Abstract
Aim
Prostate contouring using CT alone is difficult. To overcome the uncertainty, CT/MRI registration using a fiducial marker is generally performed. However, visualization of the marker itself can be difficult with MRI. This study aimed to determine the optimal MRI pulse sequence for defining the marker as well as the prostate outline among five sequences.
Materials and methods
A total of 21 consecutive patients with prostate cancer were enrolled. Two gold fiducial markers were placed before CT/MRI examination. We used the following five sequences: T1-weighted spin-echo (T1WI; TR/TE, 400–650/8 ms); T2-weighted fast spin-echo (T2WI; 4000/80); T2*-2D-weighted gradient echo (T2*2D; 700/18); T2*-3D-weighted gradient echo (T2*3D; TR/TE1/deltaTE, 37/14/7.3); and contrast-enhanced T1-weighted spin-echo (CE-T1WI; 400–650/8). Qualitative image analysis of the sequences was performed by three observers. These observers subjectively scored all images on a scale of 1–3 (1 = unclear, 2 = moderate, 3 = well visualized). A higher score indicated better visualization.
Results
T2WI was significantly superior to the other sequences in terms of prostate definition. T2*2D and T2*3D were strongly superior to the other sequences and were significantly superior in terms of fiducial marker definition.
Conclusions
T2*2D and T2*3D are superior to the other sequences for prostate contouring and marker identification. Therefore, we recommend initial T2*3D and T2*2D examinations.
Keywords: IMRT, Prostate cancer, MRI, Fiducial marker, Registration
1. Background
External beam radiotherapy (EBRT) is being more widely used for prostate cancer owing to the development of computed tomography (CT) and magnetic resonance imaging (MRI). However, prostate contouring using CT alone is difficult.1, 2, 3, 4, 5, 6 To overcome the uncertainty of prostate contouring with CT images alone, CT/MRI registration using a fiducial marker is generally performed. However, visualization of the marker itself tends to be difficult using MRI. T2-weighted spin-echo (T2WI) and two-dimensional T2*-weighed gradient echo (T2*2D) sequences can clearly show the prostate outline.1, 2, 3 T2*2D has been reported to sensitively detect fiducial markers as well as T2WI.2, 3 However, T2*3D-weighted gradient echo (T2*3D) and contrast-enhanced T1-weighted spin-echo (CE-T1WI) sequences have not been evaluated in this setting. T2*2D is better than T2WI and is an accepted sequence for detecting fiducial markers. Additionally, it provides soft tissue contrast for the prostate; however, no precise comparison study has been conducted previously.
We previously reported that CE-T1WI is the best sequence to precisely detect both the seeds and prostate outline in postimplant dosimetry of low-dose-rate I-125 prostate brachytherapy.7 Recently, three-dimensional T2-weighted images (T2*3D) have been used for pelvic examinations.
This study aimed to determine the optimal MRI pulse sequence for defining the marker, as well as the prostate outline, among five sequences, including T2*3D and CE-T1WI.
2. Materials and methods
Between April 2015 and September 2015, 21 consecutive patients with prostate cancer were treated with intensity-modulated radiation therapy (IMRT) at our hospital. All patients provided written informed consent to participate in this study. Additionally, all patients were pathologically diagnosed with prostate adenocarcinoma and were classified as having low-to-high risk according to the D’Amico classification system.8 This study was registered in the UMIN Clinical Trial Registry. IMRT was performed in the low-risk, intermediate-risk, and high-risk groups at 74 Gy, 76 Gy, and 78 Gy, respectively. Patients allergic to the MRI contrast agent were excluded from this study.
Two gold fiducial markers (VISICOIL, RadioMed Corporation, Bartlett, TN, USA) were placed on the prostate before CT/MRI examination at three weeks. We placed two gold markers on the central meddle of the prostate, but sometimes in a different place. Two, and not three, gold markers were used because markers were 10 mm linear, and their ends are treated as points. To decrease artifacts in the CT image and the amount of bleeding in the prostate, we selected markers measuring 0.35 mm in diameter and 10 mm in length for all patients. We selected the smallest marker with the thinnest 22 G (in Japan) needle to prevent bleeding, tumor seeding, and pain. The markers were well visualized on cone-beam CT using the Novalis Tx system (Varian Medical Systems, Inc., Palo Alto, CA, USA).
2.1. Image acquisition
All patients ingested 200 ml of water 30 min before CT/MRI to accumulate a certain volume of urine in the bladder. External beam planning CT (Optima CT580, GE Medical Systems, Milwaukee, WI, USA) and MRI (Intera 1.5 Nova, Philips Medical Systems, Eindhoven, The Netherlands) scans were performed. MRI was performed within 20 min after CT. MRI was performed using a 5-channel sense cardiac coil (3-mm section thickness with no intersection gap and a 16-cm field of view for all sequences). The approximate scan time for the sequences was 4–6 min in all cases.
2.2. MRI sequences
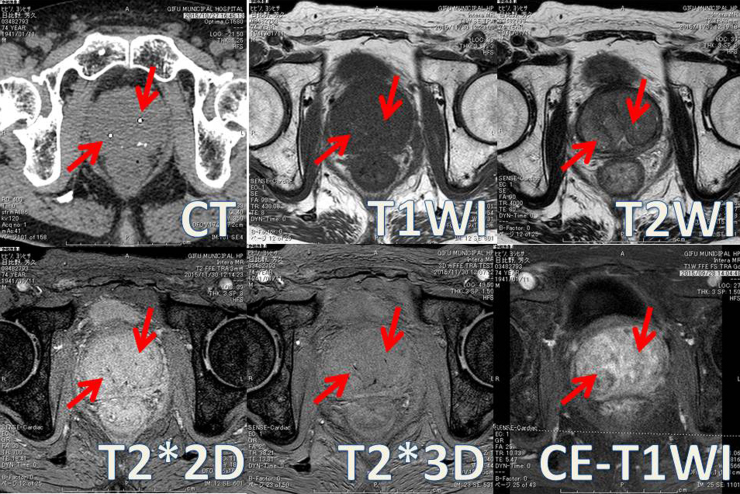
We used the following five MRI sequences in all patients: (Fig. 1) T1WI, T2WI, T2*2D, T2*3D, and CE-T1WI. The phase-encoding direction was right–left in all sequences. CE-T1WI MRI was performed using gadopentetate dimeglumine (Magnevist; Bayer, Berlin, Germany). Quality comparison of the five sequences was conducted by one radiation oncologist and two radiation technologists.
Fig. 1.
CT images were acquired as follows: 16-row detector CT; thickness: 1.25 mm; field of view: 40 cm × 40 cm; 120 kV; and 460 mA. MRI; (1) T1WI [repetition time (TR)/echo time (TE)], 400–650/8 ms; number of average signals (NAS), 4; number of phase encoding steps (PES), 192; number of frequency encoding steps (FES), 240; typical pixel size (TPS; frequency/phase), 0.67/0.83]; (2) T2WI (TR/TE, 4000/80 ms; NAS, 4; PES, 205; FES, 256; and TPS, 0.63/0.80); (3) T2*2D (TR/TE, 700/18 ms; NAS, 2; PES, 205; FES, 256; and TPS, 0.63/0.78); (4) T2*3D (TR/TE1/deltaTE, 37/14/7.3 ms; NAS, 2; PES, 218; FES, 272; and TPS, 0.55/0.54); and (5) CE-T1WI (TR/TE, 400–650/8 ms; NAS, 4; PES, 192; FES, 240; and TPS, 0.67/0.83). The phase-encoding direction was right–left in all sequences. CE-T1WI MRI was performed using gadopentetate dimeglumine (Magnevist; Bayer, Berlin, Germany).
2.3. Image scoring
First, we checked the two fiducial markers in CT images for all cases. We did not used blind scoring systems for MR images. Because we do not make a treatment plan only with an MR image in clinical situation, we confirm a position of marker with a CT image earlier. Thus, we thought that it was not necessary to make blindness. Additionally, we assessed the markers on MRI with the guidance of CT images. The three observers (observer 1: brachytherapy radiation oncologist with 15 years of experience; observer 2: radiation oncologist with 20 years of experience; observer 3: radiological technologist with 10 years of experience) subjectively scored all images according to the following five evaluation items: definition of the prostate outline; apex vs. soft tissue; base vs. bladder; base vs. seminal vesicle; and gold fiducial marker detection. A score from 1 to 3 (1 = unclear, 2 = moderate, 3 = well visualized) was assigned to all items. A higher score indicated better visualization. We then compared the mean scores for each item.
2.4. Statistical validation
Statistical analysis was performed using analysis of variance. Among the five sequences, the sequences that scored better than the other sequences were studied, and a p-value < 0.05 was considered statistically significant. The Excel statistics 2015 (Social Survey Research Information Co., Ltd. Japan) software was used for statistical analysis.
3. Results
The study included 21 consecutive patients with prostate cancer. Our findings are shown in Table 1 and Fig. 1. The evaluation of image quality varied among the three observers. T2WI was significantly superior to the other sequences in terms of prostate definition (p < 0.05). T2*2D and T2*3D were strongly superior to the other sequences and were significantly superior to the other sequences in terms of fiducial marker definition (p < 0.05). There were no significant differences between T2*2D and T2*3D. T1WI and CE-T1WI were not superior to the other sequences.
Table 1.
Mean imaging score of magnetic resonance imaging (MRI) pulse sequence to define and gold fiducial marker.
| Outline of prostate | Apex vs. soft tissue | Base vs. bladder | Base vs. SV | Fiducial marker definition | |
|---|---|---|---|---|---|
| Observer 1 | |||||
| T1WI | 1.6 | 1.1 | 1.3 | 1.2 | 1.2 |
| T2WI | 2.0 | 1.8 | 1.5 | 2.0† | 1.2 |
| T2*2D | 2.0 | 2.2 | 2.4‡ | 1.0 | 2.4‡ |
| T2*3D | 2.5 | 1.2 | 2.4‡ | 1.1 | 2.6‡ |
| CE-T1WI | 2.4 | 2.0 | 1.6 | 1.1 | 1.6 |
| Observer 2 | |||||
| T1WI | 2.6 | 1.0 | 1 | 2.4 | 1.0 |
| T2WI | 3.0 | 2.7† | 2.4 | 3.0 | 1.1 |
| T2*2D | 2.8 | 1.1 | 1.3 | 1.2 | 2.2‡ |
| T2*3D | 2.6 | 1.3 | 1.1 | 1.4 | 2.4‡ |
| CE-T1WI | 2.9 | 1.7 | 1.9 | 2.6 | 1.0 |
| Observer 3 | |||||
| T1WI | 1.7 | 1.1 | 1.6 | 1.3 | 1.0 |
| T2WI | 2.7 | 2.4† | 2.4† | 2.3† | 1.1 |
| T2*2D | 1.8 | 1.1 | 1.1 | 1.1 | 2.4‡ |
| T2*3D | 1.9 | 1.2 | 1.1 | 1.2 | 2.7‡ |
| CE-T1WI | 2.4 | 1.9 | 1.3 | 1.3 | 1.0 |
Abbreviations: T1WI, T1-weighted spin-echo (repetition time [TR]/echo time [TE] in ms): (range 400–650/8); T2WI, T2-weighted fast spin-echo (4000/80); T2*2D, T2*_T2-weighted gradient echo (700/18); T2*3D, T2*_3-dimentional T2_weighted gradient echo [TR/TE1/deltaTE](37/14/7.3); CE-T1WI, contrast enhanced T1-weighted spin-echo (range 400–650/12); SV, seminal vesicle.
One radiation oncologist and two radiation technologists subjectively scored the images based on 5 evaluation items. A score from 1 to 3 (1: poor, 2: moderate, 3: good) was assigned to all items. The higher score was regarded as the greater definition of the prostate edge and gold fiducial markers.
The significantly (p < 0.05) highest score among four sequences.
The significantly (p < 0.05) higher score than that in other three sequences.
4. Discussion
T2WI and T2*2D are the gold standard for prostate contouring and detecting the intraprostatic fiducial marker and I-125 seed after low-dose-rate brachytherapy. For advanced EBRT, a fiducial marker is necessary to contour the prostate using CT/MRI registration. We previously reported that fat-suppressed CE-T1WI is the best sequence to detect I-125 seeds (with a diameter of 1 mm), as well as the prostate outline, using CT/MRI registration.7 We additionally compared CT/MRI fusion-based and MRI-only-based postimplant dosimetry, and MRI-only-based postimplant dosimetry is clinically used as well.7 Summary of recommended sequences is shown in Table 2.
Table 2.
MRI sequences recommended in the literature to detect fiducial marker in the prostate.
| No. of patients | Recommend MRI sequence | |
|---|---|---|
| Ours | 21 | T2*3D-weighted gradient echo image |
| Tanaka et al.7 | 52 | CE-T1weighted fast spin echo image (seed*) |
| Kapeman et al.9 | 30 | T1/T2*-weighted gradient echo image |
| Lim et al.10 | Pictorial review | T1-3D dual gradient echo image |
| Ghose et al.11 | 15 | T1/T2*-weighted gradient echo image |
Seed*: diameter of 1 mm for low dose rate brachytherapy for prostate cancer.
Kapanen et al. reported MRI-only-based EBRT for prostate cancer and demonstrated that the distinction of the seeds may be difficult in the T2- and T2/T2*-weighted images. To increase the quality of seed and fiducial marker detection, the susceptibility effect was used, and T2/T1/T2*-weighted images were acquired with a 3D steady-state echo gradient sequence FIESTA-C® (flip angle = 35°).9
Lim et al. summarized that the T2-3D fast spin-echo sequence has the advantage of increased signal-to-noise ratio but is vulnerable to motion degradation. Additionally, multi-echo gradient-recalled echo increased the optimal depiction of fiducial markers vulnerable to motion degradation.10
T2*2D is generally the most useful sequence to depict fiducial markers; therefore, we evaluated the use of new sequences (CE-T1WI and T2*3D). We hypothesized that CE-T1WI was the optimal sequence, similar to postimplant dosimetry; however, this sequence was worse than the other sequences. Potential explanations for the poor marker visualization are the marker size, I-125 seed diameter (1 mm), and fiducial marker diameter (0.35 mm). A larger implanted device may be visualized on MRI as a susceptibility artifact owing to the fact that marker visualization in the prostate depends on the marker size, magnetic permeability, and pulse sequences. If we use a 1-mm marker, the results may differ from those in the present study.
In the present series, the results of the three observers varied. The primary reason of this was the importance of the clinical position. Observer 1, a prostate brachytherapist, emphasized all items. Observers 2 and 3 were actually involved in clinical EBRT using CT and MRI registration. The results of image scoring showed that T2*3D was statistically similar to T2*2D; however, we may mistake black dots as the markers we speculate that this phenomenon may be more frequently found in T2*2D than in T2*3D. Fiducial markers demonstrate a high density in CT images. If a CT image is not used, T2*3D may be superior to all of the sequences. Both T2*2D and T2*3D were strongly affected by bowel movement. In this study, we did not use butylscopolamine to reduce bowel movement. A clinical study on the effect of butylscopolamine for bowel movement during MRI is currently underway in our institution.
Ghose et al. investigated a template-matching approach for the detection of these seeds in MRI. A fiducial detection accuracy of 95% was obtained compared to manual observations.11 We used the smallest fiducial marker (0.35 mm in diameter) to decrease the artifact on CT, but such markers need to be clearly visualized on MRI. Therefore, T2*2D and T2*3D are suitable for detecting both the marker and the prostate outline. We recommend to initially obtain T2*3D-WI to detect fiducial marker and prostate outline. If T2*3D-WI is inadequate, then T2*2D-WI should be used.
5. Conclusion
T2*2D and T2*3D are superior to the other sequences. T2WI exhibits the greatest precision for detecting the prostate outline, but we could not precisely identify the fiducial markers in the prostate. T2*2D and T2*3D are superior to the other sequences with regard to prostate contouring and marker identification, and there are no significant differences between the performances of T2*2D and T2*3D for detecting fiducial markers. However, misleading signal voids might be more frequent with T2*2D than with T2*3D. Therefore, we recommend that imaging be initially performed with T2*3D, followed by T2*2D. Additionally, our findings suggest that T1WI and CE-T1WI are unsuitable for depicting the outline of the prostate and the fiducial markers.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Huisman H.J., Fütterer J.J., van Lin E.N. Prostate cancer: precision of integrating functional MR imaging with radiation therapy treatment by using fiducial gold markers. Radiology. 2005;236:311–317. doi: 10.1148/radiol.2361040560. [DOI] [PubMed] [Google Scholar]
- 2.Susil R.C., Ménard C., Krieger A. Transrectal prostate biopsy and fiducial marker placement in a standard 1.5 T magnetic resonance imaging scanner. J Urol. 2006;175:113–120. doi: 10.1016/S0022-5347(05)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol A.M., Brock K.K., Lockwood G.A. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007;67:48–56. doi: 10.1016/j.ijrobp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin P.W., Evans C., Feng M. Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate contouring accuracy. Int J Radiat Oncol Biol Phys. 2010;76:369–378. doi: 10.1016/j.ijrobp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Riches S.F., Payne G.S., Desouza N.M. Effect on therapeutic ratio of planning a boosted radiotherapy dose to the dominant intraprostatic tumour lesion within the prostate based on multifunctional MR parameters. Br J Radiol. 2014;87:20130813. doi: 10.1259/bjr.20130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieda N., Avruch L., Shabana W.M. Multi-echo gradient recalled echo imaging of the pelvis for improved depiction of brachytherapy seeds and fiducial markers facilitating radiotherapy planning and treatment of prostatic carcinoma. J Magn Reson Imaging. 2015;41:715–720. doi: 10.1002/jmri.24590. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka O., Hayashi S., Matsuo M. Comparison of MRI-based and CT/MRI fusion-based postimplant dosimetric analysis of prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;66:597–602. doi: 10.1016/j.ijrobp.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 9.Kapanen M., Collan J., Beule A. Commissioning of MRI-only based treatment planning procedure for external beam radiotherapy of prostate. Magn Reson Med. 2013;70:127–135. doi: 10.1002/mrm.24459. [DOI] [PubMed] [Google Scholar]
- 10.Lim C., Malone S.C., Avruch L. Pictorial review. Magnetic resonance for radiotherapy management and treatment planning in prostatic carcinoma. Br J Radiol. 2015;88(1054):20150507. doi: 10.1259/bjr.20150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghose S., Mitra J., Rivest H. MRI-alone radiation therapy planning for prostate cancer: automatic fiducial marker detection. Med Phys. 2016;43:2218–2228. doi: 10.1118/1.4944871. [DOI] [PubMed] [Google Scholar]