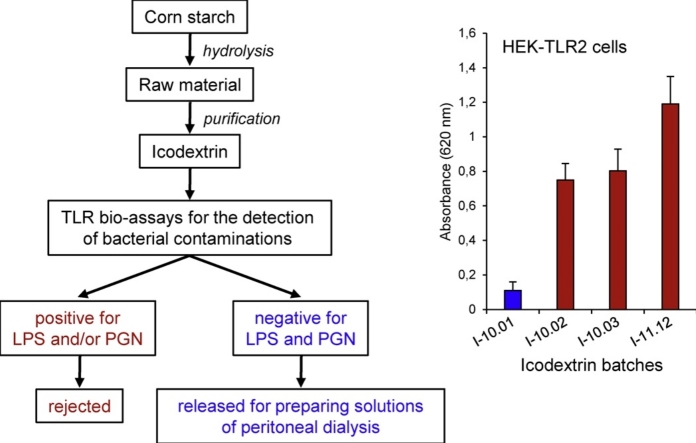
Graphical abstract
Abbreviations: dp, degree of polymerization; FCS, fetal calf serum; HEK, human embryonic kidney; IL, interleukin; LAL, limulus amoebocyte lysate; LPS, lipopolysaccharides; LTA, lipoteichoic acid; PBS, phosphate buffered saline; PGN, peptidoglycans; SEAP, secreted embryonic alkaline phosphatase; SLP, Silkworm Larvae plasma; TLR, Toll-like receptor; TNF, tumor necrosis factor-α
Keywords: Icodextrin, Peritoneal dialysis, Peptidoglycans, Lipopolysaccharides, Toll-like receptors, Inflammation
Highlights
-
•
Monocyte test assays were poorly efficient for the detection of little bacterial contamination in icodextrin batches.
-
•
TLR2- and TLR4-transfected cell lines were appropriate for detecting PGN and LPS contaminations.
-
•
Mutanolysin treatment of icodextrins enhanced the response of TLR2-transfected cells to contaminating PGN.
-
•
Using TLR-transfected cell lines is a valuable approach for selecting icodextrin batches for peritoneal dialysis solutions.
Abstract
Icodextrin is a starch derivative used for preparing solutions of peritoneal dialysis. Unfortunately, peptidoglycans (PGN) and lipopolysaccharides (LPS) have been reported to contaminate certain icodextrin batches and to contribute to the development of sterile peritonitis. The decision of selecting or rejecting icodextrin batches is however difficult, because of limitations in the detection of these bacterial contaminants. Besides monocyte activation tests of cytokine release, a number of bio-assays using stably TLR-transfected cell lines have been developed. Here, we compared the efficacy of TLR2- and TLR4-transfected cells to detect bacterial contamination with the responses of monocytes exposed to the same icodextrin samples. In contrast to monocyte models of cytokine release, we found that TLR2- and TLR4-transfected cell lines are highly sensitive to detect little PGN and LPS contaminations in the presence of icodextrin. With the intent to increase PGN reactivity, mutanolysin was used to generate soluble fragments in icodextrin samples. We found that such an enzymatic treatment led to an enhanced response of TLR2-transfected cells, even though parental icodextrin samples were poorly reactive. Altogether, these findings indicate that the use of TLR2- and TLR4-transfected cell lines is a valuable approach for helping to the decision of selecting icodextrin batches for peritoneal dialysis.
1. Introduction
During the procedure of peritoneal dialysis, peritoneal cavity of patients is filled with an osmotic fluid, which is discharged after the filtration process [1], [2]. Although glucose has been commonly used as an osmotic agent, its rapid absorption leads however to a loss of pressure gradient after short time and its partial degradation during heat sterilization of dialysis solutions results in the formation of cytotoxic products [3], [4]. To avoid these drawbacks, osmotic agents such as icodextrin are now used as an alternative [5], [6]. Icodextrin is a starch-derived, water-soluble glucose polymer, with an average molecular weight of 15 kDa. Given that it cannot be absorbed as fast as glucose, the osmotic pressure gradient and consequent transfer across the peritoneum remain stable for a longer time [3], [7], [8], [9].
Peritoneal dialysis solutions released for human use have to comply with European and US Pharmacopoeias, which provide standards for drugs, nutritional supplements and health-care products. Over the past years, some standards of concern have been to apply tests for the detection of lipopolysaccharides (LPS), e.g., Limulus Amoebocyte lysate (LAL) assay, as these substances most frequently contaminate manufactured products [10]. However, these standards have a lot of shortcomings, because a number of substances other than endotoxins can potentially cause acute inflammation in humans [11]. For example, peptidoglycans (PGN) have been identified as contaminating inflammatory substances in a series of icodextrin-containing solutions for dialysis. This was associated to an increase in the number of reported cases of aseptic peritonitis, thus highlighting how manufactured products free of LPS contamination could be incorrectly considered safe under current pharmacopoeia tests [12], [13], [14], [15]. In the light of needs for other tests, a number of cellular models, in which the production of inflammatory molecules was used as a read-out of pyrogenic activation, have been developed and validated. The first ones have used peripheral blood mononuclear cells as a source of monocytes to detect endotoxin contamination [16], [17]. Subsequently, other cellular models, using either whole blood cells, primary monocytes or monocytic cell lines, have been established to detect LPS and non-endotoxin contaminations by monitoring the release of cytokines, including interleukin-6 (IL-6), IL-1β or tumor necrosis factor-α (TNF-α) [11], [18], [19], [20], [21], [22], [23].
LPS and other pathogen-associated molecules are recognized by a number of cellular sensors of the innate immunity, which include Toll-like receptors (TLRs). While TLR4 mediates the inflammatory response to LPS, TLR2 is more specifically involved in the detection of PGN, lipoteichoic acid (LTA) or lipoproteins derived from gram positive bacteria [24], [25]. Thus, a number of enzymatic bio-assays have been developed, based on the use of stably TLR-transfected cell lines, e.g. HEK (Human embryonic kidney)-293 cell lines, and designed to provide a sensitive and reliable method for the detection of TLR agonists [26], [27], [28]. Among them, HEK-Blue™/hTLR2 and HEK-Blue™/hTLR4 are commercially available cell lines (marketed by InvivoGen company), which co-express a specific TLR gene and a TLR-inducible reporter gene encoding the secreted embryonic alkaline phosphatase (SEAP). In this system, TLR stimulation can be conveniently monitored by using a phosphatase detection assay.
In the present study, we decided to test the efficacy of TLR-transfected cell lines to detect PGN and LPS in icodextrin batches, because these bacterial components have been identified as potential sources of contamination in peritoneal dialysis solutions. Thus, we compared the responses induced by the abundance of TLR2 and TLR4 agonists in icodextrin samples, as quantified by using TLR transfected-cell based bioassays, with the capacity of the same samples to induce cytokine secretion from monocytes.
2. Materials and methods
2.1. Materials
Icodextrin was produced from hydrolyzed Waxy corn starch and manufactured by Roquette Frères (Lestrem, France). It is a completely water-soluble glucose polymer with a number-average molecular weight (Mn) of less than 8 kDa and a weight-average molecular weight (Mw) between 12 and 20 kDa. It contains less than 3% of glucose and polymers having a degree of polymerization (dp) ≤ 3 and less than 0.5% of glucose polymers having a dp >600. Checking of possible contamination of the icodextrin generating circuit is routinely carried out by analysis of the final product. The contents usually measured are as follows (expressed per gram of glucose polymer): yeasts and molds, 0; aerobic microorganisms, 0; Alicyclobacillus acidocaldarius, <1; LPS, <0.3 EU, as measured by the Endosafe Gel-Clot LAL test (Charles River, Margate, UK); PGN, <2 ng, as measured by the Silkworm Larvae plasma (SLP) test (Wako, Osaka, Japan) [29]. Among icodextrin batches produced in the years 2009–2010, we selected four samples, named I-10.01, I-10.02, I-10.03 and I-11.12, on the basis of their levels of PGN and LPS contaminations; two others samples, named M-10.05 and M-10.07, were obtained from parental raw materials (Table 1). With the exception of I-10.01, other icodextrin samples were not suitable for making up solutions for therapeutic use in humans, because of the presence of LPS and/or PGN contaminations. All samples were prepared in solution in sterile phosphate buffer (PBS: Na phosphate 20 mM, NaCl 150 mM, pH 7.4). PGN, LTA (both from Staphylococcus aureus) and LPS (Escherichia coli 055B5; one nanogram of endotoxin is equivalent to 5 EU in LAL assay) were from Sigma-Aldrich (St Louis, MO, USA). Recombinant human TNF-α was from Peprotech (Rocky Hill, NJ, USA).
Table 1.
LPS and PGN contaminations in icodextrin (I-10.01, I-10.02, I-10.03 and I-11.12) and raw material (M-10.05 and M10.07) samples, as determined by LAL and SLP assays.
| I-10.01 | I-10.02 | I-10.03 | I-11.12 | M-10.05 | M-10.07 | |
|---|---|---|---|---|---|---|
| LPS (EU/g) | <0.3 | 0.3 | <0.3 | 0.6 | 9.6 | 38.4 |
| PGN (ng/g) | <2 | 253 | 11 | 393 | 501 | 645 |
2.2. Cellular models
Human citrated venous blood samples were obtained from the local blood transfusion center (Lille, France). Experiments were undertaken with the understanding and written consent of each subject (NT/18/2015/092) and methodologies were approved by the local ethic committee. Primary monocytes were collected by density centrifugation on Lymphoprep medium (Eurobio-Abcys, Courtaboeuf, France) and purified with magnetic beads coupled to CD14 (BD Biosciences, New Jersey, USA). Monocyte purity was >95% when assessed by flow cytometry. They were cultured at 0.8 × 106 per mL in RPMI 1640 medium supplemented with 10% heated fetal calf serum (FCS) and 2 mM L-glutamine (Lonza, Verviers, Belgium), in a 5% CO2 enriched atmosphere. Human leukemia THP1 cell line was purchased from the ECACC (no 88081201, Porton Down, Salisbury, UK). Cells were routinely cultured at 37 °C in complete RPMI 1640 medium completed with 20 μM β-mercaptoethanol. Before use, they were suspended at 0.6 × 106 per mL in RPMI medium without β-mercaptoethanol and differentiated for 72 h with phorbol 12-myristate 13-acetate to enhance cytokine production via TLR agonists. HEK-Blue™ cell lines were obtained from InvivoGen (San Diego, CA, USA). They are stably transfected with a reported gene encoding SEAP, for which transcriptional activation is under the control of a TLR-inducible gene promoter. In contrast to immune cells, parental HEK-293 cells do not express TLRs on plasma membrane. In order to obtain cells responsive to TLR agonists, HEK-Blue™ cell lines have been co-transfected with an expression plasmid encoding one TLR. In the current study, we used HEK-Blue™/hTLR2 and HEK-Blue™/hTLR4 (termed here HEK-Blue-2 and HEK-Blue-4) for the detection of TLR2 and TLR4 agonists, respectively, and HEK-Blue™/Null1 (HEK-Null) as a negative control. Thus, the way by which icodextrin samples induced the production of SEAP in each cell line has been informative on the contamination by LPS, PGN or both. According to the instructions of the manufacturer, HEK-Blue cells were routinely cultured in DMEM medium (Lonza) supplemented with 10% FCS, 2 mM L-glutamine, 0.5% penicillin/streptomycin. In addition, each cell line was cultured with a specific antibiotic mixture: 0.4% HEK-Blue Selection™ plus 0.2% Normocin™ for HEK-Blue-2 and HEK-Blue-4 cells; 0.2% Normocin™ plus 0.1% Zeocin™ for HEK-Null cells (all from InvivoGen).
2.3. Cell stimulation
Primary monocytes and THP1 cells were seeded into 96-well culture plates (180 μL per well) and stimulated by the addition of 20 μL of the solutions of glucose polymer samples. The final cellular concentrations were 3 × 106 cells/mL and 0.75 × 106 cells/ml for primary monocytes and THP1 cells, respectively. After 16 h of incubation at 37 °C, the plates were centrifuged (5 min, 900g) and 100 μL of the supernatants were collected for analysis of cytokine secretion. Production of IL-6, TNF-α and CCL5/RANTES was quantified by ELISA (Eurobio-Abcys). According to the recommendations of the manufacturer, HEK-Blue-2 and HEK-Null cells were seeded into 96-well culture plates (180 μL per well in DMEM/10% heat inactivated FCS containing medium without antibiotics). Thereafter, 20 μL of the solutions of glucose polymers were added in each well, to obtain a final cellular concentration of 0.25 × 106 cells/mL. After 16 h of incubation at 37 °C, 50 μL of the supernatants were collected and mixed with 150 μL of Quanti-Blue™ solution, which contains a chromogenic substrate of SEAP (InvivoGen). After 1 h-incubation at 37 °C, the absorbance was measured at 620 nm. In order to improve their responsiveness, HEK-Blue-4 cells were seeded at 10,000 per well and cultured for three days. After wash, 180 μL of fresh culture medium was added in each well, and cells were stimulated by the addition of 20 μL of the solutions of glucose polymers. The production of SEAP was assayed after 16 h of incubation by mixing 50 μL of culture supernatants and 150 μL of Quanti-Blue™, as described.
2.4. Treatment with mutanolysin
Mutanolysin is a muralytic enzyme that cleaves the N-acetylmuramyl-N-acetylglucosamine linkage within PGN structure. Prior analysis of its potential to enhance the inflammatory responses triggered by the glucose polymer samples, we checked that the commercial enzyme from Streptomyces globisporus (ATCC 21553, Sigma) was efficient to depolymerize PGN. To this end, standard PGN from Staphylococcus aureus (1 μg/mL in sterile PBS) was either untreated or treated with high concentration of mutanolysin (25,000 U/mL) for 24 h at 37 °C. Thereafter, samples were tested for their ability to stimulate HEK-Blue-2 cells. As expected, we found that enzymatically-treated sample was no more reactive, which confirmed that mutanolysin has efficiently cleaved the PGN polymer. In order to define the optimal conditions for partial depolymerization, standard PGN (1 μg/mL) was solubilized in sterile solution in the absence or presence of I-10.01 icodextrin. Samples were then treated in the presence of various concentrations of mutanolysin for incubation times varying from 4 to 24 h, after which HEK-Blue-2 cells were stimulated by the addition of 20 μL of each sample to 180 μL of cell supernatant. We retained a treatment with mutanolysin at 2500 U/mL for 16 h, as it was the most efficient to enhance the ability of the samples to stimulate HEK-Blue-2 cells.
2.5. Statistical analysis
Results are representative of at least three independent experiments conducted with primary monocytes isolated from different donors or with cultured cells obtained from three distinct cell preparations. Statistical significance between the different values was analysed by Student’s t-test, with a threshold of P < 0.05 considered as significant.
3. Results
3.1. Detection of bacterial contaminants with cellular models of cytokine release
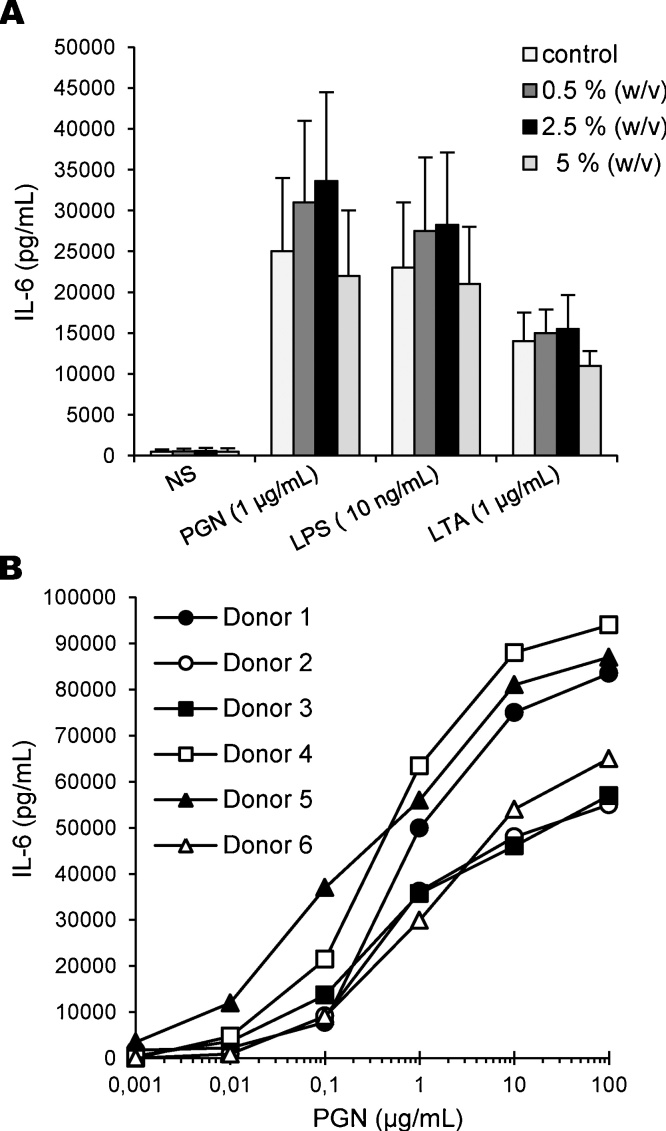
Prior analysis of the responses triggered by bacterial contaminants in monocytes, we optimized the conditions of cell culture and stimulation. To this end, I-10.01 icodextrin was solubilized in sterile PBS solution and then artificially loaded with standard inflammatory molecules, i.e. PGN, LPS and LTA. Thereafter, primary monocytes (0.6 × 106 cells per well) were stimulated by the addition of 20 μL of each solution to 180 μL of cell suspension, in order to obtain glucose polymer concentrations of 0.5, 2.5 and 5% (w/v) in culture. The final concentrations of PGN, LPS and LTA were of 1 μg/mL, 10 ng/mL and 1 μg/mL respectively, which are known from the literature to trigger a full activation of monocytes. As shown in Fig. 1A, incubation of primary monocytes with I-10.01 icodextrin alone did not induce any inflammatory response. The background production of IL-6 from non-stimulated monocytes was indeed identical in the absence or presence of the glucose polymer. In line with the findings in the literature, all the three pro-inflammatory molecules induced a high production of IL-6 from monocytes. Moreover, the presence of the glucose polymer at concentrations less or equal to 25 mg/mL did not hamper the cellular responses. Nevertheless, we also observed a large variation in the amplitude of the responses between each preparation of monocytes, which may reflect the inter-individual variability in the responses to TLR agonists [30], [31]. To know whether this variability could confound detection of bacterial contaminants in icodextrin samples, we compared individual responses of primary monocytes from six distinct donors exposed to increasing concentrations of PGN. As shown in Fig. 1B, large inter-individual differences in the dose-response curves were observed. While monocytes from donor 5 were able to release a significant amount of IL-6 in response to less than 10 ng/mL of PGN, a 10-fold higher concentration was required to trigger a similar response in monocytes from other donors. In addition, PGN concentrations giving a response halfway between baseline and maximal response (EC50) varied from 0.5 μg/mL to >10 μg/mL, which clearly illustrates the high and low responder phenotypes of monocytes. To overcome this inter-individual variability, we decided to test the ability of THP1 cells to release cytokines when cultured in the presence of icodextrin samples. To this end, THP1 cells were exposed to PGN, LPS and LTA in sterile PBS solution containing I-10.01 icodextrin for 16 h, after which the production of cytokines, including IL-6 and RANTES/CCL5, was measured by ELISA. As already described by others, we found that the production of IL-6 from THP1 cells was very low. Then, we retained the chemokine RANTES, because it was produced in higher amount when compared to other cytokines. As expected, incubation of THP1 cells with I-10.01 icodextrin did not induce any inflammatory response. Moreover, the presence of icodextrin at 25 mg/mL did not hamper the response of THP1 cells exposed to PGN, LPS or LTA. We then determined the concentration-dependency of the response of THP1 cells exposed to PGN. A release of significant amount of RANTES was measured at 50 ng/mL of PGN, which corresponds to a threshold of detection of 2 μg of PGN per gram of glucose polymer (2.5% w/v). Moreover, EC50 was estimated at ∼1 μg/mL (n = 3 separate experiments), which was in the middle of the values obtained with primary monocytes.
Fig. 1.
Production of IL-6 by primary monocytes in the presence of icodextrin. A: Stimulation of monocytes by optimal doses of PGN, LPS or LTA in the absence or presence of I-10.01 icodextrin at finale concentrations of 0.5, 2.5 and 5% (w/v). After 16 h of incubation, the secretion of IL-6 was measured in cell-free supernatants by ELISA. Data of IL-6 production are expressed as means values ± SEM from three experiments conducted with monocytes from distinct donors. B: Inter-individual variability in PGN-induced IL-6 production from monocytes. Human primary monocytes from six distinct blood donors were stimulated with increasing concentrations of PGN in the presence of the I-10.01 icodextrin at the finale concentration of 2.5% (w/v). After 16 h of stimulation, IL-6 production was quantified in cell-free supernatants by ELISA. Data are means from triplicates for each donor.
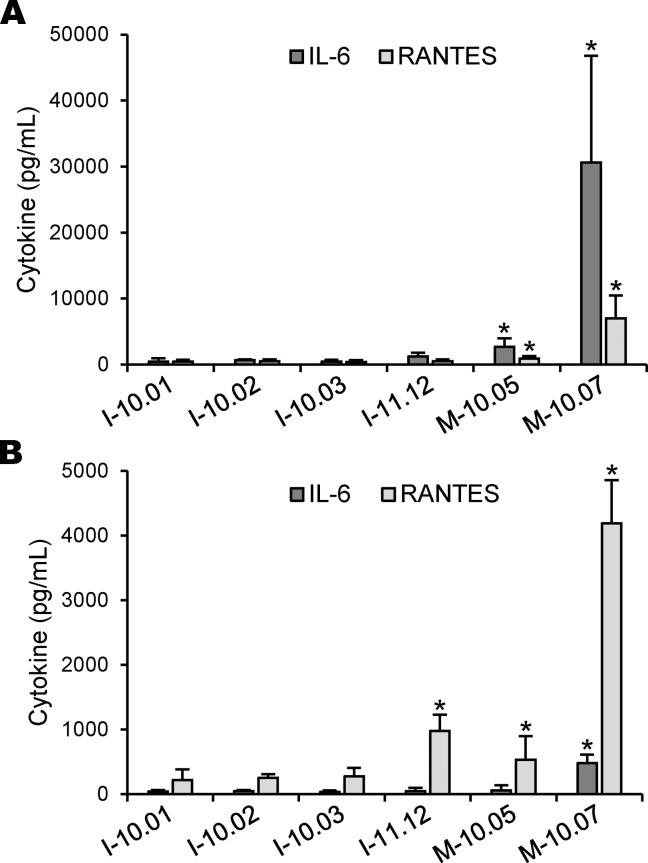
We then tested the efficacy of both cellular models to detect bacterial contamination in a series of icodextrin and raw material samples (Table 1). As described by others [11], [18], [19], [20], [21], [22], [23], we found that the production of IL-6 from primary monocytes was remarkably greater than that of RANTES, which explains that it has been currently used as a read-out of monocyte activation. Among the samples, only M-10.05 and M.10-07 induced a release of significant amount of IL-6 and RANTES from primary monocytes (Fig. 2A). Actually, both samples were raw materials and contained the highest levels of PGN and LPS contaminations, as measured by SLP and LAL assays. In contrast, icodextrin samples contained low amounts of LPS (<1 EU/g), but two of them, namely I-10.02 and I-11-12, were also contaminated with considerable amounts of PGN. Nevertheless, they did not induce any significant release of IL-6 from primary monocytes, which indicates that this cellular model was not reactive enough for the detection of small amount of LPS and/or PGN. We have then used THP1 cells for measuring the presence of bacterial contaminants in our samples (Fig. 2B). In line with the findings in the literature, we found that the production of IL-6 was very low, while RANTES was produced in higher amounts. When compared to primary monocytes, we found that THP1 cells were similarly responsive to M-10.05 and M-10.07 samples. In addition, we found a significant production of RANTES induced by I-11.12 sample. Unfortunately, other icodextrin samples did not induce any release of significant amount of the cytokine, which leads us to conclude that bio-assays based on cellular models of cytokine release are not suitable for the detection of little bacterial contamination in icodextrin batches.
Fig. 2.
Induction of cytokine production by icodextrins and raw materials. Human primary monocytes (A) and THP1 cells (B) were stimulated with icodextrins (I-10.01, I-10.02, I-10.03 and I-11.12) and raw materials (M-10.05 and M-10.07), all samples at the finale concentration of 2.5% (w/v). After 16 h of incubation, production of IL-6 and RANTES was measured in cell-free supernatants by ELISA. Data are expressed as means values ± SEM from three separate experiments conducted with primary monocytes isolated from different donors or with THP1 cells obtained from three distinct cell preparations (*P < 0.05, significantly different by comparison with non-stimulated cells).
3.2. Detection of bacterial contaminants with enzymatic bio-assays
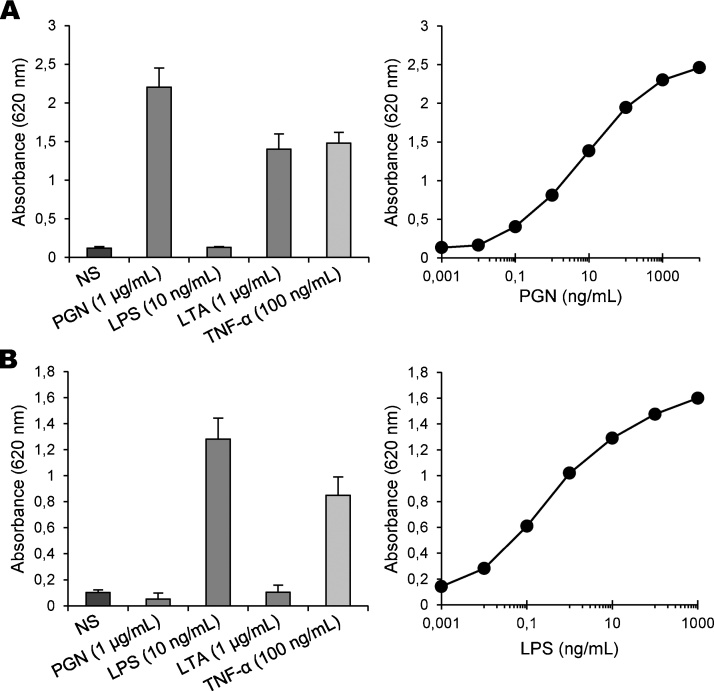
In next experiments, we addressed the possibility that enzymatic bio-assays might be more convenient and sensitive than the models of cytokine release. Thus, we tested the ability of HEK-Blue-2 and HEK-Blue-4 cells to detect bacterial contaminants in our glucose polymer samples. As a prerequisite, we checked that icodextrin alone did not induce any activation of the cells. To this end, cells were exposed for 16 h to standard TLR agonists solubilized in a solution containing the I-10.01 icodextrin at finale concentrations varying from 2.5 to 5% (w/v), after which time the activity of SEAP was measured. Because HEK cell lines express the cognate receptor of TNF-α, the inflammatory cytokine was used as a positive control in our experiments. We found that concentrations up to a value equal to 37.5 mg/mL (3.75% w/v) did not significantly interfere with the production of SEAP from cells exposed to PGN, LPS or LTA. As expected, HEK-Blue-2 cells were responsive to both PGN and LTA. LPS, which is a TLR4 agonist, did not induce any release of SEAP from these cells when compared to non-stimulated cells. Concentration-dependency analysis revealed that a significant amount of SEAP activity was measured in cell supernatant from 0.1 ng/mL of PGN (2.5 ng of PGN/g of glucose polymer), and the EC50 was estimated at 5 ng/mL (Fig. 3A), which makes this bio-assay almost 100-fold more sensitive that the cellular models of cytokine release. As shown in Fig. 3B, HEK-Blue-4 cells were strongly responsive to LPS, while both TLR2 agonists did not trigger any significant production of SEAP, as expected. A significant response of HEK-Blue-4 cells was measured from 0.003 ng/mL of LPS (0.08 ng/g of glucose polymer), thus confirming the high sensitivity of this cellular model. Altogether, these observations indicate that enzymatic bio-assays are promising for the detection of small amounts of PGN and LPS in icodextrin batches.
Fig. 3.
Stimulation of TLR-expressing HEK-Blue cells by pro-inflammatory stimuli. HEK-Blue-2 (A) and HEK-Blue-4 (B) cells were either non-stimulated or stimulated by the addition of optimal doses of PGN, LPS, LTA and TNF-α (left panels) in the presence of the I-10.01 icodextrin at the finale concentration of 3.75% (w/v). After 16 h of incubation, the production of SEAP related to reporter gene activation was quantified by measuring the phosphatase activity released in cell-free supernatants with a chromogenic substrate. Data of SEAP activity are expressed as means values ± SEM of absorbance at 620 nm from three separate experiments. Typical dose-response curves of the activity of HEK-Blue-2 cells relative to PGN and of the activity of HEK-Blue-4 cells relative to LPS are also shown (right panels).
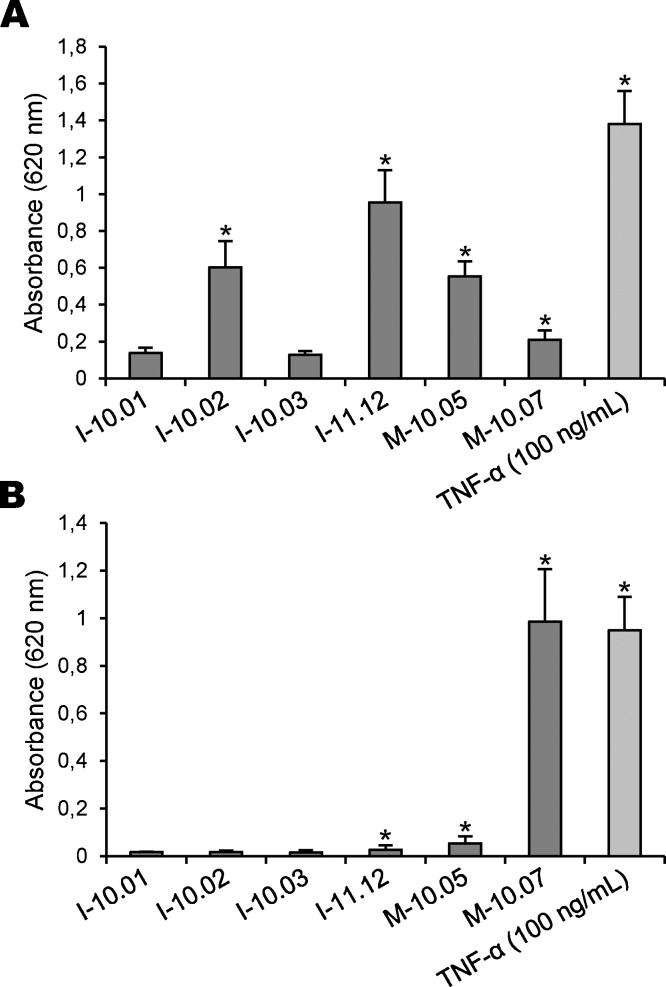
We then analysed the efficacy of HEK-Blue cells to detect bacterial contamination in our contaminated samples of icodextrin and raw material. A significant phosphatase activity was measured in the supernatants of HEK-Blue-2 cells exposed to 1-10.02, I-11.12, M-10.05 and M-10.07 samples (Fig. 4A). However, we did not found a correlation between the amplitude of the cellular responses and the values of PGN contamination measured with SLP assay (Table 1). Although PGN contamination in M-10.07 sample was indeed estimated at 645 ng/g, this raw material triggered the release of a small amount of SEAP in cell supernatant. Conversely, the highest phosphatase activity was measured with I-11.12 icodextrin, while its PGN content was estimated at 393 ng/g by SLP assay. Thus, this discrepancy suggests that the detection of PGN contamination could be different in both assays. In contrast, the levels of phosphatase activity measured in the supernatants of HEK-Blue-4 cells exposed to icodextrin and raw material samples are consistent with the values of LPS determined by LAL assay (Table 1). Indeed, the reactivity of HEK-Blue-4 cells towards glucose polymer samples was as follows: M-10.07>> M-10.05> I-11.12 (Fig. 4B), which led us to conclude that both models would be equally appropriate for the detection of little amounts of LPS.
Fig. 4.
Stimulation of TLR-expressing HEK-Blue cells by icodextrins and raw materials. HEK-Blue-2 (A) and HEK-Blue-4 (B) cells were stimulated by the addition of icodextrins (I-10.01, I-10.02, I-10.03 and I-11.12) and raw materials (M-10.05 and M-10.07), all samples at the finale concentration of 3.75% (w/v). After 16 h of incubation, production of SEAP was quantified by measuring the phosphatase activity released in cell-free supernatants. Data are expressed as means values ± SEM from three separate experiments (*P < 0.05, significantly different by comparison with non-stimulated cells cultured in the absence of glucose polymer sample).
3.3. Enhancement of PGN detection by mutanolysin treatment of icodextrin samples
It is well-known from the literature that the size of PGN fragments determines the relative severity of inflammatory responses in the host. While the largest fragments elicit a negligible acute inflammation, smaller fragments induce severe acute inflammation [32], [33], [34]. In accordance with these findings, PGN obtained by sonic degradation of streptococcal cell wall (MW >5 × 106 Da) failed to elicit an acute inflammatory response. Conversely, treatment of these PGN fragments by bacterial cell wall-lytic enzymes such as mutanolysin resulted in the production of soluble, low-molecular-weight PGN (MW∼30,000 Da), which exhibited a strong pro-inflammatory activity [35]. Thus, these observations prompted us to utilize mutanolysin to generate a greater amount of soluble PGN fragments in glucose polymer samples and therefore increase their reactivity in enzymatic bio-assays. Optimal conditions of mutanolysin treatment were first determined with standard PGN solubilized in a sterile solution of I-10.01 icodextrin. We found that treatment with mutanolysin at 2500 U/mL for 16 h increased by at least 20% the response of HEK-Blue-2 cells, when compared to the release of SEAP from cells exposed to intact PGN.
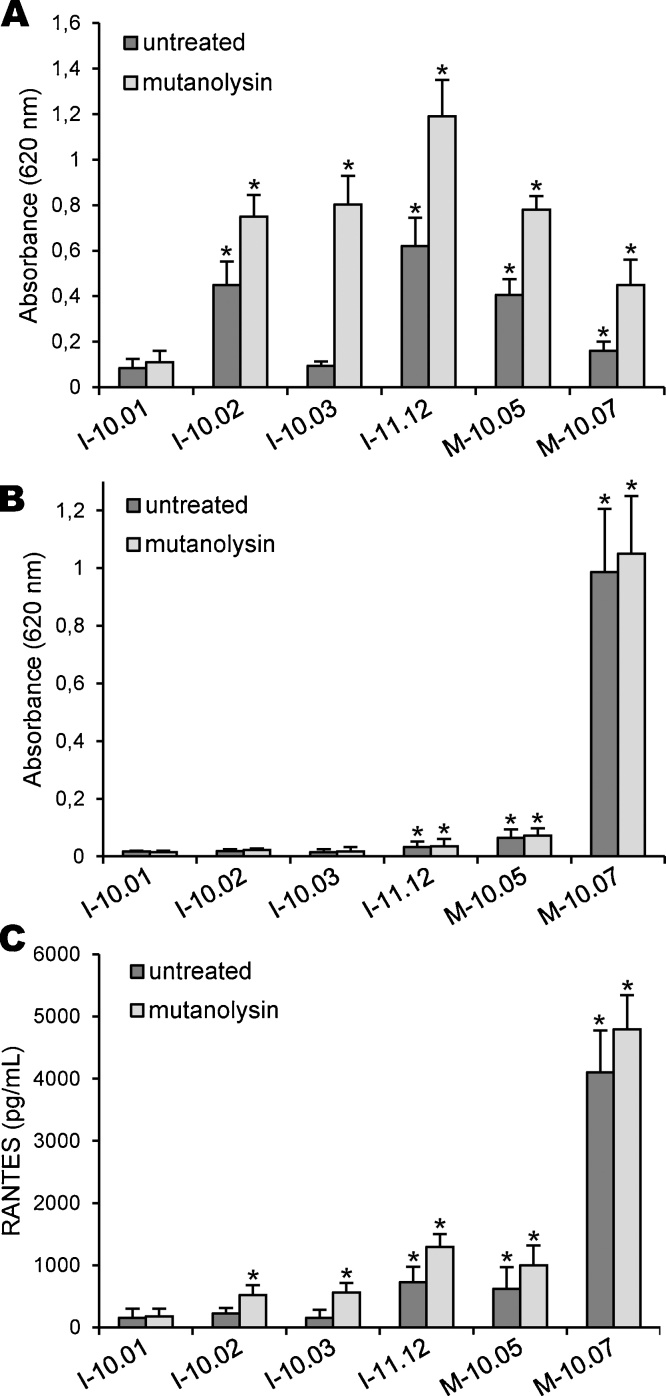
The same treatment was then applied to our samples of icodextrin and raw material. As expected, we found that mutanolysin treatment increased the responses of HEK-Blue-2 cells exposed to I-10.02, I-11.12, M-10.05 and M-10.07 samples (Fig. 5A). Indeed, the levels of phosphatase activity were approximately increased by a factor of 2 in cell supernatants, when compared to the responses induced by untreated parental samples. These results confirmed that mutanolysin has partially depolymerized large PGN fragments, which in turn led to the liberation of more soluble and active fragments. Most interestingly, we found that the enzymatic treatment led to a strong response of HEK-Blue-2 cells exposed to I-10.03 sample, which was close to that obtained with I-10.02 sample. This was unexpected since the parental untreated I-10.03 sample was unable to elicit the release of a significant amount of SEAP from TLR2-expressing cells. Moreover, PGN contamination I-10.03 sample had been estimated at only 11 ng/g by SLP assay versus 253 ng/g in I-10.02 sample. Thus, our findings suggest that I-10.03 icodextrin probably contains masked PGN in insoluble aggregates, which could not be reactive without mutanolysin treatment. These results are of great interest to detect icodextrin batches that might be considered free of contamination without liberation of soluble and active PGN fragments. As shown in Fig. 5B, treatment with mutanolysin did not modify the release of SEAP in the supernatant of HEK-Blue-4 cells exposed to icodextrin and raw material samples. These results demonstrated that the enzymatic treatment had not altered the reactivity of LPS in our assays, and further confirmed that mutanolysin was free of LPS contamination. We have then analysed the responses triggered by enzymatically-treated samples in THP1 cells (Fig. 5C). We observed a moderate increase in the production of RANTES from THP1 cells exposed to I-11.12, M-10.05 and M-10.07 samples, when compared to the responses induced by untreated parental samples. Moreover, treatment with mutanolysin induced the release of a significant amount of RANTES in the supernatant of THP1 cells exposed to I-10.02 and I-10.03 samples. These results further indicate that mutanolysin has efficiently promoted the liberation of soluble and active PGN fragments in these samples. Nevertheless, the responses of THP1 cells remained low by comparison with HEK-Blue cells. Therefore, these last results reinforce the idea that cell-based assays with TLR-expressing cells are valuable methods for the detection of PGN and LPS contaminations in icodextrin batches.
Fig. 5.
Effect of mutanolysin treatment on the inflammatory activity of PGN. Icodextrins and raw materials (37.5%, w/v in sterile PBS) were pre-treated in the absence or presence of mutanolysin (2500 U/mL) for 16 h at 37 °C. Samples were then added to HEK-Blue-2 cells (A), HEK-Blue-4 cells (B) or THP1 cells (C) (dilution 1/10). After 16 h of stimulation, the production of SEAP from HEK-Blue-2 and HEK-Blue-4 cells was quantified by measuring the phosphatase activity released in the supernatants. The production of RANTES from THP1 cells was measured in cell-free supernatants by ELISA. Data are expressed as means values ± SEM from three separate experiments (*P < 0.05, significantly different by comparison with non-stimulated cells cultured in the absence of glucose polymer sample).
4. Discussion
Peritonitis is a major complication of peritoneal dialysis, which can be usually diagnosed by positive bacterial cultures. However, a number of well-documented cases of sterile peritonitis have also been attributed to contamination by bacterial products [12], [13], [14], [15]. In addition to LPS, which is the more concerning contaminant that causes adverse effects in patients [36], [37], PGN were reported to contaminate some icodextrin batches and to contribute to the development of sterile peritonitis [14], [37]. Thus, the bacteria Alicyclobacillus acidocaldarius requires acid medium and high temperature for optimal growth, which are the conventional conditions for hydrolysis of starch to produce icodextrin. Consequently, PGN may remain at the end of the processing line, even though heat and sterile filtration applied to the final product has eliminated bacteria. Although less potent than LPS, PGN can induce cytokine production in a wide variety of immune cells [25]. Unfortunately, PGN detection in icodextrin batches is difficult, because of its relatively low concentration by comparison with the icodextrin itself, which behaved as an interfering substance. Moreover, routine monitoring for PGN with SLP assay has some shortcomings, because of limitation in the detection [38] and cross-reactivity with other substances, such like β1-3 glucans [29]. In the light of needs for other tests, we decided to compare the efficacy of different bio-assays to detect bacterial contamination in glucose polymer samples.
In first experiments, we analysed the responses of primary monocytes by measuring the production of IL-6 as a read-out of cell activation. Although exposure of monocytes to commercial PGN induced IL-6 production, we observed a large inter-individual variation in the amplitude of the responses. In addition, only raw materials induced a release of a significant amount of IL-6, which may be due to the presence of LPS in these samples. In contrast, icodextrin samples did not induce any significant activation of primary monocytes, even though two of them were contaminated with considerable amounts of PGN. Thus, the large inter-individual variation in the responses of monocytes and the lack of sensitivity for PGN could hamper the detection of little bacterial contaminations and consequently interfere with the decision of selecting or rejecting icodextrin batches. To overcome these drawbacks, we decided to test the ability of THP1 cells to release RANTES when exposed to glucose polymer samples. In addition to the raw materials, only one icodextrin induced however a significant production of RANTES, while the others did not trigger any response. Thus, these results led us to the conclusion that monocyte activation tests of cytokine release are poorly efficient for the detection of little PGN contamination in icodextrin batches.
We then addressed the possibility that enzymatic bio-assays might be more sensitive than cellular models of cytokine release. In our hands, stimulation of HEK-Blue-2 cells led to a significant response from 2.5 ng of PGN per g of icodextrin, indicating that this bio-assay is highly sensitive to this bacterial product. To a similar extent, HEK-Blue-4 cells were strongly responsive to LPS, with an estimated threshold of 0.08 ng per g of glucose polymer, which corresponds to 0.4 EU/g (LPS from E.coli 055B5: 5 EU/ng). Together, these results indicate that enzymatic bio-assays with TLR2- and TLR4-expressing HEK cells are as sensitive as SLP and LAL assays for the detection of small amounts of PGN and LPS in glucose polymer samples (PGN: <2 ng/g, as measured by SLP assay; LPS: <0.3 EU/g, as measured by LAL assay). We then analysed the efficacy of HEK-Blue cells to detect bacterial contaminations in icodextrin and raw material. We found that the responses of HEK-Blue-4 cells exposed to contaminated samples were consistent with the values of LPS contamination determined by LAL assay, confirming that this bio-assay may be as suitable as LAL assay for the detection of endotoxin. A significant release of SEAP was also measured in the supernatants of HEK-Blue-2 cells exposed to samples containing PGN contaminations. Nevertheless, no correlation was found between the amplitude of the cellular responses and the values of SLP assay. This discrepancy could however be due to differences in PGN reactivity. The SLP reagent was indeed reported to react with free PGN, but also with trace of living or inactivated bacteria [29], [38]. In contrast, the size of PGN fragments was shown to determine the responses triggered by inflammatory cells in human and animal models. While the largest fragments elicit a negligible response, smaller fragments induce a strong acute inflammation. Thus, low-molecular-weight PGN are required to induce a pro-inflammatory response via the activation of TLR2, while larger PGN fragments and bacterial cell wall debris are poorly active [32], [33], [34]. Consistent with these findings, the discrepancy between the reactivity of SLP reagent and TLR2-expressing cells is likely to be due to differences in their dependence on the molecular size of PGN fragments. In contrast to icodextrin, raw material is not clarified and contains died bacteria and cell wall debris. These contaminants were probably capable of eliciting a response in SLP assay, but they were not soluble enough to induce the activation of TLR2-expressing cells. Moreover, it may be speculated that M-10.07 contained large amounts of insoluble PGN. On the contrary, M-10.05 is probably enriched in smaller fragments, explaining the higher response observed in TLR2-expressing cells. Most of high-molecular-weight contaminants can be removed from raw material by ultrafiltration during the process of icodextrin purification. Nevertheless, soluble PGN fragments are in the same range of molecular size as those of icodextrin, which may explain why they could still be present in the icodextrin batches. Thus, I-11.12 probably contained a greater amount of soluble PGN, explaining why it has induced the highest response in HEK-TLR2 cells, while being less reactive in SLP assay.
Finally, we decided to use mutanolysin to generate more reactive PGN fragments [32], [34], [35]. In line with the expected increase, we found that enzymatic treatment with mutanolysin is a valuable method for improving the detection of PGN in our icodextrin samples. Moreover, such a treatment may be of great importance to liberate active PGN fragments in icodextrin batches that could have been considered free of contamination on the basis of SLP assay. Indeed, we found that mutanolysin treatment of I-10.03 sample resulted in a strong activation of HEK-Blue-2 cells, while the parental untreated sample was poorly reactive in SLP assay. Thus, these last results are clearly in favour of using an approach based on mutanolysin treatment of samples followed by the detection of active PGN with TLR2-expressing cells for helping to the decision of selecting or rejecting icodextrin batches for therapeutic use.
In conclusion, we have currently evaluated in vitro bio-assays for the detection of LPS and PGN contaminants in glucose polymers used as osmotic agent in peritoneal dialysis solutions. Although only a few aspects of the immune response are modelled, i.e activation of TLR receptors, this approach has several positive features. These include the sensitivity of the responses and the fact that it is based on stable cell lines, which are easily available and do not need complicated procedures of isolation and maintenance. It might be also helpful for other applications, as it would allow in vitro detection of bacterial contaminants in a number of other manufactured drugs and chemicals released for human health and therapies.
Conflicts of interest
The authors have no conflict of interest to declare. AH, PD and PL are employed by ROQUETTE FRERES which produces notably the icodextrin. HHG, AD, MC, PL and FA are designed inventors of the two patent applications WO 2012/143647 and WO 2014/154651.
Acknowledgements
This work was supported by the University of Lille, the CNRS, and ROQUETTE FRERES.
References
- 1.Jain A.K., Blake P., Cordy P., Garg A.X. Global trends in rates of peritoneal dialysis. J. Am. Soc. Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxena A.B. Recent advances in the management of peritoneal dialysis patients. F1000 Prime Rep. 2015;7:57. doi: 10.12703/P7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frampton J.E., Plosker G.L. Icodextrin: a review of its use in peritoneal dialysis. Drugs. 2003;63:2079–2105. doi: 10.2165/00003495-200363190-00011. [DOI] [PubMed] [Google Scholar]
- 4.Linden T., Cohen A., Deppisch R., Kjellstrand P., Wieslander A. 3,4-dideoxyglucosone-3-ene (3,4-DGE): a cytotoxic glucose degradation product in fluids for peritoneal dialysis. Kidney Int. 2002;62:697–703. doi: 10.1046/j.1523-1755.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra D., Nash K. Icodextrin: an alternative peritoneal dialysis fluid. Expert. Opin. Drug Metab. Toxicol. 2008;4:1455–1464. doi: 10.1517/17425255.4.11.1455. [DOI] [PubMed] [Google Scholar]
- 6.Johnson D.W., Agar J., Collins J., Disney A., Harris D.C., Ibels L., Irish A., Saltissi D., Suranyi M. Recommendations for the use of icodextrin in peritoneal dialysis patients. Nephrology. 2003;8:1–7. doi: 10.1046/j.1440-1797.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 7.Krediet R.T., Ho-Dac-Pannekeet M.M., Imholz A.L., Struijk D.G. Icodextrin’s effects on peritoneal transport. Perit. Dial. Int. 1997;17:35–41. [PubMed] [Google Scholar]
- 8.García-López E., Lindholm B. Icodextrin metabolites in peritoneal dialysis. Perit. Dial. Int. 2009;29:370–376. [PubMed] [Google Scholar]
- 9.Mistry C., Mallick N., Gokal R. Ultrafiltration with an isosmotic solution during long peritoneal dialysis exchanges. Lancet. 1987;330:178–182. doi: 10.1016/s0140-6736(87)90764-1. [DOI] [PubMed] [Google Scholar]
- 10.Mascoli C.C., Weary M.E. Limulus amebocyte lysate (LAL) test for detecting pyrogens in parenteral injectable products and medical devices: advantages to manufacturers and regulatory officials. J. Parenter. Drug Assoc. 1979;33:81–95. [PubMed] [Google Scholar]
- 11.Hartung T. The human whole blood pyrogen test – lessons learned in twenty years. ALTEX. 2015;32:79–100. doi: 10.14573/altex.1503241. [DOI] [PubMed] [Google Scholar]
- 12.Goffin E., Cosyns J.-P., Pirson F., Devuyst O. Icodextrin-associated peritonitis: what conclusions thus far? Nephrol. Dial. Transplant. 2003;18:2482–2485. doi: 10.1093/ndt/gfg368. [DOI] [PubMed] [Google Scholar]
- 13.Goffin E. Aseptic peritonitis and icodextrin. Perit. Dial. Int. 2006;26:314–316. [PubMed] [Google Scholar]
- 14.Martis L., Patel M., Giertych J., Mongoven J., Taminne M., Perrier M.A., Mendoza O., Goud N., Costigan A., Denjoy N., Verger C., Owen W.F., Jr. Aseptic peritonitis due to peptidoglycan contamination of pharmacopoeia standard dialysis solution. Lancet. 2005;365:588–594. doi: 10.1016/S0140-6736(05)17908-2. [DOI] [PubMed] [Google Scholar]
- 15.Toure F., Lavaud S., Mohajer M., Lavaud F., Canivet E., Nguyen P., Chanard J., Rieu P. Icodextrin-induced peritonitis: study of five cases and comparison with bacterial peritonitis. Kidney Int. 2004;65:654–660. doi: 10.1111/j.1523-1755.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello C.A., O’Connor J.V., LoPreste G., Swift L. Human leukocytic pyrogen test for detection of pyrogenic material in growth hormone produced by recombinant Escherichia coli. J. Clin. Microbiol. 1984;20:323–329. doi: 10.1128/jcm.20.3.323-329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duff G.W., Atkins E. The detection of endotoxin by in vitro production of endogenous pyrogen: comparison with limulus amebocyte lysate gelation. J. Immunol. Methods. 1982;52:323–331. doi: 10.1016/0022-1759(82)90004-7. [DOI] [PubMed] [Google Scholar]
- 18.Daneshian M., von Aulock S., Hartung T. Assessment of pyrogenic contaminations with validated human whole-blood assay. Nat. Protoc. 2009;4:1709–1711. doi: 10.1038/nprot.2009.159. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann S., Peterbauer A., Schindler S., Fennrich S., Poole S., Mistry Y., Montag-Lessing T., Spreitzer I., Löschner B., van Aalderen M., Bos R., Gommer M., Nibbeling R., Werner-Felmayer G., Loitzl P., Jungi T., Brcic M., Brügger P., Frey E., Bowe G., Casado J., Coecke S., de Lange J., Mogster B., Naess L.M., Aaberge I.S., Wendel A., Hartung T. International validation of novel pyrogen tests based on human monocytoid cells. J. Immunol. Methods. 2005;298:161–173. doi: 10.1016/j.jim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa Y., Maeda H., Murai T. Evaluation of the in vitro pyrogen test system based on proinflammatory cytokine release from human monocytes: comparison with a human whole blood culture test system and with the rabbit pyrogen test. Clin. Diagn. Lab. Immunol. 2002;9:588–597. doi: 10.1128/CDLI.9.3.588-597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole S., Mistry Y., Ball C., Gaines Das R.E., Opie L.P., Tucker G., Patel M. A rapid one-plate in vitro test for pyrogens. J. Immunol. Methods. 2003;274:209–220. doi: 10.1016/s0022-1759(02)00519-7. [DOI] [PubMed] [Google Scholar]
- 22.Schindler S., von Aulock S., Daneshian M., Hartung T. Development, validation and applications of the monocyte activation test for pyrogens based on human whole blood. ALTEX. 2009;26:265–277. doi: 10.14573/altex.2009.4.265. [DOI] [PubMed] [Google Scholar]
- 23.Wunderlich C., Schumacher S., Kietzmann M. Pyrogen detection methods: comparison of bovine whole blood assay (bWBA) and monocyte activation test (MAT) BMC Pharmacol. Toxicol. 2014;15:50. doi: 10.1186/2050-6511-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii K.J., Koyama S., Nakagawa A., Coban C., Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Moreillon P., Majcherczyk P.A. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 2003;35:632–641. doi: 10.1080/00365540310016259. [DOI] [PubMed] [Google Scholar]
- 26.Burger-Kentischer A., Abele I.S., Finkelmeier D., Wiesmüller K.-H., Rupp S. A new cell-based innate immune receptor assay for the examination of receptor activity ligand specificity, signalling pathways and the detection of pyrogens. J. Immunol. Methods. 2010;358:93–103. doi: 10.1016/j.jim.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Erridge C. The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br. J. Nutrition. 2011;105:15–23. doi: 10.1017/S0007114510003004. [DOI] [PubMed] [Google Scholar]
- 28.Huang L.-Y., DuMontelle J.L., Zolodz M., Deora A., Mozier N.M., Golding B. Use of Toll-like receptor assays to detect and identify microbial contaminants in biological products. J. Clin. Microbiol. 2009;47:3427–3434. doi: 10.1128/JCM.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya M., Asahi N., Suzuoki F., Ashida M., Matsuura S. Detection of peptidoglycan and beta-glucan with silkworm larvae plasma test. FEMS Immunol. Med. Microbiol. 1996;15:129–134. doi: 10.1111/j.1574-695X.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 30.Wurfel M.M., Park W.Y., Radella F., Ruzinski J., Sandstrom A., Strout J., Bumgarner R.E., Martin T.R. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J. Immunol. 2005;175:2570–2578. doi: 10.4049/jimmunol.175.4.2570. [DOI] [PubMed] [Google Scholar]
- 31.Yaqoob P., Newsholme E.A., Calder P.C. Comparison of cytokine production in cultures of whole human blood and purified mononuclear cells. Cytokine. 1999;11:600–605. doi: 10.1006/cyto.1998.0471. [DOI] [PubMed] [Google Scholar]
- 32.Dziarski R., Gupta D. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect. Immun. 2005;73:5212–5216. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox A., Brown R.R., Anderle S.K., Chetty C., Cromartie W.J., Gooder H., Schwab J.H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect. Immun. 1982;35:1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald C., Inohara N., Nuñez G. Peptidoglycan signaling in innate immunity and inflammatory disease. J. Biol. Chem. 2005;280:20177–20180. doi: 10.1074/jbc.R500001200. [DOI] [PubMed] [Google Scholar]
- 35.Chetty C., Klapper D.G., Schwab J.H. Soluble peptidoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation. Infect. Immun. 1982;38:1010–1019. doi: 10.1128/iai.38.3.1010-1019.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karanicolas S., Oreopoulos D.G., Izatt S., Shimizu A., Manning R.F., Sepp H., de Veber G.A., Darby T. Epidemic of aseptic peritonitis caused by endotoxin during chronic peritoneal dialysis. N. Engl. J. Med. 1997;296:1336–1337. doi: 10.1056/NEJM197706092962309. [DOI] [PubMed] [Google Scholar]
- 37.Mangram A.J., Archibald L.K., Hupert M., Tokars J.I., Silver L.C., Brennan P., Arduino M., Peterson S., Parks S., Raymond A., McCullough M., Jones M., Wasserstein A., Kobrin S., Jarvis W.R. Outbreak of sterile peritonitis among continuous cycling peritoneal dialysis patients. Kidney Int. 1998;54:1367–1371. doi: 10.1046/j.1523-1755.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma M., Rice T.A., Percopo C.M., Rosenberg H.F. Silkworm larvae plasma (SLP) assay for detection of bacteria: false positives secondary to inflammation in vivo. J. Microbiol. Methods. 2017;132:9–13. doi: 10.1016/j.mimet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]