Abstract
The availability of reproducible regeneration system through tissue culture is a major bottleneck in wheat improvement program. The present study has considered to develop an efficient callus induction and regeneration system using mature and immature embryos as explants in recently released agronomically superior spring wheat varieties. An efficient sterilization process was standardized using 0.1% HgCl2 and 70% ethanol for both seeds and embryos. The maximum possible combinations of plant growth regulators (PGRs) were evaluated for their effect on different wheat regeneration processes through tissue culture starting from callus to root induction. Picloram is found as an effective auxin with 87.63–98.67% callus induction efficiency in both explants. Supplementation of CuSO4 along with 2,4-D, zeatin in regeneration medium significantly enhanced the multiple shoot induction. The shoot development was achieved using full strength Murashige and Skoog’s (MS) medium and root induction using half MS medium without PGRs. The optimized medium and method has resulted up to 100% regeneration irrespective of the genotype used with high reproducibility. Thus, the standardized regeneration system can be used in the regeneration of healthy plants from embryos rescued from interspecies crosses, transgenic production, induced mutation breeding and recently developed genome editing techniques for the procreation of wheat plants having novel traits.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0463-6) contains supplementary material, which is available to authorized users.
Keywords: Triticum aestivum; Mature/immature embryos; 2,4-D; Picloram; Dicamba; Zeatin; Regeneration
Introduction
Wheat globally contributes about 30% of total grain cereals production (FAO 2016). The current world population of 7.3 billion is expected to be about 9 billion by 2050 (UN 2015). Hence, there is an urgent need for a second green revolution in cereal crops worldwide to fulfill food requirement for this tremendously growing global population. It is essential to adapt both conventional and genetic engineering based breeding strategies in crop improvement programs to achieve this food requirement in the shortest possible time. Tissue culture is an integral part of biotechnology breeding and provides an added advantage to crop improvement programmes. The hindrance of different cereal crops to regeneration through callus is a major bottleneck in any crop improvement program including wheat (Parmar et al. 2012).
A good and efficient callus induction system in wheat highly depends on sterilization process, type of explants, genotypes, media composition and its pH, growth hormones, inducers and incubation conditions (Parmar et al. 2012; Mamrutha et al. 2014). Different explants have been tested as starting material for wheat callus cultures, such as immature embryos (Hakam et al. 2015), leaf segment (Yu et al. 2012), anthers (Redha and Suleman 2011), immature inflorescence (Kavas et al. 2008), microspores (Shariatpanahi et al. 2006) and mature embryos (Parmar et al. 2012) which showed the variable response for callus induction and regeneration. Immature embryos have been found most suitable explant source due to its high callus induction and regeneration capabilities (Redway et al. 1990). However, the immature embryos have limited seasonal availability and difficult to obtain during off-season. Furthermore, the most suitable stage for their efficient culture is also strictly restricted to 12–20 days post-anthesis (Zale et al. 2004), limiting their application for in vitro culture and genetic transformation. A regeneration system based on mature embryos may overcome these limitations (Zale et al. 2004).
The presence of plant growth regulators (PGRs) and their concentration in the culture media highly affect the callus induction and regeneration. In general, the PGRs concentration varies for optimum callus growth, development, and regeneration (Kothari et al. 2004) for particular plant species and specific explant. The first successful regeneration in wheat was reported by Zhou and Lee (1984). They investigated the effect of different auxins on mature embryo culture. Most frequently used auxin to induce callus in wheat is 2,4-dichlorophenoxyacetic acid (2,4-D), a synthetic auxin and potent herbicide, at a concentration of 1–2 mg/l. It is followed by 3,6-dichloro-2-methoxybenzoic acid (dicamba), another PGR and herbicide that shares similarities in structure and activity to 2,4-D (Bahieldin et al. 2000; Ren et al. 2010) and 4-amino-3,5,6-trichloro-2-pyridinecarboxylic acid (picloram) (Mendoza and Kaeppler 2002; Satyavathi et al. 2004). Till date, many regeneration media were optimized by adding cytokinins [kinetin, 6-benzylaminopurine (BAP), thidiazuron (TDZ), zeatin (6-(4-hydroxy-3-methylbut-2-enylamino)purine)] along with auxins indole-3-acetic acid (IAA), 1-naphthaleneacetic acid (NAA), 2,4-D, picloram (Fennell et al. 1996; Fahmy et al. 2006; Benderradji et al. 2012). Additionally, some inducers, such as CuSO4, AgNO3, were found to be useful to enhance the regeneration rate (Yu et al. 2008). Przetakiewicz et al. (2003) reported good regeneration from callus culture on hormone-free full strength MS medium. Further, some studies were carried out to improve the existing regeneration protocol in wheat (Parmar et al. 2012; Hakam et al. 2015). The wheat cv. Bobwhite and Chinese spring are world recognized wheat genotypes and are extensively used in tissue culture and genetic engineering (Zale et al. 2004; Agarwal et al. 2009). However, these genotypes are agronomically inferior and developed protocols are highly genotype dependent (Zale et al. 2004). Limited information exists on established regeneration protocol in agronomically superior wheat genotypes.
Hence, the present study was undertaken in agronomically superior Indian wheat genotypes to develop a robust, reproducible and genotype independent regeneration system. The sterilization conditions for both seeds and embryos were standardized and the effect of different PGRs in various concentrations and combinations were optimized for regeneration.
Materials and methods
Plant material
The seeds of six recently released high yielding Indian wheat (T. aestivum L.) genotypes (DBW 88, DBW 90, DBW 93, DPW 621-50, HD 3086, and WH 1105) were procured from the germplasm unit of the Indian Council of Agricultural Research-Indian Institute of Wheat and Barley Research (ICAR-IIWBR), Karnal (India). The genotypes were grown in the experimental field of ICAR-IIWBR. Spikes of all the genotypes were collected after 18–20 days of post-anthesis (Zadok’s scale 75–85) for immature embryos.
Explants sterilization
Mature and immature embryos of wheat were used as explants in the present study. Mature/immature seeds of wheat were washed with sterile distilled water (SDW) twice and sterilized with 0.1% HgCl2 followed by two SDW washing, then with 70% ethanol and rinsed with SDW three times. The different treatment durations 30, 60, 120, 180, 240 and 300 s for each disinfectant viz. 0.1% HgCl2 and 70% ethanol were performed. After sterilization, mature seeds were soaked in SDW in a sterilized petri plate, sealed with parafilm and incubated overnight at 10 °C (not required for immature seeds). Embryos were excised from mature/immature seeds using a sterile needle under laminar air flow. Excised embryos were sterilized in similar manner as for the seeds, but the durations for treatment were 10, 15, 20, 30, 60, 120 and 180 s for both disinfecting agents. Finally, these embryos were washed thrice with SDW to remove the excess disinfectants and retained in SDW until they were placed in the culture medium.
Callus induction
Murashige and Skoog (MS) medium (supplementary Table S1) (34.41 g/l) was used for various media preparations supplemented with different PGRs of variable concentrations (Table 1) and was solidified using 8 g/l agar (HI-MEDIA®, India, catalogue no. PCT0901). About 40–60 embryos with scutellum side-up were placed per petri plate for individual callus induction medium having different concentrations of auxins (2,4-D, picloram, dicamba, NAA) (Table 1) ranging from 1.5 to 4.0 mg/l. The petri plates were sealed with parafilm and incubated in the dark at 24 ± 1 °C for 20 days.
Table 1.
Composition of different culture media used for callus induction, regeneration and root induction
| Medium name | PGRs (mg/l) | Medium name | PGRs (mg/l) |
|---|---|---|---|
| Callus Induction medium (CI) (full MS mediuma 34.14 g/l, agar 8 g/l, pH 5.8) | |||
| CI1 | Full MS without any PGRs | CI7 | Picloram (2.5) |
| CI2 | 2,4-D (1.5) | CI8 | Dicamba (1.5) |
| CI3 | 2,4-D (2.0) | CI9 | Dicamba (2.0) |
| CI4 | 2,4-D (2.5) | CI10 | Dicamba (2.5) |
| CI5 | Picloram (1.5) | CI11 | 2,4-D (4.0) + NAA (2.0) |
| CI6 | Picloram (2.0) | CI12 | 2,4-D (2.0) + Picloram (2.0) |
| Regeneration medium (RM) (full MS medium 34.14 g/l, agar 8 g/l, pH 5.8) | |||
| RM1 | Full MS without any PGRs | RM21 | BAP (1.5) + Kinetin (1.5) + IAA (0.5) |
| RM2 | BAP (1.5) | RM22 | BAP (1.0) + Kinetin (2.0) + IAA (0.5) |
| RM3 | BAP (2.0) | RM23 | BAP (0.5) + Kinetin (2.5) + IAA (0.5) |
| RM4 | BAP (2.5) | RM24 | 2,4-D (0.1) |
| RM5 | Kinetin (1.5) | RM25 | 2,4-D (0.1) + Zeatin (1.0) |
| RM6 | Kinetin (2.0) | RM26 | 2,4-D (0.1) + Zeatin (2.0) |
| RM7 | Kinetin (2.5) | RM27 | 2,4-D (0.1) + Zeatin (3.0) |
| RM8 | BAP (2.0) + IAA(0.1) | RM28 | 2,4-D (0.1) + Zeatin (4.0) |
| RM9 | BAP (2.0) + IAA(0.2) | RM29 | 2,4-D (0.1) + Zeatin (5.0) |
| RM10 | BAP (2.0) + IAA(0.3) | RM30 | 2,4-D (0.1) + CuSO4 (12) |
| RM11 | BAP (2.0) + IAA(0.4) | RM31 | 2,4-D (0.1) + CuSO4 (15) |
| RM12 | BAP (2.0) + IAA(0.5) | RM32 | 2,4-D (0.1) + CuSO4 (18) |
| RM13 | BAP (2.0) + 2,4-D(0.2) | RM33 | 2,4-D (0.1) + CuSO4 (21) |
| RM14 | BAP (2.5) + Kinetin (0.5) + IAA (0.1) | RM34 | 2,4-D (0.1) + CuSO4 (25) |
| RM15 | BAP (2.0) + Kinetin (1.0) + IAA (0.1) | RM35 | 2,4-D (0.1) + Zeatin (5.0) + CuSO4 (12) |
| RM16 | BAP (1.5) + Kinetin (1.5) + IAA (0.1) | RM36 | 2,4-D (0.1) + Zeatin (5.0) + CuSO4 (15) |
| RM17 | BAP (1.0) + Kinetin (2.0) + IAA (0.1) | RM37 | 2,4-D (0.1) + Zeatin (5.0) + CuSO4 (18) |
| RM18 | BAP (0.5) + Kinetin (2.5) + IAA (0.1) | RM38 | 2,4-D (0.1) + Zeatin (5.0) + CuSO4 (21) |
| RM19 | BAP (2.5) + Kinetin (0.5) + IAA (0.5) | RM39 | 2,4-D (0.1) + Zeatin (5.0) + CuSO4 (25) |
| RM20 | BAP (2.0) + Kinetin (1.0) + IAA (0.5) | ||
| Rooting medium (RTM) (half MS medium 17.21 g/l, agar 8 g/l, pH 5.8) | |||
| RTM1 | Half MS without any PGRs | RTM5 | NAA (0.2) |
| RTM2 | IAA (0.1) | RTM6 | IAA (0.1) + NAA (0.1) |
| RTM3 | IAA (0.2) | RTM7 | IAA (0.2) + NAA (0.2) |
| RTM4 | NAA (0.1) | ||
a MS medium composition and preparation (see supplementary Table S1)
Regeneration
Twenty days calli were obtained from mature and immature embryos from all six genotypes using callus induction (CI6) medium containing picloram 2.0 mg/l and transferred to regeneration media (RM) having different PGRs in various combinations. A total thirty-nine different regeneration media containing several combinations of PGRs, cytokinins (BAP, kinetin, zeatin) ranging from 0.5 to 5 mg/l and auxins (IAA and 2,4-D) ranging from 0.1 to 0.5 mg/l were tested. These media were supplemented with or without different concentration of CuSO4 (12–25 mg/l) as listed in Table 1. The incubation conditions for regeneration were relative humidity (RH) with 55–65%, temperature 24 ± 1 °C and 16/8 h photoperiod with a cool-fluorescent light 10 μmol m−2 s−1 for the first week. In the second week, each individual shoots were separated out from regenerated calli and placed in the tube (25 × 150 mm) having full MS medium (RM1) without any growth hormone for shoot development. The incubation conditions for the second week remained same except the light intensity was increased to 52 μmol m−2 s−1. The number of multiple shoots developed per callus was recorded after 15 days of culture.
Root induction
The developed shoots were placed in 300 ml culture bottles containing 50 ml half MS supplemented with different combinations of IAA and NAA. A total of seven root induction media (RTM) were used (Table 1). The incubation conditions for root induction were RH 55–65%, temperature 24 ± 1 °C and light 52 μmol m−2 s−1 for 1-week duration.
Hardening and acclimatization
The plantlets having well-established shoot and root network were transferred to 4″ plastic pots having an equal proportion of soil-peat mix and watered with 50 ml Hoagland solution (Supplementary Table S2). The plantlets were covered with transparent polythene bags for 10 days under the same conditions as of root induction to harden the plantlets. Subsequently, the polythene bags were punctured and removed after 2 days. Later, the hardened plants were transferred to 8″ sized pots, and grown up to maturity in controlled lab conditions.
Data analysis
A completely randomized design with three replications per treatment of each genotype was used in all experiments. The actual callus induction efficiency was calculated as calli without precocious germination divided by total explants and multiplied by 100. The morphological characters like callus growth rate, colour, size, weight, were also recorded. Callus size was measured using vernier calliper micrometer and callus weight was recorded using electronic microbalance (Mettler Toledo, India). The data was analysed using the SAS statistical software program, PROC GLM, SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) at different statistical significance level using ANOVA and Tukey–Kramer’s test.
Results
Explants sterilization
The explant sterilization is a crucial step in tissue culture, which affects both explants viability and regeneration efficiency. Different durations of sterilization were tested for both seeds and embryos using 0.1% HgCl2 and 70% ethanol to find out optimum sterilization period. The viability of mature seed was reduced after exceeding the sterilization time more than 120 s for both sterilizing agents (0.1% HgCl2 and 70% ethanol). Bacterial contamination was observed in 60 s and less than 60 s sterilization of seeds. For mature and immature embryos, complete sterilization was achieved at 20 s sterilization with 0.1% HgCl2 followed by 20 s of 70% ethanol treatment without affecting explants viability. Hence, the optimum duration for seeds and embryos sterilization was 120 and 20 s, respectively for both disinfectants.
Callus induction
The callus induction was tested on twelve combinations of media and all tested callus induction media showed variable embryogenic callus induction efficiency in all six wheat genotypes (DBW 88, DBW 90, DBW 93, DPW 621-50, HD 3086, and WH 1105) along with different percentage of precocious germination (Table 2). The results showed optimum embryogenic callus induction obtained from callus induction 6 (CI6) medium (picloram 2.0 mg/l) ranging from 87.63 to 97.73% and 95.4 to 98.67% for mature and immature embryos respectively (Table 3). Though, other CI media showed up to 100% callus induction, but was with slow growth rate or with high percentage of precocious germination. CI1 without PGRs showed only precocious germination in both explants (Fig. 2b). There was no callus induction using CI1 (Table 2). In the comparative analysis of three auxins viz. 2,4-D, picloram and dicamba in callus induction, dicamba showed very fast callus growth rate in both explants with increase in concentration along with high precocious germination. 2,4-D showed minimum precocious germination but with slow rate of callus induction. Picloram showed optimum callus induction with less precocious germination than dicamba. The higher concentration, i.e. 2.5 mg/l of each growth hormone showed maximum callus induction rate as well as high precocious germination than their respective lower concentrations (1.5 and 2.0 mg/l). Embryogenic calli developed on callus induction media containing 2,4-D and dicamba were of white colour while media having picloram were of light yellow. The picloram at 2.0 mg/l was found to be optimum to achieve the required callus growth rate along with good quality callus in both types of explants (Table 2). The other tested CI media were CI11 [2,4-D (4.0 mg/l) + NAA (2.0 mg/l)] and CI12 [2,4-D (2.0 mg/l) + Picloram (2.0 mg/l)]. The callus induction was not satisfactory in these media also as compared to picloram 2.0 mg/l. The results obtained for all tested twelve callus induction media were collinear for both the explants. However, the embryogenic callus induction efficiency was found higher and precocious germination was low in case of immature embryos compared to mature embryos.
Table 2.
The response of wheat genotypes for embryogenic callus induction
| Medium | Mature embryos | Immature embryos | ||||||
|---|---|---|---|---|---|---|---|---|
| Callus induction (%) | Precocious germination (%) | Callus size (cm) | Callus weight (mg) | Callus induction (%) | Precocious germination (%) | Callus size (cm) | Callus weight (mg) | |
| CI1 | 0 | 100a | 0 | 0 | 0 | 100a | 0 | 0 |
| CI2 | 97.96a | 2.04g | 0.41j | 38i | 100a | 0 | 0.50h | 42j |
| CI3 | 96.79ab | 3.21fg | 0.50h | 43h | 99.67a | 0.33e | 0.50h | 43j |
| CI4 | 95.00b | 5.00f | 0.52gh | 47g | 99.37a | 0.63e | 0.50h | 46i |
| CI5 | 92.51c | 7.49e | 0.58f | 74e | 96.37b | 3.63d | 0.58f | 75f |
| CI6 | 92.52c | 7.48e | 0.68e | 85d | 96.53b | 3.47d | 0.77e | 86e |
| CI7 | 90.98cd | 9.03de | 0.73d | 87d | 95.64bc | 4.36cd | 0.80d | 87d |
| CI8 | 87.71e | 12.29c | 0.82c | 108c | 92.26d | 7.75b | 0.90c | 114c |
| CI9 | 85.76ef | 14.24bc | 0.97b | 120b | 91.54d | 8.46b | 1.00b | 124b |
| CI10 | 85.26f | 14.74b | 1.07a | 127a | 92.67d | 7.33b | 1.10a | 136a |
| CI11 | 92.21c | 7.79e | 0.45i | 50g | 96.27bc | 3.73cd | 0.47i | 50h |
| CI12 | 89.96d | 10.04d | 0.53g | 54f | 94.53c | 5.47c | 0.57g | 53g |
Data of each trait represents the average values of six wheat genotypes (DBW 88, DBW 90, DBW 93, DPW 621-50, HD 3086 and WH 1105)
The figure followed by same superscript letters are not significantly different according to Tukey’s multiple comparison test at P < 0.01. CI6 gave the optimal callus induction
Table 3.
Callus induction response of six Indian wheat genotypes in CI6
| Genotype | Mature embryos | Immature embryos | ||||||
|---|---|---|---|---|---|---|---|---|
| Callus induction (%) | Precocious germination (%) | Callus size (cm) | Callus weight (mg) | Callus induction (%) | Precocious germination (%) | Callus size (cm) | Callus weight (mg) | |
| DBW 88 | 97.73a | 2.27c | 0.70a | 86a | 96.77a | 3.23a | 0.77a | 86a |
| DBW 90 | 94.93ab | 5.07bc | 0.67a | 86a | 96.17a | 3.83a | 0.73a | 85a |
| DBW 93 | 89.13c | 10.87a | 0.63a | 86a | 96.63a | 3.37a | 0.80a | 86a |
| DPW 621-50 | 90.30bc | 9.70ab | 0.70a | 85a | 95.53a | 4.47a | 0.73a | 85a |
| HD 3086 | 95.37a | 4.63c | 0.70a | 85a | 95.40a | 4.60a | 0.77a | 86a |
| WH 1105 | 87.63c | 12.37a | 0.70a | 85a | 98.67a | 1.33a | 0.80a | 86a |
The figure followed by same superscript letters are not significantly different according to Tukey’s multiple comparison test at P < 0.01
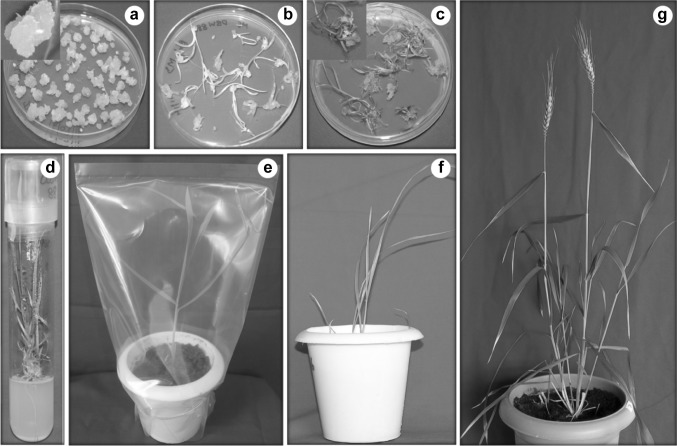
Fig. 2.
Schematic representation of standardized protocol, a embryogenic calli in CI6, b precocious germination in CI1 (only full MS) having no PGRs, c regeneration in RM36, d shoot development of plantlet in RM1, e hardening of recovered plantlet, f plant acclimatization in soil and peat mix containing pot, g plant generated through optimized protocol grown up to maturity
Regeneration
A total 39 different hormonal combination media tested for regeneration showed good regeneration, but with a variable growth rate and efficiency (Table 4). The effect of BAP alone and with kinetin and IAA in different concentrations was tested. RM14 containing BAP at 2.5 mg/l, kinetin 0.5 mg/l and IAA 0.1 mg/l showed regeneration efficiency ranging from 80 to 84.5% and 83.4 to 87.9% using mature and immature embryos, respectively. RM24 containing only 2,4-D (0.1 mg/l) also showed good regenerations of about 80.73% (mature embryos) and 89.60% (immature embryos) on an average in all genotypes with less number of shoots per callus. The concentrations of zeatin (1, 2, 3, 4 and 5 mg/l) and CuSO4 (12, 15, 18, 21, 25 mg/l) were tested individually and together with 2,4-D (0.1 mg/l) in regeneration medium. Zeatin showed its inductive effect on shoot regeneration, but found suitable at 5 mg/l in regeneration medium. Less than 5 mg/l zeatin showed less shoot induction in both mature and immature embryo explants in all tested genotypes. A linear increasing trend of multiple shoot induction was observed with increase in CuSO4 concentration in regeneration medium. The maximum regeneration frequency was observed in regeneration medium RM35 [2,4-D (0.1 mg/l) + zeatin (5 mg/l) + CuSO4 (12 mg/l)], and onwards media having 2,4-D, zeatin, and CuSO4 in different concentration in case of mature embryos. From RM32 medium onwards, the 100% regeneration was observed using immature embryos and RM32 showed the less number of multiple shoots in calli compared to others (Fig. 1). The regeneration medium RM36 [(2,4-D (0.1 mg/l) + zeatin (5 mg/l) + CuSO4 (15 mg/l)] was found as an optimum regeneration medium as it showed more individual healthy plantlets compared to RM39. The RM39 showed more rosette leaves with high CuSO4 concentration (Fig. 1). Mature and immature embryos derived calli from six genotypes gave 100% regeneration with variable number of shoots per explant in RM36 medium (Supplementary Table S3). The plantlet recovered from different regeneration media showed good shoot development in RM1 (full strength MS) having no plant growth hormones. The immature embryos gave more shootlets as compared to mature embryos from all six genotypes.
Table 4.
The response of wheat genotypes for regeneration in different RM media
| Medium | Mature embryos | Immature embryos | Medium | Mature embryos | Immature embryos | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reg (%) | SH | Reg (%) | SH | Reg (%) | SH | Reg (%) | SH | ||
| RM1 | 51.51m | 1 | 61.61l | 1 | RM21 | 71.68fghij | 1 | 69.98k | 1 |
| RM2 | 21.28q | 1 | 20.88o | 1 | RM22 | 71.28ghij | 1 | 73.47ijk | 1 |
| RM3 | 38.93n | 1 | 40.71mn | 1 | RM23 | 72.45fghi | 1 | 60.53l | 1 |
| RM4 | 57.10lm | 1 | 47.83m | 1 | RM24 | 80.73ed | 1 | 89.60cde | 1 |
| RM5 | 31.91op | 1 | 37.18n | 1 | RM25 | 81.82ed | 1 | 83.23defgh | 1 |
| RM6 | 34.47no | 1 | 41.35mn | 1 | RM26 | 83.03cbde | 1 | 86.47defg | 1 |
| RM7 | 27.81p | 1 | 37.15n | 1 | RM27 | 85.09cbd | 1 | 87.77def | 1 |
| RM8 | 66.34jk | 1 | 73.74ijk | 2 | RM28 | 87.83cb | 2 | 90.63bcd | 2 |
| RM9 | 64.95k | 1 | 73.99ijk | 1 | RM29 | 88.41b | 2 | 96.80abc | 3 |
| RM10 | 66.68ijk | 1 | 73.67ijk | 1 | RM30 | 96.72a | 3 | 97.41ab | 2 |
| RM11 | 65.91jk | 1 | 72.77jk | 1 | RM31 | 98.23a | 3 | 99.17a | 3 |
| RM12 | 67.68hijk | 1 | 77.14hijk | 1 | RM32 | 97.89a | 3 | 100a | 3 |
| RM13 | 61.91kl | 1 | 73.02ijk | 1 | RM33 | 96.52a | 4 | 100a | 4 |
| RM14 | 82.30bed | 1 | 85.60defg | 2 | RM34 | 97.59a | 5 | 100a | 4 |
| RM15 | 71.68fghij | 2 | 79.37ghij | 2 | RM35 | 100a | 8 | 100a | 8 |
| RM16 | 74.57fg | 1 | 80.41fghi | 2 | RM36 | 100a | 9 | 100a | 11 |
| RM17 | 72.29fghi | 1 | 76.91hijk | 1 | RM37 | 99.48a | 11 | 100a | 13 |
| RM18 | 66.91ijk | 2 | 79.97ghij | 1 | RM38 | 100a | 12 | 100a | 15 |
| RM19 | 77.28ef | 2 | 74.49ijk | 2 | RM39 | 100a | 14 | 100a | 17 |
| RM20 | 73.23fgh | 2 | 82.32efgh | 2 | |||||
Data of each trait represents the average values of six wheat genotypes (DBW 88, DBW 90, DBW 93, DPW 621-50, HD 3086 and WH 1105)
Reg regeneration, SH average number of shoots per embryo
The figure followed by same superscript letters are not significantly different according to Tukey’s multiple comparison test at P < 0.01. RM36 gave the optimal regeneration
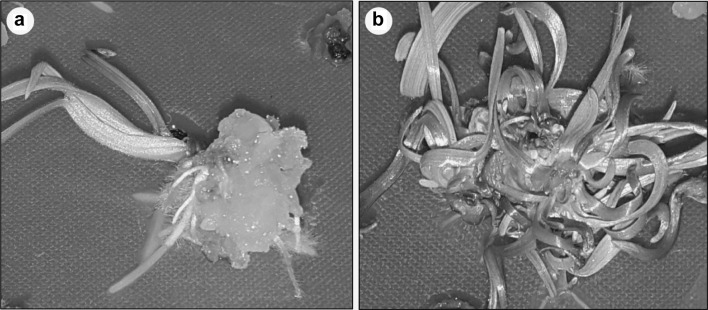
Fig. 1.
Effect of different concentrations of CuSO4 in regeneration media along with 2,4-D 0.1 mg/l and zeatin 5 mg/l, a RM29 (without CuSO4), and, b RM39 (CuSO4 25 mg/l)
Root induction of regenerated plantlets
All tested root induction media showed root initiation. However, the optimum root network was observed within a week in rooting medium 1 (RTM1) i.e. medium without any PGRs, for all tested genotypes in both immature and mature embryos. The root initiation rate was higher in RTM1 followed by RTM7 having IAA (0.2 mg/l) and NAA (0.2 mg/l).
Hardening and acclimatization
All plants recovered from RTM1 survived under hardening process and acclimatized in controlled conditions. Morphologically healthy wheat plants were regenerated from this protocol. The complete regeneration of wheat plant from the explants to hardening took about 52–60 days as shown in Fig. 2.
Discussion
A robust genotype independent regeneration system for wheat is the need of the hour to transfer desired genes using Agrobacterium-mediated method or by biolistic method or with other tissue culture based methods. This study emphasized on evaluation of the PGRs influence on callus induction and regeneration and to find out their optimal concentrations using both immature and mature embryos in agronomically superior Indian wheat genotypes. The explants sterilization is the most important step in tissue culture. Earlier reported sterilizing agents such as sodium hypochlorite, calcium hypochlorite, mercuric chloride, ethanol and their long term treatment with explants caused explant death and consequently reduced the regeneration efficiency (Fillipov et al. 2006; Chauhan et al. 2007). In the present study, the higher regeneration efficiency was maintained by sterilization using 0.1% HgCl2 and 70% ethanol for 120 s for seed and 20 s (each disinfectant) for embryos followed by SDW washing after each step. Only the seeds sterilization was reported in previous studies for wheat (Parmar et al. 2012; Hakam et al. 2015). To our best knowledge, this is the first report on embryos sterilization before their use in callus induction. Hence, the optimized sterilization process for both seeds and embryos will reduce both duration and contamination risk in wheat tissue culture.
Some studies have already reported the effect of growth regulators on callus induction and regeneration in wheat (Fillipov et al. 2006; Chauhan et al. 2007). But these studies were carried out in limited number of wheat genotypes and with less number of PGR combinations in the media. Earlier reports, claims 2,4-D has optimum callus induction capacity in wheat (Yu et al. 2008). However, our results indicated that 2,4-D has the slowest rate of callus induction as compared to dicamba followed by picloram. Dicamba showed high callus induction rate among the tested auxins but it also has highest precocious germination. Some reports (Hunsinger and Schauz 1987; Papenfus and Carman 1987; Redway et al. 1990) also claims that the dicamba shows rapid callus induction than 2,4-D. These reports suggest that dicamba is consumed very rapidly by cellular metabolism in wheat tissue. In contrast, 2,4-D is a highly stable auxin and shows strong resistance to enzymatic degradation and conjugation in the plant cell (Moore 1989).The rapid rate of dicamba consumption from callus induction medium than 2,4-D induces the precocious germination of somatic embryos (Mendoza and Kaeppler 2002). In our study, by considering maximum combinations and different concentration of auxins, picloram was identified as suitable auxin at optimum concentration of 2.0 mg/l for good quality embryogenic callus induction within 20 days using mature and immature embryos irrespective of the genotypes used.
Shoot regeneration is another crucial step after callus induction in tissue culture. BAP and kinetin were tested at different concentration and combinations. The absence of 2,4-D in regeneration medium showed the shoot induction as well as the root induction. The root induction suppressed to a large extent by supplying 2,4-D alone in regeneration medium or with other growth regulators, only shoot induction occurred. It was evident that the inclusion of 2,4-D in combination with cytokinins is valuable for regeneration (Chauhan et al. 2007). From the current study, RM36 [2,4-D (0.1 mg/l) + zeatin (5 mg/l) + CuSO4 (15 mg/l)] showed optimal regeneration within 1 week among thirty-nine tested regeneration media. Chauhan et al. (2007) used zeatin and TDZ individually and in different combination and found zeatin as superior to TDZ. CuSO4 acts as stress inducing agent in regeneration medium and promotes shoots. The Cu2+ is known to be a cofactor of many important enzymes implicated in biological processes, suggesting these Cu enzymes could play an important role in plant tissue culture (Sparks et al. 2014). As CuSO4 concentration increased, the amount of green tissue per callus was increased. The excessive concentration of CuSO4 showed more wrinkled plantlets with rosette leaves. Hence, the optimum concentration was 15 mg/l which gave individual plantlets having good and healthy leaves. The immature embryos showed fast callus induction rate and higher regeneration than mature embryos, which conferred immature embryos as a good source of explants for wheat tissue culture. Nevertheless, the mature embryos also showed the good callus induction and regeneration in the standardized media. The accessibility of immature embryos throughout the year is a very major hurdle due to limited seasonal availability. Results also indicate that the selected genotypes have not much significant influence on callus induction and regeneration. However, most of the earlier published protocols were genotype dependent (Zale et al. 2004; Yu et al. 2008). Overall, the standardized regeneration method showed 100% regeneration efficiency irrespective of the genotype used in both mature and immature embryos as explant. Hence, a robust and reproducible regeneration protocol to our best knowledge has been reported in agronomically superior wheat genotypes for the first time. And these genotypes immediately after gene transfer can be easily used in the breeding program with the added advantage of a gene.
Conclusion
Establishment of a reproducible regeneration system is the essential prerequisite for effective transgenics development in wheat improvement. In the current research, the sterilization process was effectively standardized using 0.1% HgCl2 and 70% ethanol for both seed and embryos. The effect of different PGRs on callus induction, regeneration and root induction was also analyzed. Among the tested auxins, picloram (2.0 mg/l) was found to be most suitable for embryogenic callus induction. A combination of 2,4-D + zeatin + CuSO4 showed good regeneration. The MS medium without any PGRs gave good rooting. The optimized media showed good callus induction and regeneration in both mature and immature embryos as explant irrespective of the genotype used. All these standardized media and protocol can be effectively used in different wheat genotypes, for faster introgression of useful transgenes through Agrobacterium or by any other tissue culture based method for rapid wheat improvement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work is financially supported by the Indian Council of Agricultural Research, New Delhi, India under the project entitled ICAR Network Project “Transgenic in crops (NPTC)” (Project No. 1006474).
Abbreviations
- 2,4-D
2,4-Dichlorophenoxyacetic acid
- BAP
6-Benzylaminopurine
- Dicamba
3,6-Dichloro-2-methoxybenzoic acid
- IAA
Indole-3-acetic acid
- NAA
1-Naphthaleneacetic acid
- PGRs
Plant growth regulators
- Picloram
4-Amino-3,5,6-trichloro-2-pyridinecarboxylic acid
- RH
Relative humidity
- TDZ
Thidiazuron
- Zeatin
6-(4-Hydroxy-3-methylbut-2-enylamino)purine
Authors’ contribution
MHM conceived the project and designed the experiments with AG. RK conducted experiments. AK assisted RK during Research. RK, MHM, and KV wrote the manuscript. RajK and VT guided and extended facilities for research. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0463-6) contains supplementary material, which is available to authorized users.
References
- Agarwal S, Loar S, Steber C, Zale J. Floral transformation of wheat. Methods Mol Biol. 2009;478:105–113. doi: 10.1007/978-1-59745-379-0_6. [DOI] [PubMed] [Google Scholar]
- Bahieldin A, Dyer WE, Qu R. Concentration effects of dicamba on shoot regeneration in wheat. Plant Breed. 2000;119:437–439. doi: 10.1046/j.1439-0523.2000.00523.x. [DOI] [Google Scholar]
- Benderradji L, Brini F-A, Kellou K, Ykhlef N, Djekoun A, Masmoudi K, Bouzerzour H. Callus induction, proliferation, and plantlets regeneration of two bread wheat (Triticum aestivum L.) genotypes under saline and heat stress conditions. ISRN Agron. 2012 [Google Scholar]
- Chauhan H, Desai SA, Khurana P. Comparative analysis of the differential regeneration response of various genotypes of Triticum aestivum, Triticum durum and Triticum dicoccum. Plant Cell Tissue Org. 2007;91:191–199. doi: 10.1007/s11240-007-9285-5. [DOI] [Google Scholar]
- Fahmy AH, El-Shafy YH, El-Shihy OM, Madkour MA. Highly efficient regeneration via somatic embryogenesis from immature embryos of Egyptian wheat cultivars (Triticum aestivum L.) using different growth regulators. World J Agric Sci. 2006;2(3):282–289. [Google Scholar]
- FAO (2016) FAO cereal supply and demand brief. http://www.fao.org/worldfoodsituation/csdb/en/. Accessed 25 Oct 2016
- Fennell S, Bohorova N, Ginkel M-V, Crossa J, Hoisington D. Plant regeneration from immature embryos of 48 elite CIMMYT bread wheats. Theor Appl Genet. 1996;92:163–169. doi: 10.1007/BF00223371. [DOI] [PubMed] [Google Scholar]
- Fillipov M, Miroshnichenko D, Vernikovskaya D, Dolgov S. The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell Tissue Org. 2006;84:213–222. doi: 10.1007/s11240-005-9026-6. [DOI] [Google Scholar]
- Hakam N, Udupa SM, Rabha A, Ibriz M, Iraqi D. Efficient callus induction and plantlets regeneration in bread wheat using immature and mature embryos. Int J Biotechnol Res. 2015;3(1):001–009. [Google Scholar]
- Hunsinger H, Schauz K. The influence of Dicamba on somatic embryogenesis and frequency of plant regeneration from cultured immature embryos of wheat (Tritium aestivum L.) Plant Breed. 1987;98:119–123. doi: 10.1111/j.1439-0523.1987.tb01103.x. [DOI] [Google Scholar]
- Kavas M, Öktem HA, Yücel M. Factors affecting plant regeneration from immature inflorescence of two winter wheat cultivars. Biol Plantarum. 2008;52(4):621–626. doi: 10.1007/s10535-008-0122-4. [DOI] [Google Scholar]
- Kothari SL, Agarwal K, Kumar S. Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet (Elusine coracana L. gaertn.) In Vitro Cell Dev Biol Plant. 2004;40:515–519. doi: 10.1079/IVP2004564. [DOI] [Google Scholar]
- Mamrutha HM, Kumar R, Venkatesh K, Sharma P, Kumar R, Tiwari V, Sharma I. Genetic transformation of wheat—present status and future potential. J Wheat Res. 2014;6(2):1–13. [Google Scholar]
- Mendoza MG, Kaeppler HF. Auxin and sugar effects on callus induction and plant regeneration frequencies from mature embryos of wheat (Triticum aestivum L.) In Vitro Cell Dev Biol Plant. 2002;38:39–45. doi: 10.1079/IVP2001250. [DOI] [Google Scholar]
- Moore TC. Biochemistry and physiology of plant hormones. Berlin: Springer; 1989. [Google Scholar]
- Papenfus JM, Carman JG. Enhanced regeneration from wheat callus cultures using dicamba and kinetin. Crop Sci. 1987;27:588–593. doi: 10.2135/cropsci1987.0011183X002700030035x. [DOI] [Google Scholar]
- Parmar SS, Sainger M, Chaudhary D, Jaiwal PK. Plant regeneration from mature embryo of commercial Indian bread wheat (Triticum aestivum L.) cultivars. Physiol Mol Biol Plants. 2012;18(2):177–183. doi: 10.1007/s12298-012-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przetakiewicz A, Orczyk W, Nadolska-Orczyk A. The effect of auxin on plant regeneration of wheat, barley and triticale. Plant Cell Tiss Org. 2003;73:245–256. doi: 10.1023/A:1023030511800. [DOI] [Google Scholar]
- Redha A, Suleman P. Effects of exogenous application of polyamines on wheat anther cultures. Plant Cell Tissue Org. 2011;105:345–353. doi: 10.1007/s11240-010-9873-7. [DOI] [Google Scholar]
- Redway FA, Vasıl V, Lu D, Vasıl IK. Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.) Theor Appl Genet. 1990;79:609–617. doi: 10.1007/BF00226873. [DOI] [PubMed] [Google Scholar]
- Ren J-P, Wang X-G, Yin J. Dicamba and sugar effects on callus induction and plant regeneration from mature embryo culture of wheat. Agric Sci China. 2010;9(1):31–37. doi: 10.1016/S1671-2927(09)60064-X. [DOI] [Google Scholar]
- Satyavathi VV, Jauhar PP, Elias EM, Rao MB. Effects of growth regulators on vitro plant regeneration in durum wheat. Crop Sci. 2004;44:1839–1846. doi: 10.2135/cropsci2004.1839. [DOI] [Google Scholar]
- Shariatpanahi ME, Belogradova K, Hessamvaziri L, Heberle-Bors E, Touraev A. Efficient embryogenesis and regeneration in freshly isolated and cultured wheat (Triticum aestivum L.) microspores without stress pretreatment. Plant Cell Rep. 2006;25:1294–1299. doi: 10.1007/s00299-006-0205-7. [DOI] [PubMed] [Google Scholar]
- Sparks CA, Doherty A, Jones H. Genetic transformation of wheat via Agrobacterium-mediated DNA delivery. In: Henry RJ, Furtado A, editors. Cereal genomics: methods and protocols. Series Methods in Molecular Biology. New York: Springer; 2014. pp. 235–250. [DOI] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division (2015) World Population Prospects: The 2015 Revision, World Population 2015 Wallchart. ST/ESA/SER.A/378
- Yu Y, Wang J, Zhu M-L, Wei Z-M. Optimization of mature embryo-based high frequency callus induction and plant regeneration from elite wheat cultivars grown in China. Plant Breed. 2008;127:249–255. doi: 10.1111/j.1439-0523.2007.01461.x. [DOI] [Google Scholar]
- Yu H, Wang W, Wang Y, Hou B. High frequency wheat regeneration from leaf tissue explants of regenerated plantlets. Adv Biosci Biotechnol. 2012;3:46–50. doi: 10.4236/abb.2012.31008. [DOI] [Google Scholar]
- Zale JM, Borchardt-Wier H, Kidwal KK, Stebar CM. Callus induction and plant regeneration from mature embryos of a diverse set of wheat genotypes. Plant Cell Tiss Org. 2004;76:277–281. doi: 10.1023/B:TICU.0000009248.32457.4c. [DOI] [Google Scholar]
- Zhou MD, Lee TT. Selectivity of auxin for induction and growth of callus from excised embryo of spring and winter wheat. Can J Bot. 1984;62:1393–1397. doi: 10.1139/b84-189. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.