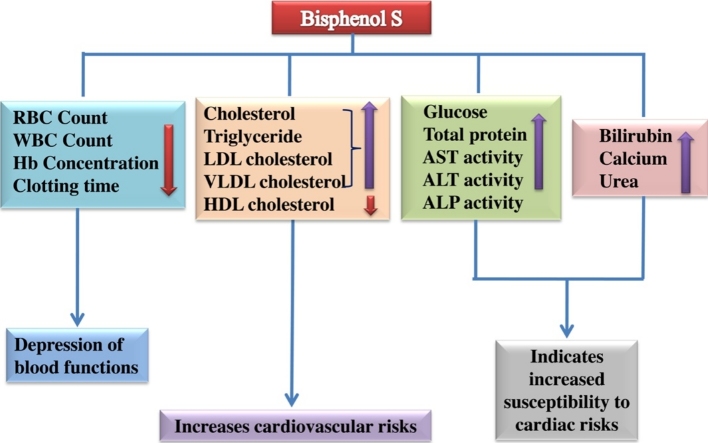
Graphical abstract
Abbreviations: BPS, bisphenol S; BPA, bisphenol A; DMSO, dimethyl sulphoxide; RBC, red blood cells; WBC, white blood cells; Hb, hemoglobin; MCH, mean corpuscular hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein
Keywords: Bisphenol S, Red blood cell count, White blood cell count, Clotting time, LDL cholesterol, HDL cholesterol, Cardiovascular risks
Highlights
-
•
Bisphenol S alters blood homeostasis.
-
•
Bisphenol S is probably a cardiac risk augmenting chemical.
-
•
Bisphenol S is a haemolysis promoting chemical.
Abstract
Bisphenol S (BPS) is an industrial chemical which is recently used to replace the potentially toxic Bisphenol A (BPA) in making polycarbonate plastics, epoxy resins and thermal receipt papers. The probable toxic effects of BPS on the functions of haemopoietic and cardiovascular systems have not been reported till to date. We report here that BPS depresses haematological functions and induces cardiovascular risks in rat. Adult male albino rats of Sprague-Dawley strain were given BPS at a dose level of 30, 60 and 120 mg/kg BW/day respectively for 30 days. Red blood cell (RBC) count, white blood cell (WBC) count, Hb concentration, and clotting time have been shown to be significantly (*P < 0.05) reduced in a dose dependent manner in all exposed groups of rats comparing to the control. It has also been shown that BPS increases total serum glucose and protein concentration in the exposed groups of rats. We have observed that BPS increases serum total cholesterol, triglyceride, glycerol free triglyceride, low density lipoprotein (LDL) and very low density lipoprotein (VLDL) concentration, whereas high density lipoprotein (HDL) concentration has been found to be reduced in the exposed groups. BPS significantly increases serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities dose dependently. Moreover, serum calcium, bilirubin and urea concentration have been observed to be increased in all exposed groups. In conclusion, BPS probably impairs the functions of blood and promotes cardiovascular risks in rats.
1. Introduction
The practice of employing different chemicals in consumer goods, without knowing the possible harmful effects, has become a common occurrence. Bisphenol S (BPS) is one of such chemicals, which is substituting the potentially toxic Bisphenol A (BPA). Bisphenol S (BPS; 4,4′-Sulfonyldiphenol; CAS 80-09-1) is made up of two phenol groups, attached one on each side to the central sulphur atom of a sulfonyl group. BPS was first manufactured in 1869 as a dye. BPS has been used as a substitute for BPA in some consumer products at an increasing rate [1]. It has increased stability against high temperature range and more resistant to sunlight; hence there is a possibility of less biodegradation in the environment. The plastic products coined as ‘BPA free’ are often made of BPS [2]. BPS has been used in manufacturing plastics, thermal receipt papers, currency bills, food cartoons etc [3], [4]. In some recent studies it has been reported that BPS shows adverse effects in both ex vivo and in vivo experiments.
BPA has been reported to have widespread accumulation in human body [5], [6]. BPS is also being biomonitored in human population. BPS has been detected in 81% of the urine samples, collected from United States and seven Asian countries. The highest BPS concentration has been observed in Japan, trailed by United States [7]. Russo G et al,. reported that in Italian market the dermal absorption of BPS for general population is 0.0244 μg/day, whereas for occupational exposure it is 15.6 μg/day [8].
BPS has been reported to possess estrogenic activity and genotoxic potential [9], [10]. It has been observed that BPS resulted in decreased egg production and hatching and increases the 17β estradiol concentration in female zebra fish. Considerable decrease in testosterone concentration has also been observed in male zebra fish [11]. BPS has been also reported to induce testicular oxidative damage associated with altered morphology of the testis. Intra-testicular testosterone concentration also reduced at higher BPS concentration [12]. According to Rui Zhang et al., BPS can bind to catalase via hydrogen and hydrophobic bonds leading to altered secondary structure and activity [13].
Wang Y et al., reported that the secondary and tertiary structures of trypsin and pepsin were altered by Bisphenol S binding, which resulted in the loosening of the skeletons of trypsin and pepsin [14]. Another study reported that BPS induced an increase in the lipid content in the 3T3-L1 cell line and more moderately in hepatic cells [15]. According to Vin҃as R et al., BPS interrupts E2 induced cell signaling, which ultimately leads to altered cell proliferation, cell death, PRL release in rat pituitary cell line study [16]. BPS affects mammary gland morphology, maternal and nursing behaviour at the time of pregnancy and lactation [17], [18]. Meiotic process of pig oocyte is disrupted by BPS administration [19].
The in vivo studies on toxic effects of BPS are only some. Although the effect of BPS on haematological and cardiovascular variables have not been reported till date. Hence this study has been deliberated to evaluate the probable toxic effects of BPS on some haematological and serum biochemical variables in rat model.
2. Materials & methods
2.1. Experimental model
The experimental animals chosen for the study were adult male albino rats of Sprague-Dawley strain. Adult male rats weighing about 100–140 gm and 14–16 weeks of age were purchased from authorised breeder. The animals were housed in polypropylene cages, each containing 3- 4 rats, in Molecular Neurotoxicology Laboratory of Department of Physiology, University of Kalyani. Animal handling was done with great care, following the guidelines of Kalyani University Animal Ethics Committee. The experimental animals were maintained at a temperature range of 21−25° C and 12 h light-dark cycle. Rats were provided with standard rodent feed and abundant supply of water.
2.2. Experimental design
After a week of acclimatization in the new environment the rats were divided into 4 groups containing 15 rats each. The first group of rats received 0.5 ml of 20% DMSO by oral gavage for 30 days and subsequently designated as vehicle control group. The remaining groups of rats received graded doses of Bisphenol S (BPS) and consecutively named as exposed groups (Table 1).
Table 1.
Showing experimental design of vehicle control group and BPS exposed groups of rats.
| Groups | Exposure to test element | Duration |
|---|---|---|
| Group I (Vehicle Control) | Received 20% of DMSO | 30 days |
| Group II | Received 30 mg BPS/Kg Body weight/Day | 30days |
| Group III | Received 60 mg BPS/Kg Body weight/Day | 30days |
| Group IV | Received 120 mg BPS/Kg Body weight/Day | 30days |
2.3. Chemicals & reagents
All the essential chemicals utilized in this experiment are of analytical grade. Bisphenol S (CAS No. 80-09-1, Purity 98%) was purchased from Sigma Aldrich, USA. Dimethyl sulphoxide (DMSO) was procured from Merck. Diagnostic test kits were purchased from ARKRAY Healthcare Pvt. Ltd.
2.4. Sample collection
At the end of the test duration, the rats were sacrificed by cervical dislocation, 24 h after the last applied dose. An incision was made on abdomen, thoracic cage has been exposed and blood was drawn directly from the heart. The blood sample was centrifuged at 4000 rpm for 10 min and serum was preserved at −20 °C for further biochemical assay.
2.5. Haematological study
Red blood cell (RBC) and white blood cell (WBC) of whole blood were viewed under Olympus light microscope (Model CH20iBIMF) in 40X magnification and counted with the help of improved neubauer counting chamber [20]. Hemoglobin concentration (Hb) was measured by Sahli’s Hemoglobinometer following the Acid Hematin method [20]. Estimation of clotting time was done by Capillary Tube method whereas mean corpuscular hemoglobin (MCH) was calculated by the formula mentioned by Gk Paul et al. [20].
2.6. Biochemical study
Serum glucose, cholesterol, triglyceride, glycerol free triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL), and very low density lipoprotein (VLDL) were measured following the instruction provided in the commercial diagnostic test kits [Glucose test kit, Autospan, REF Old- B0112, New- 93DP100-74, LOT- 4000017073; Cholesterol test kit, Autospan, REF Old- LG051, New- 71LS200-40, LOT- 4000017118; Triglyceride test kit, Autospan, REF Old-LG061, New- 72LS100-40, LOT- 4000016487]. Serum total protein level was determined as per the protocol of Lowry et al. [21].
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activity were estimated as per the protocol of diagnostic test kits [GOT (AST) test kit, Code No.- 25706, New Code No.- 77MB101-50, LOT- 4000017045; GPT (ALT) test kit, Code No.- 25707, New Code- 76MB101-50, LOT- 4000016730; ALP test kit, Old Code- 25904-A, New Code- 75MB 100-40, LOT- 4000016800].
Serum calcium, bilirubin and urea concentration were estimated by test kit instruction [Calcium, Autospan, REF Old LG0811, New- 87LS100-60, LOT- 4000016857; Urea, Autospan, REF Old- B1611, New- 81DP300-72, LOT- 4000017225; Bilirubin, REF Old-LG1712, New- 78LS200-66, LOT- 4000014920].
2.7. Statistical analysis
All the data were presented as Mean ± SEM. Statistical analysis of the data obtained from control and exposed groups was carried out by Student’s t-test or one way analysis of variance (ANOVA), by using GraphPaD Prism 5.03 (GraphPaD Software Inc.). *p < 0.05 was considered as significant level.
3. Results
3.1. Effects of bisphenol S on serum haematological variables
From the study it has been observed that BPS has significantly reduced the red blood cell (RBC) and white blood cell (WBC) count of whole blood in all exposed groups of rats comparing to the (Table 2) control group of rats (Table 2). BPS also decreases hemoglobin (Hb) concentration in exposed rats (Table 2). The clotting time duration of BPS exposed rats showed marked reduction in comparison to the vehicle control group of rats. Although no significant alteration in the mean corpuscular hemoglobin (MCH) concentration has been found in the exposed groups of rats (Table 2).
Table 2.
Tabular representation of RBC count, WBC count, Hb concentration, clotting time, mean corpuscular hemoglobin (MCH) value in control and exposed groups of rats after 30 days exposure duration. Values are presented as Mean ± SEM (n = 7).*p 0.05 vs. Control, **p 0.01 vs. Control, ***p ≤ 0.001 vs. Control.
| Group I (Control) | Group II | Group III | Group IV | |
|---|---|---|---|---|
| RBC Count (million/mm3) | 10.030 ± 0.607 | 9.229 ± 0.723 | 7.440 ± 0.496** | 6.606 ± 0.735** |
| WBC Count (thousand/mm3) | 6.864 ± 0.220 | 6.793 ± 0.307 | 6.471 ± 0.351 | 5.850 ± 0.311* |
| Hb Concentration (g/dl) | 12.114 ± 0.567 | 11.057 ± 0.635 | 8.371 ± 0.558*** | 7.571 ± 0.613*** |
| Clotting time (min) | 2.214 ± 0.065 | 1.857 ± 0.092** | 1.321 ± 0.090*** | 0.964 ± 0.065*** |
| MCH Value (pg/cell) | 12.156 ± 0.258 | 12.175 ± 0.566 | 11.331 ± 0.477 | 11.735 ± 0.485 |
3.2. Effects of bisphenol S on serum biochemical variables
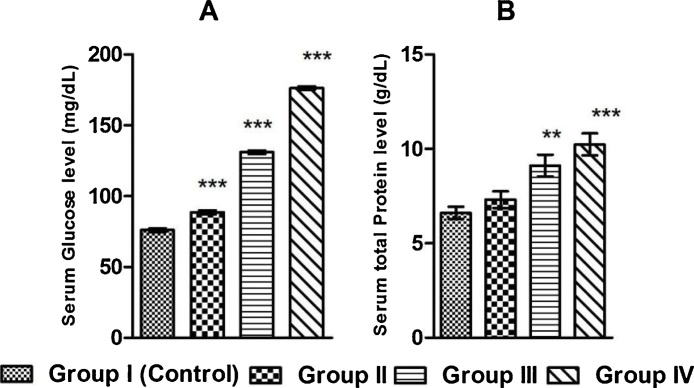
BPS has been observed to significantly increase the serum glucose level in the exposed groups of rats in a dose dependent manner comparing to the control group of rats. Serum total protein level has been increased due to BPS exposure, proportionately with graded doses (Fig. 1).
Fig. 1.
Graphical representation of the serum level of (A) glucose, (B) total protein in control and BPS exposed rats after 30 days exposure duration. Values are presented as Mean ± SEM (n = 8). **p < 0.01 vs. Control, ***p < 0.001 vs. Control.
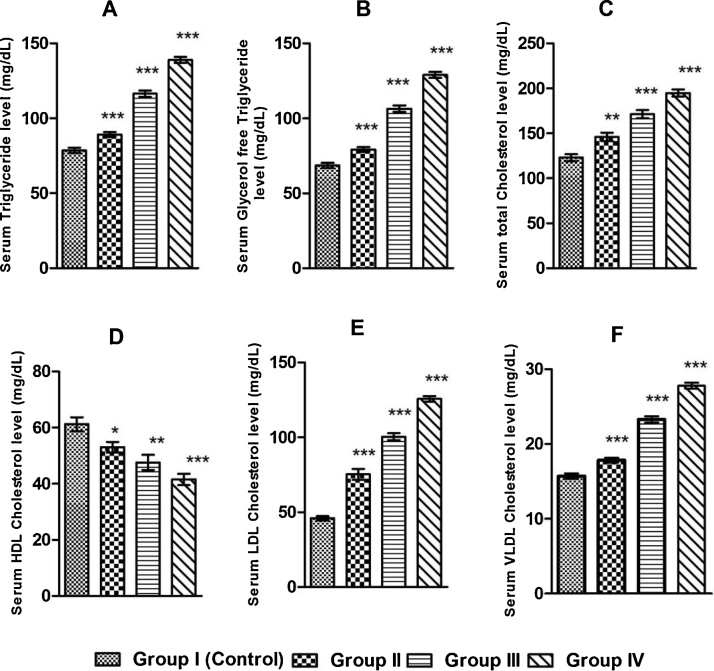
BPS has significantly increased the serum cholesterol, triglyceride, glycerol free triglyceride, low density lipoprotein (LDL), very low density lipoprotein (VLDL) level in BPS exposed groups of rats, whereas the high density lipoprotein level (HDL) level was markedly reduced comparing to the control group of rats (Fig. 2).
Fig. 2.
Graphical representation of serum levels of (A) triglyceride, (B) glycerol free triglyceride, (C) cholesterol, (D) HDL cholesterol, (E) LDL cholesterol, (F) VLDL cholesterol in control and BPS exposed groups of rats after 30 days exposure duration. Values are presented as Mean ± SEM (n = 8).*p < 0.05 vs. Control, **p < 0.01 vs. Control, ***p < 0.001 vs. Control.
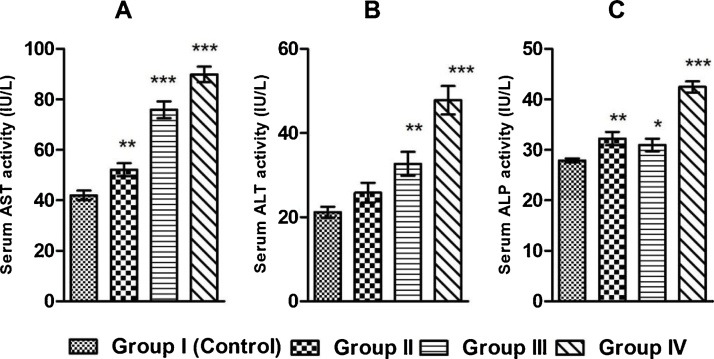
It has also been found that BPS significantly increases serum aspartate aminotranferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities in the exposed rats in a dose dependent manner comparing to the control group of rats (Fig. 3).
Fig. 3.
Graphical representation of serum activity of (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), (C) alkaline phosphatase (ALP) in control and BPS exposed groups of rats after 30 days exposure duration. Values are presented as Mean ± SEM (n = 8).*p < 0.05 vs. Control, **p < 0.01 vs. Control, ***p < 0.001 vs. Control.
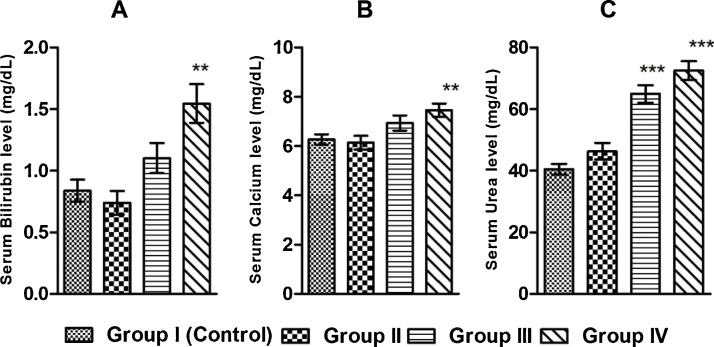
BPS also significantly increases serum bilirubin, calcium, and urea concentration in the exposed groups of rats when compared to the control rats (Fig. 4).
Fig. 4.
Graphical representation of serum level of (A) bilirubin, (B) calcium, (C) urea in control and exposed groups of rats after 30 days exposure duration. Values are presented as Mean ± SEM (n = 8). **p 0.01 vs. Control, ***p ≤ 0.001 vs. Control.
4. Discussion
Red blood cells (RBC) are responsible for gaseous transport, carrying oxygen to the cellular level and carbon dioxide from the cells to the lungs [22], [23], [24]. To find out the effects of BPS on the ability of RBC to carry oxygen and carbon dioxide in the blood, the RBC count has been studied and total hemoglobin concentration, and mean corpuscular hemoglobin (MCH) concentration have been measured in BPS exposed groups of rats. In our study, it has been found that BPS significantly decreases RBC count in all exposed groups of rats comparing to the control group of rats (Table 2). These results suggest that BPS probably induces hypoxia, and carbon dioxide toxicity in the tissues by lowering the number of circulating red blood cells and depressing the synthesis of hemoglobin. The BPS induced decrease in the number of RBC in the circulating blood might be due to the inhibition of RBC formation (erythropoiesis) from the committed stem cells of the bone marrow and/or promotion of haemolysis. RBC production in vivo is regulated by several factors including the principal regulatory role of erythropoietin, a circulating glycoprotein. Erythropoietin is secreted from kidney and inactivated at liver. Erythropoietin increases the number of erythropoietin sensitive committed stem cell of the bone marrow that are converted to red blood cell precursors and subsequently to mature RBC. So, BPS induced decrease in number of RBC in the circulation might be due to the inhibition on the secretion of erythropoietin, promotion of the inactivation of erythropoietin at the liver and/or inhibition of the erythropoietin signaling in the committed stem cells by the BPS. Tiwari D et al., reported that BPA which has been previously used in the industries before the substitution by BPS induces oxidative damage of the bone marrow [25]. As BPS is a structural analogue of BPA, so we can suggest from our results that alternatively, BPS might inhibit the RBC production by inducing stress in bone marrow precursor cells. One or all of the above mechanisms might be involved in BPS induced decrease in RBC count.
Hemoglobin (Hb) is the iron containing metalloprotein, present in the RBCs [22]. Hb concentration of the whole blood was measured to examine the BPS induced possible effects on blood hemoglobin. We have observed a marked reduction in hemoglobin concentration due to BPS exposure (Table 2). The decrease in the amount of Hb might be due to decrease in the number of circulating RBCs due to the inhibition of erythropoiesis and/or promotion of haemolysis and/or inhibition of the synthesis of Hb. To examine the BPS induced haemolysis we have measured the serum bilirubin concentration in exposed groups of rats. In our study it has been observed that BPS significantly increases the bilirubin concentration in all exposed groups of rats (Fig. 4). Bilirubin is the end products of Hb catabolism [26], [27]. After haemolysis of the RBC in the circulating blood the cellular Hb is coming out to the plasma. Then it is normally catabolised to the bilirubin for excretion by the kidney or through faeces. So our results ascertain that BPS might promote the haemolysis of RBC in circulating blood.
Clotting time is the time required for the formation of blood clot in vitro under standard conditions. To examine the probable effect of BPS on intravascular clot formation, the clotting time in all BPS exposed rats and controlled rats have been measured. In our study the clotting time has been decreased (Table 2). BPS impairs the clotting time possibly by augmenting the activity of several intrinsic clotting factors, principally formation of fibrin from fibrinogen and polymerisation of fibrin monomers and increase in the concentration of calcium ions. Any one or all of the above mechanisms might be involved in the BPS induced decrease in clotting time. Calcium ions play an important role in the blood clotting process. From our study it has also been found that BPS increases serum calcium ion level significantly in the exposed rats comparing to the control group of rats (Fig. 4). This increase in blood calcium level might facilitate the blood clotting process by involving in the intrinsic and extrinsic pathways of coagulation. Not only that, calcium ion is essential for cardiac rhythmicity. Littledike et al. reported that alteration in plasma calcium concentration induces cardiac arrhythmia via changes in the vagal activity [28]. Therefore our result on calcium ion determination indicates that BPS might promote cardiac arrhythmia probably by augmenting the activity of cardiac vagus.
White blood cells (WBC) are the chief warriors of body’s defence system. Polymorphonuclear leucocytes family, containing granular cytoplasm kills invading pathogens and antigens by phagocytosis or by releasing histamine leading to inflammatory response. On the other hand T lymphocyte is related to cell mediated immunity and B lymphocyte is associated with humoral immunity [22], [29]. WBC count of whole blood was studied to observe the action of BPS on the immune functions. From the experiment we found that BPS significantly decreases total WBC count in the exposed groups of rats comparing to the control (Table 2). The BPS induced decrease in WBC count in the circulating blood might suppress the immune system and defence functions by decreasing the number of WBC. BPS decreases the WBC count probably by impairing the production of WBC (leucopoiesis) from the committed haematopoietic stem cells in the bone marrow. The suppression of the function of committed WBC precursor cells in the bone marrow might be due to BPS induced oxidative stress [25].
Glucose is the main carbohydrate fuel for the cellular metabolism. To evaluate the BPS induced effect on blood glucose level, we have measured glucose level in all exposed and control group of rats. From the results it has been observed that BPS significantly increases blood glucose level in the exposed groups of rats in comparison with the control group of rats (Fig. 1). The BPS induced hyperglycemia gradually rises in the exposure groups proportionately with the higher doses. BPS induced hyperglycemia in rats might be due to inhibition of cellular glucose uptake and/or augmentation of glycogen breakdown in liver and muscle tissues. BPS probably inhibits the cellular glucose uptake by inhibiting the secretion of insulin from the β cells of islets of langerhans of the pancreas and inhibiting the tyrosine kinase mediated cell signaling. Our result is supported by the work of Zhao F et al., where BPS increases the blood glucose level by enhancing gluconeogenesis and glycogenolysis in liver, and by decreasing insulin level [30].
Protein present in the serum of the blood carries hormones from the source of origin to the target sites and also the immunoglobulin (antibody) involved in humoral immunity are found in the serum fraction of the blood. Different metals of biological importance and lipids are carried to the sites of assimilation of tissues through the plasma proteins. If the plasma protein level is decreased, the physiological systems depending on hormones, immunoglobulin, micro and macronutrients and essential lipids, will be impaired. Whereas increase in the concentration of protein may indicates tissue damage. To examine the possible effects of BPS on protein involved physiological functions, the level of serum plasma proteins in BPS exposed rats has been examined. BPS significantly increases serum total protein in all exposed groups of rats above the controlled normal range (Fig. 1). So the result indicates that BPS promotes hyperproteinemia probably by promoting the tissue damage. To ascertain the BPS induced tissue damage serum aspartate aminotransferase (AST) activity (the marker of the ischemic tissue damage of the heart), the activity of serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) (which are bio indicators of liver function probably linked with the cardiac tissue damage), have been studied. In our study we have observed that BPS significantly increases AST, ALT and ALP activity in the exposed rats comparing to the control groups of rats (Fig. 3). So the results indicate that BPS probably decreases that cardiac function by inducing damage of cardiac tissue. Though these enzymes are liver function enzymes but reports suggest that liver abnormalities are often associated with cardiac problems [31].
Majority of lipids are insoluble in aqueous solution and need carriers to be transported. Cholesterol and triglyceride are carried in the blood in the form of lipoprotein particles [32], [33]. Low density lipoprotein (LDL) is the main source of cholesterol and for transporting cholesterol from the liver to peripheral tissues for de novo cholesterol synthesis. High density lipoprotein (HDL) has antiatherogenic characters, one of which is the removal of cholesterol from the dying cells to be excreted through bile [34], [35]. We have examined the effect of BPS on the level of some lipid variables linked to cardiovascular risks. We have observed that BPS increases the serum triglyceride level in BPS exposure groups in comparison to control rats (Fig. 2). BPS also increases serum glycerol free triglyceride, cholesterol, low density lipoprotein (LDL) cholesterol, very low density lipoprotein (VLDL) cholesterol in exposed groups of rats. On the other hand, high density lipoprotein (HDL) cholesterol concentration was significantly decreased (Fig. 2). Lipoproteins, including the LDL, VLDL and HDL, are synthesized and secreted by liver and intestinal cells. So, the result indicates that BPS might increase the synthesis of LDL and VLDL and decrease the synthesis of HDL. On the other hand concentration of total cholesterol, both free and bound, has been increased in exposed groups of rats in our study. Similar findings have been reported, in case of BPA exposure [36], [37]. BPS might increase the cholesterol concentration by inducing the peripheral cholesterol biosynthesis, increasing the degeneration of cell membranes in the process of cell apoptosis, and/or decreasing the level of HDL lipoprotein which carries cholesterol from dying cells to the liver for excretion. It has been reported that BPA causes overexpression of the genes like Mvd, Lss, Hmgcr, and Sqle which are principal genes of cholesterol biosynthesis [38]. Our suggestion about BPS induced increase in synthesis of cholesterol in the peripheral tissues might be corroborated by Marmugi A et al. The increase in the level of glycerol free triglyceride and triglyceride might be due to BPS induced augmentation of cell membrane degenerations. So, our results suggest that BPS might induce the cardiovascular risks by increasing the levels of lipid profile variables which promotes the formation of atherosclerotic plaque and by decreasing the level of lipid profile variable which antagonises the formation of atherosclerotic plaque in the vasculature.
BPS also increases the serum urea concentration in BPS exposed rats comparing to the control group of rats (Fig. 4). Urea is the end product of protein metabolism in ureotelic animals including humans [39]. Urea is a harmful metabolite and filtered from blood by kidney. So, the increased urea concentration in blood generally indicates poor kidney function. Elevated urea concentration sometimes is also associated with heart failure and acts as an indicator of cardiac failure. Elevated level of urea indicates renal response due to decompensated heart failure [40]. Hence, the increased blood urea concentrations in BPS exposed rats in our study suggests that BPS induced cardiac risk might be also linked to the increased concentration of urea.
5. Conclusion
Considering the results obtained from the study it can be concluded that BPS impairs the haematological functions and induces cardiovascular risks in rats. BPS impairs the haematological functions in rat probably by promoting hypoxia, carbon dioxide induced toxicity in tissues, and suppressing the WBC mediated immunity; reduces cellular glucose uptake and/or induces glycogenolysis; causes damage of blood corpuscles, cardiac and blood vessel wall tissues. BPS also induces cardiac risks probably by increasing cholesterol, triglyceride, LDL, and VLDL cholesterol concentration and decreasing the level of HDL cholesterol.
Conflict of interest
There is no conflict of interest.
Acknowledgement
The INSPIRE Fellowship of Sanghamitra Pal (No. DST/INSPIRE Fellowship/IF150101), Department of Science and Technology, Ministry of Science and Technology of the Government of India under INSPIRE program for pursuing full time doctoral (PhD) program at University of Kalyani is gratefully acknowledged for financial support for the research work.
References
- 1.Mathew M., Sreedhanya S., Manoj P., Aravindakumar U.K., Aravind C.T. Exploring the interaction of bisphenol-S with serum albumins: a better or worse alternative for bisphenol A? J. Phys. Chem. B. 2014;118(14):3832–3843. doi: 10.1021/jp500404u. [DOI] [PubMed] [Google Scholar]
- 2.Grignard E., Lapenna S., Bremer S. Weak estrogenic transcriptional activities of bisphenol a and bisphenol S. Toxicol. In Vitro. 2012;26(5):727–731. doi: 10.1016/j.tiv.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Liao C., Liu F., Kannan K., Bisphenol S. a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 2012;46(12):6515–6522. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- 4.Viñas P., Campillo N., Martínez-Castillo N., Hernández-Córdoba M. Comparison of two derivatization-based methods for solid-phase microextraction?gas chromatography?mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal. Bioanal. Chem. 2010;397:115–125. doi: 10.1007/s00216-010-3464-7. [DOI] [PubMed] [Google Scholar]
- 5.Tzatzarakis M.N., Vakonaki E., Kavvalakis M.P., Barmpas M., Kokkinakis E.N., Xenos K., Tsatsakis A.M. Biomonitoring of bisphenol A in hair of Greek population. Chemosphere. 2015;118:336–341. doi: 10.1016/j.chemosphere.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez M.F., Arrebola J.P., Taoufiki J., Navalón A., Ballesteros O., Pulgar R., Vilchez J.L., Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007;2:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Liao C., Liu F., Alomirah H., Loi V.D., Mohd M.A., Moon H.B., Nakata H., Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ. Sci. Technol. 2012;12:6860–6866. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- 8.Russo G., Barbato F., Grumetto L. Monitoring of bisphenol A and bisphenol S in thermal paper receipts from the Italian market and estimated transdermal human intake: a pilot study. Sci. Total Environ. 2017:599–600. doi: 10.1016/j.scitotenv.2017.04.192. 68–75. [DOI] [PubMed] [Google Scholar]
- 9.Lee S., Liu X., Takeda S., Choi K. Genotoxic potentials and related mechanisms of Bisphenol A and other Bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere. 2013;93(2):434–440. doi: 10.1016/j.chemosphere.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Kuruto-Niwa R., Nozawa R., Miyakoshi T., Shiozawa T., Terao Y. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ. Toxicol. Pharmacol. 2005;1:121–130. doi: 10.1016/j.etap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Ji K., Hong S., Kho Y., Choi K. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013;47(15) doi: 10.1021/es400329t. 8893–8800. [DOI] [PubMed] [Google Scholar]
- 12.Ullah H., Jahan S., Ain Q.U., Shaheen G., Ahsan N. Effect of bisphenol s exposure on male reproductive system of rats: a histological and biochemical study. Chemosphere. 2016;152:383–391. doi: 10.1016/j.chemosphere.2016.02.125. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R., Liu R., Zong W. Bisphenol S interacts with catalase and induces oxidative stress in mouse liver and renal cells. J. Agric. Food Chem. 2016;64:6630–6640. doi: 10.1021/acs.jafc.6b02656. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.Q., Zhang H.M. Effects of bisphenol S on the structures and activities of trypsin and pepsin. J. Agric. Food Chem. 2014;62:11303–11311. doi: 10.1021/jf504347w. [DOI] [PubMed] [Google Scholar]
- 15.Héliès-Toussaint C., Peyre L., Costanzo C., Chagnon M.C., Rahmani R. Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol. Appl. Pharmacol. 2014;280(2):224–235. doi: 10.1016/j.taap.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Vin҃as R., Watson C.S. Bisphenol S disrupts estradiol-Induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ. Health Perspect. 2013;121(3):352–358. doi: 10.1289/ehp.1205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPlante C.D., Catanese M.C., Bansal R., Vandenberg L.N. Bisphenol S alters the lactating mammary gland and nursing behaviors in mice exposed during pregnancy and lactation. Endocrinology. 2017;10:3448–3461. doi: 10.1210/en.2017-00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catanese M.C., Vandenberg L.N. Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during Pregnancy/Lactation and their daughters. Endocrinology. 2017;3:516–530. doi: 10.1210/en.2016-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Žalmanová T., Hošková K., Nevoral J., Adámková K., Kott T., Šulc M., Kotíková Z., Prokešová S., Jílek F., Králíčková M., Petr J. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci. Rep. 2017;1:485. doi: 10.1038/s41598-017-00570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal G.K., Pal P. 3rd edition. 36-51. University Press Private Limited.; India: 2014. pp. 101–102. (Text Book of Practical Physiology). [Google Scholar]
- 21.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.I. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Kim E. Barrett, Susan M. Barman, Scott Boitano, Heddwen L. Brooks Ganong’s Review of Medical Physiology. Mc Graw Hill Education. 25th Edition. ISBN- 13: 978-93-392-2328-1, ISBN-10: 93-392-2328-4; 553-566.
- 23.Bruce M. Koeppen, Bruce A. Stanton. Berne & Levy Physiology. Mosby Elsevier. 6th Edition. ISBN- 978-0-8089-2412-8; 460-467.
- 24.Kuhn V., Diederich L., Keller 4th T.C.S., Kramer C.M., Lückstädt W., Panknin C., Suvorava T., Isakson B.E., Kelm M., Cortese-Krott M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism. Anemia. Antioxid. Redox Signal. 2017;13:718–742. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari D., Vanage G. Bisphenol an induces oxidative stress in bone marrow cells, lymphocytes, and reproductive organs of holtzman rats. Int. J. Toxicol. 2017;2:142–152. doi: 10.1177/1091581817691224. [DOI] [PubMed] [Google Scholar]
- 26.Tenhunen R., Marver H.S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 1968;2:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inayagi T., Emi Y., Ikushiro S. Biochemical and molecular aspects of genetic disorders of bilirubin metabolism. Biochim. Biophys. Acta. 1998;1407(3):173–184. doi: 10.1016/s0925-4439(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 28.Littledike E.T., Glazier D., Cook H.M. lectrocardiographic changes after induced hypercalcemia and hypocalcemia in cattle: reversal of the induced arrhythmia with atropine. Am. J. Vet. Res. 1976;4:383–388. [PubMed] [Google Scholar]
- 29.Arthur C. Guyton, John E. Hall Text book of Medical Physiology. Elsvier Inc. 11th Edition. ISBN- 0-7216-0240-1; 429-437.
- 30.Zhao F., Jiang G., Wei P., Wang H., Ru S. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio. Ecotoxicol. Environ. Saf. 2017;147:794–802. doi: 10.1016/j.ecoenv.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez A.M., Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int. J. Angiol. 2011;20(3):135–142. doi: 10.1055/s-0031-1284434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rye K.A., Bursill C.A., Lambert G., Tabet F., Barter P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 2009;(50 Suppl):S195–S200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havel R.J. Triglyceride-rich lipoproteins and plasma lipid transport. Arterioscler. Thromb. Vasc. Biol. 2010;30(1):9–19. doi: 10.1161/ATVBAHA.108.178756. [DOI] [PubMed] [Google Scholar]
- 34.Lewis G.F., Rader D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;12:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 35.Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto, Jr. Lubert Stryer. Biochemistry. Freeman Macmillan. 8th Edition. ISBN-13:978-1-4641-2610-9, ISBN-10: 1-4641-2612-0; 782-787.
- 36.Moustafa G.G., Ahmed A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: genotoxic and immunohistochemical implications. Toxicol. Rep. 2016;3:685–695. doi: 10.1016/j.toxrep.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel B.B., Di Iorio M., Chalifour L.E. Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicol. Rep. 2014;1:522–532. doi: 10.1016/j.toxrep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marmugi A., Lasserre F., Beuzelin D., Ducheix S., Huc L., Polizzi A., Chetivaux M., Pineau T., Martin P., Guillou H., Mselli-Lakhal L. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology. 2014;325:133–143. doi: 10.1016/j.tox.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Murray R, Granner D, Rodwell V. Harper’s Illustrated Biochemistry. Mc Graw Hill. 27th Edition. ISBN- 007-125300-9; 134-135.
- 40.Aronson D., Hammerman H., Beyar R., Yalonetsky S., Kapeliovich M., Markiewicz W., Goldberg A. Serum blood urea nitrogen & long term mortality in acute ST- elevation myocardial infarction. Int. J. Cardiol. 2008;127(3):380–385. doi: 10.1016/j.ijcard.2007.05.013. [DOI] [PubMed] [Google Scholar]