Abstract
Purpose
The incidence of neuroendocrine neoplasms is increasing. This work aimed at: (i) establishing worldwide incidence trend of low-grade neuroendocrine neoplasms; (ii) defining the incidence and temporal trend of high-grade neuroendocrine neoplasms in USA utilizing the Surveillance Epidemiology and End Results database; (iii) comparing trends for low-grade vs. high-grade neuroendocrine neoplasms.
Methods
We conducted a literature search on MEDLINE and Scopus databases and incidence trends were plotted for 1973-2012. The Surveillance Epidemiology and End Results database was used to identify incidence rates in USA for 1973-2012. Incidence rates were stratified according to histological grade, gender and ethnicity. Trends were summarized as annual percent change and corresponding 95% confidence interval.
Results
11 studies were identified involving 72,048 cases; neuroendocrine neoplasm incidence rates increased over time in all countries for all sites, except for appendix. In Surveillance Epidemiology and End Results low-grade neuroendocrine neoplasm incidence rate increased from 1.09 in 1973 to 3.51 per 100,000 in 2012. During this interval, high-grade neuroendocrine neoplasm incidence rate increased from 2.54 to 10.52 per 100,000. African Americans had the highest rates of digestive neuroendocrine neoplasms with male prevalence in high-grade.
Conclusions
Our data indicate an increase in the incidence of neuroendocrine neoplasms as a worldwide phenomenon, affecting most anatomical sites and involving both low-grade and high-grade neoplasms.
Keywords: Neuroendocrine, Cancer, Low-grade, High-grade, Incidence
Introduction
Neuroendocrine define those neoplasms exclusively made by cells with a neuroendocrine phenotype, i.e., expressing markers of neuroendocrine differentiation like chromogranin A, synaptophysin, neuron specific enolase and others including hormones. As such, neuroendocrine neoplasms (NENs) may develop at any anatomical site [1]. The present paper focuses on NENs of the gastroenteropancreatic (GEP) tract. The current World Health Organization (WHO) classification of GEP NENs defines neuroendocrine tumor (NET) as well differentiated low to intermediate grade, and neuroendocrine carcinomas (NEC) as high grade neoplasms, poorly differentiated in phenotype [2]. Recent evidences also identified well differentiated NENs of high grade (for review and definition see [3]), now endorsed by the American Joint Cancer Committee Cancer Staging Manual [4, 5] and heralding a yet-to-come WHO classification change.
Global incidence of GEP NENs appears to be increasing. In the last four decades, incremental trends were reported in various populations for NENs overall and for specific primary sites [6–16]. Data were obtained from different population-based registries, which varied in completeness and time periods. Seminal papers were generated from the Surveillance, Epidemiology, and End Results (SEER) program of the US collecting cancer information since 1973, probably one of the most complete cancer registry publicly available in western countries [9, 17].
Published investigations focused broadly on NENs and were generated utilizing general “neuroendocrine” ICD-O codes potentially mixing cancers with different biology. Available data are thus difficult to interpret, and are commonly intended as referring to NENs of low to intermediate grade (from now on low-grade NENs), either defined as carcinoids, atypical carcinoids or, more recently, neuroendocrine tumors (NETs). Limited epidemiological data is available for poorly differentiated, high-grade NENs (from now on high-grade NENs) [18, 19].
Aims of this work were: (i) establishing worldwide trends in the incidence of low-grade NENs; (ii) defining the incidence and temporal trend of high-grade NENs in USA utilizing the Surveillance Epidemiology and End Results (SEER) database; (iii) comparing trends for low- vs. high-grade NENs.
Materials and methods
Published studies data analysis
Search strategy
We conducted a systematic review according to PRISMA guidelines [20] aiming at identifying studies on NEN incidence. We searched MEDLINE and Scopus with the following keywords: “neuroendocrine”, “carcinoid”, “epidemiology”, “trend” and “incidence”. The search was limited to English studies on human subjects till October 1, 2015. References of included articles were screened for any additional eligible study.
Inclusion and exclusion criteria
Inclusion criteria required that the study: (i) reported incidence estimates for NENs overall or for site-specific NENs; (ii) reported incidence estimates for at least 10-years with at least two time points; (iii) used data series from population-based surveillance systems. If articles reported data on overlapping regions or time intervals, the study with the most recent information was included. Studies reporting NEN incidence estimates in USA were excluded as we generated results for this country based on the most recent data available from the SEER database.
Data extraction
Titles and abstracts of articles obtained from the literature search were reviewed for eligibility by two authors (EL and KA). Papers successfully meeting the inclusion criteria were selected for data extraction. The investigators independently extracted the following information: first author’s name, publication year, study location, study period, cancer site (including topographical and morphological codes), number of cases, incidence rates, and standard population used for adjustment. We extracted data according to sex or racial/ethnic group, whenever available.
Summarization of data
International trends in incidence were plotted for all NENs combined and for most common primary sites for the period 1973–2012. Measures of incidence presented are those reported in the individual studies. Any measure also depends on the standard population used for the adjustment. When studies reported incidence rates separately for men and women, an average incidence rate was computed.
NEN incidence in the United States
We used the SEER database to identify NEN incidence rates in the United States [21]. The SEER program, started in 1973, currently registers cancer incidence and subsequent cause-specific mortality in 30% US population.
In order to provide comparable data with published incidence data, but also to be as much as possible consistent with the current classification systems for pure NENs, records were selected based on the following ICD-O-3 (International Classification of Diseases for Oncology) morphological codes: M8150/3 (Islet cell carcinoma), M8151/3 (insulinoma), M8152/3 (glucagonoma), M8153/3 (gastrinoma), M8155/3 (vipoma), M8156/3 (somatostatinoma), M8240/3 (carcinoid tumor, not appendix), M8241/3 (carcinoid tumor, argentaffin), M8242/3 (enterochromaffinlike-cell tumor), M8246/3 (neuroendocrine carcinoma), M8249/3 (atypical carcinoid).
Low-grade NENs were selected based on the following ICD-O-3 codes: M8150/3, M8151/3, M8152/3, M8153/3, M8155/3, M8156/3, M8240/3, M8241/3, M8242/3, M8249/3; whereas high-grade NENs were selected based on the following codes: M8013/3 (large cell neuroendocrine carcinoma), M8041/3 (small cell carcinoma), M8246/3 (neuroendocrine carcinoma). Codes identifying cancer types of mixed neuroendocrine/non-neuroendocrine phenotype (e.g., goblet cell carcinoid, mixed adeno-neuroendocrine carcinoma) were excluded.
Statistical analysis
Age-adjusted incidence rates of NENs for the most common primary sites (lung and bronchus, stomach, pancreas, small intestine, colon, appendix, rectum) and for all sites combined for the 40 years period 1973–2012, were reported. Incidence data were obtained from the SEER 9 registries for years 1973–1991, from the SEER 13 registries for years 1992–1999 and from the SEER 18 registries for the years 2000–2012.
Incidence rates according to histological classification of malignancy grade and by gender and by ethnicity (white, black, Asian Pacific/Islander) for 2000 through 2012 were collected from the SEER 18 registries were reported to further assess whether overall changes in incidence rates were uniform or more marked for specific groups. All rates are expressed per 100,000 persons and age-adjusted according to the 2000 US standard population.
Trends for the period 2000–2012 were summarized with the annual percent change (APC) (Joinpoint Regression Program, Version 4.3.1.0 - April 2016; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute) and corresponding 95% confidence interval (CI) [22]. A sensitivity analysis of trends according to histological grade restricted to the SEER 9 registries with complete data during the period 1975–2012, was done.
Male-to-female (M:F) ratios, based on the most recently available incidence data from the 2010–2012 SEER, were also computed. Stata software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) was used for data management and to produce graphs.
Results
Review of worldwide incidence of NEN
The results of the bibliographic search are summarized in Fig. 1. A total of 4114 articles were considered. After excluding duplicates, reviews and studies unrelated to the search topic, we assessed for eligibility of 31 full-text articles. Of these, 20 articles were excluded after full review. Table 1 shows the main characteristics of the studies included and the SEER Program. The 11 studies were published between 2000 and 2015 and involved a total of 72,048 cases. Nine were conducted in Europe [6–12, 15], one in Canada [16], and one in Taiwan [13] (Fig. 2). In five countries (Denmark, England, The Netherlands, Norway, Taiwan), the incidence data covered the entire national population, while in the other six countries, covered 1% to 30% of the population.
Fig. 1.
Flow diagram of study selection
Table 1.
Summary of the studies reporting NEN incidence and the SEER Program of the National Cancer Institute
| First author, publication year | Study period | Country | Study area | Total cases | Incidence rates, type of adjustment | Subsites of NEN incidence |
|---|---|---|---|---|---|---|
| North America | ||||||
| SEER [present series] | 1973–2012 | USA | 1973–1991: 9 Registries, 1992–1999: 13 Registries, 2000–2012: 18 Registries | na | Age-standardized, 2000 US Standard Population | Overall, lung, stomach, pancreas, small intestine, colon, appendix, rectum |
| Hallet, 2015 | 1994–2009 | Canada | Ontario | 5619 | Crude | Overall, lung, stomach, pancreas, small intestine, colon, appendix, rectum |
| Europe | ||||||
| Korse, 2013 | 1990–2010 | Netherlands | Nationwide | 47,800 | Age-standardized, European Standard Population | Overall, lung, stomach, pancreas, small intestine, appendix, rectum |
| Scherubl, 2013 | 1976–2006 | Germany | Mecklenburg-Western Pomerania, Saxony, Brandenburg or Thuringi | 2821 | Age-standardized, Population of Germany in 1987 | Stomach, pancreas, small intestine, colon, appendix, rectum |
| Caldarella, 2011 | 1985–2005 | Italy | Firenze and Prato | 455 | Age-standardized, 2000 European Standard Population | Overall |
| Ellis, 2011 | 1971–2006 | England | Nationwide | 10,324 | Crude | Stomach, small intestine, colon, appendix, rectum |
| Landerholm, 2010 | 1960–2005 | Sweden | Jonkoping County | 145 | Age-standardized, Population of Sweden in 2005 | Small intestine |
| Hauso, 2008 | 1993–2004 | Norway | Nationwide | 2030 | Age-standardized, 2000 US Standard Population | Overall, lung, stomach, pancreas, small intestine, colon, appendix, rectum |
| Lepage, 2006 | 1976–2001 | France | Burgundy | 102 | Age-standardized, World standard population | Small intestine |
| Skuladottir, 2002 | 1978–1997 | Denmark | Nationwide | 347 | Age-standardized, Population of Denmark in 1980 | Lung |
| Levi, 2000 | 1974–1997 | Switzerland | Vaud | 218 | Age-standardized, World Standard Population | Overall, lung, stomach, small intestine |
| Asia | ||||||
| Tsai, 2013 | 1996–2008 | Taiwan | Nationwide | 2187 | Age-standardized, 2000 US Standard Population | Overall, lung, stomach, small intestine, pancreas, rectum |
NEN neuroendocrine neoplasm, ICD-O International Classification of Disease for Oncology, SEER Surveillance, Epidemiology, and End Results
Fig. 2.
Countries with information available on trends in incidence of NENs
Seven studies reporting details on ICD-O codes included the ambiguous neuroendocrine carcinoma (M8246/3; ambiguous since could have comprised both low-grade and high-grade NENs), six included codes of mixed neuroendocrine–non neuroendocrine cancer (e.g. M8243 goblet cell carcinoid), and two included the high-grade large cell neuroendocrine carcinoma (Table 2). Thus, the published data were not fully comparable and homogenous for neuroendocrine cancer types collected, either non-purely neuroendocrine and/or of different grades.
Table 2.
NEN-related ICD-O codes used in SEER database extraction and in 11 studies included in the systematic review
| USA [present series], SEER, 2015 | SWITZERLAND, Levi, 2000 | DENMARK, Skuladottir, 2002 | FRANCE, Lepage, 2006 | NORWAY, Hauso, 2008 | USA, Hauso, 2008 | SWEDEN, Landerholm, 2010 | UNITED KINGDOM, Ellis, 2010 | ITALY, Caldarella, 2011 | GERMANY, Scherubl, 2013 | TAIWAN, Tsai, 2013 | NETHERLANDS, Korse, 2014 | Canada, Hallet, 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M8013/3 Large cell neuroendocrine carcinoma (§) | Not reported | Not reported | Not reported | ● | Not reported | ● | |||||||
| M8041/3 Small cell carcinoma | ●(#) | ||||||||||||
| M8150/3 Islet cell carcinoma | ● | ● | ● | ● | ● | ||||||||
| M8151/3 Insulinoma | ● | ● | ● | ● | ● | ||||||||
| M8152/3 Glucagonoma | ● | ● | ● | ● | ● | ||||||||
| M8153/3 Gastrinoma | ● | ● | ● | ● | ● | ||||||||
| M8154 Mixed islet cell and exocrine adenocarcinoma | ● | ● | |||||||||||
| M8155/3 Vipoma | ● | ● | ● | ● | ● | ||||||||
| M8156/3 Somatostatinoma | ● | ● | ● | ● | ● | ||||||||
| M8157 Enteroglucagonoma | ● | ● | ● | ||||||||||
| M8240/1 Carcinoid tumor, appendix | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| M8240/3 Carcinoid tumor, not appendix | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| M8241/3 Carcinoid tumor, argentaffin | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| M8242/3 Enterochromaffinlike-cell tumor (§) | ● | ● | ● | ● | ● | ● | ● | ||||||
| M8243 Goblet cell carcinoid | ● | ● | ● | ● | ● | ● | ● | ||||||
| M8244 Composite carcinoid | ● | ● | ● | ● | ● | ● | |||||||
| M8245 Adenocarcinoid tumor | ● | ● | ● | ● | ● | ● | |||||||
| M8246/3 Neuroendocrine carcinoma | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| M8248/3 Apudoma | ● | ||||||||||||
| M8249/3 Atypical carcinoid (§) | ● | ● | ● | ● | ● | ● | ● | ||||||
| M8574/3 Adenocarcinoma with neuroendocrine differentiation (§) | ● | ● | ● | ||||||||||
| ICD-O edition | ICD-O-3 | ICD-O-1 | ICD-O-1 | ICD-O-2 | ICD-O-2 | ICD-O-2 and 3 | Not reported | ICD-O-1 and 2 | ICD-O-3 | ICD-O-3 | ICD-O-Field trial and 3 | ICD-O-1,2 and 3 | ICD-O-3 |
NEN neuroendocrine neoplasm, ICD-O International Classification of Disease for Oncology, SEER Surveillance, Epidemiology, and End Results
(§) New histology codes (ICD-O-3) for NETs, in use since 2001.
(#) In the present study we report the trends in incidence of NEN in The Netherlands excluding small cell neuroendocrine carcinoma (G3-SCNEC) to make data comparable with other studies
NEN incidence in the United States
Trends in incidence for NENs overall and for common primary sites are shown in Fig. 3a and detailed in Table 3. Incidence data of all NENs combined were available for seven countries. In USA and EU countries for which trend data was available, the incidence of NENs increased steadily. The incidence also increased in Taiwan (from 0.30 in 1996 to 1.51 in 2008), where it was relatively low compared to other countries. In the United States, NEN incidence increased from 1.52 to 7.41 cases per 100,000 from 1973 to 2012; this represented a 4.88-fold increase.
Fig. 3.
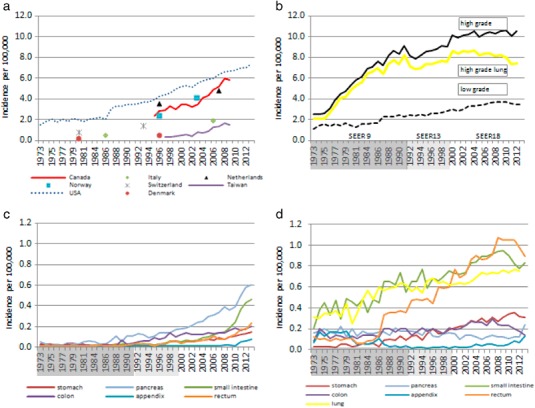
a International incidence of NENs overall per 100,000 persons; b Incidence of high-grade lung NEN, and low-grade and high-grade NENs per 100,000 persons in the United States, 1973–2012; c Incidence of low-grade NENs per 100,000 persons by primary site, in the United States, 1973–2012; d Incidence of high-grade NENs per 100,000 persons by primary site, in the United States, 1973–2012
Table 3.
NEN incidence per 100,000 by country and tumor site
| NEN incidence per 100,000 | All sites average increase (cases per 100,000 per year) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Year | Lung | Stomach | Pancreas | Small intestine | Colon | Appendix | Rectum | All sites | |
| North America | ||||||||||
| USA | 1973 | 0.31 | 0.03 | 0.16 | 0.21 | 0.08 | 0.07 | 0.12 | 1.52 | 0.16 |
| 2012 | 1.61 | 0.45 | 0.82 | 1.28 | 0.30 | 0.22 | 1.10 | 7.41 | ||
| Canada | 1994 | 0.83 | 0.07 | 0.10 | 0.42 | na | na | 0.22 | 2.46 | 0.23 |
| 2009 | 1.28 | 0.29 | 0.60 | 1.01 | na | na | 0.96 | 5.86 | ||
| Europe | ||||||||||
| The Netherlands | 1990–00 | 0.68 | 0.15 | 0.19 | 0.32 | 0.13 | 0.58 | 0.19 | 3.57 | 0.11 |
| 2001–10 | 1.21 | 0.19 | 0.32 | 0.47 | 0.19 | 0.59 | 0.29 | 4.76 | ||
| Germany | 1976–78 | na | 0.01 | 0.06 | 0.13 | 0.05 | 0.28 | 0.01 | na | nc |
| 2004–06 | na | 0.25 | 0.25 | 0.52 | 0.24 | 0.35 | 0.25 | na | ||
| Italy | 1985 | na | na | na | na | na | 0.00 | na | 0.50 | 0.07 |
| 2005 | na | na | na | na | na | 0.30 | na | 1.90 | ||
| England | 1971–78 | na | 0.01 | na | 0.12 | 0.18 | 0.04 | 0.01 | na | nc |
| 2000–06 | na | 0.16 | na | 0.39 | 0.17 | 0.50 | 0.11 | na | ||
| Sweden | 1960–75 | na | na | na | 0.58 | na | na | na | na | nc |
| 1991–05 | na | na | na | 1.33 | na | na | na | na | ||
| Norway | 1993–97 | 0.49 | 0.15 | 0.15 | 0.60 | 0.19 | 0.10 | 0.22 | 2.35 | 0.24 |
| 2000–04 | 0.90 | 0.20 | 0.30 | 1.01 | 0.33 | 0.23 | 0.25 | 4.06 | ||
| France | 1976 | na | na | na | 0.07 | na | na | na | na | nc |
| 2001 | na | na | na | 0.21 | na | na | na | na | ||
| Denmark | 1978 | 0.20 | na | na | na | na | na | na | na | nc |
| 1997 | 0.49 | na | na | na | na | na | na | na | ||
| Switzerland | 1974–85 | 0.33 | 0.01 | na | 0.16 | na | na | na | 0.77 | 0.05 |
| 1986–97 | 0.47 | 0.05 | na | 0.44 | na | na | na | 1.39 | ||
| Asia | ||||||||||
| Taiwan | 1996 | 0.05 | 0.02 | 0.02 | 0.03 | 0.02 | na | 0.07 | 0.30 | 0.11 |
| 2008 | 0.27 | 0.13 | 0.13 | 0.06 | 0.09 | na | 0.38 | 1.51 | ||
na not available, nc not computable
Incidence rates of all site-specific NENs increased over time in all countries for all sites, except for appendix (supplementary Fig. 1). Rates of appendix NENs have been increasing over time in England and Norway, while remained stable in Germany, the Netherlands and the United States.
NEN incidence in the United States by grade
The age-adjusted incidence rates of NENs according to histological grade from 1973 to 2012 are illustrated in Fig. 3b–d. The overall incidence rate of low-grade NENs increased from 1.09 per 100,000 in 1973 to 3.51 in 2012 (Fig. 3b); this represented a 3.2-fold increase. During the same interval, the overall incidence rate of high-grade NENs increased from 2.54 per 100,000 to 10.52 (Fig. 3b); this represented a 4.1-fold increase.
During 2000–2012, the incidence rate of low-grade NENs (Fig. 3c and supplementary Fig. 2) increased in the lung (0.63 per 100,000 in 1973 vs 0.75 per 100,000 in 2012; APC: 1.74, 95% CI: 1.21, 2.27), stomach (0.22 per 100,000 in 1973 vs 0.31 per 100,000 in 2012; APC: 3.03, 95% CI: 1.65, 4.43), appendix (0.02 per 100,000 in 1973 vs 0.13 per 100,000 in 2012; APC: 12.07, 95% CI: 6.86, 17.54), and rectum (0.69 per 100,000 in 1973 vs 0.89 per 100,000 in 2012; APC: 2.52, 95% CI: 0.90, 4.17). By converse, the incidence rate of pancreas and small intestine tumors remained stable, while for colon it decreased (0.20 per 100,000 in 1973 vs 0.14 per 100,000 in 2012; APC: −2.65, 95% CI: −5.26, −0.03). In 2012, the highest incidence rate of low-grade NENs was observed for rectum (0.89 per 100,000), followed by the small intestine (0.83 per 100,000), and lung (0.75 per 100,000). Overall, the incidence of digestive low-grade NENs was 2.54 per 100,000.
During 2000–2012, the incidence rates of high-grade NENs (Fig. 3d and supplementary Fig. 2), increased over time for all sites, except for lung, for which a decreasing trend was observed (8.62 per 100,000 in 1973 vs 7.47 per 100,000 in 2012; APC: −1.14, 95% CI: −1.63, −0.64) (Fig. 3b and supplementary Fig. 2). During the same period, the small intestine showed the largest APC (0.07 per 100,000 in 1973 vs 0.46 per 100,000 in 2012; APC 20.68, 95% CI: 17.8, 23.7), while the colon showed the lowest APC (0.14 per 100,000 in 1973 vs 0.20 per 100,000 in 2012; APC 4.09, 95% CI: 2.56, 5.64) (Fig. 3d and supplementary Fig. 2). Lung cancer was the most frequently diagnosed high-grade NEN (7.47 per 100,000), accounting for 71% of the total new high-grade NEN cases in 2012. Overall the incidence of digestive high-grade NENs was 1.72 per 100,000.
Incidence rates of NENs from 2000 to 2012 according to grade by gender are presented overall and by anatomical site in Supplementary Figs. 3 and 4. Incidence rates of low-grade and high-grade NENs demonstrated a similar pattern in both males and females. When taking into account all the NEN cases over the last 3 years, the overall M:F ratio was 0.9 and 1.2 for low-grade and high-grade NENs, respectively.
Incidence rates of NENs from 2000 to 2012 according to grade for three ethnic populations are presented overall and by anatomical site in Supplementary Figs. 5 and 6. In the period 2010–2012, within the low-grade NENs, African Americans showed the highest overall incidence rate (5.57 per 100,000), followed by whites (3.28 per 100,000), and Asians/Pacific Islanders (2.21 per 100,000). In the same period, within the high-grade NENs, whites had the highest overall incidence rate (11.02 per 100,000), followed by African Americans (10.56 per 100,000), and Asians/Pacific Islanders (4.75 per 100,000).
Sensitivity analyses restricted to SEER 9 registries with complete data (period 1975–2012) revealed incidence trends for NEN according to histological grade, comparable to those obtained from different combinations of SEER registries in terms of both magnitude and statistical significance (data not shown).
Discussion
This work aimed at defining the current pattern and trend in the incidence of NENs worldwide, and at analyzing the SEER database for more detailed results. Based on the codes here utilized, only pure neuroendocrine cancers were investigated. Additionally, of the recently recognized high grade NEN types (G3 NET and NEC), the present investigation applies to NEC only [3, 23–28]. Our data indicate that (i) the reported incremental trend observed for low-grade NENs is confirmed as a worldwide phenomenon, (ii) that this trend occurs at most anatomical sites and (iii) it is paralleled by an increase in the incidence of high-grade NENs.
The worldwide incremental trend for NENs is here confirmed at most anatomical sites investigated. No explanation for this phenomenon is apparent. A mix of better understanding of biology and clinical features of NEN and better diagnosis was offered as the most likely interpretation [17]. Though this could be the case, the link to potential NEN-specific promoting agents cannot be excluded. As an example and in line with this hypothesis the widespread access to proton-pump inhibitors has been proposed as possible risk factor for gastric NEN development [29]. Recent data from Norway however suggest a true increase of NENs [18, 19].
The observed rapid increases in incidence rates associated with significant variations in national NEN incidence rates. Our data indicate that geographic variations remained stable over time, the highest incidence rates observed in North America and relatively low rates in Taiwan. These variations may well reflect heterogeneity in disease classification since classification changes occurred in recent years [2]. Nonetheless little is known of environmental, ethnic/genetic or other risk/predisposing factors that could be involved [30].
Here we report also the age-adjusted incidence rates of NENs according to histological grade in US. Our data show that the incidence of high-grade NENs is overall significantly higher when compared to low-grade NENs (10.52 vs. 3.51 per 100,000 in 2012). However, this reflects the very high incidence observed for lung, as compared to digestive NENs (7.47 vs. 1.72 per 100,000 in 2012). These site-dependent features further confirm significant site-specific differences.
In the period 2000–2012, the incidence rates of high-grade NENs in SEER increased over time for all sites, except for lung, where a decreasing trend was observed. This latter observation may well reflect the change in smoking habits in US over the last decades [31]. An increase in the incidence rate of low-grade NENs was also observed at various anatomical sites (lung, rectum, stomach, and appendix), whereas for others it remained stable (pancreas and small intestine) or decreased (colon). However, when comparing the trends overall, the incidence increase observed for high-grade NENs was only slightly higher, and substantially in the same order of magnitude to that observed for low-grade (high-grade 4.1-fold increase vs low-grade 3.2).
Our data are the first to report an incremental incidence trend for digestive high-grade NENs. Very few epidemiological data are available for this highly aggressive neuroendocrine cancer group, often excluded from epidemiology investigation on NENs [7, 32]. This incremental trend was consistently observed at all digestive sites investigated, grossly paralleling that observed for low-grade NENs.
Our findings raise the hypothesis that NENs share susceptibility factors independently of cancer grade, a phenomenon observed for other cancer types. As an example, in the upper and lower airways, smoking habits associate with various carcinomas, no matter the histological type and the cancer grade [33, 34]. High-grade cancer associates with severe genetic somatic abnormalities, usually involving genes controlling key cell proliferation pathways, and this is true for NENs too [35]. As for NENs, it could be hypothesized that the same, yet unknown factor(s) promote neuroendocrine carcinogenesis, and may then result in grade differences depending on the adding-on of key genetic or epigenetic alterations (either synchronous or metachronous).
As for gender and ethnicity, the general picture emerging from our data is that the patient with low-grade NENs is more frequently African–American of either sex, though prevalently female in case of NEN from the lung or the stomach. Similarly, high-grade NENs were more frequently observed in male African Americans, with the notable exception of the lung (and at lesser extent the appendix) for which NENs were predominantly observed in male white patients. So, male gender and African American ethnicity appear to play a role in determining NEN development risk.
Finally, the incidence of NEN in the 2012 in 18 SEER registries was 14.02 per 100,000, low-grade NEN being approximately 25% of all, and up to 57% when the high-grade NEN of the lung were excluded. In US, a cancer is rare for an incidence lower than 15 in 100,000 people; while in EU, a cancer is rare when lower than six in 100,000 people [36–38]. Our data suggest that NEN are close to outgrow the current US categorization, and well above the EU definition. In reality, when the high-grade NENs of the lung are excluded, NEN incidence is considerably lower (6.55 per 100,000) and far below the US cut-off definition for rare cancer, but still higher than the EU definition. A homogenous worldwide definition of rare cancer is probably needed, at least to uniform data analysis and to promote common cancer-specific policies.
Though we aimed at being as accurate as possible, some methodological limits are present. In specific, different morphological codes were used to classify NENs, and different standard populations were used to adjust for differences between countries in the age structure of the various populations. So, comparisons among different geographical areas or populations were subject to some bias. In addition, SEER data refer to tumors of proven malignancy only, and since “carcinoids” for long have been considered benign, this may have hampered data collection, especially between 1973–2000. Despite such limits, this study is based on data from 11 countries with a total population of over 100 million. Also, SEER was used as referral database since containing information on over 7.5 million cancer cases, providing the large number of events required for reliable estimation of incidence in rare cancers as NENs.
In conclusion, our data indicate that NENs overall are stably increasing independently of grade. This yet poorly understood phenomenon would require a major investigation effort to answer the expected rising demand for cure of the increased NEN cancer patients, as well as prevention of this group of neoplasms.
Electronic supplementary material
Acknowledgements
E.L. was visiting scientist at Mount Sinai School of Medicine. Funding in part was supported by internal university grants (Università Cattolica line D.1/2013-70201056 and D.1 2014-70201266) and by the Associazione Italiana Ricerca sul Cancro-AIRC IG 2013 14696 to GR. The funders had no role in the study design and data analysis.
Author contributions
E.L., P.B., M.S. and K.A. contributed to data generation and analysis; E.L., P.B., S.B. and G.R. contributed to data interpretation and manuscript writing; E.L., S.B. and G.R. gave the study design.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Stefania Boccia and Guido Rindi contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s12020-017-1273-x) contains supplementary material, which is available to authorized users.
References
- 1.Inzani F, Rindi G. Classification of Neuroendocrine Neoplasms. In: Pacak K, Taïeb D, editors. Diagnostic and Therapeutic Nuclear Medicine for Neuroendocrine Tumors. Contemporary Endocrinology. Cham: Springer; 2017. pp. 1–14. [Google Scholar]
- 2.Rindi G, Arnold R, Capella C, Klimstra DS, Klöppel G, Komminoth P, Solcia E. Nomenclature and classification of digestive neuroendocrine tumours. In: Bosman F, Carneiro F, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC; 2010. pp. 10–12. [Google Scholar]
- 3.Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, Pisa E, Barberis M, Vanoli A, Buzzoni R, Pusceddu S, Concas L, Sessa F, Solcia E, Capella C, Fazio N, La Rosa S. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104(1):85–93. doi: 10.1159/000445165. [DOI] [PubMed] [Google Scholar]
- 4.M.B. Amin, S.B. Edge, F.L. Greene, D.R. Byrd, D.B. Brookland, M.K. Washington, J.E. Gershenwald, C.C. Compton, K.R. Hess, D.C. Sullivan, J.M. Jessup, J.D. Brierley, L.E. Gaspar, R.L. Schilsky, C.M. Balch, D.P. Winchester, E.A. Asare, M. Madera, D.M. Gress, L.R. Meyer (eds.), AJCC Cancer Staging. Manual, 8th ed. (Springer, New York, Philadelphia, 2017)
- 5.E.A. Woltering, E.K. Bergsland, D.T. Beyer, T.M. O’Dorisio, G. Rindi, D.S. Klimstra, L.H. Tang, D. Reidy-Lagunes, J.R. Strosberg, E.M. Wolin, A.I. Vinik, E.K. Nakakura, E.A. Asare, D.L. Bushnell, R.L. Schilsky, Y.-Z. Wang, M.K. Kim, E.H. Liu, R.T. Jensen, R.K.S. Wong, J.K. Ramage, K. Mallin, R.F. Pommier: Neuroendocrine tumors of the jejunum and ileum. In: M.B. Amin, S.B. Edge, F.L. Greene, D.R. Byrd, D.B. Brookland, M.K. Washington, J.E. Gershenwald, C.C. Compton, K.R. Hess, D.C. Sullivan, J.M. Jessup, J.D. Brierley, L.E. Gaspar, R.L. Schilsky, C.M. Balch, D.P. Winchester, E.A. Asare, M. Madera, D.M. Gress, L.R. Meyer (eds.) AJCC Cancer Staging Manual. (Springer, New York, Philadelphia, 2017), pp. 375–387
- 6.Levi F, Te VC, Randimbison L, Rindi G, La Vecchia C. Epidemiology of carcinoid neoplasms in Vaud, Switzerland, 1974-97. Br. J. Cancer. 2000;83(7):952–955. doi: 10.1054/bjoc.2000.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skuladottir H, Hirsch FR, Hansen HH, Olsen JH. Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer. 2002;37(2):127–135. doi: 10.1016/S0169-5002(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 8.C. Lepage, A.M. Bouvier, S. Manfredi, V. Dancourt, J. Faivre, Incidence and management of primary malignant small bowel cancers: a well-defined French population study. The American journal of gastroenterology 101(12), 2826–2832 (2006). doi:10.1111/j.1572-0241.2006.00854.x [DOI] [PubMed]
- 9.Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, Modlin IM. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113(10):2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 10.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am. J. Gastroenterol. 2010;105(12):2563–2569. doi: 10.1038/ajg.2010.341. [DOI] [PubMed] [Google Scholar]
- 11.Landerholm K, Falkmer S, Jarhult J. Epidemiology of small bowel carcinoids in a defined population. World J. Surg. 2010;34(7):1500–1505. doi: 10.1007/s00268-010-0519-z. [DOI] [PubMed] [Google Scholar]
- 12.Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol. Oncol. Res. 2011;17(3):759–763. doi: 10.1007/s12253-011-9382-y. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. 2013;8(4):e62487. doi: 10.1371/journal.pone.0062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.C.M. Korse, B.G. Taal, M.L. van Velthuysen, O. Visser, Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. European journal of cancer 49(8), 1975–1983 (2013). doi:10.1016/j.ejca.2012.12.022 [DOI] [PubMed]
- 15.Scherubl H, Streller B, Stabenow R, Herbst H, Hopfner M, Schwertner C, Steinberg J, Eick J, Ring W, Tiwari K, Zappe SM. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: epidemiological changes in Germany. World J. Gastroenterol. 2013;19(47):9012–9019. doi: 10.3748/wjg.v19.i47.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 17.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 18.Sandvik OM, Soreide K, Gudlaugsson E, Kvaloy JT, Soreide JA. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br. J. Surg. 2016;103(3):226–232. doi: 10.1002/bjs.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, Tretli S, Bergestuen DS, Hansen S. Trends in incidence of neuroendocrine neoplasms in Norway: a report of 16,075 cases from 1993 through 2010. Neuroendocrinology. 2017;104(1):1–10. doi: 10.1159/000442207. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008;19(10):1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19(3):335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Velayoudom-Cephise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D, Debaere T, Caramella C, Schlumberger M, Planchard D, Elias D, Ducreux M, Scoazec JY, Baudin E. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer. 2013;20(5):649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 24.H. Sorbye, S. Welin, S.W. Langer, L.W. Vestermark, N. Holt, P. Osterlund, S. Dueland, E. Hofsli, M.G. Guren, K. Ohrling, E. Birkemeyer, E. Thiis-Evensen, M. Biagini, H. Gronbaek, L.M. Soveri, I.H. Olsen, B. Federspiel, J. Assmus, E.T. Janson, U. Knigge: Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. (2012). doi:10.1093/annonc/mds276 [DOI] [PubMed]
- 25.Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, Barriuso J, Pavel M, O’Toole D, Walter T, other Knowledge Network, m. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer. 2015;22(4):657–664. doi: 10.1530/ERC-15-0119. [DOI] [PubMed] [Google Scholar]
- 26.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am. J. Surg. Pathol. 2015;39(5):683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: New insights and treatment implications. Cancer Treat. Rev. 2016;50:61–67. doi: 10.1016/j.ctrv.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic well-differentiated grade 3 neuroendocrine tumors: review and position statement. Oncol. 2016;21(10):1191–1199. doi: 10.1634/theoncologist.2015-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jianu CS, Fossmark R, Viset T, Qvigstad G, Sordal O, Marvik R, Waldum HL. Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment. Pharmacol. Ther. 2012;36(7):644–649. doi: 10.1111/apt.12012. [DOI] [PubMed] [Google Scholar]
- 30.Leoncini E, Carioli G, La Vecchia C, Boccia S, Rindi G. Risk factors for neuroendocrine neoplasms: a systematic review and meta-analysis. Ann. Oncol. 2016;27(1):68–81. doi: 10.1093/annonc/mdv505. [DOI] [PubMed] [Google Scholar]
- 31.Prevention, C.f.D.C.a.: Current Cigarette Smoking Among Adults–United States, in Morbidity and Mortality Weekly Report, 2005–2014, Vol. 64. (Prevention C.f.D.C.a. Washington, DC, 2015), pp. 1233–1240 [DOI] [PubMed]
- 32.Naalsund A, Rostad H, Strom EH, Lund MB, Strand TE. Carcinoid lung tumors--incidence, treatment and outcomes: a population-based study. Eur. J. Cardiothorac. Surg. 2011;39(4):565–569. doi: 10.1016/j.ejcts.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, Franceschi S, Hayes RB, Herrero R, Koifman S, La Vecchia C, Lazarus P, Levi F, Mates D, Matos E, Menezes A, Muscat J, Eluf-Neto J, Olshan AF, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Zaridze D, Zatonski W, Zhang ZF, Berthiller J, Boffetta P. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 34.Steliga MA, Dresler CM. Epidemiology of lung cancer: smoking, secondhand smoke, and genetics. Surg. Oncol. Clin. N. Am. 2011;20(4):605–618. doi: 10.1016/j.soc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjaer A, Knigge U. Neuroendocrine carcinomas of the gastroenteropancreatic system: a comprehensive review. Diagnostics (Basel) 2015;5(2):119–176. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European parliament and council of the European communities. Decision no. 1295/1999/EC of the European parliament and of the council of 29 April 1999 adopting a programme of community action on rare diseases within the framework for action in the field of public health (1999–2003). (1999)
- 37.National Institutes of Health, Annual report on the rare diseases research activities at the National Institutes of Health, FY in 2005 (National Institutes of Health, Bethesda, MD, 2006)
- 38.National Cancer Institute Epidemiology and Genetics Research, Synergizing epidemiologic research on rare cancers. Workshop, May 10-11 2007, Bethesda, MD.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


