Abstract
Low-temperature storage is generally used to extend postharvest lifetime and to inhibit decay of cucumber fruit, but it also enhances the intensity of chilling injury. The capability of γ-aminobutyric acid to enhance antioxidant enzyme activities and reduce chilling injury was studied in cucumber (Cucumis sativus L.) fruit stored at 1 °C for 5 weeks. The purpose of this study was to define if the GABA-induced modification in antioxidant system and phospholipase activity is linked to the reduced chilling injury in cold-stored cucumber fruit. Alleviation of chilling injury by GABA treatment was related to increased content of proline, endogenous GABA and enhanced activities of CAT and SOD, together with reduced activities of PLC, PLD and LOX. We suggest that PLC, LOX and PLD are associated with chilling injury initiation by involvement in a signaling pathway and membrane deterioration. Therefore the results obtained in this study suggest GABA’s potential for postharvest applications for reducing chilling injury symptom in cucumber fruit.
Keywords: GABA, Cucumber fruit, Antioxidant enzyme, LOX, PLC, PLD
Introduction
Horticultural plants are used for food, either as edible products, or for culinary ingredients, and for medicinal use. They are genetically very diverse group and play a major role in modern society end economy. They are considered an important component of traditional food, and are also central to healthy diets of urban population (Benjak et al. 2005; Celik et al. 2007; Ercisli et al. 2010; Rop et al. 2014; Canan et al. 2016; Hricova et al. 2016; Zorenc et al. 2016).
Chilling injury (CI) is a physiological disturbance that restricts the storage of chilling-sensitive cucumber fruit at low temperatures (Lurie and Crisosto 2005). Two main assumptions, which are not pairwise exclusive, have been forwarded to clarify the injury effect of low temperature in this fruit. The first assumption proposes that chilling results in cell membrane stabilizations, which induces inhibition of membrane-bound enzymes or carrier activities (Wolfe 2006; Zhang et al. 2010). This stability is dependent on the fatty acid composition of the membranes. Some evidence in favor of the membrane rigidification hypothesis has been found (Nishida and Murata 1996; Promyou et al. 2008), but other reports found no obvious relationship between membrane fatty acid compound and CI sensitivity (Prasad 2001; Wongsheree et al. 2009; Sirikesorn et al. 2013). The second assumption refers to generation of active oxygen species (AOS). AOS consist of superoxide, hydroxyl radicals and hydrogen peroxide (H2O2). AOS rapidly react with different molecules, including proteins and DNA, and cause membrane lipid peroxidation (Rice-Evans et al. 1997). This leads to cellular damage or cell death (Blokhina et al. 2003). In presence of transition metal ions (such as Zn, Cu and Fe) hydrogen peroxide may react with superoxide radical (O−2·) to hydroxyl radicals (·OH). Among the primary produced radicals, the hydroxyl radicals are the most reactive and hence the most noxious (Apel and Hirt 2004).
GABA (γ-Aminobutyric acid) is a non-protein amino acid, conserved from bacteria through yeasts to vertebrates. GABA was detected in plants almost 60 years ago (Malekzadeh et al. 2012). As an intrinsic signal molecule, GABA is very useful in adjustment of stress reactions (Kinnersley and Turano 2000; Malekzadeh et al. 2014).
GABA synthesis system regulates H+ in cytosol, functioning as a pH-stat (Sawaki et al. 2009). GABA can mitigate oxidative damage induced by aluminium and proton stresses on barley seedlings (Song et al. 2010). Shi et al. (2010) reported that GABA regulates expression of genes involved in generation of H2O2 in Caragana intermedia roots under salinity. Exogenous GABA mitigates CI in tomato and wheat plants under chilling stress (Malekzadeh et al. 2012, 2014). A deeper understanding of GABA in decreasing CI needs further investigation.
CI affects cell membrane integrity (Rui et al. 2010). Lipid peroxidation is known to be responsible for damage to cell membrane integrity which can be measured by the malonyl-dialdehyde (MDA) generation (Wise and Naylor 1987). The peroxidation of membrane fatty acids generates MDA and its level is utilized as a marker of oxidative stress. A rise in MDA production indicates the occurrence of lipid peroxidation, which results in damage to the cell membrane (Hodges et al. 1999). MDA and electrolyte leakage (EL) content are popular physiological markers of membrane lipid peroxidation and loss of membrane semi-permeability. These markers are used to indirectly calculate cell membrane integrity (Malekzadeh et al. 2014).
This study aimed to evaluate whether GABA-affected changes in the antioxidant system and MDA, proline and EL content are linked to the enhanced tolerance to CI in cold-stored cucumber fruit.
Materials and methods
Plant material and treatments
Hydroponic cluster cucumber (Cucumis sativus L.) was obtained from a commercial greenhouse in Tehran, Iran. Fruits were harvested and immediately transported to the laboratory. The fruit surface was disinfected in 0.1% sodium hypochlorite solution for 2 min, thoroughly washed in tap water and air-dried at 25 °C prior to use. Based on previous studies (Kinnersley and Turano 2000; Deewatthanawong et al. 2010a, b; Shang et al. 2011; Malekzadeh et al. 2012, 2014), the first group was immersed in 5 mM solution for 10 min, whereas the second group of fruit was soaked in sterile deionized water for 10 min and served as controls. All fruits were then air-dried for approximately 30 min and stored at 1 °C and 80–90% relative humidity (RH). Samples were collected from fruit after 3 or 5 weeks of storage at 1 °C for measurements of levels of CI, EL, H2O2, MDA, GABA and proline content, and the activities of SOD, CAT, APX, LOX, PLC and PLD. Each experiment was replicated three times.
Untreated and treated fruits were sampled on day 0, after 3 and 5 weeks of cold storage. Equatorial slices of sampled fruit were diced, frozen in liquid nitrogen and stored at −80° C for enzyme analysis. For CI evaluation, fruit of each treatment was sampled weekly from cold storage and held at 20 °C for 3 days.
CI index
The degree of CI was visually assessed on the mesocarp surface, following a double cut parallel to the axial diameter (Ding et al. 2002). The extent of flesh browning was divided into 4 classes: 0 = no browning; 1 = browning covering < 25% of the fruit surface; 2 = browning covering < 50%, but > 25% of surface; 3 = browning covering > 50%. The average extent of cold damage was expressed as a CI index calculated using the following formula:
Determination of MDA and proline contents
MDA content was measured by the thiobarbituric acid method described by Chen et al. (2008). The MDA concentration was calculated according to the following formula:
where A532, A600 and A450 represent the absorbance of the mixture at 532, 600, and 450 nm, respectively.
Proline content was measured using the acid-ninhydrin method described by Shan et al. (2007). Proline content was expressed as μg proline g−1 FW.
Electrolyte leakage
The rate of electrolyte leakage was measured according to the method described by Chen et al. (2008) with modification. Cylinders of cucumber mesocarp tissue were excised with a 10-mm diameter stainless steel cork borer. Two pieces of 4 mm thickness were cut from each cylinder. After being rinsed three times (2–3 min) with deionized water, 10 pieces were put into 50 mL of deionized water and shaken at 100 cycles per min for 30 min. Conductivity was measured using a Conductance Bridge (DDS-11A, Yamei Electron Instrument Factory, Hangzhou, China). Total conductivity was obtained after keeping the flasks boiling for 10 min, and electrolyte leakage was expressed as percentage of total conductivity.
Enzyme assays
The activity of Superoxide dismutase was determined using the slightly modified method of Xu et al. (2008). Fresh samples (0.5 g) were rapidly extracted in a pre-chilled mortar on an ice bath with 5 mL of ice-cold phosphate buffer (100 mM, pH 7.8) containing 1 mM EDTA and 5% (w/v) PVP. After centrifugation at 10,000×g for 30 min at 4 °C, the supernatant was used for SOD analysis. One hundred μL of the enzyme extract was mixed with 2.5 mL of 100 mM phosphate buffer (pH 7.8), 75 μL of 55 mM methionine, 300 μL of 0.75 mM nitroblue tetrazolium (NBT) and 60 μL of 0.1 mM riboflavin in a test tube. The test tubes containing the reaction solution were irradiated under 2 fluorescent light tubes (40 μmol m−2 s−1) for 10 min. The absorbance measured at 560 nm with a UV/visible spectrophotometer (HACH, USA). Blanks and controls were run in the same manner, but without illumination and enzyme, respectively. One unit of SOD activity was determined by the method of Rao et al. (1996). The reaction medium contained 50 mmol L−1 sodium phosphate buffer (pH 7.8), 14 mmol L−1 methionine, 3 μmol L−1 EDTA, 1 μmol L−1 nitroblue tetrazolium (NBT), 60 μmol L−1 riboflavin and 0.1 mL of SOD extract. The formation of blue formazan was monitored by recording the absorbance at 560 nm. One unit of SOD activity was defined as the amount of enzyme that caused 50% inhibition of NBT.
CAT activity was assayed according to the method of Chance and Maehly (1955). This involved monitoring the disappearance of H2O2 by recording the decrease in absorbance at 240 nm of a reaction mixture containing 50 mmol L−1 sodium phosphate buffer (pH 7), 12.5 mmol L−1 H2O2 and 20 μL of CAT extract. One unit of CAT activity was defined as the amount of enzyme that decomposed 1 μmol H2O2 min−1 at 30 °C. Ascorbate peroxidase measurement was adapted from the method of Vicente et al. (2006). 1 g frozen tissue was ground with 5 mL of 50 mM sodium phosphate buffer (pH 7.0), containing 0.1 mM EDTA, 1 mM ascorbic acid and 1% polyvinyl-pyrrolidone (PVP). The homogenate was centrifuged at 10,000g for 20 min at 4 °C and the supernatant was used to determine APX activity. One unit of APX activity is defined as the amount of enzyme that oxidized 1 μmol ascorbate per minute at 30 °C.
LOX activity was assayed using the method of Todd et al. (1990). The standard assay mixture contained 200 μL of Tween 20 and 40 μL of linoleic acid (Aldrich, Milwaukee, WI, USA) in 40 mL of 0.1 mL sodium phosphate buffer (pH 7). To 1 mL of standard assay mixture in a cuvette, 0.2 mL of LOX extract was added. One unit of LOX activity was defined as the amount of enzyme that caused an increase in absorption at 234 nm of 0.01 min−1 at 25 °C with linoleic acid as substrate.
PLC and PLD activities were determined by the procedures of Kurioka and Matsuda (1976) and Gupta and Wold (1980) respectively using D-nitrophenylphosphorylcholine (NPPC; Aldrich) as substrate. The reaction mixture for PLC consisted of 1 mL Tris–HCl buffer (0.25 mol L−1, pH 7.2 containing 20 mmol L−1 NPPC and 600 g L−1 d-sorbitol), and 0.3 mL of enzyme extract. For PLD, 0.9 mL Ca-acetate (50 mmol L−1, pH 5.6) containing 27.4 mM NPPC was mixed with 0.1 mL (0.4 U) of acid phosphatase (Aldrich) dissolved in 50 mM Ca-acetate (pH 5.6) along with 0.3 mL of enzyme extract. In both cases, after incubation for 60 min at 37 °C, 0.1 mL of 50 mM NaOH was added and the D-nitrophenol content was determined at 400 nm. One unit of PLC or PLD activity was defined as the amount of enzyme that catalyzed the formation of 1 nmol d-nitrophenol h−1.
H2O2 analysis
For H2O2 determination, 2 g of frozen tissue was homogenized with 5 mL of chilled pure acetone and centrifuged at 10,000×g for 20 min at 4 °C. The supernatant was collected for H2O2 analysis by a method based on titanium oxidation (Patterson et al. 1984). The acetone extract (supernatant) was mixed with hydrochloric acid containing 200 mL L−1 TiCl4 and 17 mol L−1 ammonia solution. The precipitate was washed with acetone and dissolved in 2 M sulfuric acid for spectrophotometric measurement at 410 nm. H2O2 concentration was determined from a standard graph (from 5 to 1 mM H2O2, constructed by direct addition of H2O2 to the titanium solution) and expressed as nmol g−1 FW (Cao et al. 2009).
GABA determination
The GABA concentration in fruit pulp was estimated by the method of Zhang and Bown (1997). GABA was determined on the basis of the increase in A340 after 30 min following supplier recommendations for commercially available of GABase (Aldrich), a spectrophotometric-coupled enzyme assay system for GABA. The resulting values were compared with a standard curve constructed using known amounts of GABA and expressed as μg GABA/g fresh weight (FW).
Statistical analysis
Five cucumber fruits from each treatment were taken at each sampling. All the assays were carried out in triplicate. Data analyses were performed using SPSS software version 16. Results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Correlation between various parameters was also investigated. Significance was determined at p < 0.05 and the results were expressed as mean values and standard error (SE) of the means.
Results
According to ANOVA results, the interaction effects of week and GABA treatments were significant for all the traits (Table 1).
Table 1.
Analysis of variance (ANOVA) of the effect of week and GABA on some growth, enzymes, biochemical and mineral nutrient parameters cucumber fruit
| Source | df | CI | EL | Prolin | MDA | H2O2 | CAT |
|---|---|---|---|---|---|---|---|
| Week | 2 | 200.1** | 3.5** | 87.6** | 35.8** | 126.8** | 136.2** |
| GABA | 1 | 33.4** | 5.7** | 58.8** | 18.33 | 40.9** | 89.2** |
| Week* GABA | 2 | 9.2** | 2.3** | 14.0** | 3.0** | 16.3** | 17.9** |
| CV % | 78.94 | 35.96 | 42.6 | 40.71 | 54.58 | 33.68 |
| df | SOD | APX | PLC | PLD | LOX | GABA content | |
|---|---|---|---|---|---|---|---|
| Week | 2 | 954.5** | 214.1** | 152.1** | 561.8** | 47.2** | 309.7** |
| GABA | 1 | 63.8** | 1.9** | 43.4** | 89.3** | 46.0** | 210.3** |
| Week* GABA | 2 | 10.1** | 1.3* | 12.8* | 27.9** | 16.3** | 62.7** |
| CV % | 53.90 | 31.53 | 40.38 | 28.23 | 30.11 | 41.87 |
*, ** Effects significant at probability of 0.05 and 0.01 respectively; ns non-significant (p > 0.05)
Effect of GABA treatment on CI in cucumber fruit
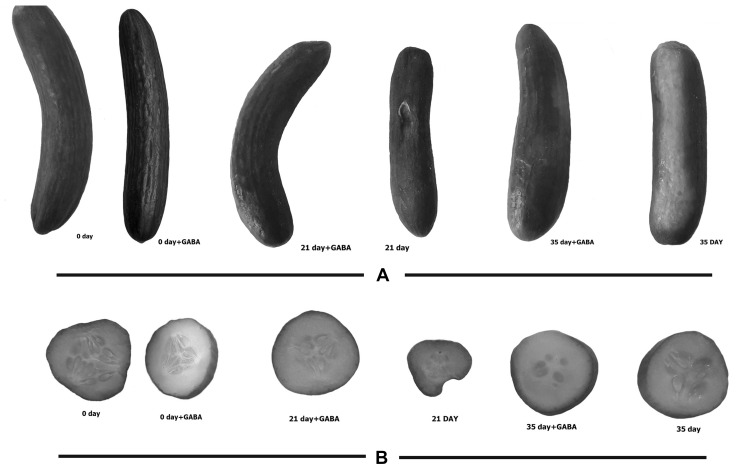
Index of CI in cucumber fruit increased during storage period, this increasing was delayed when treated with exogenous GABA application. Chilling injury symptoms after 10 days storage at 1 °C were visible in cucumber fruit. Significant difference(s) in the incidence of CI between control fruit and GABA-treated fruit were observed (Fig. 1). Exogenous GABA treatment reduced CI symptoms and maintained quality of fruit (Fig. 2).
Fig. 1.
Appearances and transects of cucumber fruits stored at 1 ± 0.5 °C for 5 weeks. Fruits were treated with 5 mM GABA for 10 min and then stored. The fruit in the control was stored without GABA treatment. Appearances (a) and transects (b)
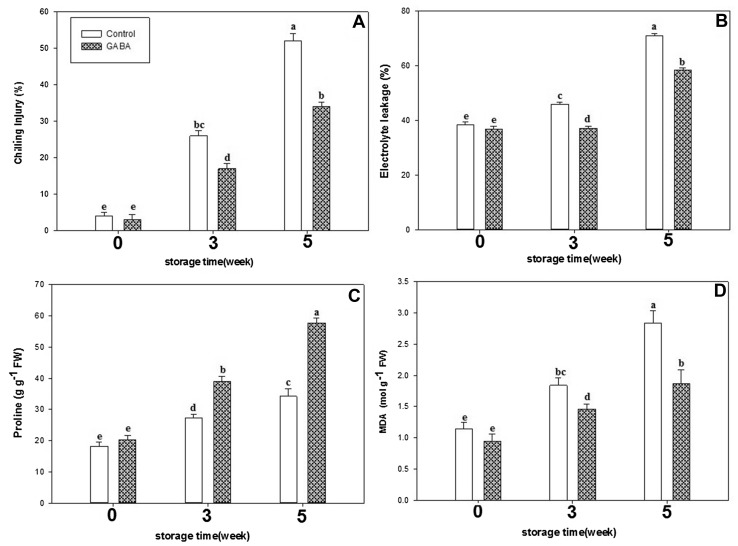
Fig. 2.
The effects of exogenous GABA on CI (a), electrolyte leakage (b), proline (c) and MDA (d) content in cucumber fruit during storage period. Vertical bars represent standard error of the means. Different letters indicate statistically different means at p < 0.05
Levels of MDA and Electrolyte leakage in cucumber fruit
MDA and electrolyte leakage content in cucumber fruit increased during storage (Fig. 2). Levels of MDA and EL in control fruit were higher than that of GABA-treated fruit during storage. GABA-treated fruit had 23% less EL than control fruit after 3 weeks of storage. In line with this decreased EL content, the amount of MDA in GABA-treated fruit was 34% less than control fruit after 5 weeks of storage. Exogenous GABA treatment reduced MDA content and EL in cucumber fruit during storage.
Effect of GABA treatment on proline content in cucumber fruit
Proline content increases in both control as well as GABA treated fruits (Fig. 2). The proline content in the GABA-treated fruit was significantly (p < 0.05) higher than in control fruit. Also, the proline content in GABA-treated fruit was 168.7% higher than that in control after storage for 5 weeks (Fig. 2).
Effect of GABA treatment on SOD, APX and CAT activities and H2O2 content in cucumber fruit
As shown in Fig. 3, activity of ROS-scavenging system in cucumber significantly affected by postharvest GABA treatment during storage at 1 °C.
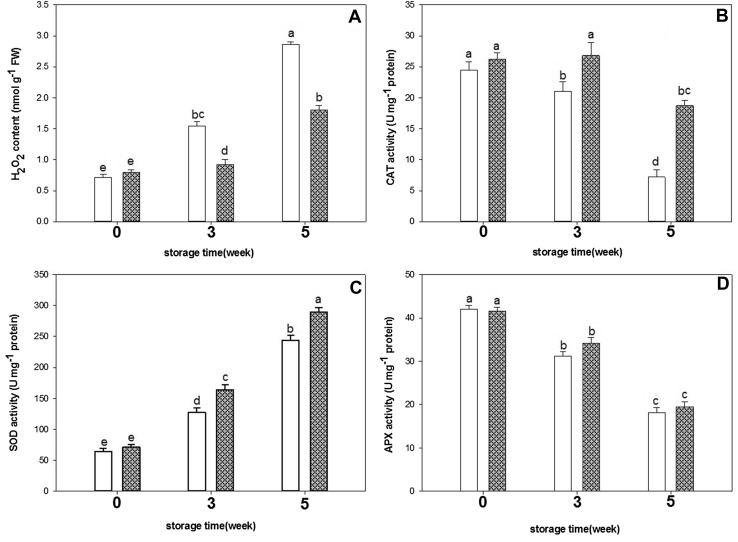
Fig. 3.
The effects of exogenous GABA on H2O2 content (a), CAT (b) SOD (c) and APX (d) activities in cucumber fruit during storage period. Vertical bars represent standard error of the means. Different letters indicate statistically different means at p < 0.05
H2O2 content and SOD activity increased during storage, while APX and CAT activities decreased. GABA treatment resulted in increased SOD and CAT activities, inhibited the increase in H2O2 levels and had no significant impact on APX activity when compared to control cucumber fruit (Fig. 3).
The results indicate that, SOD activity in the cucumber fruit increased with storage period and reached the peak after 5 weeks of storage at 1 °C.
SOD activity in GABA-treated cucumber fruit was 19% higher than in the control cucumber fruit at 5 weeks of storage period.
Exogenous GABA treatment maintained higher CAT activity compared with the control fruits over the whole storage period.
Although CAT decreased with storage time, the activity in GABA-treated cucumber fruit was 63% higher than control at 5 weeks of storage.
APX activity significantly decreased during the storage period, reduced to half of the 0 day after 5 weeks of storage with no significant difference between GABA-treated and control fruit.
Levels of H2O2 in cucumber fruit enhanced gradually during storage. However, the rise in H2O2 level was significantly slower with GABA treatment. As could be seen in Fig. 3, the content of H2O2 in GABA-treated fruit was 26 and 37% lower than that of control fruit at week 3 and 5, respectively.
Effect of GABA treatment on Lipoxygenase, PLC and PLD activities in cucumber fruit
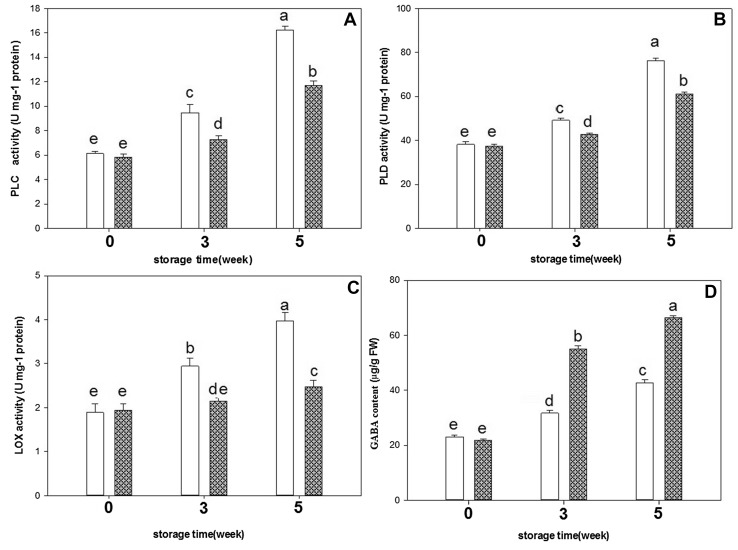
LOX, PLC and PLD activities increased during storage (Fig. 4). Like SOD, these activities were also much lower in GABA treatment than control. LOX, PLD and PLC activities in exogenous GABA-treated fruit were 38, 20 and 28% lower than that of control fruit at week 5 of storage, respectively.
Fig. 4.
The effects of exogenous GABA on PLC (a), PLD (b), LOX (c) activities and endogenous GABA content (d) in cucumber fruit during storage period. Vertical bars represent standard error of the means. Different letters indicate statistically different means at p < 0.05
Discussion
GABA levels increase rapidly in response to environmental stress in plants, which in turn, strengthens the defense system of plants. Increase in GABA content was also observed due to low oxygen and high CO2 storage in strawberry (Deewatthanawong et al. 2010a) and tomato (Deewatthanawong et al. 2010b). GABA concentrations in soybean leaves increased 20–40 folds within 5 min of cold-treatment (Wallace et al. 1984). Chilling injury symptoms of wheat and tomato seedlings during 15 days of storage at 2 °C, were alleviated by exogenous GABA treatments (Malekzadeh et al. 2012, 2014). In this study, treatment of 5 mM exogenous GABA alleviated symptoms of chilling in cucumber fruit stored at 1 °C for 5 weeks and delayed CI percent increase (Fig. 2).
A significant increase in EL in cucumber tissue exposed to low temperatures is a good marker of cold stress. The results shown that the amount of electrolyte leakage significantly increased in cucumber fruit after 3 weeks (Fig. 2). Accompanying this rapid rise in EL of control fruit, the increase was delayed in GABA-treated cucumbers. Environmental stresses induce reactive oxygen species (ROS) generation that causes cell oxidative damage and lipid peroxidation, so that MDA (a final product of lipid peroxidation) can be used as an assay of cell oxidative damage (Xu et al. 2009). In this study, the change in level of MDA and electrolyte leakage shared similar patterns. The accumulation of MDA under chilling stress resulted in lipid peroxidation (Fig. 2). These results revealed that chilling condition might mediate catabolic reactions targeting cell membranes.
To limit damage to cells by reactive oxygen species, plant cells employ the reactive oxygen species (ROS) scavenger system. In a previous study, Chongchatuporn et al. (2013), observed that overall chilling injury induced enhancement in the activities of APX, SOD and CAT and the content of MDA, H2O2 and EL in mango fruit.
Our finding also revealed that, level of MDA content was increased and SOD activity declined in fruit. Further, the results show that GABA treatment significantly delayed the MDA increase in cucumber fruits (Fig. 1), together with significant increase in the activity of SOD (Fig. 2). Therefore, the results suggest that the peripherally administered GABA can scavenge ROS and protect the tissue against active carbonyl harm.
Apel and Hirt (2004) reported that, the generation of O−2· catalyzed by several enzymes including LOX and NADPH oxidase. The application of GABA resulted in lower activity of LOX (Ding et al. 2007; Hatamnia et al. 2016). Our results also show that, the activity of SOD, the O−2· scavenging enzyme, did increase by GABA treatment. SOD has been demonstrated as a target of ROS (Qin et al. 2009). The effect of GABA treatment on H2O2 may attribute to the GABA-induced higher activity of SOD.
Membrane damage during stress condition is initiated by a lipolytic cascade, with PLD and LOX being critical to the phospholipid catabolism (Bargmann et al. 2009). PLD-mediated hydrolysis increased under environmental stresses such as chilling and salt stresses. The higher activities of PLD and LOX were shown to be associated with their gene expression (Zhao et al. 2010; Malekzadeh 2015) and increases of these two enzyme activities were accompanied by the induction of CI (Mao et al. 2007; Rui et al. 2010). LOX plays an important role in peroxidative damage in membrane lipids in plants. Lower LOX activity was shown to be related to chilling tolerance of plants as membrane fluidity and lipid unsaturation was enhanced by LOX (Lee et al. 2005).
PLD, PLC and LOX activities induced by chilling might stimulate corresponding physiological reactions related to deterioration and senescence during the storage period.
In this study also, development of chilling injury was accompanied by an increase in PLC, PLD and LOX activities, which reached the maximum at week 5 at 1 °C. Activities of PLC, PLD and LOX increased during storage, but to a lesser extent in GABA-treated cucumbers suggesting that chilling resistance was enhanced by exogenous GABA application probably due to inhibition of these enzymes.
In summary, results of this investigation showed that GABA treatment can improve the chilling- tolerance of cucumber fruit by improving antioxidant enzyme activities, and decreasing the level of ROS, thus protecting membranes from chilling stress. Together with other studies (Trobacher et al. 2013; Yu et al. 2014), it is suggested that the exogenous GABA treatment can prove to be a promising and safe postharvest technology for increasing the shelf life of harvested cucumber fruit and thereby maintaining its quality. Further studies are needed to elucidate the role of exogenous GABA in chilling tolerance at the molecular level.
Abbreviations
- AOS
Active oxygen species
- APX
Ascorbate peroxidase (EC 1.11.1.11)
- CAT
Catalase (EC 1.11.1.6)
- CI
Chilling injury
- EL
Electrolyte leakage
- GABA
γ-Aminobutyric acid
- LOX
Lipoxygenases (EC 1.13.11)
- MDA
Malonyl-dialdehyde
- PLC
Phospholipase C (EC 3.1.4.11)
- SOD
Superoxide dismutase (EC 1.15.1.1)
References
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Bargmann BOR, Laxalt AM, Ter Riet B, Testerink C, Merquiol E, Mosblech A, Leon-Reyes A, Pieterse CMJ, Haring MA, Heilmann I, Bartels D, Munnik T. Reassessing the role of phospholipase D in the Arabidopsis wounding response. Plant Cell Environ. 2009;32(7):837–850. doi: 10.1111/j.1365-3040.2009.01962.x. [DOI] [PubMed] [Google Scholar]
- Benjak A, Ercisli S, Vokurka A, Maletic E, Pejic I. Genetic relationships among grapevine cultivars native to Croatia, Greece and Turkey. Vitis. 2005;44(2):73–77. [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation, stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canan I, Gundogdu M, Seday U, Oluk CA, Karasahin Z, Eroglu EC, Yazici E, Unlu M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk J Agric For. 2016;40:894–899. doi: 10.3906/tar-1606-83. [DOI] [Google Scholar]
- Cao SF, Zheng YH, Wang KT, Rui HJ, Tanga SS. Effects of 1-methylcyclopropene on oxidative damage, phospholipases and chilling injury in loquat fruit. J Sci Food Agric. 2009;89:2214–2220. doi: 10.1002/jsfa.3710. [DOI] [Google Scholar]
- Celik A, Ercisli S, Turgut N. Some physical, pomological and nutritional properties of kiwifruit cv. Hayward. Int J Food Sci Nutr. 2007;58(6):411–418. doi: 10.1080/09637480701252518. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidase. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- Chen JY, He LH, Jiang YM, Wang Y, Joyce DC, Ji ZL, Lu WJ. Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol Plant. 2008;132:318–328. doi: 10.1111/j.1399-3054.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- Chongchatuporn U, Ketsa S, van Doorn WG. Chilling injury in mango (Mangifera indica) fruit peel: relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biol Technol. 2013;86:409–417. doi: 10.1016/j.postharvbio.2013.07.023. [DOI] [Google Scholar]
- Deewatthanawong R, Nock JF, Watkins CB. γ-Aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest Biol Technol. 2010;57:92–96. doi: 10.1016/j.postharvbio.2010.03.003. [DOI] [Google Scholar]
- Deewatthanawong R, Rowell P, Watkins CB. γ-Aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biol Technol. 2010;57:97–105. doi: 10.1016/j.postharvbio.2010.03.007. [DOI] [Google Scholar]
- Ding CK, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- Ding ZS, Tian SP, Zheng XL, Zhou ZW, Xu Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol Plant. 2007;130:112–121. doi: 10.1111/j.1399-3054.2007.00893.x. [DOI] [Google Scholar]
- Ercisli S, Tosun M, Duralija B, Voca S, Sengul M, Turan M. Phytochemical content of some black (Morus nigra L.) and purple (Morus rubra L.) mulberry genotypes. Food Technol Biotechnol. 2010;48(1):102–106. [Google Scholar]
- Gupta MN, Wold F. A convenient spectrophotometric assay for phospholipase D using p-nitrophenyl-phosphocholine as substrate. Lipids. 1980;15:594–596. doi: 10.1007/BF02534185. [DOI] [Google Scholar]
- Hatamnia AA, Rostamzad A, Hosseini M, Abbaspour N, Darvishzadeh R, Malekzadeh P, Aminzadeh BM. Antioxidant capacity and phenolic composition of leaves from ten Bene (Pistacia atlantica subsp. kurdica) genotypes. Nat Prod Res. 2016;30(5):600–604. doi: 10.1080/14786419.2015.1028060. [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Hricova A, Fejer J, Libiakova G, Szabova M, Gazo J, Gajdosova A. Characterization of phenotypic and nutritional properties of valuable Amaranthus cruentus L. mutants. Turk J Agric For. 2016;40:761–771. doi: 10.3906/tar-1511-31. [DOI] [Google Scholar]
- Kinnersley AM, Turano FJ. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci. 2000;19:479–509. doi: 10.1016/S0735-2689(01)80006-X. [DOI] [Google Scholar]
- Kurioka S, Matsuda M. Phospholipase C assay using p-nitrophenylphosphorylcholine together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976;75:281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ahn SJ, Im YJ, Cho K, Chung GC, Cho BH, Han O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in fig leaf gourd and cucumber roots. Biochem Biophys Res Commun. 2005;330:1194–1198. doi: 10.1016/j.bbrc.2005.03.098. [DOI] [PubMed] [Google Scholar]
- Lurie S, Crisosto CH. Chilling injury in peach and nectarine. Postharvest Biol Technol. 2005;37:195–208. doi: 10.1016/j.postharvbio.2005.04.012. [DOI] [Google Scholar]
- Malekzadeh P. Influence of exogenous application of glycinebetaineon antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.) Physiol Mol Biol Plants. 2015;20:133–137. doi: 10.1007/s12298-013-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekzadeh P, Khara J, Heydari R. Effect of exogenous gama-aminobutyric acid on physiological tolerance of wheat seedlings exposed to chilling stress. Iran J Plant Physiol. 2012;3(1):611–617. [Google Scholar]
- Malekzadeh P, Khara J, Heydari R. Alleviating effects of exogenous Gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol Mol Biol Plants. 2014;20(1):133–137. doi: 10.1007/s12298-013-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LC, Pang HG, Wang GZ, Zhu CG. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol Technol. 2007;44:42–47. doi: 10.1016/j.postharvbio.2006.11.009. [DOI] [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Patterson BD, Mackae EA, Ferguson IB. Estimation of hydrogen peroxide in plant extracts using titanium. Anal Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- Prasad TK. Mechanisms of chilling injury and tolerance. In: Basra AR, editor. Crop responses and adaptations to temperature. Binghamton: Food Products Press; 2001. pp. 1–53. [Google Scholar]
- Promyou S, Ketsa S, van Doorn WG. Hot water treatments delay cold-induced banana peel blackening. Postharvest Biol Technol. 2008;48:132–188. doi: 10.1016/j.postharvbio.2007.09.006. [DOI] [Google Scholar]
- Qin GZ, Meng XH, Wang Q, Tian SP. Oxidative damage of mitochondrial proteins contributes to fruit senescence: a redox proteomics analysis. J Proteome Res. 2009;8:2449–2462. doi: 10.1021/pr801046m. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Rop O, Ercisli S, Mlcek J, Jurikova T, Hoza I. Antioxidant and radical scavenging activities in fruits of 6 sea buckthorn (Hippophae rhamnoides L.) cultivars. Turk J Agric For. 2014;38(2):224–232. doi: 10.3906/tar-1304-86. [DOI] [Google Scholar]
- Rui H, Cao S, Shang H, Jin P, Wang K, Zheng Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J Sci Food Agric. 2010;90:1557–1561. doi: 10.1002/jsfa.3993. [DOI] [PubMed] [Google Scholar]
- Sawaki Y, Iuchi S, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, Koyama H. STOP1 regulates multiple genes that protect arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150(1):281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao ZG, Zheng CC. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- Shang H, Cao SH, YangZ Cai Y, Zheng Y. Effect of exogenous γ-Aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem. 2011;59(4):1264–1268. doi: 10.1021/jf104424z. [DOI] [PubMed] [Google Scholar]
- Shi SQ, Shi Z, Jiang Z, Qi L, Sun X, Li C. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010;33:149–162. doi: 10.1111/j.1365-3040.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Sirikesorn L, Ketsa S, van Doorn WG. Low temperature-induced water-soaking of Dendrobium inflorescences: relation with phospholipase D activity, thiobarbaturic-acid-staining membrane degradation products, and membrane fatty acid composition. Postharvest Biol Technol. 2013;80:47–55. doi: 10.1016/j.postharvbio.2013.01.007. [DOI] [Google Scholar]
- Song HM, Xu XB, Wang H, Wang HZ, Tao YZ. Exogenous γ-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J Sci Food Agric. 2010;90:1410–1416. doi: 10.1002/jsfa.3951. [DOI] [PubMed] [Google Scholar]
- Todd JF, Paliyath G, Thompson JE. Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiol. 1990;94:1225–1232. doi: 10.1104/pp.94.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobacher CP, Clark SM, Bozzo GG, Mullen RT, DeEll JR, Shelp BR. Catabolism of GABA in apple fruit: subcellular localization and biochemical characterization of two γ-aminobutyrate transaminases. Postharvest Biol Technol. 2013;75:106–113. doi: 10.1016/j.postharvbio.2012.08.005. [DOI] [Google Scholar]
- Vicente AR, Martínez GA, Chaves AR, Civello PM. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol Technol. 2006;40:116–122. doi: 10.1016/j.postharvbio.2005.12.012. [DOI] [Google Scholar]
- Wallace W, Secor J, Schrader LE. Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical stress. Plant Physiol. 1984;175:170–175. doi: 10.1104/pp.75.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR, Naylor AW. Chilling-enhanced photophylls, chilling-enhanced photooxidation—the peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol. 1987;83:272–277. doi: 10.1104/pp.83.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. Chilling injury in plants—the role of membrane lipid fluidity. Plant Cell Environ. 2006;1:241–247. doi: 10.1111/j.1365-3040.1978.tb02036.x. [DOI] [Google Scholar]
- Wongsheree T, Ketsa S, van Doorn WG. The relationship between chilling injury and membrane damage in lemon basil (Ocimum × citriodorum) leaves. Postharvest Biol Technol. 2009;51:91–96. doi: 10.1016/j.postharvbio.2008.05.015. [DOI] [Google Scholar]
- Xu PL, Guo YK, Bai JG, Shang L, Wang XJ. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol Plant. 2008;132:467–478. doi: 10.1111/j.1399-3054.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Xu WT, Peng X, Luo YB, Wang J, Guo X, Huang KL. Physiological and biochemical responses of grapefruit seed extract dip on ‘Redglobe’ grape. LWT Food Sci Technol. 2009;42:471–476. doi: 10.1016/j.lwt.2008.09.002. [DOI] [Google Scholar]
- Yu C, Zeng L, Sheng K, Chen F, Zhou T, Zheng X, Yu T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014;159:29–37. doi: 10.1016/j.foodchem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang G, Bown AW. The rapid determination of γ-aminobutyric acid. Phytochemistry. 1997;44:1007–1009. doi: 10.1016/S0031-9422(96)00626-7. [DOI] [Google Scholar]
- Zhang C, Fei SZ, Arora R, Hannapel DJ. Ice recrystallization inhibition proteins of perennial rye-grass enhances freezing tolerance. Planta. 2010;232:155–169. doi: 10.1007/s00425-010-1163-4. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qian C, Chen J, Peng Y, Mao L. Responses of phospholipase D and lipoxygenase to mechanical wounding in postharvest cucumber fruits. J Zhejiang Univ Sci B. 2010;11(6):443–450. doi: 10.1631/jzus.B0900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorenc Z, Veberic R, Stampar F, Koron D, Mikulic-Petkovsek M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk J Agric For. 2016;40:855–867. doi: 10.3906/tar-1607-57. [DOI] [Google Scholar]