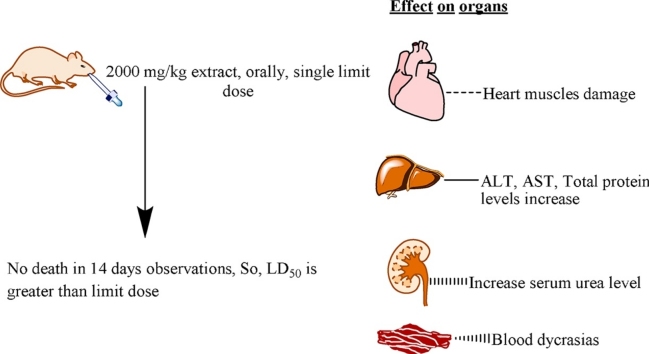
Graphical abstract
Keywords: S. munja roots, LD50, Acute oral toxicity
Highlights
-
•
Saccharum munja Roxb. roots extract was subjected to acute oral toxicity evaluation.
-
•
No animal died at limit dose (2000 mg/kg), this is indicative of LD50 is greater than 2000 mg/kg.
-
•
But extract showed moderate toxicity signs on vital organs.
Abstract
Background
S. munja roots have been used in ethno medicines for the treatment of different ailments. Despite its beneficial uses no studies on its toxicity potential have been reported.
Objective
The study was designed to evaluate acute toxic potential of aqueous ethanolic extract of S. munja roots according to OECD TG No. 425.
Material and methods
Female mice were divided into two groups (n = 5). One group served as control while the other as treated group that received 2000 mg/kg b.w. of S. munja roots ethanolic extract orally. Then both groups were observed for 14 days. Then the blood samples were collected by cardiac puncture, under chloroform general anesthesia and were subjected to hematological and biochemical analyses. The vital organs of anesthetized animals were preserved for histopathological examination.
Results
The the data revealed that LD50 of the extract was greater than 2000 mg/kg b.w. There was no significant alteration found in body weight and organ to body mass index. In comparison with control group, there was significant increase in levels of ALT, AST, total proteins, globulin levels, serum urea, cholesterol, triglycerides, LDL, platelet count, MCV, MCH, WBC count and lymphocytes whereas ALP and MCHC levels were reduced significantly.
Conclusions
From the data obtained in this study, It can be concluded that though LD50 is greater than 2000 mg/kg b.w. but moderate toxicity signs appeared in liver, kidney, lipid profile and CBC also showed blood dyscresias at limit dose.
1. Introduction
Before the development of synthetic or semi-synthetic medicines, folklore use of herbals was very common in rural areas, the use of herbal preparations for the treatment of various ailments is still very common [1]. According to a survey reports in United States (US), herbal medicines were used by almost 12% of the population in 1993 as an alternative and complementary source which is significantly higher than 1990′s report [2], [3]. Since natural herbal remedies are being used on large scale, it is now the major focus of the researchers to conduct studies on efficacy and safety of medicinal plants [4]. The plants having medicinal activity should have low toxicity because of their long-term use in humans. However, various medicinal plants used in folklore medicines have been reported to exhibit toxic effects [5], [6]. Paracelsus, known as father of toxicology, has given a statement which is often quoted: “All substances are poisons; there is none which is not a poison. It is the right dose which differentiates remedy from poison” [7]. A large number of modern medicines are produced from the natural sources. Out of them many preparations rely on the use of agents in traditional medicines [8].
Saccharum munja (S. munja) is the common name of a wild grass found in Pakistan, Afghanistan and India along the river banks and in arid areas [9].The plant is commonly known as sarkanda, Kana or moonja. The flowering and fruiting season is on annual basis which starts from October and ends in January. The buffalos and cattles use the young leaves of the plant as a fodder because of the large tufted grass [9], [10], [11]. The roots of the plant are used to treat dysuria, giddiness and vertigo. Fever and inflammation is also treated with its roots. S. munja grass is used in the form of gauze-pad to stop blood flow [12].
2. Materials and methods
2.1. Collection of plant material
S. munja was collected from suburbs of Southern Punjab- Pakistan. After identification and authentication by a taxonomist Prof. Dr. Zaheer-ud-Din Khan, Botany Department, Government College University, Lahore- Pakistan, a voucher specimen (Ref. No. 2937) containing leaves, flowers and roots was deposited to herbarium. The roots were separated, cleaned and washed with tap water and dried under shade for seven days. The dried roots were ground to fine powder.
2.2. Preparation of crude extract
Roots powder was soaked in 70% ethanol and 30% distilled water for seven days. Extract was filtered through muslin cloth and then passed through whatman’s filter paper for getting clear filtrate. The excess solvent was removed with rotary evaporator at 40 °C.
2.3. Approval from animals ethics committee
The study was performed after getting approval from Animals Ethics Committee of Riphah International University. Ref. No. REC/RIPS/2017/002.
2.4. Acute toxicity assay
In accordance to OECD Test Guidelines 425 (Up and Down Procedure), nulliparous and non-pregnant female albino mice, weighing 28 ± 4 g having age 8–10 weeks were randomly selected. Animals were kept under standard conditions for five days. Limit test was performed at 2000 mg/kg p.o. as single dose and mice were kept without food for 3–4 h prior to dosing but had access to water ad libitum. The dose was administered to a single female mice according to body weight. The animals were closely observed for first 30 min, then for 4 h. Food was provided after 1–2 h of dosing. After survival of treated mouse, 4 additional mice were administered with the same dose under same conditions. The same procedure was followed for vehicle treated control group of 5 mice to whom 1% Carboxymethyl cellulose (CMC) gel was administered in same volume as that of treated group. Both the groups were observed closely for any toxic effect within first 6 h and then at regular intervals for a total period of 14 days. Surviving mice were observed to determine the toxic reactions onset. Weights of animals were monitored and documented as well. At the end of study, animals were weighed and blood samples were collected by cardiac puncture under anesthesia with isoflurane and serum was separated for biochemical and hematological evaluations. Vital organs were excised after killing mice by cervical dislocation; weight of organs was noted and preserved in 10% formalin for histopathological evaluation.
2.5. Biochemical analysis
Urea, creatinine, cholesterol, triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL), bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate, total protein, albumin, globulins were measured by using Randox kits.
2.6. Hematological analysis
The blood samples from animals (both treated and vehicle control groups) were collected in EDTA containing tubes for hematological study. CBC parameters, hemoglobin (Hb), total RBC, packed cell volume (PVC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, white blood cells (WBC) count, neutrophils (N), lymphocytes (L), monocytes (M), and eosinophils (E) were determined with humalyzer.
2.7. Histopathological study
The vital organs isolated from sacrificed mice were fixed in 10% formalin, then after processing embedded in paraffin wax. Paraffin sections were made at 5 mm and stained with hematoxylin and eosin. The slides were studied under a light microscope and captured the magnified images of tissues structure for further study.
2.8. Statistical analysis
Experimental results were presented as mean ± SEM and the statistical significance between the groups was analyzed by means of one way ANOVA followed by Tukey’s multiple comparison test. P ≤ 0.05 was considered as statistically significant.
3. Results
When limit test was conducted with dose of 2000 mg/kg b.w. of S. munja roots extract by using 1% CMC gel as a vehicle, no mortality was observed. Test animal was observed with special attention for first 30 min and then for 4 h. Observations were recorded at regular time intervals throughout the study period i.e. 14 days. All results are as follows:
3.1. Behavioral pattern and body weight
The body weights of test animals of both control and S. munja roots extract treated groups were increased progressively throughout the study period as showed in Table 1. Behavioral observation of the test animals after dosing showed elevated respiration rate for first 30 min in the extract treated group and also an increase in somatomotor activity was observed for first 2 h in this group. Drowsing and sleepy effects were noted in both groups at intervals for first 4 h. Convulsions and tremors were observed in the extract treated group frequently in first 4 h and then at intervals for 48 h, itching and shivering were also noticed. Sometimes itching in both groups was observed in first week of this study. Behavioral observations are summarized in Table 2.
Table 1.
Effects of the extract on body weight of mice in acute toxicity study.
| Groups | 1st Day | 7th Day | 14th Day |
|---|---|---|---|
| Body Weight (gm) | Body Weight (gm) | Body Weight (gm) | |
| Vehicle control | 26.11 ± 0.495 | 27.68 ± 0.590 | 28.76 ± 0.691 |
| 2000 mg/kg SMRE | 25.94 ± 0.624 | 27.57 ± 0.575 | 29.32 ± 0.690 |
SMRE: S. munja roots extract; Values are presented as mean ± SEM; N = 5.
Table 2.
Behavioral patterns of mice in extract treated (2000 mg/kg p.o.) and vehicle treated groups.
| Parameters | Observations of vehicle control and S. munja roots extract treated groups |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30minutes |
4 h |
24 h |
48 h |
7 days |
14 days |
|||||||
| CG | TG | CG | TG | CG | TG | CG | TG | CG | TG | CG | TG | |
| Fur & skin | N | N | N | N | N | N | N | N | N | N | N | N |
| Eyes | N | N | N | N | N | N | N | N | N | N | N | N |
| Salivation | N | N | N | N | N | N | N | N | N | N | N | N |
| Respiration | N | ↑ | N | N | N | N | N | N | N | N | N | N |
| Urination(color) | N | N | N | N | N | N | N | N | N | N | N | N |
| Faeces consistency | N | N | N | N | N | N | N | N | N | N | N | N |
| Somatomotor activity & behavior pattern | N | ↑ | N | ↑ | N | N | N | N | N | N | N | N |
| Sleep | N | N | ↑ | ↑ | N | N | N | N | N | N | N | N |
| Mucous membrane | N | N | N | N | N | N | N | N | N | N | N | N |
| Convulsions & tremors | N.F | P | N.F | P | N.F | P | N.F | N.F | N.F | N.F | N.F | N.F |
| Itching | P | P | P | P | N.F | P | N.F | N.F | N.F | N.F | N.F | N.F |
| Coma | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F |
| Mortality | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F | N.F |
Key: CG = Vehicle Control group, TG = S. munja roots extract treated groups, N = Normal, P = Present, ↑ = Increased, N.F = Not found
3.2. Organ to body weight index
No lesion was found on examination of isolated vital organs such as heart, kidney and liver from testing animals. Organ to body weight index was calculated and summarized in Table 3 which shows that there was no significant variation present among the groups.
Table 3.
Effects on organ to body weight indices in mice of extract (at limit dose 2000 mg/kg b.w. p.o.) treated and vehicle treated groups.
| Organs | Vehicle control group | Acute Toxicity Group |
|---|---|---|
| (CMC 1%gel) | (SMRE 2000 mg/kg) | |
| Heart | 0.714 ± 0.029 | 0.630 ± 0.029 |
| Kidney | 1.484 ± 0.020 | 1.572 ± 0.022 |
| Liver | 6.560 ± 0.257 | 7.10 0.201 |
Values are presented as mean ± SEM, N = 5; CMC 1%gel = 1% Carboxymethyl cellulose gel, SMRE = S. munja roots extract; organ-to-body weight index = (organ weight × 100)/body weight.
3.3. Biochemical analysis
No change in serum creatinine level was observed whereas serum urea level in acute toxicity group was higher when compared to vehicle control group (Table 4). There were significant (p <0.05) changes in biochemical markers of liver function test as summarized in Table 5. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein and globulin levels were raised while alkaline phosphatase decreased in acute toxicity group as compared to vehicle control group. No significant changes were observed in total bilirubin, albumin and albumin to globulin ratio among the groups. It was observed that when the extract treated group at limit dose was compared with vehicle control group, H.D.L, V.L.D.L and cholesterol to H.D.L ratio among the groups have no variations whereas there was significant (p <0.05) increase in cholesterol, triglycerides and L.D.L (Table 6).
Table 4.
Effect of the extract and vehicle treatment on renal function tests of mice.
| Parameters | Unit | Vehicle control group |
Acute Toxicity Group |
|---|---|---|---|
| (CMC 1%gel) | (SMRE 2000 mg/kg) | ||
| Creatinine (Serum) | mg/dl | 0.4 ± 0.017 | 0.4 ± 0.023 |
| Urea (Serum) | mg/dl | 18 ± 0.231 | 21 ± 0.433* |
SMRE = S. munja roots extract; CMC 1%gel = 1% Carboxymethyl cellulose gel; Values are presented as mean ± SEM, N = 5.
p < 0.05 when compared with the control group.
Table 5.
Effect of extract (given at limit dose) and vehicle treated groups on liver function test in mice.
| Parameters | Unit | Vehicle Control group |
Acute Toxicity Group |
|---|---|---|---|
| (CMC 1%gel) | (SMRE 2000 mg/kg) | ||
| S.G.P.T (A.L.T) | U/L | 211 ± 3.180 | 269 ± 5.774* |
| S.G.O.T (A.S.T) | U/L | 338 ± 4.041 | 371 ± 3.464* |
| Alkaline phosphatase | U/L | 164 ± 2.887 | 122 ± 1.732* |
| Bilirubin total | mg/dl | 0.90 ± 0.012 | 1.03 ± 0.035 |
| Total protein | G/dl | 6.8 ± 0.087 | 7.4 ± 0.052* |
| Albumin | G/dl | 4.5 ± 0.098 | 4.5 ± 0.231 |
| Globulins | G/dl | 2.3 ± 0.173 | 2.9 ± 0.012* |
| A/G Ratio | 2.03 ± 0.035 | 1.6 ± 0.115 |
SMRE = S. munja roots extract; CMC 1%gel = 1% Carboxymethyl cellulose gel; Values are presented as mean ± SEM, N = 5.
p < 0.05 when compared with the vehicle control group.
Table 6.
Effects of the extract (given at limit dose) and vehicle treatment on lipid profile in mice.
| Parameters | Unit | Vehicle Control group |
Acute Toxicity Group |
|---|---|---|---|
| (CMC 1%gel) | (SMRE 2000 mg/kg) | ||
| Cholesterol | mg/dl | 165 ± 2.309 | 196 ± 1.528* |
| Triglycerides | mg/dl | 125 ± 0.981 | 151 ± 1.732* |
| H.D.L (Cholesterol) | mg/dl | 30 ± 0.693 | 34 ± 0.531 |
| L.D.L (Cholesterol) | mg/dl | 110 ± 1.386 | 131 ± 0.882* |
| V.L.D.L | mg/dl | 25 ± 0.577 | 30 ± 1.155 |
| Cholesterol/HDL Ratio | 5.5 ± 0.115 | 5.7 ± 0.058 |
SMRE = S. munja roots extract; CMC 1%gel = 1% Carboxymethyl cellulose gel; Values are presented as mean ± SEM, N = 5.
p < 0.05 when compared with the control group.
3.4. Hematological analysis
The data in Table 7 presents the acute toxicity potential of hematological profile. It can be seen that no remarkable alterations in levels of Hb, total RBC, MCH, neutrophils, monocytes and eosinophils when compared to vehicle control group. Nevertheless there were significant (P < 0.05) elevations in levels of HCT, MCV, MCHC, platelet count, WBC count and lymphocytes in comparison with normal control (Fig. 1).
Table 7.
Effects of the extract (given at limit dose) and vehicle treated groups on CBC in mice.
| Parameters | Unit | Vehicle Control group |
Acute Toxicity Group |
|---|---|---|---|
| (CMC 1%gel) | (SMRE 2000 mg/kg) | ||
| Hb | g/dl | 11.8 ± 0.075 | 12.8 ± 0.121 |
| Total RBC | x10^12/l | 7.45 ± 0.173 | 7.91 ± 0.058 |
| HCT | % | 33.7 ± 0.693 | 42.91 ± 0.577* |
| MCV | Fl | 45.2 ± 0.035 | 54 ± 1.155* |
| MCHC | g/dl | 35 ± 0.254 | 29.9 ± 1.193* |
| Platelet Count | x10^12/l | 245 ± 4.619 | 487 ± 8.660* |
| WBC Count (TLC) | x10^9/l | 3.2 ± 0.115 | 5.16 ±0.208* |
| Neutrophils | % | 10 ± 0.058 | 10.23 ± 0.052 |
| Lymphocytes | % | 86 ± 2.309 | 88 ± 0.745* |
| Monocytes | % | 3 ± 0.144 | 4 ± 0.098 |
| Eosinophils | % | 1 ± 0.115 | 2 ± 0.040 |
| MCH | Pg | 16 ± 0.577 | 16.2 ± 0.017 |
SMRE = S. munja roots extract; CMC 1%gel = 1% Carboxymethyl cellulose gel; Values are presented as mean ± SEM.
p < 0.05 when compared with the vehicle control group.
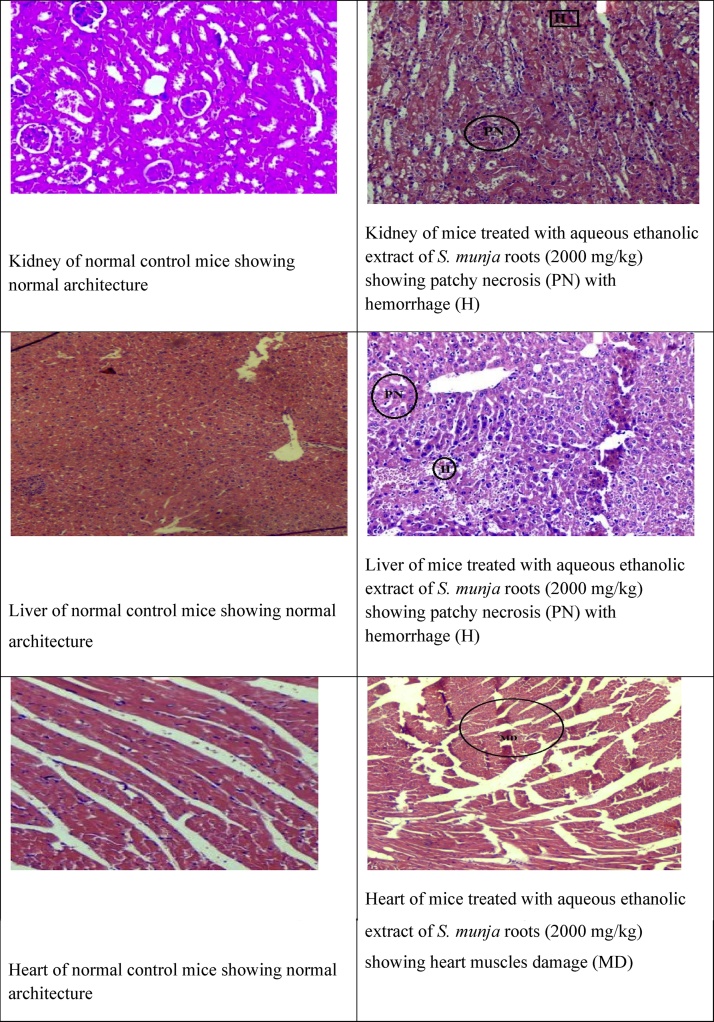
Fig. 1.
Histopathology of control and aqueous ethanol extract treated groups at limit dose (2000 mg/kg).
4. Discussion
Medicinal plants are being used since centuries to treat different diseases [13]. Phytotherapy is gaining popularity as WHO encourages the appropriate ethnomedicinal use and signifies safety evaluation of herbal medicines [14], [15], [16], [17]. FDA and WHO emphasize the validation of efficacious and safe use of herbal therapies through conduction of scientific based studies [14], [18]. Preliminary toxicological evaluation is necessary for authentication of safety of herbal medications. Although Saccharum munja Roxb. roots have valuable pharmacological effects, the comprehensive awareness about its toxicity potential has been lacking. Therefore, the current study was conducted to assess the acute toxicity of aqueous ethanolic extract of S. munja roots in animal model by following OECD guidelines 425 [19] as the acute oral toxicity study is necessary to determine the safer dose range to manage the clinical signs and symptoms of the drugs [20]. In this study, mice rather than rats were used because it is scientifically documented that lethal dose data collected from mice might be more appropriately to anticipate the toxic effects in human beings [21].
The toxic outcomes of drugs on vital body organs are exposed by clinical signs and symptoms which are principal observations among various other toxicity indicators [22]. No animal was found dead while some changes in behavioral pattern like increased respiration, increased somatomotor activity, convulsion, tremor and itching were observed in treatment group in first 24 h (Table 2). During 14 days of acute toxicity evaluation period, it was observed that food and water intake were normal with non- significant body weight variations. It suggests the normal processing of lipids, carbohydrates and protein metabolism inside animals body because these nutrients play a major role in different physiological functions of the body [23], [24], [25]. Liver, kidney, heart, lungs and spleen are the vital organs of our body which are the major targeted area of any toxic substance metabolically [26]. When animals were sacrificed at the end of study, there were no lesions found on macroscopic examination of heart, kidney and liver in comparison with vehicle control group. Statistically, no significant variations were found in organ to body weight index of mice in treatment group when compared with vehicle control group (Table 3). According to globally harmonized classification system, chemicals are divided into five groups on their LD50 basis [27]. The ethanolic root extract of Saccharum munja can be put in group 5 (LD50 > 2000 mg/kg), falling in lower toxicity class.
In acute toxicity evaluation of S. munja roots extract, the health status of the body was evaluated by other biological parameters including serum biomarkers measurement. Liver injury caused by hepatotoxic drugs can result in elevated ALT, AST and total proteins levels [28], [29], [30]. Statistically significant elevation in ALT, AST, total proteins and globulin levels were observed in this study (Table 5). The present study data are in agreement with findings of Adedapo et al. and Adeoye et al. [31], [32]. Hepato cellular damage may results in increased cell membrane permeability and cause release of amino transferases into blood stream [28], [33], [34]. ALP is considered as the standard marker of biliary tract obstruction [35]. In this study, there was significant decrease found in ALP levels (Table 5) which is indicative of plant hepato protective effect [20]. Multiple hyperlipidemias are often secondary to many factors e.g. diet, alcohol intake, therapies or to diseases such as nephrosis, diabetes, hypothyroidism or tumors [36]. Increased levels of cholesterol, triglycerides and LDL (Table 6) were found in the treated group suggesting multiple hyperlipidemic effects of plant under study [37]. Renal function impairment is indicated by elevated levels of serum creatinine and urea [38]. In the present study, serum urea levels were found elevated (Table 4) showing that there is mild renal injury which is supported by patchy necrosis seen during histopathological evaluation [39].
Hematological parameters are sensitive markers of the physiological changes in response to any environmental pollutant or toxic stress in animals [40]. Blood platelets have a vital role in the process of blood coagulation. This study showed remarkable elevated levels of platelet count (Table 7) indicating hemostatic activity of tested extract sample [41]. In this study, increase in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) levels and decline in mean corpuscular hemoglobin concentration (MCHC) was observed (Table 7). Statistically, significant elevated WBC count and lymphocytes (Table 7) suggest its defending potential against the microorganisms and also its contribution to enhance cellular inflammatory process. These results are supported by the study of different researchers [31], [42], [43].
5. Conclusion
In the light of findings of acute toxicity testing it was concluded that aqueous ethanolic extract of Saccharum munja Roxb. roots is not devoid of toxic effects as it elevates LFT, RFT, lipid profile parameters and also showed blood dyscrasias. However, the preliminary results suggested that it should be further evaluated for long term use and repeated dose effects to ensure safety of this herb.
References
- 1.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., William P.D., Duncan J.S. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg D.M., Roger B.D., Susan L.E., Appel S., Wilkey S., Rompay M.V., Kessler R.C. Trends in alternative medicine use in the united states, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. http://jamanetwork.com/journals/jama/fullarticle/188148 [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg D.M., Kessler R.C., Foster C., Norlock F.E., Calkins D.R., Delbanco T.L. Unconventional medicine in the united states?prevalence, costs, and patterns of use. N. Eng. J. Med. 1993;328(4):246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 4.Chen X., Zhou H., Liu Y.B., Wang J.F., Hu Li, Ung C.Y., Han L.Y., Cao Z.W., Chen Y.Z. Database of traditional chinese medicine and its application to studies of mechanism and to prescription validation. Br. J. Pharmacol. 2006;149(8):1092–1103. doi: 10.1038/sj.bjp.0706945. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2014641/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ertekin V., Selimoğlu M.A., Altinkaynak S. A combination of unusual presentations of datura stramonium intoxication in a child: rhabdomyolysis and fulminant hepatitius. J. Emerg. Med. 2005;28(2):227–228. doi: 10.1016/j.jemermed.2004.11.006. http://www.jem-journal.com/article/S0736-4679(04)00343-9/abstract [DOI] [PubMed] [Google Scholar]
- 6.Koduru D.S. Antimicrobial activity of solanum aculeastrum. Pharm. Biol. 2006;44(4):283–286. http://www.tandfonline.com/doi/abs/10.1080/13880200600714145 [Google Scholar]
- 7.Hunter P. A toxic brew we cannot live without. EMBO Rep. 2008;9(1):15–18. doi: 10.1038/sj.embor.7401148. http://onlinelibrary.wiley.com/doi/10.1038/sj.embor.7401148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi M.M.A., El. Hassadi I.M.G., Younis S.B. Bioefficacies of cassia fistula: an indian labrum. Afr. J. Pharm. Pharmacol. 2009;3(6):287–292. http://www.academicjournals.org/journal/AJPP/article-abstract/28E7B7C34856 [Google Scholar]
- 9.Rahar S., Nagpal N., Swami G., Nagpal M.A., Kapoor R. Pharmacognostical studies of saccharum munja roxb. Root. Int. J. Pharm. Tech. Res. 2011;3(2):792–800. [Google Scholar]
- 10.Gautam A. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007;110(2):200–234. doi: 10.1016/j.jep.2006.12.031. https://www.ncbi.nlm.nih.gov/pubmed/17276637 [DOI] [PubMed] [Google Scholar]
- 11.Basu K.R., Basu K., Singh B., Singh M.P. 1991. Indian Medicinal Plants: Plates. (Available from: https://books.google.co.in/books?id=24NiQwAACAAJ) [Google Scholar]
- 12.Sandeep R., Navneet N., Gaurav S., Manisha A., Suraj B., Sandeep G., Shwali S., Preeti S., Reni K. Medicinal aspects of saccharum munja. RJPT. 2010;3(3):636–639. [Google Scholar]
- 13.Ridtitid W., Sae-Wong C., Reanmongkol W., Wongnawa M. Antinociceptive activity of the methanolic extract of kaempferia galanga linn. In experimental animals. J Ethnopharmacol. 2008;118(2):225–230. doi: 10.1016/j.jep.2008.04.002. http://europepmc.org/med/18486374 [DOI] [PubMed] [Google Scholar]
- 14.WHO . World Health Organization; 1993. Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines; p. 94. (Available from: http://apps.who.int/medicinedocs/en/d/Jh2946e/4.7.3.html) [Google Scholar]
- 15.Daswani G.P., Brijesh S., Birdi J.T. reclinical testing of medicinal plants: advantages and approaches. Workshop Proceedings on Approaches Towards Evaluation of Medicinal Plants Prior to Clinical Trial Citeseer. 2006 [Google Scholar]
- 16.Ogbonnia S.O., Mbaka G.O., Anyika E.N., Osegbo O.M., Igbokwe N.H. Evaluation of acute toxicity in mice and subchronic toxicity of hydroethanolic extract of chromolaena odorata (l.) king and robinson (fam. Asteraceae) in rats. ABJNA. 2010;1(5):859–865. https://www.cabdirect.org/cabdirect/abstract/20113247718 [Google Scholar]
- 17.Vaghasiya Y.K., Shukla V.J., Chanda S.V. Acute oral toxicity study of pluchea arguta boiss extract in mice. J. Pharmacol. Toxicol. 2011;6(2):113–123. [Google Scholar]
- 18.Setzer R.W., Kimmel C.A. Use of noael, benchmark dose, and other models for human risk assessment of hormonally active substances. Pure Appl. Chem. 2003;75(11-12):2151–2158. https://pdfs.semanticscholar.org/2713/54311b3365c088bbbd007a37edb9acef2ec3.pdf [Google Scholar]
- 19.OECD . Vol. 425. OECD; 2008. Acute oral toxicity: Up and down procedure; pp. 1–2. (Guideline for the Testing of Chemicals). [Google Scholar]
- 20.Saleem U., Ahmad B., Ahmad M., Erum A., Hussain K., Bukhari N.I. Is folklore use of euphorbia helioscopia devoid of toxic effects? Drug Chem. Toxicol. 2016;39(2):233–237. doi: 10.3109/01480545.2015.1092040. http://www.tandfonline.com/doi/abs/10.3109/01480545.2015.1092040?journalCode=idct20 [DOI] [PubMed] [Google Scholar]
- 21.Walum E., Nilsson M., Clemedson C., Ekwall B. The meic program and its implications for the prediction of acute human systemic toxicity. Altern. Methods Toxicol. 1995;11:275–282. [Google Scholar]
- 22.Subramanion L.J., Zakaria Z., Chen Y., Lau Y.L., Latha L.Y., Sasidharan S. Acute oral toxicity of methanolic seed extract of cassia fistula in mice. Molecules. 2011;16(6):5268–5282. doi: 10.3390/molecules16065268. http://www.mdpi.com/1420-3049/16/6/5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iversen P.O., Nicolaysen G. Water–for life. Tidsskrift for Den Norske Laegeforening: tidsskrift for praktisk medicin, ny raekke. 2003;123(23):3402–3405. http://europepmc.org/med/14713981 [PubMed] [Google Scholar]
- 24.Stevens K.R., Mylecraine L. Issues in chronic toxicology. Principles Methods Toxicol. 1994;3:673. [Google Scholar]
- 25.Gregus Z., Klaassen C.D. Vol. 6. 2001. Mechanisms of toxicity; pp. 35–82. (Casarett and Doull’s toxicology: The Basic Science of Poisons). [Google Scholar]
- 26.Auletta C.S. CRC Press; London: 1995. Acute, Subchronic and Chronic Toxicology. [Google Scholar]
- 27.Secretariat United Nations . United Nations Publications; 2009): 2017. Economic Commission for Europe. Globally Harmonized System of Classification and Labelling of Chemicals (ghs) [Google Scholar]
- 28.Friedman L.S., Martin P., Munoz S.J. Vol. 1. 1996. Liver function tests and the objective evaluation of the patient with liver disease; pp. 791–833. (Hepatology: A Textbook of Liver Disease). [Google Scholar]
- 29.Ramaiah S.K. Preclinical safety assessment: current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clin. Lab. Med. 2011;31(1):161–172. doi: 10.1016/j.cll.2010.10.004. http://www.labmed.theclinics.com/article/S0272-2712(10)00146-0 [DOI] [PubMed] [Google Scholar]
- 30.Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. http://europepmc.org/med/18291570 [DOI] [PubMed] [Google Scholar]
- 31.Adedapo A.A., Abatan M.O., Olorunsogo O.O. Toxic effects of some plants in the genus euphorbia on haematological and biochemical parameters of rats. Veterinarski arhiv. 2004;74(1):53–62. http://www-staro.vef.unizg.hr/vetarhiv/papers/74-1/adedapo.pdf [Google Scholar]
- 32.Adeoye G.O., Alimba C.G., Oyeleke O.B. The genotoxicity and systemic toxicity of a pharmaceutical effluent in wistar rats may involve oxidative stress induction. Toxicol. Rep. 2015;2:1265–1272. doi: 10.1016/j.toxrep.2015.09.004. http://www.sciencedirect.com/science/article/pii/S2214750015300585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogunlana O.O., Ogunlana O.E., Adeneye A.A., Udo-Chijioke O.A.C., Dare-Olipede T.I., Olagunju J.A., Akindahunsi A.A. Evaluation of the toxicological profile of the leaves and young twigs of caesalpinia bonduc (linn) roxb. Afr. J. Tradit. Complement Altern. Med. 2013;10(6):504–512. doi: 10.4314/ajtcam.v10i6.20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali T., Bhalli J.A., Rana S.M., Khan Q.M. Cytogenetic damage in female pakistani agricultural workers exposed to pesticides. Environ. Mol. Mutagen. 2008;49(5):374–380. doi: 10.1002/em.20395. http://onlinelibrary.wiley.com/doi/10.1002/em.20395 [DOI] [PubMed] [Google Scholar]
- 35.Manjunatha B.K., Vidya S.M., Dhiman P., Pallavi R., Mankani K.L. 2005. Hepatoprotective Activity of Leucas Hirta Against Ccl 4 Induced Hepatic Damage in Rats.http://imsear.li.mahidol.ac.th/jspui/handle/123456789/59445 [PubMed] [Google Scholar]
- 36.Havel R.J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv. Intern. Med. 1969;15:117. [PubMed] [Google Scholar]
- 37.Goldstein J.L., Schrott H.G., Hazzard W.R., Bierman E.L., Motulsky A.G. Hyperlipidemia in coronary heart disease ii. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Invest. 1973;52(7):2544. doi: 10.1172/JCI107332. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC302426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Travlos G.S., Morris R.W., Elwell M.R., Duke A., Rosenblum S., Thompson M.B. Frequency and relationships of clinical chemistry and liver and kidney histopathology findings in 13-week toxicity studies in rats. Toxicology. 1996;107(1):17–29. doi: 10.1016/0300-483x(95)03197-n. https://www.ncbi.nlm.nih.gov/pubmed/8597028 [DOI] [PubMed] [Google Scholar]
- 39.Alimba C.G., Bakare A.A., Aina O.O. Liver and kidney dysfunction in wistar rats exposed to municipal landfill leachate. Resources and Environment. 2012;2(4):150–163. http://article.sapub.org/10.5923.j.re.20120204.04. html [Google Scholar]
- 40.Jain N., Sharma P., Sharma N., Joshi S.C. Haemato-biochemical profile followi g sub acute toxicity of malathio i male albi o rats. Avicenna J. Phytomed. 2009;2:500–506. http://pharmacologyonline.silae.it/files/archives/2009/vol2/050.NISHA.pdf [Google Scholar]
- 41.Li M., Jia Z., Hu Z., Zhang R., Shen T. Experimental study on the hemostatic activity of the tibetan medicinal herb lamiophlomis rotata. Phytother. Res. 2008;22(6):759–765. doi: 10.1002/ptr.2359. http://onlinelibrary.wiley.com/doi/10.1002/ptr.2359 [DOI] [PubMed] [Google Scholar]
- 42.Chunlaratthanaphorn S., Lertprasertsuke N., Srisawat U., Thuppia A., Ngamjariyawat A., Suwanlikhid N., Jaijoy K. Acute and subchronic toxicity study of the water extract from root of citrus aurantifolia (christm. Et panz.) swingle in rats. Songklanakarin J. Sci. Technol. 2007;29(1):25–136. https://www.researchgate.net/publication/26469453_Acute_and_subchronic_toxicity_study_of_the_water_extract_from_root_of_Citrus_aurantifolia_Christm_et_Panz_Swingle_in_rats [Google Scholar]
- 43.Sillanaukee P. Laboratory markers of alcohol abuse. Alcohol. 1996;31(6):613–616. doi: 10.1093/oxfordjournals.alcalc.a008199. https://academic.oup.com/alcalc/article-pdf/31/6/613/439952/31-6-613.pdf [DOI] [PubMed] [Google Scholar]