed as well as normal conditions (Saleh and Madany 2015). Barley exposed to water/salinity stress accumulated phenols significantly higher resulting in quick removal of toxic radicals like superoxide (Ahmed et al. 2015).
Pretreatment of cucumber seedlings with ferulic acid enhanced the water stress tolerance by enhancing the activity of antioxidant enzymes resulting in the quick scavenging of ROS and reduced oxidative stress. Ferulic acid treatment in conjunction with PEG stress given to seedlings results in higher transcript levels of the antioxidant genes as compared to the treatments of PEG alone. Increased accumulation of osmotic constituents like proline, free sugars due to ferulic acid treatment mediated the active water uptake resulting in the maintainence of RWC (Li et al. 2013).
Potassium and plant growth
K availability, transport and transporters
Plants require relatively large amounts of macroelements to carry out various cellular functions. Potassium is one amongst the macroelements standing third in the list of importance and requirement to plants. Plants absorb the important elements from the soil solution in the form of ions and potassium is available to plants as K+. Nearly 2.6% potassium is present on the earth’s crust and in soil, majority of the K+ remains either dehydrated or coordinated to oxygen atoms which is unavailable to plants (see Maathuis 2009). Both nutrient dynamics as well as the total soil potassium pool affect the K+ availability to plants for absorption in addition to the physico-chemical properties and type of the soil (see Agarwal et al. 2009; Zorb et al. 2014).
Extraction of K+ from soil solution and its subsequent distribution within the plant tissue system requires efficient membrane bound transport proteins. Molecular studies have demonstrated the complexity of K+ transport and within last few years, a number of transporters mediating K uptake and compartmentalization have been identified. Physiological roles of these transport proteins in influx, efflux, compartmentation and transport of K within the plant have been characterized to certain degree. Transporter proteins involved in maintaining the homeostasis within the plant cell include (a) transport proteins mainly operating at the soil: root interface like AKT, CNGC, KUP/HAK and channel mediated K+ release (GORK), (b) transporters involved in xylem loading which is mainly mediated by K+ selective (SKOR) or less operative non selective cation channels (NSCC), (c) K+ loading in phloem involving AKT channel and (d) vacuolar accumulation and release is driven primarily by H+-coupled antiporters such as NHX and tonoplast TPK1 respectively. Under potassium starvation conditions, vacuolar K+ is released by the active participation of H+ coupled high affinity K+ (KUP/HAK) transporters (see Ahmad and Maathuis 2014; Shabala et al. 2016). Under K deficient and water stressed conditions expression of high affinity K transporter (KUP/HAK) genes is enhanced contributing towards maintenance of differential homeostasis in plant tissues (Song et al. 2015).
Role of K in plants and impact of K deficiencey
Low K status triggers expression of high affinity K transporters and associated signalling cascades. ROS molecules and phytohormones like auxin, ethylene, jasmonic acid may have important role in sensing the K deficiency (Ashley et al. 2006). Potassium deficiency can reduce plant resistance to both biotic as well as abiotic stresses and sufficient potassium promotes pathways and mechanisms including increased accumulation of compatible organic osmolytes, enhancing enzyme activities and also helping in maintainence of higher K/Na ratio thus providing strategy for maintenance of growth and yied (Tiwari et al. 1998; Sharma et al. 2006; Tomar and Agarwal 2013; Jatav et al. 2014; Ahanger et al. 2015).
In addition to its critical role in photoassimilate loading into the phloem tissues, potassium has profound role in maintaining the activity of key metabolic enzymes (Anschutz et al. 2014; see Erel et al. 2015). Under potassium deficient conditions, K ions are also effluxed from the guard cells into the apoplast because keeping stomata open may be difficult in such conditions (Ahmad and Maathuis 2014). Potassium salts induce positive changes in photosynthetic attributes like net CO2 assimilation rate, stomatal conductance, intercellular CO2 concentrations, mesophyll conductance (Tiwari et al. 1998), chloroplastic CO2 concentrations and electron transport system (Xiao-guang et al. 2015).
Roots are the first main organs to experience and sense the deficiency of potassium. Root hair and epidermal cell plasma membranes sense the K deficiency and transduce subsequently to the cell cytosol. Membrane potential, ROS and phytohormones are involved in short term responses after hours of exposure to K deficiency as compared to long term responses that take much time to express at the metabolic and morphological levels (Wang et al. 2013).
Potassium and stress tolerance
Studies conducted by employing electrophysiological techniques in tobacco have indicated threshold requirement of K for the cell cycle progression (Sano et al. 2007). K starved plants are often accompanied by increased non-photochemical quenching of Chl fluorescence and reduced efficiency of excitation transfer as well as the photochemical quenching coefficient (Weng et al. 2007). Under water stress conditions K deficiency causes restriction in stomatal movements as has been observed in sunflower (Benlloch-Gonzalez et al. 2010). Adequate K levels are important for preventing oxidative damage through regulation of stomatal movements, osmoregulation and water use efficiency (Shabala and Pottosin 2014). Gimeno et al. (2014) have demonstrated enhanced drought stress tolerance in K treated Citrus macrophylla L. plants. Crop cultivars sensitive to water stress exhibit less phosynthetic rates due to their reduced K uptake potential (Song et al. 2015). Martineau et al. (2017) have reported significant role of K in optimizing Zea mays yield through its active involvement in the improvement of growth attributes like leaf area, height, water potential maintenance, photosynthetic characteristics, WUE and the grain filling.
Under short and long term drought stress conditions, K and Ca efflux from the leaf mesophyll can serve as chemical signal indicating the intensity of stress. Such efflux of K has also been observed in salinity stressed barley plants (Britto et al. 2010). K starvation directly affects water status of plants by reducing the activity of root aquaporins and suppressing hydraulic conductivity. Reduced hydraulic conductivity results in decreased cellular expansion by restricting sufficient water supply to growing tissues and to leaf for transpiration (Kanai et al. 2011). K reduces ROS generation by inhibiting the NADPH-oxidase (Cakmak 2005) therefore leading to reduced lipid peroxidation (Hernandez et al. 2012). Under salinity (Zheng et al. 2008) and water stress (Ahanger and Agarwal 2017a), K improves growth strengthening antioxidant system and bringing down the oxidative-stress-induced peroxidation of membrane lipids.
Improved salt tolerance is linked with enhanced ability to discriminate between K+ and Na+ in the soil solution during their transport to the shoot. Preferential accumulation of K+ and exclusion or sequestration of Na+ into the less sensitive organelles or cell spaces like vacuole or apoplast is also important. Chen and Polle (2010) have identified the key mechanisms favoring salt tolerance in plants. In root cells, compartmentalisation of chloride (Cl) ions into the vacuole, reduced NaCl loading in xylem, Na+ extrusion into the soil solution and prevention or reduction of excessive loss of K+ by regulating the depolarisation-activated cation channels contribute to salt tolerance. Shoot, leaf cells preferably compartmentalize Na ions into vacuole or apoplast and such mechanisms improve K+/Na+ balance, an important prerequisite for increased salt tolerance. Ability of plant cultivars to retain the leaf mesophyll K by regulating its excessive efflux through plasma membrane is an important trait of salt tolerance (Wu et al. 2013). Plants prefer to maintain concentrations of Na below the toxic levels particularly in the upper plant parts as compared to root (Iqbal et al. 2015; Ahanger et al. 2015; Ahanger and Agarwal 2017b).
Maintaining high K+/Na+ ratio is much important that to maintain the low concentrations of Na+ (Cuin et al. 2003) and high K+/Na+ ratio has been reported to be closely related to salt resistance in chickpea (Ozcan et al. 2000), Brassica napus (Qasim and Ashraf 2006), rice and finger millet (Vijayalakshmi et al. 2014). During xylem loading plants show selectivity for K+ over Na+ and this capacity together with the potential to redirect Na+ from leaves to roots, are important tolerance strategies for accommodating mild saline stress (Attia et al. 2009).
Contribution of osmolytes to stress tolerance
The mechanisms which allow a species to tolerate prolonged periods of stress can involve several attributes and accumulation of osmotically active solutes during periods of water and salinity stress can be one of the strategies of stress tolerance. Many compatible solutes are used in osmotic adjustment because even at high concentration, they do not pose deleterious effects to macromolecular processes (Hayat et al. 2012). Compatible solutes include both organic as well as inorganic components. Osmolyte accumulation helps plants maintain cell volume homeostasis, protein folding, protein disaggregation, and protein–protein interactions. Osmolytes shift equilibrium toward natively-folded conformations by raising the free energy of the unfolded state. As osmolytes predominantly affect the protein backbone, the balance between interactions of osmolyte with the protein backbone and the amino acid side chain and the solvent determines the fate of protein folding (Khan et al. 2010). Osmotic adjustment has positive associations with grain yield under limited water environments in several plants like sorghum (Singh and Kuhad 2005), barley (Gonzalez et al. 2008) and wheat (Jatav et al. 2014). Potassium is a major contributor to osmotic adjustment in the beginning of the water deficit which however, is overtaken by organic osmolytes with the growth transitions (Nio et al. 2011). Potassium supplementation results in greater amino acid accumulation, mainly the ones serving as precursors for important protective molecules (Sharma et al. 2006). Ahmed et al. (2013) has attributed the improved tolerance to combined effect of water and salinity stress to higher K+ accumulation leading to reduced Na/K ratio, accumulation of osmolytes including proline, sugars and GB resulting in greater WUE.
Potassium-induces proline accumulation contributing to better tissue water maintenance (Ahanger and Agarwal 2017a, b; Zain and Ismail 2016). Potassium-induced enhancement in proline accumulation may probably help plants to recover quickly after stress release and accumulated proline may provide energy for growth and transition phase (Kishor and Sreenivasulu 2014). However, potassium-induced regulation of proline metabolism, at transcriptional or translational levels is still to be evaluated.
Under potassium deficient growth conditions, feeding potassium salt promotes degradation of glucose, fructose and sucrose concomitant with enhancement in their synthesis. Such impact of potassium shall reflect certain effects on the cellular respiration rate by maintaining the optimal supply of glucose and fructose (Sugiyama and Goto 1966). Ibrahim et al. (2012) have reported considerable increase in the activity of invertase (acid as well as alkaline) resulting in significant improvement in sugar accumulation in potassium supplemented Labisia pumila.
Conclusion and future prospects
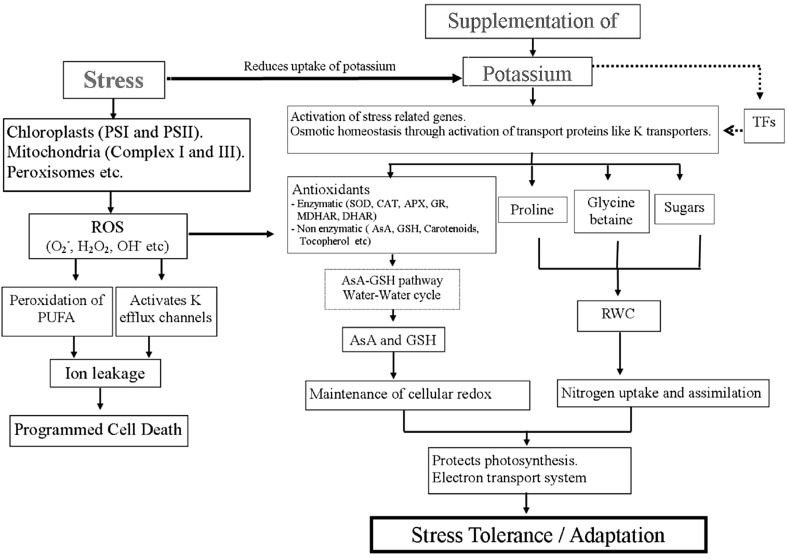
Environmental stresses enhance the production and accumulation of ROS causing oxidative stress and peroxidation of PUFA resulting in loss of membrane functioning. Though the research pertaining to the understanding of tolerance strategies like antioxidant system, osmoregulation and the K nutrition has significantly added to the current knowledge, but further substantiation is required regarding its relevance to complex ROS scavenging pathways. Use of transgenic technique in improvement in metabolic pathways linked to ROS scavenging and up-regulation of antioxidant system need to be taken as prime target. K nutrition enhances growth performance of plant under normal as well as stress conditions by modulating the metabolism and the changes are triggered at genetic and molecular levels (Fig. 2). Therefore, identifying the gene products expressed under K deficient conditions can be useful in identifying the definite set of markers involved in K induced stress tolerance.
Fig. 2.
Stress induced changes in metabolism as influenced by potassium. Potassium can regulate the plant growth by reducing the ROS generation and strengthening the antioxidant metabolism and the accumulation of osmolytes (arrows indicate positive or negative regulation and dotted lines indicate possible regulation)
Acknowledgements
Thanks are due to Head, School of Studies in Botany, Jiwaji University, Gwalior, MP for providing necessary facilities and thanks to Dr Shahid Umar, Hamdard University for sharing some of the literature. First author is highly thankful to Jiwaji University, Gwalior and MPCST, Bhopal for financial assistance. Funding was provided by Jiwaji University (Grant No. F/DEV/2013-14/33) and MPCST, Bhopal (Grant No. 1466/CST/R&D(BS)/2015).
Abbreviations
- ROS
Reactive oxygen species
- PCD
Programmed cell death
- ABA
Abscisic acid
- SAR
Sodium adsorption ratio
- ESP
Exchangeable sodium percentage
- EC
Electrical conductivity
- PSII
Photosystem II
- HKT
High affinity potassium transporter
- SOS
Salt overly sensitive
- QTL
Quantitative trait loci
- H2O2
Hydrogen peroxide
- O2−
Superoxide ion
- 1O2
Singlet oxygen
- O22−
Peroxide
- O2H
Perhydroxyl radical
- OH−
Hydroxyl radical
- RO−
Alkoxy radicals
- RNS
Reactive nitrogen species
- NO
Nitric oxide
- NO2
Nitric dioxide
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- APX
Ascorbate peroxidase
- MDHAR
Monodehydroascorbate reductase
- DHAR
Dehydroascorbate reductase
- GR
Glutathione reductase
- GST
Glutathione-S-transferase
- AsA
Ascorbic acid
- MDHA
Monodehydroascorbate; oxidised ascorbate
- GSH
Reduced glutathione
- GSSG
Glutathione disulphide; oxidised glutathione
- PAL
Phenylalanine ammonia lyase
- AKT
Arabidopsis shaker type
- CNGC
Cyclic nucleotide gated channel
- KUP/HAK
High affinity potassium transporter
- NSCC
Non selective cation channels
- NHX
Sodium proton exchanger
- TPK1
Tonoplast two pore K+ type channel
- GORK
Guard cell outward-rectifying K+ channel
- SKOR
Stelar K+ outward-rectifying channel
- HPODEs
Hydroperoxy octadecadienoates
References
- Agarwal RM, Pandey R, Gupta S. Certain aspects of water stress induced changes and tolerance mechanisms in plants. J Indian Bot Soc. 1999;78:255–269. [Google Scholar]
- Agarwal RM, Tomar NS, Jatav KS, Sharma GL (2009) Potassium induced changes in flowering plants. In: Flower Retrospect and Prospect (Professsor Vishwambhar Puri Birth Centenary Volume). SR Scientific Publication, Delhi, pp 158-186.
- Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent) J Plant Int. 2015;10:211–223. [Google Scholar]
- Ahanger MA, Agarwal RM. Potassium improves antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.) Protoplasma. 2017;254:1471–1486. doi: 10.1007/s00709-016-1037-0. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Agarwal RM. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Maathuis FJM. Cellular and tissue distribution of potassium: physiological relevance, mechanisms and regulation. J Plant Physiol. 2014;171:708–714. doi: 10.1016/j.jplph.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Ahmed IM, Dai H, Zheng W, Cao F, Zhang G, Sun D, Wu F. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol Biochem. 2013;63:49–60. doi: 10.1016/j.plaphy.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Ahmed IM, Nadira UA, Bibi N, Cao F, He X, Zhang G, Wu F. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ Exp Bot. 2015;111:1–12. doi: 10.1016/j.envexpbot.2014.10.003. [DOI] [Google Scholar]
- Alam MM, Nahar K, Hasanuzzaman M, Fujita M. Alleviation of osmotic stress in Brassica napus, B. campestris and B. juncea by ascorbic acid application. Biol Plant. 2014;58:697–708. doi: 10.1007/s10535-014-0447-0. [DOI] [Google Scholar]
- Anjaneyulu E, Reddy PS, Sunita MS, Kishor PBK, Meriga B. Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. J Plant Physiol. 2014;171:789–798. doi: 10.1016/j.jplph.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Anschutz U, Becker D, Shabala S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol. 2014;171:670–687. doi: 10.1016/j.jplph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Arora A, Byrem TM, Nari MG, Strasburg GM. Modulation of liposomal membranes fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000;373:102–109. doi: 10.1006/abbi.1999.1525. [DOI] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MK, Grant M, Grabov A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot. 2006;57:425–436. doi: 10.1093/jxb/erj034. [DOI] [PubMed] [Google Scholar]
- Attia H, Karray N, Ellili A, Msilini N, Lachaal M. Sodium transport in Basil. Acta Physiol Plant. 2009;31:1045–1051. doi: 10.1007/s11738-009-0324-1. [DOI] [Google Scholar]
- Bacha H, Rodenas R, Lopez-Gomez E, Garcia-Legaz MF, Nieves-Cordones M, Rivero RM, Martinez V, Angeles Botella A. High Ca2+ reverts the repression of high-affinity K+ uptake produced by Na+ in Solanum lycopersicum L. (var. microtom) plants. J Plant Physiol. 2015;180:72–79. doi: 10.1016/j.jplph.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Baisakh N, Rao MNR, Rajasekaran K, Subudhi P, Janda J, Galbraith D, Vanier C, Pereira A. Enhanced salt stress tolerance of rice plants expressing a vacuolar H+-ATPase subunit c1 (SaVHAc1) gene from the halophyte grass Spartina alterniflora Loisel. Plant Biotechnol J. 2012;10:453–464. doi: 10.1111/j.1467-7652.2012.00678.x. [DOI] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot. 2014;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- Benlloch-Gonzalez M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M. K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot. 2010;61:1139–1145. doi: 10.1093/jxb/erp379. [DOI] [PubMed] [Google Scholar]
- Blum A. Crop response to drought and the interpretation of adaptation. J Plant Growth Regul. 1996;20:135–148. doi: 10.1007/BF00024010. [DOI] [Google Scholar]
- Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ. 42 K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytol. 2010;186:373–384. doi: 10.1111/j.1469-8137.2009.03169.x. [DOI] [PubMed] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci. 2005;168:521–530. doi: 10.1002/jpln.200420485. [DOI] [Google Scholar]
- Cao F, Cai Y, Liu L, Zhang M, He X, Zhang G, Wu F. Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul. 2015;75:715–723. doi: 10.1007/s10725-014-9973-1. [DOI] [Google Scholar]
- Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol. 2012;35:1011–1019. doi: 10.1590/S1415-47572012000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro D, Parra A, Moreno JM. Reproductive output, seed anatomy and germination under water stress in the seeder Cistus ladanifer subjected to experimental drought. Environ Exp Bot. 2016;123:59–67. doi: 10.1016/j.envexpbot.2015.11.002. [DOI] [Google Scholar]
- Chen S, Polle A. Salinity tolerance of Populus. Plant Biol. 2010;12:317–333. doi: 10.1111/j.1438-8677.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- Cherel I, Lefoulon C, Boeglin M, Sentenac H. Molecular mechanisms involved in plant adaptation to low K+ availability. J Exp Bot. 2014;65:833–848. doi: 10.1093/jxb/ert402. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh RA. Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot. 2003;54:657–661. doi: 10.1093/jxb/erg072. [DOI] [PubMed] [Google Scholar]
- Deeba F, Pandey AK, Ranjan S, Mishra A, Singh R, Sharma YK, Shirke PA, Pandey V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol Biochem. 2012;53:6–18. doi: 10.1016/j.plaphy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- del-Rio LA. ROS and RNS in plant physiology: an overview. J Exp Bot. 2015;66:2827–2837. doi: 10.1093/jxb/erv099. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Straltsova V, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot. 2014;65:1259–1270. doi: 10.1093/jxb/eru004. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Edwards R. Roles for glutathione transferases in plant secondary metabolism. Phytochem. 2010;71:338–350. doi: 10.1016/j.phytochem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- El-Sayed OM, El-Gammal OHM, Salama ASM. Effect of ascorbic acid, proline and jasmonic acid foliar spraying on fruit set and yield of Manzanillo olive trees under salt stress. Sci Hortic. 2014;176:32–37. doi: 10.1016/j.scienta.2014.05.031. [DOI] [Google Scholar]
- Erel R, Yermiyahu U, Ben-Gal A, Dag A, Shapira O, Schwartz A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J Plant Physiol. 2015;177:1–10. doi: 10.1016/j.jplph.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Eskling M, Arvidsson PO, Akerlund HE. The xanthophyll cycle, its regulation and components. Physiol Plant. 1997;100:806–816. doi: 10.1111/j.1399-3054.1997.tb00007.x. [DOI] [Google Scholar]
- Fan W, Deng G, Wang H, Zhang H, Zhang P. Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas) Physiol Plant. 2015;154:560–571. doi: 10.1111/ppl.12301. [DOI] [PubMed] [Google Scholar]
- Fatma M, Asgher M, Masood A, Khan NA. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot. 2014;107:55–63. doi: 10.1016/j.envexpbot.2014.05.008. [DOI] [Google Scholar]
- Fayaz KA, Bazaid SA. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agric Sci. 2014;13(1):45–55. [Google Scholar]
- Flores-Caceres ML, Hattab S, Hattab S, Boussetta H, Banni M, Hernandez LE. Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 2015;233:165–173. doi: 10.1016/j.plantsci.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracasso A, Trindade L, Amaducci S. Drought tolerance strategies highlighted by two Sorghum bicolor races in a dry-down experiment. J Plant Physiol. 2016;190:1–14. doi: 10.1016/j.jplph.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot. 2013;64:433–443. doi: 10.1093/jxb/ers330. [DOI] [PubMed] [Google Scholar]
- Garg N, Manchanda G. ROS generation in plants: boon or bane? Plant Biosyst. 2009;143:8–96. doi: 10.1080/11263500802633626. [DOI] [Google Scholar]
- Ghanta S, Datta R, Bhattacharyya D, Sinha R, Kumar D, Hazra S, Mazumdar AB, Chattopadhyay S. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J Plant Physiol. 2014;171:940–950. doi: 10.1016/j.jplph.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gimeno V, Diaz-Lopez L, Simon-Grao S, Martinez V, Martinez-Nicolas JJ, Garcia-Sanchez F. Foliar potassium nitrate application improves the tolerance of Citrus macrophylla L. seedlings to drought conditions. Plant Physiol Biochem. 2014;83:308–315. doi: 10.1016/j.plaphy.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Giorgi A, Mingozzi M, Madeo M, Speranza G, Cocucci M. Effect of nitrogen starvation on the phenolic metabolism and antioxidant properties of yarrow (Achillea collina Becker ex Rchb.) Food Chem. 2009;114:204–211. doi: 10.1016/j.foodchem.2008.09.039. [DOI] [Google Scholar]
- Gols R. Direct and indirect chemical defences against insects in a multitrophic framework. Plant, Cell Environ. 2014;37:1741–1752. doi: 10.1111/pce.12318. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Martin I, Ayerbe L. Yield and osmotic adjustment capacity of barley under terminal water stress conditions. J Agron Crop Sci. 2008;194:81–91. doi: 10.1111/j.1439-037X.2007.00289.x. [DOI] [Google Scholar]
- Goufo P, Pereira J, Moutinho-Pereira J, Correia CM, Figueiredoc N, Carranca C, Rosa EAS, Trindade H. Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ Exp Bot. 2014;99:28–37. doi: 10.1016/j.envexpbot.2013.10.021. [DOI] [Google Scholar]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav. 2012;7:1–11. doi: 10.4161/psb.7.1.18574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Fernandez-Garcia N, Garcia-Garma J, Rubio-Asensio JS, Rubio F, Olmos E. Potassium starvation induces oxidative stress in Solanum lycopersicum L. roots. J Plant Physiol. 2012;169:1366–1374. doi: 10.1016/j.jplph.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Himabindu Y, Chakradhar T, Reddy MC, Kanygin A, Redding KE, Chandrasekhar T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in Glycophytes. Environ Exp Bot. 2016;124:39–63. doi: 10.1016/j.envexpbot.2015.11.010. [DOI] [Google Scholar]
- Horemans N, Foyer CH, Asard H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000;5:263–267. doi: 10.1016/S1360-1385(00)01649-6. [DOI] [PubMed] [Google Scholar]
- Ibrahim MH, Jaafar HZE, Karimi E, Ghasemzadeh A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian herb kacip fatimah (Labisia pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int J Mol Sci. 2012;13:15321–15342. doi: 10.3390/ijms131115321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Shahid Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea) J Plant Physiol. 2015;178:84–91. doi: 10.1016/j.jplph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jatav KS, Agarwal RM, Tomar NS, Tyagi SR. Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J Indian Bot Soc. 2014;93:177–189. [Google Scholar]
- Kagan VE, Tyurina YY. Recycling and redox cycling of phenolic antioxidants. Ann N Y Acad Sci. 1998 doi: 10.1111/j.1749-6632.1998.tb09921.x. [DOI] [PubMed] [Google Scholar]
- Kaiser WM. Correlation between changes in photosynthetic activity and changes in total protoplast volume in leaf tissue from hygro, meso and xerophytes under osmotic stress. Planta. 1982;154:538–545. doi: 10.1007/BF00402997. [DOI] [PubMed] [Google Scholar]
- Kanai S, Moghaieb RE, El-Shemy HA, Panigrahid R, Mohapatra PK, Ito J, Nguyen NT, Saneoka H, Fujita K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011;180:368–374. doi: 10.1016/j.plantsci.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Karimi S, Yadollahi A, Arzani K, Imani A, Aghaalikhani M. Gas-exchange response of almond genotypes to water stress. Photosynthetica. 2015;53:29–34. doi: 10.1007/s11099-015-0070-0. [DOI] [Google Scholar]
- Khan NA, Nazar R, Anjum NA. Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hortic. 2009;122:455–460. doi: 10.1016/j.scienta.2009.05.020. [DOI] [Google Scholar]
- Khan SH, Ahmad N, Ahmad F, Kumar R. Naturally occurring organic osmolytes: from cell physiology to disease prevention. Life. 2010;62:891–895. doi: 10.1002/iub.406. [DOI] [PubMed] [Google Scholar]
- Khan MIR, Asgher M, Fatma M, Per TS, Khan NA. Drought stress vis-a-vis plant functions in the era of climate change. Clim Change Environ Sustain. 2015;3:13–25. doi: 10.5958/2320-642X.2015.00002.2. [DOI] [Google Scholar]
- Kishor PBK, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37(2):300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Lavid N, Schwartz A, Yarden O, Tel-Or E. The involvement of polyphenols and peroxidase activities in heavy metal accumulation by epidermal glands of water lily (Nymphaeceaea) Planta. 2001;212:323–331. doi: 10.1007/s004250000400. [DOI] [PubMed] [Google Scholar]
- Li DM, Nie YX, Zhang J, Yin JS, Li Q, Wang XJ, Bai JG. Ferulic acid pretreatment enhances dehydration-stress tolerance of cucumber seedlings. Biol Plant. 2013;57:711–717. doi: 10.1007/s10535-013-0326-0. [DOI] [Google Scholar]
- Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJ. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012;135:1065–1070. doi: 10.1016/j.foodchem.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu L, Tong J, Ding J, Wang R, Lu Y, Xiao Y. Tiller number is altered in the ascorbic acid-deficient rice suppressed for l-galactono-1,4-lactone dehydrogenase. J Plant Physiol. 2013;170:389–396. doi: 10.1016/j.jplph.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Lopez-Huertas E, Charlton WL, Johnson B, Graham IA, Baker A. Stress induces peroxisome biogenesis genes. EMBO J. 2000;19:6770–6777. doi: 10.1093/emboj/19.24.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM. Physiological functions of mineral macronutrients. Curr Opin Plant Biol. 2009;12:250–258. doi: 10.1016/j.pbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Martineau E, Domec JC, Bosc A, Denoroy P, Fandino VA, Jrb JL, Jordan-Meille L. The effects of potassium nutrition on water use in field-grown maize (Zea mays L.) Environ Exp Bot. 2017;134:62–71. doi: 10.1016/j.envexpbot.2016.11.004. [DOI] [Google Scholar]
- Mittal S, Kumari N, Sharma V. Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem. 2012;54:17–26. doi: 10.1016/j.plaphy.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Breusegem FV. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Kuromori T, Takeuchi Y, Yamaji N, Yokosho K, Shimazawa A, Sugimoto E, Omote H, Ma JF, Shinozaki K, Moriyama Y. AtPHT4; 4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat Commun. 2015;6:5928. doi: 10.1038/ncomms6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C. Alternative electron flows (Water–Water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol. 2010;51:1951–1963. doi: 10.1093/pcp/pcq173. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosyn Res. 2005;86:459–474. doi: 10.1007/s11120-005-8811-8. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:581–651. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nio SA, Cawthray GR, Wade LJ, Colmer TD. Pattern of solutes accumulated during leaf osmotic adjustment as related to duration of water deficit for wheat at the reproductive stage. Plant Physiol Biochem. 2011;49:1126–1137. doi: 10.1016/j.plaphy.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH. Glutathione in plants: an integrated overview. Plant, Cell Environ. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Oh MM, Trick HN, Rajashekar CB. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J Plant Physiol. 2009;166:180–191. doi: 10.1016/j.jplph.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Ort DR, Baker NR. A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol. 2002;5:193–198. doi: 10.1016/S1369-5266(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Oukarroum A, Bussotti F, Goltsev V, Kalaji HM. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot. 2015;109:80–88. doi: 10.1016/j.envexpbot.2014.08.005. [DOI] [Google Scholar]
- Ozcan H, Turan MA, Koc O, Cikili Y, Taban S. Growth and variations in proline, sodium, chloride, phosphorus and potassium concentrations of chickpea varieties under salinity stress. Turk J Agric For. 2000;24:649–654. [Google Scholar]
- Pandey R, Agarwal RM, Jeevaratnam K, Sharma GL. Osmotic stress-induced alterations in rice (Oryza sativa L.) and recovery on stress release. Plant Growth Regul. 2004;42:79–87. doi: 10.1023/B:GROW.0000014893.45112.55. [DOI] [Google Scholar]
- Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S. Halophyte agriculture: success stories. Environ Exp Bot. 2014;107:71–83. doi: 10.1016/j.envexpbot.2014.05.006. [DOI] [Google Scholar]
- Puniran-Hartley N, Hartley J, Shabala L, Shabala S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance. Plant Physiol Biochem. 2014;83:32–39. doi: 10.1016/j.plaphy.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Purohit S, Laloraya MM, Bharti S, Nozzolillo C. Effect of phenolic compounds on ABA-induced Changes in K + concentration of guard cells and in epidermal diffusive resistance. J Exp Bot. 1992;43(246):103–110. doi: 10.1093/jxb/43.1.103. [DOI] [Google Scholar]
- Qasim M, Ashraf M. Time course of ion accumulation and its relationship with the salt tolerance of two genetically diverse lines of canola (Brassica napus L.) Pak J Bot. 2006;38:663–672. [Google Scholar]
- Qiao W, Li C, Fan LM. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot. 2014;100:84–93. doi: 10.1016/j.envexpbot.2013.12.014. [DOI] [Google Scholar]
- Rahil MH, Qanadillo A. Effects of different irrigation regimes on yield and water use efficiency of cucumber crop. Agric Water Manag. 2015;148:10–15. doi: 10.1016/j.agwat.2014.09.005. [DOI] [Google Scholar]
- Ramegowda V, Senthil-kumar M. The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol. 2015;176:47–54. doi: 10.1016/j.jplph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Rasool S, Ahmad A, Siddiqi TO. Differential response of chickpea genotypes under salt stress. J Funct Environ Bot. 2012;2:59–64. doi: 10.5958/j.2231-1742.2.1.006. [DOI] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Roy SJ, Negrao S, Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Tygai A. Physiology and molecular biology of salinity stress tolerant in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and pro-oxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- Saleh AM, Madany MMY. Coumarin pretreatment alleviates salinity stress in wheat seedlings. Plant Physiol Biochem. 2015;88:27–35. doi: 10.1016/j.plaphy.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Sano T, Becker D, Ivashikina N, Wegner LH, Zimmermann U, Roelfsema MRG, Nagata T, Hedrich R. Plant cells must pass a K+ threshold to re-enter the cell cycle. Plant J. 2007;50:401–413. doi: 10.1111/j.1365-313X.2007.03071.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Pottosin I. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant. 2014;151:257–279. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Shabala S, Bose J, Fuglsang AT, Pottosin I. On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. J Exp Bot. 2016;67(4):1015–1031. doi: 10.1093/jxb/erv465. [DOI] [PubMed] [Google Scholar]
- Shafiq S, Akram NA, Ashraf M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci Hortic. 2015;185:68–75. doi: 10.1016/j.scienta.2015.01.010. [DOI] [Google Scholar]
- Shan C, Zhao X. Effects of lanthanum on the ascorbate and glutathione metabolism of Vigna radiata seedlings under salt stress. Biol Plant. 2014;58:595–599. doi: 10.1007/s10535-014-0413-x. [DOI] [Google Scholar]
- Sharma GL, Agarwal RM, Singh RP. Potassium induced changes in certain aspects of nitrogen metabolism in chickpea (Cicer arietinum L.) Physiol Mol Biol Plants. 2006;12:157–162. [Google Scholar]
- Shrivastava M, Ahanger MA, Agarwal RM. Improved growth of Trigonella foenum-graecum L. with potassium supplementation involves physiological and biochemical implications. J Funct Environ Bot. 2016;6(2):84–101. doi: 10.5958/2231-1750.2016.00014.7. [DOI] [Google Scholar]
- Singh N, Kuhad MS. Role of potassium in alleviating the effect of water stress on yield and seed quality in chickpea (Cicer arietinum L.) Bull Natl Inst Ecol. 2005;15:219–225. [Google Scholar]
- Song ZZ, Yang Y, Ma RJ, Xu JL, Yu ML. Transcription of potassium transporter genes of KT/HAK/KUP family in peach seedlings and responses to abiotic stresses. Biol Plant. 2015;59:65–73. doi: 10.1007/s10535-014-0462-1. [DOI] [Google Scholar]
- Sugiyama T, Goto Y. Physiological role of potassium in the carbohydrate metabolism of plants (part II) Soil Sci Plant Nutr. 1966;12(6):19–23. doi: 10.1080/00380768.1966.10431962. [DOI] [Google Scholar]
- Tiwari HS, Agarwal RM, Bhatt RK. Photosynthesis, stomatal resistance and related characteristics, as influenced by potassium under normal water supply and water stress conditions in rice (Oryza sativa L.) Indian J Plant Physiol. 1998;3:314–316. [Google Scholar]
- Tomar NS, Agarwal RM. Influence of treatment of Jatropha curcas L. leachates and potassium on growth and phytochemical constituents of wheat (Triticum aestivum L.) Am J Plant Sci. 2013;4:1134–1150. doi: 10.4236/ajps.2013.45140. [DOI] [Google Scholar]
- Tomar NS, Sharma M, Agarwal RM. Phytochemical analysis of Jatropha curcas L. during different seasons and developmental stages and seedling growth of wheat (Triticum aestivum L.) as affected by extracts/leachates of Jatropha curcas L. Physiol Mol Biol Plant. 2015;21:83–92. doi: 10.1007/s12298-014-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette BW, Smith GA, Rhodes KL, Poole M. Tolerance and avoidance: two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. J Exp Mar Bio Ecol. 2009;380:106–112. doi: 10.1016/j.jembe.2009.08.015. [DOI] [Google Scholar]
- Turkan I, Demiral T. Recent developments in understanding salinity tolerance. Environ Exp Bot. 2009;67:2–9. doi: 10.1016/j.envexpbot.2009.05.008. [DOI] [Google Scholar]
- Tuteja N, Ahmad P, Panda BB, Tuteja R. Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res. 2009;681:134–149. doi: 10.1016/j.mrrev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Uzilday B, Turkan I, Ozgur R, Sekmen AH. Strategies of ROS regulation and antioxidant defense during transition from C3 to C4 photosynthesis in the genus Flaveria under PEG-induced osmotic stress. J Plant Physiol. 2014;171:65–75. doi: 10.1016/j.jplph.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Vadez V. Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Res. 2014;165:15–24. doi: 10.1016/j.fcr.2014.03.017. [DOI] [Google Scholar]
- Vijayalakshmi D, Ashok SK, Raveendran M. Screening for salinity stress tolerance in rice and finger millet genotypes using shoot Na+/K+ ratio and leaf carbohydrate contents as key physiological traits. Indian J Plant Physiol. 2014;19:156–160. doi: 10.1007/s40502-014-0090-y. [DOI] [Google Scholar]
- Wang M, Zheng Q, Shen Q, Guo S. The critical role of potassium in plant stress response. Int J Mol Sci. 2013;14:7370–7390. doi: 10.3390/ijms14047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng XY, Zheng CJ, Xu HX, Sun JY. Characteristics of photosynthesis and functions of the water–water cycle in rice (Oryza sativa) leaves in response to potassium deficiency. Physiol Plant. 2007;131:614–621. doi: 10.1111/j.1399-3054.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Wiesner R, Suzuki H, Walther M, Yamamoto S, Kuhn H. Suicidal inactivation of the rabbit 15-lipoxygenase by 15 s-HpETE is paralleled by covalent modification of active site peptides. Free Radic Biol Med. 2003;34(3):304–315. doi: 10.1016/S0891-5849(02)01244-3. [DOI] [PubMed] [Google Scholar]
- Willekens H, Villarroel R, Van Montagu M, Inze D, Van Camp W. Molecular identification of catalases from Nicotiana plumbaginifolia L. FEBS Lett. 1994;352:79–83. doi: 10.1016/0014-5793(94)00923-6. [DOI] [PubMed] [Google Scholar]
- Wu H, Shabala L, Barry K, Zhou M, Shabala S. Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiol Plant. 2013;149:515–527. doi: 10.1111/ppl.12056. [DOI] [PubMed] [Google Scholar]
- Xiao-guang W, Xin-hua Z, Chun-ji J, Chun-hong L, Shan C, Di W, Yan-qiu C, Hai-qiu Y, Chun-yan W. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max L. Merr) J Integr Agric. 2015;14:856–863. doi: 10.1016/S2095-3119(14)60848-0. [DOI] [Google Scholar]
- Yadav S, Irfan M, Ahmad A, Hayat S. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 2011;32:667–685. [PubMed] [Google Scholar]
- Yang CQ, Fang X, Wu XM, Mao YB, Wang LJ, Chen XY. Transcriptional regulation of plant secondary metabolism. J Integr Plant Biol. 2012;54(10):703–712. doi: 10.1111/j.1744-7909.2012.01161.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, You J, Ou Y, Ma J, Wu X, Xu G. Ultraviolet-B protection of ascorbate and tocopherol in plants related with their function on the stability on carotenoid and phenylpropanoid compounds. Plant Physiol Biochem. 2015;90:23–31. doi: 10.1016/j.plaphy.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Zain NAM, Ismail MR. Effects of potassium rates and types on growth, leaf gas exchange and biochemical changes in rice (Oryza sativa) planted under cyclic water stress. Agric Water Manag. 2016;164:83–90. doi: 10.1016/j.agwat.2015.09.022. [DOI] [Google Scholar]
- Zheng Y, Jia A, Ning T, Xu J, Li Z, Jiang G. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J Plant Physiol. 2008;165:1455–1465. doi: 10.1016/j.jplph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–445. doi: 10.1016/S1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Zorb C, Senbayram M, Peiter E. Potassium in agriculture—status and perspectives. J Plant Physiol. 2014;171:656–669. doi: 10.1016/j.jplph.2013.08.008. [DOI] [PubMed] [Google Scholar]