Abstract
Our study aimed to evaluate intraspecific variability of pea (Pisum sativum L.) in Al tolerance and to reveal mechanisms underlying genotypic differences in this trait. At the first stage, 106 pea genotypes were screened for Al tolerance using root re-elongation assay based on staining with eriochrome cyanine R. The root re-elongation zone varied from 0.5 mm to 14 mm and relationships between Al tolerance and provenance or phenotypic traits of genotypes were found. Tolerance index (TI), calculated as a biomass ratio of Al-treated and non-treated contrasting genotypes grown in hydroponics for 10 days, varied from 30% to 92% for roots and from 38% to 90% for shoots. TI did not correlate with root or shoot Al content, but correlated positively with increasing pH and negatively with residual Al concentration in nutrient solution in the end of experiments. Root exudation of organic acid anions (mostly acetate, citrate, lactate, pyroglutamate, pyruvate and succinate) significantly increased in several Al-treated genotypes, but did not correlate with TI. Al-treatment decreased Ca, Co, Cu, K, Mg, Mn, Mo, Ni, S and Zn contents in roots and/or shoots, whereas contents of several elements (P, B, Fe and Mo in roots and B and Fe in shoots) increased, suggesting that Al toxicity induced substantial disturbances in uptake and translocation of nutrients. Nutritional disturbances were more pronounced in Al sensitive genotypes. In conclusion, pea has a high intraspecific variability in Al tolerance and this trait is associated with provenance and phenotypic properties of plants. Transformation of Al to unavailable (insoluble) forms in the root zone and the ability to maintain nutrient uptake are considered to be important mechanisms of Al tolerance in this plant species.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0469-0) contains supplementary material, which is available to authorized users.
Keywords: Aluminium, Biodiversity, Organic acids, Nutrient uptake, Pea, Rhizosphere
Introduction
High soil acidity is a worldwide stress factor limiting productivity of agricultural crops. For the most part, phytotoxicity of acid soils is the result of elevated concentrations of mobile aluminium ions, such as Al3+ and hydroxy-Al species (Kochian 1995; Gupta et al. 2013). Plants have developed a number of mechanisms to avoid and/or tolerate Al toxicity: exudation of organic acids (malate, citrate, oxalate) from roots to complex Al in the rhizosphere and prevent its entry into the root; increased rhizosphere pH and secretion of mucilage to immobilize Al outside the root; internal detoxification involving Al chelation and complexation within plant tissues; sequestration of Al in the vacuole, and the actual efflux of accumulated Al from the root apex via transport proteins (Taylor 1991; Ma et al. 2001; Kochian et al. 2004; Delhaize et al. 2012; Liu et al. 2014). Numerous studies performed to investigate the mechanisms of Al tolerance in different plant species such as wheat, barley, maize, soybean and Arabidopsis thaliana, revealed differences in the significance of a particular mechanism for different plant species (see literature reviewed in the references above). However, little is known about Al tolerance in pea (Pisum sativum L.). An Al-tolerant pea cultivar had a higher pH value in the root zone and lower root Al content (Klimashevsky et al. 1972) compared to Al-sensitive cultivar treated with Al. Measuring root growth during a recovery period in Al-free solution distinguished differences in Al toxicity between two pea cultivars (Lazof and Holland 1999). The recovery of pea root elongation depended on the ability to resist Al-induced oxidative stress and the reduction of lignin production (Motoda et al. 2010; Matsumoto and Motoda 2012). Immobilization of Al by pectin located in the pea root border cells was shown to play an important role in protecting the root apex (Yu et al. 2009a).
The important role of calcium (Ca) in alleviating Al toxicity in plants occurs by preventing a decrease in the negative charge on the plasma membrane (PM) caused by Al, decreasing activity of Al3+ on PM surface, and maintaining normal hormonal status and Ca2+-related signalling pathways in the presence of toxicant (Rengel 1992; Kinraide et al. 2004; Kobayashi et al. 2013; Pandey et al. 2013). Magnesium (Mg) can also counteract Al phytotoxicity by increasing synthesis and exudation of organic acids, improving carbon partitioning from shoots to roots, regulating cytoplasmic pH and enhancing activity of stress-related enzymes (Rengel and Robinson 1989; Silva et al. 2001; Bose et al. 2011; Pandey et al. 2013). Zhou et al. (2015) showed that the alleviation mechanism of boron (B) could be related to changes in expression of genes involved in Al tolerance. Exudation of phosphate from maize roots might also be involved in immobilization of Al in the rhizosphere (Pellet et al. 1995, 1996; Pineros et al. 2002). In pea, treatment with Ca decreased a negative effect of Al on K uptake by roots (Matsumoto and Yamaya 1986) and treatment with B alleviated Al toxicity in root tips by decreasing Al binding in cell walls and callose formation (Yu et al. 2009b). The role of other nutrient elements in interactions of plants with toxic Al is scarcely understood.
Pea, along with other legume species, is a relatively sensitive crop compared to cereals (Aniol and Gustafson 1984; Lazof and Holland 1999; Akhter et al. 2009). Toxic Al concentrations inhibited pea root growth and injured root tissues (Lazof and Holland 1999; Motoda et al. 2010), induced oxidative stress (Yamamoto et al. 2001; Panda and Matsumoto 2010; Matsumoto and Motoda 2012) and inhibited nutrient uptake (Matsumoto and Yamaya 1986).
Significant intraspecific variability in Al tolerance was reported for various crops. In wheat cultivars, Al tolerance positively correlated with malate efflux from root apices (Ryan et al. 1995), Ca influx and transport from root to shoot (Huang et al. 1995) and shoot Ca content (Foy 1996), but negatively correlated with root Al content (Foy 1996; Khabaz-Saberi and Rengel 2010). A lower root Al content (Stass et al. 2006) and higher root citrate and phosphate exudation (Pellet et al. 1995) was observed in an Al-tolerant maize cultivar, compared to Al-sensitive one. Comparing 25 sorghum genotypes demonstrated the important role of maintaining nutrient homeostasis to alleviate Al stress (Bernal and Clark 1997). Root retention of Al, preventing its transport to the shoot, was proposed as a mechanism of Al tolerance comparing 9 rice cultivars (Jan and Pettersson 1989). Al-tolerant soybean genotypes possessed higher P efficiency (Liao et al. 2006; Liang et al. 2013), responded more actively to toxic Al by citrate, malate and oxalate exudation (Silva et al. 2001; Liao et al. 2006), and responded to Mg or Ca treatments by alleviating Al toxicity (Silva et al. 2001). However, intraspecific variability in Al tolerance of pea was not studied, except our recent report describing significant genotypic variation in root re-elongation of 19 pea genotypes following Al exposure (Vishnyakova et al. 2015).
Our study aimed to investigate intraspecific variability of pea in Al tolerance, to select contrasting genotypes and use them for revealing the mechanisms underlying genotypic differences in this trait. The selected genotypes is a promising model for the in-depth study of pea tolerance to acidic soils and for breeding highly productive Al-resistant cultivars.
Materials and methods
Plant material
Seeds of pea (Pisum sativum L.) were obtained from the Pea World Collection (PWC) of the N.I. Vavilov Research Institute of Plant Genetic Resources (VIR, Saint-Petersburg). Selection of genotypes was carried out to provide maximum coverage of the species genetic biodiversity. The following phenotypic and economic traits of pea genotypes were taken into account: geographical origin, purpose of use, morphotype, individual seed biomass, seed yield, seed color, seed surface and period of maturity (see Table 1 in Online Resource 1 for details). The catalogue collection numbers of the studied varieties are given in the text, Online Resource 1 and figures throughout this paper.
Primary screening for Al tolerance using root re-elongation assay
The recently proposed root re-elongation assay to rapidly assess Al tolerance in pea (Vishnyakova et al. 2015) was used to screen 106 genotypes. For this purpose, seeds (25 seeds per genotype) were germinated in the dark for 3 days at 21 °C in special containers filled with nutrient solution (µM): KH2PO4, 400; KNO3, 1200; MgSO4, 250; CaCl2, 60; pH 4.5. Well germinated seeds (from 10 to 20 depending on genotype) were selected, washed with water, placed in fresh nutrient solution supplemented with 110 µM AlCl3 × 6H2O and incubated for 24 h at 21 °C in growth chamber (7000 lx, 12 h photoperiod with minima/maxima temperatures of 19/21 °C respectively). Then seedlings were transferred to fresh nutrient solution without Al and incubated for 2 days as described above. The roots were removed from solution and stained with 0.1% eriochrome cyanine R for 10 min. Staining of roots with eriochrome cyanine R was repeatedly used for rapid assessment of Al tolerance in different plant species using root re-elongation assay. Recently we adapted this method to estimate root re-elongation of pea treated with Al and have already published these results (Vishnyakova et al. 2015). Eriochrome cyanine R is a dye that forms color complexes with cations such as Al and Fe. So, some staining probably could be observed on roots of plants grown in Al-free solution in the presence of Fe as a micronutrient. However, intensity of such staining is almost invisible and dramatically low as compared with Al-treated roots. Actually, if the root tip is significantly damaged by Al, it contains Al in tissues, stained purple and does not re-elongate. If the damage is less, the root re-elongates, some staining is present on damaged zone, but the re-elongating root zone is not stained because there is no Al in tissues developed after transferring the plants to Al-free solution. Root damage by Al was coloured purple and the root re-elongation zone was measured as the length of uncoloured zone from the root tip named as increment of root (IR). Genotypes having contrasting response to Al toxicity were repeatedly assessed as described above.
Plant growth conditions in hydroponic culture
Seeds were surface sterilized and scarified by treatment with 98% H2SO4 for 30 min, rinsed with tap water and germinated on filter paper in Petri dishes for 3 days at 25 °C in the dark. Seedlings were transferred to plastic pots (one pot with 5 uniform seedlings per genotype and treatment) containing 1 l of sterile nutrient solution (µM): KH2PO4, 400; KNO3, 1200; Ca(NO3)2, 60; MgSO4, 250; KCl, 250; CaCl2, 60; Fe-tartrate, 12; H3BO3, 2; MnSO4, 1; ZnSO4, 3; NaCl, 6; Na2MoO4, 0.06; CoCl2, 0,06; CuCl2, 0.06; NiCl2, 0,06. The nutrient solution was acidified up to pH 4.7 via addition of 1 M HCl and supplemented or not with 80 µM AlCl3 × 6H2O. Such Al concentration was chosen because preliminary experiments showed that during the experiment, a complete growth inhibition of Al sensitive genotypes treated with 110 µM Al occurred. Plants were cultivated for 10 days in a growth chamber (ADAPTIS-A1000, Conviron, UK) with 7000 lx, 12 h photoperiod and minima/maxima temperatures of 18/23 °C respectively. The nutrient solution was changed and where necessary the supplement was added on the 5th day after planting. Transpiration losses were compensated daily via addition of sterile deionized water to maintain constant solution volume. Root and shoot fresh weight (FW) of individual plants and pH of nutrient solution were determined. Roots were washed for 1 min in deionized water to remove unbound Al and nutrients from root surface. Aliquots of nutrient solution were centrifuged at 7000 rpm for 10 min, acidified with HNO3 up to final concentration of 0.5% to prevent microbial activity and stored for elemental analysis. The plants were dried at room temperature and stored for elemental analysis. Experiments for each pea genotype were repeated three times with 5 plants each.
Root exudation of organic acid anions
On harvest day, the nutrient solution of each pot (containing root exudates accumulated for 5 days) was centrifuged (Model 5804R, Eppendorf, USA) for 10 min at 4500 g, vacuum filtered through 0.45 µm filters (Waters, USA) and concentrated at 45 °C using a rotary vacuum evaporator (Heidolph Hei-VAP Precision, Heidolph Instruments GmbH & Co, Germany). The concentrates were passed through a column of ion exchange resin DOWEX 50Wx8, evaporated to dryness, dissolved in 0.5 ml of deionized water and filtered through 0.22 µm centrifuge tube filters (Corning Costar Spin-X, Corning Inc, USA) for subsequent chromatographic analysis using the UPLC system Waters ACQUITY H-Class (Waters, USA) as previously described (Kuzmicheva et al. 2014). Organic acids were separated on column ACQUITY CSH C18 (Waters, USA) and determined using UV detector at 210 nm. Standards comprised organic acids from analytical grade reagents of Sigma-Aldrich (USA).
Elemental analysis
The dried roots and shoots were ground and digested in a mixture (1:1) of concentrated HNO3 and 38% H2O2 at 70 °C using DigiBlock (LabTech, Italy). Contents of elements in digested plant samples (Al, B, Ca, Co, Cu, Fe, K, Mg, Mn, Mo, Ni, P, S and Zn) and concentration of Al in the acidified nutrient solutions were determined using an inductively coupled plasma emission spectrometer ICPE-9000 (Shimadzu, Japan) following manufacturer instructions.
Statistical analysis
Statistical analysis of the data was performed using the software STATISTICA version 10 (StatSoft Inc., USA) and an ANOVA software (Vorobyev et al. 2013). Fisher’s LSD test (ANOVA), correlation analysis, and cluster analysis (standardized values, Ward’s method for linkage rules, 1-Pearson-r distance measure) were applied.
Results
Screening of 106 pea genotypes showed that after treatment with Al the IR varied significantly from 0.5 to 1.5 mm for the most Al-sensitive genotypes to 13–14 mm for the most Al-tolerant genotypes (Fig. 1). The distribution of genotypes on the basis of this trait was normal: MV = 7.6; SD = 3.6; CV = 47 ± 3; As = −0.03 ± 0.23; Ex = −0.79 ± 0.46.
Fig. 1.
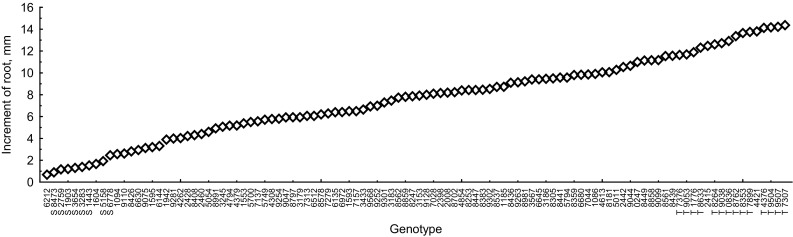
Root re-elongation (increment of root) of 106 pea genotypes. Seedlings were treated with 110 µM AlCl3 for 1 day, transferred to fresh nutrient solution without aluminum and incubated for 2 days. Standard errors are less then symbol size (n varied from 10 to 30 depending on genotype). The most Al-tolerant or the most Al-sensitive genotypes chosen for further experiments are marked by T or S, respectively
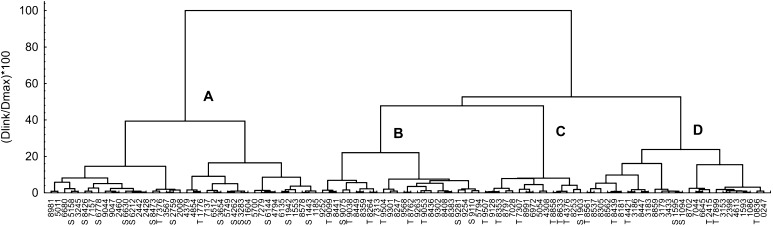
Four clusters were recognized on approximately 40% dissimilarity level when pea genotypes were clustered based on phenotypic and economic traits listed in Materials and methods section (Fig. 2). The most Al-sensitive genotypes tended to group together in cluster A, whereas most of the Al-tolerant genotypes distributed among clusters B, C and D. Indeed, mean IR value of genotypes combined in cluster A significantly differed from other clusters (Fig. 3). The IR value correlated with geographic origin (R = 0.21, P = 0.027, n = 106), purpose of use (R = 0.39, P < 0.001, n = 106) and individual seed biomass (r = −0.53, P < 0.001, n = 106) values. Analysis of these results showed that the genotypes combined in cluster A mainly originated from South America, Australia or Africa, were related to forage peas and characterized by small individual seed biomass and seed yield (Fig. 3).
Fig. 2.
Dendrogram showing relationships among the studied pea varieties based on cluster analysis of the data for phenotypic and economic traits (see “Materials and methods” section for details). Ward’s method for linkage rules, 1-Pearson-r distance measure. Capital letters indicate numbers of clusters. The 20 most Al-tolerant or 20 most Al-sensitive genotypes (accordingly to the data shown in Fig. 1) are marked by T or S, respectively. The properties used for cluster analysis are presented in supplemental Table 1
Fig. 3.
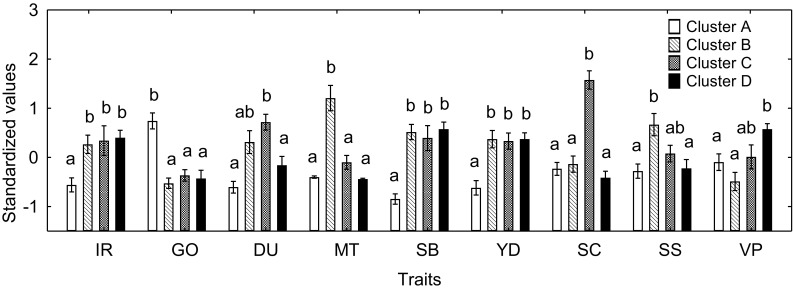
Means for the clusters combining pea genotypes according to the dendrogram shown in Fig. 2. Traits: increment of root (IR), geographic origin (GO), direction of use (DU), morphotype (MT), individual seed biomass (SB), seed yield (YD), seed color (SC), seed surface (SF), vegetation period (VP). Note that IR was not included into the cluster analysis. Vertical bars indicate standard errors (n = 40, 24, 15 and 27 for clusters 1, 2, 3 and 4, respectively). Different lowercase letters indicate significant differences between clusters for each trait (LSD test, P ≤ 0.05)
Based on the screening results, the most Al-sensitive (8473, 2759, 1903, 3654, 3283, 1443, 5158, 6778) and Al-tolerant genotypes (7376, 9053, 1776, 8633, 8264, 9038, 0836, 8762, 8353, 7899, 4376, 9504, 9507, 7307) were used for further experiments. Treatment with 80 µM AlCl3 for 10 days significantly decreased root biomass of all genotypes except 6778, 7307, 0836 and 8353 (Fig. 4a), whereas shoot biomass decreased in all genotypes except 6778, 7307 and 8357 (Fig. 4b). TI varied from 30% (genotype 3283) to 92% (genotype 8353) for root biomass and from 38% (genotype 2759) to 90% (genotypes 6778 and 8353) for shoot biomass (Fig. 4c). The IR value positively correlated with root TI (r = +0.68, P < 0.0001, n = 22), but not with shoot TI (r = +0.32, P = 0.15, n = 22), whereas root and shoot TIs were closely correlated (r = +0.73, P < 0.0001, n = 22).
Fig. 4.

Effect of aluminium on biomass of root (a) and shoot (b) and tolerance index (c) of the selected pea genotypes. Vertical bars indicate standard errors (n = 15). Asterisks indicate significant differences between untreated (control) and Al-treated (80 µM Al) plants for each genotype (LSD test, P ≤ 0.05). Genotypes are shown in order of increasing root tolerance index from left to right
Eleven genotypes (Al-sensitive 3654, 8473, 2759, 1903; Al-tolerant 8762, 9507, 8633, 6778, 7307, 0836, 8353) were selected for more detailed study. Amongst these, significant genotypic differences in Al content were found in root (Fig. 5a) and/or in shoot (Fig. 5b). These Al contents were not correlated with IR or TI values, however minimal root Al contents were observed in two Al-sensitive genotypes 2759 and 2759. Shoot Al content was similar in all genotypes, except that 3654 (Al-sensitive) and 6778 (Al-tolerant) had 6 and 3 times higher Al-contents as compared with other genotypes (Fig. 5b). At the end of experiment, Al concentration in nutrient solution significantly differed depending on pea genotype (Fig. 5c) and negatively correlated with IR (r = −0.77, P = 0.006, n = 11), root TI (r = −0.84, P = 0.001, n = 11) and shoot TI (r = −0.67, P = 0.025, n = 11). Moreover, final pH of nutrient solution negatively correlated with final solution Al concentration (r = −0.69, P = 0.020, n = 11), but positively correlated with IR (r = +0.63, P = 0.038, n = 11), root TI (r = +0.75, P = 0.007, n = 11) and shoot TI (r = −0.68, P = 0.022, n = 11). Significant correlation between final pH of nutrient solution and IR (r = +0.45, P = 0.035, n = 22) or root TI (r = +0.52, P = 0.013, n = 22) was also observed for 22 pea genotypes presented in Fig. 4. Slight turbidity of the Al treated solutions was observed for all pea genotypes, suggesting precipitation of Al.
Fig. 5.

Aluminium content in root (a) and shoot (b) of the selected pea genotypes and final solution Al concentration (c) and pH (d) in the end of experiments with Al-treated plants. Vertical bars indicate standard errors (for Al in plants n = 5; for solution Al and pH n = 3). Different lowercase letters indicate significant differences between means (LSD test, P ≤ 0.05). Genotypes are shown in order of increasing root tolerance index from left to right and the arrow differentiates Al sensitive from Al resistant genotypes
The major organic acid anions exuded by pea roots and detected in the nutrient solutions at the end of experiments were acetate, citrate, lactate, pyroglutamate, pyruvate and succinate (Fig. 6). In the absence of Al, maximum exudation of organic acids was observed for Al-tolerant genotypes such as 6778 (acetate, citrate and succinate), 0836 (acetate, citrate and pyruvate) and 8353 (acetate, lactate and pyroglutamate). However, in the presence of Al, maximum exudation was observed in Al-sensitive genotypes 8473 (acetate and pyruvate), 2759 (citrate, pyroglutamate and pyruvate) and 1903 (acetate and succinate), as well as in Al-tolerant genotypes 8353 (lactate) and 6778 (pyruvate and succinate). Malate was detected in solutions of Al-untreated 2759 (67 ± 16 µg g−1 DW) and 0836 (380 ± 139 µg g−1 DW) genotypes only. In general, when summing all organic acids, Al treatment increased exudation of these compounds, particularly in Al-sensitive genotypes 3654, 2759 and 1903, as well as in Al-tolerant genotypes 6778 and 7307 (Fig. 6). However, in some cases Al-treatment decreased organic acid exudation, namely lactate (genotypes 3654, 2759, 8633 and 8353) and pyroglutamate (3654, 1903, 8762, 9507, 8633 and 8353). No correlations were found between organic acid exudation and TI or Al content in plants, except that root Al content negatively correlated with pyroglutamate (r = −0.72, P = 0.013, n = 11) or the sum of all organic acids (r = −0.65, P = 0.032, n = 11) exuded by Al-treated plants.
Fig. 6.

Exudation of organic acid anions by roots of the selected pea genotypes. The plants were incubated in nutrient solution for 5 days. White and black columns indicate untreated control and Al-treated plants, respectively. Vertical bars indicate standard errors (n = 3). Asterisks show not detected. Different lowercase letters indicate significant differences between means (LSD test, P ≤ 0.05). Genotypes are shown in order of increasing root tolerance index from left to right and the arrow differentiates Al sensitive from Al resistant genotypes
Treatment with Al significantly decreased contents of macronutrients K, Mg and S in roots of all genotypes, except for Al-tolerant genotypes 7307 and 0836 (Table 2 in Online Resource 1). Root Ca content also decreased in Al-treated plants, except for Al-tolerant genotypes 7307, 0836 and 8353. In contrast, root P content was increased in all genotypes, except for Al-tolerant genotype 8633. Significant decrease in shoot K, Ca, Mg and S contents was found in all genotypes, except for Al-tolerant genotype 7307, which had increased content of these elements, and genotype 0836, which had increased S content (Table 3 in Online Resource 1). Shoot P content was decreased in genotypes 8473, 8762, 8633 and 6778.
Al treatment generated large variation in plant micronutrient uptake, depending on element, genotype and plant organ (root or shoot). Several Al-treated genotypes, particularly those which were Al-sensitive, had increased content of B, Fe and Mn in roots (Table 4 in Online Resource 1) and B and Fe in shoots (Table 5 in Online Resource 1). Molybdenum content of Al-sensitive genotypes increased in roots, but was not affected or even decreased in shoots, mostly of Al-tolerant genotypes. Majority of the genotypes, except for Al-tolerant 7307 and 0836, had decreased content of Co, Mn, Ni and Zn in both root (Table 4 in Online Resource 1) and shoot (Table 5 in Online Resource 1).
Two Al-tolerant genotypes 7307 and 0836 significantly differed from other genotypes by maintaining nutrient uptake in the presence of toxic Al (Tables 4 and 5 in Online Resource 1). When root TI was plotted against the effects of Al on root nutrient (B, Ca, Co, Fe, K, Mg, Mn and Mo) concentrations, the more tolerant genotypes consistently showed smaller percent changes in root element composition, independently whether the element content was decreased or increased by Al treatment (Fig. 7). Similar relationships were found between root TI and shoot Ca, Co, K, Mg, Mn, Ni and Zn contents, as well as between shoot TI and shoot Ca, K and Mn contents (data not shown).
Fig. 7.

Correlations between Al tolerance index of roots and effects of Al on nutrient element contents. Pea genotypes: 1—3654; 2—8473; 3—2759; 4—1903; 5—8762; 6—9507; 7—8633; 8—6778; 9—7307; 10—0836; 11—8353. Genotypes are listed in order of increasing root tolerance index. Dashed line shows linear regression. Element symbol, correlation coefficient (r) and probability (P) are shown in each part of the figure (n = 11)
Discussion
Significant variation among 106 pea genotypes was found in response to toxic Al estimated by the root re-elongation assay and by the root and shoot growth responses to Al treatment. This is in agreement with substantial intraspecific variability in this trait previously found in other legumes such as cowpea (Horst 1987; Kolawole et al. 2000; Jemo et al. 2007), clover (Baligar et al. 1987) and soybean (Liao et al. 2006; Villagarcia et al. 2001).
For the first time, we detected relationships between Al tolerance and provenance or phenotypic traits of pea varieties such as geographic origin, purpose of use, individual seed biomass and seed yield. The only previous report described correlation between Al tolerance of sorghum varieties with their agronomic traits like biomass, height and days to flowering (Anas and Yoshida 2004). Previously, we also found that tolerance to Cd negatively correlated with seed number, individual seed weight and seed N content of 99 pea genotypes (Belimov et al. 2015b). Interestingly, Al-tolerant or Cd-tolerant pea genotypes were less productive (that is characteristic of forage peas) and probably less domesticated. Although better understanding of the observed relationships is required, these results seem useful for elucidating the mechanisms underlying genotype-related Al tolerance and for breeding purposes.
It was repeatedly shown in experiments with bean (Shen et al. 2002), soybean (Silva et al. 2001), wheat (Foy 1996; Khabaz-Saberi and Rengel 2010) and maize (Stass et al. 2006) that root Al content is lower in Al tolerant genotypes. The Al-sensitive pea mutant E107 (brz) actively accumulated Al in roots, seriously inhibiting root growth (Guinel and LaRue 1993). On the other hand, opposite observations were obtained when comparing cowpea (Jemo et al. 2007), wheat (Kikui et al. 2007) or rice (Jan and Pettersson 1989) genotypes. Aluminum tolerance did not correlate with root Al content in sorghum genotypes (Bernal and Clark 1997). Similarly, in our experiments Al tolerance was not correlated with root or shoot Al contents in pea, despite of significant genotypic differences in these traits. While we agree that low root Al content is important to counteract Al toxicity in various plant species, but propose that this mechanism does not play a crucial role in Al tolerance of pea. In our previous experiments with pea, growth and metabolic responses to Cd toxicity did not correlate with root Cd content (Metwally et al. 2005), but genotypic differences in Cd tolerance were related to decreased Cd transport from root to shoot (Belimov et al. 2003). However, the pea mutant SGECdt, characterized by increased tolerance to Cd and Co, and decreased tolerance to Hg, accumulated more Cd, equal Co, but less Hg in roots and shoots (Belimov et al. 2015a). Thus, our present results give new evidence concerning partial independence of traits related to tolerance and uptake of toxic metals by plants.
For the first time we showed that Al tolerance was negatively correlated with residual Al concentration in the nutrient solution, suggesting that Al tolerant genotypes were better able to actively exclude Al from the root zone, probably due to precipitation with phosphates and other compounds. Further studies are needed to investigate the nature and the role of such compounds in more detail. However, one mechanism of the observed phenomenon can be associated with changes in solution pH, because it is known that Al mobility decreases with increased pH (Kochian 1995) and in our experiments the final solution pH positively correlated with Al tolerance of pea genotypes. Previously, the Al-tolerant pea cultivar Success had higher solution pH compared to the Al sensitive cultivar Tulunsky Green (Klimashevsky et al. 1972). Similarly, solution pH and Al tolerance were positively correlated in winter (Taylor and Foy 1985a) and spring (Taylor and Foy 1985b) wheat cultivars, although it was not possible to explain genotypic differences in Al tolerance solely by the ability to maintain a high solution pH (Taylor 1988). Increased H+ influx by roots, leading to increased root surface pH, was observed in Al-tolerant A. thaliana mutant alr-104 (Degenhardt et al. 1998). However in other reports Al tolerance did not correlate with solution pH in wheat (Miyasaka et al. 1989) and barley (Wagatsuma and Yamasaku 1985) cultivars. Such contradictory results indicate that lowering pH is not a sole reason for decreased mobile Al concentration in the root zone, and that the latter parameter depends on plant genotype. Indeed, in our case solution pH of Al tolerant pea genotype 0836 was similar to those of Al sensitive genotypes, but the final Al concentration in the solution was similar to Al tolerant genotypes (Fig. 5).
Aluminium tolerant genotypes of cowpea (Jemo et al. 2007), soybean (Silva et al. 2001; Liao et al. 2006), bean (Shen et al. 2002) and lima bean (Mimmo et al. 2013) were characterized by high root exudation of the organic acid anions such as malate and/or citrate. Addition of malate or citrate to the nutrient solution increased Al tolerance of wheat (Kikui et al. 2007) or bean (Shen et al. 2002), respectively. Treatment of pea with malate and citrate restored activity of membrane-associated Mg2+-ATPase in the presence of toxic Al (Matsumoto and Yamaya 1986). These reports showed that active exudation of malate and citrate to complex Al ions in root zone is an important mechanism of Al tolerance. However, Al treatment of Al tolerant cowpea genotypes did not increase citrate exudation (Jemo et al. 2007). No correlation was found between Al tolerance and organic acid exudation by oat (Zheng et al. 1998) and maize varieties (Kidd et al. 2001; Pineros et al. 2005) or by closely related signalgrass species (Wenzl et al. 2001), suggesting that other mechanisms were involved in this trait. In our study, root or shoot TIs were not correlated with concentrations of detected organic acids in the nutrient solution, and only five of eleven Al treated genotypes exuded more organic acids than control plants. Moreover, malate was detected only in exudates of one Al tolerant (0836) and one Al sensitive (2759) genotype. At the same time, root Al content was negatively correlated with exudation of pyroglutamate and the sum of all organic acids by Al-treated plants. These results demonstrated that organic acid exudation is not a major determinant of Al tolerance in pea, but is involved in decreasing Al uptake by pea roots. An important observation was that the main components of the exuded organic acids by pea were not malate and citrate, but succinate, pyruvate, acetate and lactate. Interestingly, lactate exudation correlated with final solution pH. The role of these acid anions in plant Al tolerance has not been investigated and it would be worthwhile to elucidate it.
It was reported that Al treatment inhibited uptake of Ca in cowpea (Horst 1987) and wheat (Huang et al. 1995), uptake of Mg in ryegrass (Rengel and Robinson 1989) and uptake of K in pea (Matsumoto and Yamaya 1986). Aluminium toxicity significantly decreased root Ca, Mg, K, Fe, S, Mn and Zn contents in sorghum (Bernal and Clark 1997). On the other hand, Ca and Mg treatments alleviated Al toxicity, probably due to competition for the binding sites in the apoplast, and these nutrient elements were repeatedly shown to be important in enhancing Al tolerance in various plant species (Rengel 1992; Silva et al. 2001; Bose et al. 2011; Kobayashi et al. 2013; Pandey et al. 2013), although pea has not been studied in this respect. Supplemental boron (B) enhanced Al tolerance in several plant species (see references cited by Zhou et al. 2015), including pea (Yu et al. 2009b). For the first time, we have shown that Al toxicity not only inhibits uptake of many nutrient elements and Na, but increases content of several nutrients (P, B, Fe and Mo) and Cr in roots and/or in shoots. Thus, Al treatment perturbed nutrient homeostasis and translocation of elements from root to shoot. Our important observation was that Al tolerance of the selected pea genotypes negatively correlated with percent changes in root and/or shoot element contents caused by Al toxicity. Among these elements, B, Ca and Mg are known to be important agents for plant Al tolerance. The role of other elements such as K, Co, Fe, Mn and Mo in alleviating Al toxicity is worthy of further study. We propose that maintaining nutrient uptake per se is an important mechanism of Al tolerance in pea. This trait is likely related to the ability of tolerant genotypes to counteract negative effects of Al on function and permeability of plasma membranes, which in its turn depends on Ca and Mg nutrition (Kinraide 1998; Kinraide et al. 2004; Silva et al. 2001). The importance of balanced nutrient uptake and transport for pea subjected to toxic metals such as Cd, Co and Hg was previously shown in our experiments with mutant SGECdt (Tsyganov et al. 2007; Belimov et al. 2015a).
Conclusion
Using a representative range of pea genotypes, we revealed significant intraspecific variability in Al tolerance and Al content of plants subjected to Al stress. Moreover, for the first time we found relationships between Al tolerance and provenance or phenotypic traits of plants. The selected pea genotypes are useful model for elucidating the mechanisms underlying genotype-related Al tolerance and for breeding purposes. The absence of correlations between Al tolerance and Al content in plants or organic acid exudation suggests that low uptake, root-to-shoot transport and exclusion of Al from plant tissues and root exudation of organic acids do not play a crucial role in Al tolerance in pea. However, the role of the major organic acids found in pea root exudates (acetate, lactate, pyroglutamate, pyruvate) in response to toxic Al requires further study. The important mechanism of Al tolerance in pea found here is related to active exclusion of Al from the root zone, most probably due to increased solution pH and transformation of mobile Al into insoluble forms via precipitation with phosphates and/or other unknown compounds. The ability to counteract negative effects of Al on uptake and transport of nutrient elements from root to shoot, and thereby maintain nutrient uptake, is a second mechanism of Al tolerance in pea. Thus, genotype dependent and multi-factorial reactions including rhizosphere processes and internal mechanisms modulate Al tolerance in this plant species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. Dodd I.C. for critically reading the manuscript and help in improving the English language. This work was supported by the Russian Science Foundation (14-16-00137 and only for UPLC analysis 14-26-0094).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0469-0) contains supplementary material, which is available to authorized users.
References
- Akhter A, Wagatsuma T, Khan MSH, Tawaraya K. Comparative studies on aluminum tolerance screening techniques for sorghum, soybean and maize in simple solution culture. Am J Plant Physiol. 2009;4:1–8. doi: 10.3923/ajpp.2009.1.8. [DOI] [Google Scholar]
- Anas A, Yoshida T. Heritability and genetic correlation of Al-tolerance with several agronomic characters in sorghum assessed by hematoxylin staining. Plant Prod Sci. 2004;7:280–282. doi: 10.1626/pps.7.280. [DOI] [Google Scholar]
- Aniol A, Gustafson P. Chromosome location of genes controlling aluminum tolerance in wheat, rye and triticale. Can J Genet Cytol. 1984;26:701–705. doi: 10.1139/g84-111. [DOI] [Google Scholar]
- Baligar VC, Wright RJ, Kinraide TB, Foy CD, Elgin JH. Aluminum effects on growth, mineral uptake, and efficiency ratios in red clover cultivars. Agron J. 1987;79:1038–1044. doi: 10.2134/agronj1987.00021962007900060018x. [DOI] [Google Scholar]
- Belimov AA, Safronova VI, Tsyganov VE, Borisov AY, Kozhemyakov AP, Stepanok VV, Martenson AM, Gianinazzi-Pearson V, Tikhonovich IA. Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.) Euphytica. 2003;131:25–35. doi: 10.1023/A:1023048408148. [DOI] [Google Scholar]
- Belimov AA, Dodd IC, Safronova VI, Malkov NV, Davies WJ, Tikhonovich IA. The cadmium tolerant pea (Pisum sativum L.) mutant SGECdt is more sensitive to mercury: assessing plant water relations. J Exp Bot. 2015;66:2359–2369. doi: 10.1093/jxb/eru536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belimov AA, Puhalsky IV, Safronova VI, Shaposhnikov AI, Vishnyakova MA, Semenova EV, Zinovkina NY, Makarova NM, Wenzel W, Tikhonovich IA. Role of plant genotype and soil conditions in symbiotic plant-microbe interactions for adaptation of plants to cadmium polluted soils. Water Air Soil Pollut. 2015;226:1–15. doi: 10.1007/s11270-015-2537-9. [DOI] [Google Scholar]
- Bernal JH, Clark RB. Mineral acquisition of aluminum-tolerant and sensitive sorghum genotypes grown with varied aluminum. Commun Soil Sci Plant Anal. 1997;28:49–62. doi: 10.1080/00103629709369771. [DOI] [Google Scholar]
- Bose J, Babourina O, Rengel Z. Role of magnesium in alleviation of aluminium toxicity in plants. J Exp Bot. 2011;62:2251–2264. doi: 10.1093/jxb/erq456. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ma JF, Ryan PR. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012;17:341–348. doi: 10.1016/j.tplants.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Foy CD. Tolerance of durum wheat lines to an acid, aluminum toxic subsoil. J Plant Nutr. 1996;19:1381–1394. doi: 10.1080/01904169609365206. [DOI] [Google Scholar]
- Guinel FC, LaRue TA. Excessive aluminium accumulation in the pea mutant El07 (brz) Plant Soil. 1993;157:75–82. doi: 10.1007/BF02390229. [DOI] [Google Scholar]
- Gupta N, Gaurav SS, Kumar A. Molecular basis of aluminium toxicity in plants: a review. Am J Plant Sci. 2013;4:21–37. doi: 10.4236/ajps.2013.412A3004. [DOI] [Google Scholar]
- Horst WJ. Aluminium tolerance and calcium efficiency in cowpea genotypes. J Plant Nutr. 1987;10:1121–1129. doi: 10.1080/01904168709363640. [DOI] [Google Scholar]
- Huang JW, Grunes DL, Kochian LV. Aluminium and calcium transport interactions in intact roots and root plasmalemma vesicles from aluminium-sensitive and tolerant wheat cultivars. Plant Soil. 1995;171:131–135. doi: 10.1007/BF00009575. [DOI] [Google Scholar]
- Jan F, Pettersson S. Varietal diversity of upland rice in sensitivity to aluminium. J Plant Nutr. 1989;12:973–993. doi: 10.1080/01904168909364017. [DOI] [Google Scholar]
- Jemo MR, Abaidoo C, Nolte C, Horst WJ. Aluminum resistance of cowpea as affected by phosphorus-deficiency stress. J Plant Physiol. 2007;164:442–451. doi: 10.1016/j.jplph.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Khabaz-Saberi H, Rengel Z. Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J Plant Nutr Soil Sci. 2010;173:461–468. doi: 10.1002/jpln.200900316. [DOI] [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) J Exp Bot. 2001;52:1339–1352. [PubMed] [Google Scholar]
- Kikui S, Sasaki T, Osawa H, Matsumoto H, Yamamoto Y. Malate enhances recovery from aluminum-caused inhibition of root elongation in wheat. Plant Soil. 2007;290:1–15. doi: 10.1007/s11104-006-9068-5. [DOI] [Google Scholar]
- Kinraide TB. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998;118:513–520. doi: 10.1104/pp.118.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Pedler JF, Parker DR. Relative effectiveness of calcium and magnesium in the alleviation of rhizotoxicity in wheat induced by copper, zinc, aluminum, sodium, and low pH. Plant Soil. 2004;259:201–208. doi: 10.1023/B:PLSO.0000020972.18777.99. [DOI] [Google Scholar]
- Klimashevsky EL, Markova YA, Malysheva AS. Genotypic specify in localization of Al3+ in cells of pea roots. Dokl Akad Nauk SSSR. 1972;203:711–713. [Google Scholar]
- Kobayashi Y, Kobayashi Y, Watanabe T, Shaff JF, Ohta H, Kochian LV, Wagatsuma T, Kinraide TB, Koyama Y. Molecular and physiological analysis of Al3+ and H+ rhizotoxicities at moderately acidic conditions. Plant Physiol. 2013;163:180–192. doi: 10.1104/pp.113.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Phys. 1995;46:237–260. doi: 10.1146/annurev.pp.46.060195.001321. [DOI] [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kolawole GO, Tian G, Singh BB. Differential response of cowpea lines to aluminum and phosphorus application. J Plant Nutr. 2000;23:731–740. doi: 10.1080/01904160009382055. [DOI] [Google Scholar]
- Kuzmicheva YV, Shaposhnikov AI, Azarova NS, Petrova SN, Naumkina TS, Borisov AY, Belimov AA, Kravchenko LV, Parakhin NV, Tikhonovich IA. Composition of root exometabolites of the symbiotically effective pea cultivar triumph and its parental forms. Russ J Plant Physiol. 2014;61:112–118. doi: 10.1134/S1021443714010087. [DOI] [Google Scholar]
- Lazof DB, Holland MJ. Evaluation of the aluminium-induced root growth inhibition in isolation from low pH effects in Glycine max, Pisum sativum and Phaseolus vulgaris. Aust J Plant Physiol. 1999;26:147–157. doi: 10.1071/PP98072. [DOI] [Google Scholar]
- Liang C, Piñeros MA, Tian J, Yao Z, Sun L, Liu J, Shaff J, Coluccio A, Kochian LV, Liao H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013;161:1347–1361. doi: 10.1104/pp.112.208934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol. 2006;141:674–684. doi: 10.1104/pp.105.076497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Piñeros MA, Kochian LV. The role of aluminum sensing and signaling in plant aluminum resistance. J Int Plant Biol. 2014;56:221–230. doi: 10.1111/jipb.12162. [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6:273–278. doi: 10.1016/S1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Motoda H. Review: aluminum toxicity recovery processes in root apexes. Possible association with oxidative stress. Plant Sci. 2012;185–186:1–8. doi: 10.1016/j.plantsci.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Yamaya T. Inhibition of potassium uptake and regulation of membrane-associated Mg2+-ATPase activity of pea roots by aluminium. Soil Sci Plant Nutr. 1986;32:179–188. doi: 10.1080/00380768.1986.10557495. [DOI] [Google Scholar]
- Metwally A, Safronova VI, Belimov AA, Dietz KJ. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot. 2005;56:167–178. doi: 10.1093/jxb/eri017. [DOI] [PubMed] [Google Scholar]
- Mimmo T, Ghizzi M, Cesco S, Tomasi N, Pinton R, Puschenreiter M. Aluminium–phosphate interactions in the rhizosphere of two bean species: Phaseolus lunatus L. and Phaseolus vulgaris L. J Sci Food Agric. 2013;93:3891–3896. doi: 10.1002/jsfa.6392. [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Kochian LV, Shaff JE, Foy CD. Mechanisms of aluminum tolerance in wheat: an investigation of genotypic differences in rhizosphere pH, K+, and H+ transport, and root-cell membrane potentials. Plant Physiol. 1989;91:1188–1196. doi: 10.1104/pp.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H. Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil. 2010;333:49–58. doi: 10.1007/s11104-010-0318-1. [DOI] [Google Scholar]
- Panda SK, Matsumoto H. Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals. 2010;23:753–762. doi: 10.1007/s10534-010-9342-0. [DOI] [PubMed] [Google Scholar]
- Pandey P, Srivastava RK, Dubey RS. Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology. 2013;22:656–670. doi: 10.1007/s10646-013-1058-9. [DOI] [PubMed] [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. doi: 10.1007/BF01106775. [DOI] [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros MA, Magalhaes JV, Alves VMC, Kochian LV. The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol. 2002;129:1194–1206. doi: 10.1104/pp.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros MA, Shaff JE, Manslank HS, Carvalho AVM, Kochian LV. Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol. 2005;137:231–241. doi: 10.1104/pp.104.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. Role of calcium in aluminium toxicity. New Phytol. 1992;121:499–513. doi: 10.1111/j.1469-8137.1992.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Rengel Z, Robinson DL. Competitive A13+ inhibition of net Mg2+ uptake by intact Lolium multiflorum roots. Plant Physiol. 1989;91:1407–1413. doi: 10.1104/pp.91.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Aust J Plant Physiol. 1995;22:531–653. doi: 10.1071/PP9950531. [DOI] [Google Scholar]
- Shen H, Yan XL, Wang XR, Zheng SL. Exudation of citrate in common bean in response to aluminum stress. J Plant Nutr. 2002;25:1921–1932. doi: 10.1081/PLN-120013284. [DOI] [Google Scholar]
- Silva IR, Smyth TJ, Israel DW, Raper CD, Rufty TW. Magnesium ameliorates aluminum rhizotoxicity in soybean by increasing citric acid production and exudation by roots. Plant Cell Physiol. 2001;42:546–554. doi: 10.1093/pcp/pce067. [DOI] [PubMed] [Google Scholar]
- Stass A, Wang Y, Eticha D, Horst WJ. Aluminium rhizotoxicity in maize grown in solutions with Al3+ or Al(OH)4− as predominant solution Al species. J Exp Bot. 2006;57:4033–4042. doi: 10.1093/jxb/erl174. [DOI] [PubMed] [Google Scholar]
- Taylor GJ. Mechanisms of aluminum tolerance in Triticum aestivum (wheat). V. Nitrogen nutrition, plant-induced pH, and tolerance to aluminum; correlation without causality? Can J Bot. 1988;66:694–699. doi: 10.1139/b88-100. [DOI] [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. Plant Physiol Biochem. 1991;10:57–93. [Google Scholar]
- Taylor GJ, Foy CD. Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). I. Differential pH induced by winter cultivars in nutrient solutions. Am J Bot. 1985;72:695–701. doi: 10.2307/2443681. [DOI] [Google Scholar]
- Taylor GJ, Foy CD. Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). II. Differential pH induced by spring cultivars in nutrient solutions. Am J Bot. 1985;72:702–706. doi: 10.2307/2443682. [DOI] [Google Scholar]
- Tsyganov VE, Belimov AA, Borisov AY, Safronova VI, Georgi M, Dietz KJ, Tikhonovich IA. A chemically induced new pea (Pisum sativum L.) mutant SGECdt with increased tolerance to and accumulation of cadmium. Ann Bot. 2007;99:227–237. doi: 10.1093/aob/mcl261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagarcia MR, Carter TEJ, Rufty TW, Niewoehner AS, Jennette MV, Arrellano C. Genotypic rankings for aluminum tolerance of soybean roots grown in hydroponics and sand culture. Crop Sci. 2001;41:1499–1507. doi: 10.2135/cropsci2001.4151499x. [DOI] [Google Scholar]
- Vishnyakova MA, Semenova EV, Kosareva IA, Kravchuk ND, Loskutov SI, Puhalsky IV, Shaposhnikov AI, Sazanova AL, Belimov AA. Method for rapid assessment of aluminum tolerance of pea (Pisum sativum L.) Agric Biol. 2015;50:353–360. [Google Scholar]
- Vorobyev NI, Provorov NA, Sviridova OV (2013) Program for ANOVA randomized biological data. The official e-newsletter of the Federal Service for Intellectual Property “Computer programs, databases, integrated circuits”, 2, State registration certificate 2013615092, Mascow.
- Wagatsuma T, Yamasaku K. Relationship between differential aluminum tolerance and plant-induced pH change of medium among barley cultivars. Soil Sci Plant Nutr. 1985;31:521–535. doi: 10.1080/00380768.1985.10557461. [DOI] [Google Scholar]
- Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM. The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol. 2001;125:1473–1484. doi: 10.1104/pp.125.3.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Shen R, Liu J, Chen R, Xu M, Yang Y, Xiao H, Wang HD, Wang HZ, Wang C. The role of root border cells in aluminum resistance of pea (Pisum sativum) grown in mist culture. J Plant Nutr Soil Sci. 2009;172:528–534. doi: 10.1002/jpln.200800039. [DOI] [Google Scholar]
- Yu M, Shen RF, Xiao HD, Xu MM, Wang HZ. Boron alleviates aluminium toxicity in pea (Pisum sativum L.) Plant Soil. 2009;314:87–98. doi: 10.1007/s11104-008-9708-z. [DOI] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminum stress. Physiol Plant. 1998;103:209–214. doi: 10.1034/j.1399-3054.1998.1030208.x. [DOI] [Google Scholar]
- Zhou XX, Yang LT, Qi YP, Guo P, Chen SL. Mechanisms on boron-induced alleviation of aluminum-toxicity in Citrus grandis seedlings at a transcriptional level revealed by cDNA-AFLP analysis. PLoS ONE. 2015;10:e0115485. doi: 10.1371/journal.pone.0115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.