Abstract
Introduction
Inconsistent results from previous studies of exercise and cognitive function suggest that rigorously designed randomized controlled trials are urgently needed. Here, we describe the design of the Intense Physical Activity and Cognition (IPAC) study, which will assess the impact of a 6-month high-intensity exercise intervention on cognitive function and biomarkers of dementia risk, compared with a 6-month moderate-intensity exercise intervention and control group (no study-related exercise).
Methods
One-hundred and five cognitively healthy men and women aged between 60 and 80 years are randomized into a high-intensity exercise, moderate-intensity exercise, or control group. Individuals randomized to an exercise intervention undertake 6 months of cycle-based exercise twice a week, at 50 minutes per session. All participants undergo comprehensive neuropsychological testing, blood sampling, brain magnetic resonance imaging, fitness testing, and a body composition scan at baseline, 6 months (immediately after intervention), and 18 months (12 months after intervention).
Discussion
The IPAC study takes a multidisciplinary approach to investigating the role of exercise in maintaining a healthy brain throughout aging. Rigorous monitoring of exertion and adherence throughout the intervention, combined with repeated measures of fitness, is vital in ensuring an optimum exercise dose is reached. Results from the IPAC study will be used to inform a large-scale multicentre randomized controlled trial, with the ultimate aim of pinpointing the frequency, duration, and intensity of exercise that provides the most benefit to the brain, in terms of enhancing cognitive function and reducing dementia risk in older adults.
Keywords: Exercise, Cognition, Dementia, Study design, Intervention
1. Introduction
The relationship between physical activity and cognition has been the subject of consistent study over recent years [1], [2], [3], [4], [5]; yet, despite expanding literature on the topic, questions still remain regarding the effectiveness of physical activity and exercise as a protective measure against the development of cognitive decline and dementia in older adults. While exercise interventions have demonstrated benefits to cognitive function in older adults [3], [6], [7], [8], [9], [10], a recent Cochrane review published in 2015 suggested there was insufficient evidence from randomized controlled trials (RCTs) to conclude such a benefit [11]. Since this review, a number of RCTs have published data demonstrating the benefits of exercise using comprehensive batteries of cognitive tasks as outcome measures. Tamura et al. [10] reported that a 2-year exercise intervention was associated with improvements in attention shift, a positive change that was maintained 6 months after intervention. Vidoni et al. [9] evaluated the potential dose response of exercise duration on cognitive function, demonstrating increasing benefits in visuospatial processing across groups exercising 75 min wk−1, 150 min wk−1, and 225 min wk−1. Most recently, a review commissioned by The Lancet recommended the prescription of exercise for dementia prevention in older adults [12]. In contrast to the above, a recent large RCT (n = 1635) reported no benefits to cognition following a 24-month moderate-intensity (MI) exercise intervention [13]. This study, however, has received methodological criticisms, in particular regarding both the use of relatively short unsupervised exercise sessions and the inability of the investigators to ensure exercise was conducted at a moderate intensity [14]. Importantly, previous observational work indicated that high-intensity (HI) exercise provides greater benefit to cognitive health than low-intensity exercise [15], [16]. The aforementioned findings highlight the importance of rigorous methodological procedures in exercise interventions, particularly in regards to ensuring an optimum exercise dose is reached. Thus, RCTs conducting supervised and monitored exercise interventions are vital in examining the true effect of exercise on cognitive health parameters.
The aim of the Intense Physical Activity and Cognition (IPAC) study is to contribute to the growing research base linking exercise to cognition and more specifically provide clarity for researchers, practitioners, and the community in relation to the impact of exercise intensity on cognitive health, and ultimately dementia risk. The IPAC study has been rigorously designed to ensure that criticisms of previous exercise interventions in older adults are considered. More specifically, we propose to undertake repeated aerobic fitness measurements, conduct supervised HI and MI exercise interventions, and monitor participant exertion throughout each exercise session. Our primary objective is to assess whether 6 months of HI exercise is associated with improved cognitive function, compared with an MI exercise intervention or control group. Our secondary objectives are to assess the impact of a 6-month HI exercise intervention on (1) cortical gray matter (GM) volume, region of interest GM volumes (most specifically, hippocampal volume), and default mode network connectivity measured by magnetic resonance imaging (MRI) and (2) Alzheimer's disease (AD)–related blood-based biomarkers, including proteomics and gene expression, compared with an MI exercise intervention or control group. Our tertiary objectives are to evaluate mediating and moderating variables of the relationship between exercise and cognitive function, that is, evaluating the mediating effect of cardiorespiratory fitness, biomarkers, and brain volume and connectivity and also examining the moderating effect of genotypes associated with increased dementia risk, such as carriage the apolipoprotein (APOE) ε4 allele or the brain-derived neurotrophic factor (BDNF) Val66Met single nucleotide polymorphism.
2. Methods
The IPAC study is conducted as a single-centre single-blind RCT and is currently funded by the National Health and Medical Research Council National Institute of Dementia. The human research ethics committees of Edith Cowan University and Murdoch University (Western Australia) have approved this study. The study is registered with the Australian New Zealand Clinical Trials Registry (under identification number ACTRN12617000643370).
2.1. Participants and power analysis
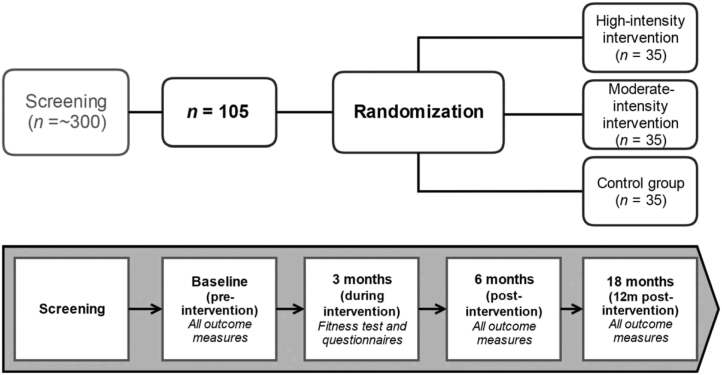
The study cohort comprised 105 cognitively healthy men and women (equal ratio) aged 60 to 80 years. Participants are randomized into one of three groups: HI exercise (n = 35), MI exercise (n = 35), or a control group (n = 35; Fig. 1). Participants will be recruited via advertisements in local community and state-wide newspaper publications, presentations to local community organizations, and word-of-mouth. Detailed inclusion and exclusion criteria are listed in Table 1.
Fig. 1.
Schematic diagram of IPAC study design. Please refer to Table 2 for detailed study outcome measures. Abbreviation: IPAQ, International Physical Activity Questionnaire.
Table 1.
Inclusion and exclusion criteria for the International Physical Activity Questionnaire study
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Our power analysis is based on our primary outcome variable, cognitive function. Using estimates of change in cognitive composite scores from Vidoni et al. [9], the sample size required to detect differences in cognition (domains assessed in Vidoni et al.: verbal memory, visuospatial processing, and simple attention) between the three groups with at least 80% power and at the 5% level of significance is 28 per group. Assuming a dropout rate of 10% during the 6-month intervention and accounting for possible variance due to covariates in our planned analyses (e.g., age, APOE ε4 allele carriage), 35 subjects will be recruited in each of the 3 groups, giving a total required sample size of 105.
2.2. Procedures
In the 4 weeks preceding the start of the exercise intervention (or control period), baseline measurements are obtained in relation to neuropsychological testing, blood sample collection, neuroimaging (MRI), completion of questionnaires, assessments of physical fitness, and a dual-energy X-ray absorptiometry (DXA) scan for the evaluation of body composition. After 3 months of the exercise intervention (or control period), all participants will undergo assessments of physical fitness, a DXA scan, and completion of questionnaires. Following completion of the 6-month intervention (or control period) and at 12-month postintervention completion, all participants will repeat all procedures undertaken at baseline. Study procedures (including fitness testing, blood sample collection, cognitive testing, MRI, and DXA) are undertaken at intervals as per the assessment schedule illustrated in Fig. 1 and described in Table 2.
Table 2.
Overview of assessments at each time point
| Measure | Screening | Baseline (after intervention) | 3 months (during intervention) | 6 months (immediately after intervention) | 18 months (12 months after intervention) |
|---|---|---|---|---|---|
| Primary objective | |||||
| Cognitive assessment | X | X | X | ||
| Secondary objectives | |||||
| Blood sample | X | X | X | ||
| Gene expression | X | X | X | ||
| Biomarkers | X | X | X | ||
| Brain MRI | X | X | X | ||
| Mediating/moderating variables, covariates, and descriptive data | |||||
| Fitness Testing (VO2max) | X | X | X | X | |
| Mini–Mental State Examination | X | ||||
| DXA | X | X | X | X | |
| Height and weight | X | X | X | X | |
| Blood pressure | X | X | X | X | |
| Questionnaires | |||||
| IPAQ | X | X | X | X | X |
| SF-36 | X | X | X | X | |
| DASS | X | X | X | X | |
| CHAMPS | X | X | X | X | |
| CCV FFQ | X | X | X | X | |
| PSQI | X | X | X | X | |
| GDS | X | ||||
| Medical history and demographic information | X | ||||
| Genotyping | X | ||||
Abbreviations: CCV FFQ, Cancer Council of Victoria Food Frequency Questionnaire; CHAMPS, Community Healthy Activities Model Program for Seniors; DASS, Depression, Anxiety and Stress Scale; DXA, dual-energy X-ray absorptiometry; GDS, Geriatric Depression Scale; IPAQ, International Physical Activity Questionnaire; MRI, magnetic resonance imaging; PSQI, Pittsburgh Sleep Quality Index; SF-36, short-form health survey; VO2max, maximal oxygen consumption.
2.3. Randomization
All participants are assigned to one of three groups: HI exercise group, MI exercise group, or control group. Assignment of individuals to each group is performed using a block randomization protocol with a block size of three. Age and gender are both associated with cognitive function, and thus, participants are stratified by age and gender before being randomly assigned to one of the three conditions: HI exercise, MI exercise, or control.
3. Intervention
All participants must demonstrate their ability to undertake upright stationary cycling before the exercise intervention (or control period). Researchers determine the most appropriate setup for each participant, and any participants who cannot adequately use the equipment (i.e. due to functional or anatomical limitations) are excluded from the study.
Participants allocated to an exercise intervention complete 6 months of either an MI or HI cycling program consisting of 100 minutes of cycling per week (two sessions at 50 minutes per session). All exercise sessions are completed within a university setting under the supervision of an Exercise and Sports Science Australia–accredited exercise physiologist and undergraduate exercise science students. Using this supervisory method, a participant-to-researcher ratio of no greater than 3:1 (i.e., 3 participants per researcher) is ensured. All exercise is completed on a cycle ergometer (WattbikePro; Wattbike, Australia) allowing accurate measurement of intensity (wattage), and radiotelemetric heart-rate monitors (Garmin HRM1G; Garmin, USA) are used to provide an assessment of physiological intensity.
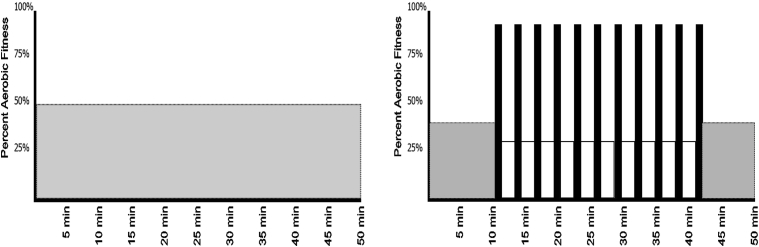
The target exercise intensity for the MI and HI conditions are set using the 6 to 20 Borg Scale of perceived exertion (6 = no exertion and 20 = maximal exertion; [17]). This method is ecologically valid and provides the ability for participants to auto regulate their exercise, thus maintaining a relative level of intensity consistent with changes in fitness over time [18]. All exercise sessions commence with verbal instruction from the supervising exercise specialist regarding the purpose and focus of the session. Participants are educated on the difference between perceived exertion and effort [19] during the first exercise session and are explicitly instructed to base exercise intensity on perceived exertion, irrespective of effort. During the MI sessions, participants exercise at a constant intensity (50%–60% aerobic capacity; 13.0 Borg Scale) for 50 minutes. Individuals in the HI group first complete a 10-minute cycling warm-up at a low intensity (30–40% aerobic capacity; 11.0 Borg Scale) after which they complete 11 intervals of 1 minute of hard exertion (>80% aerobic capacity; 18.0 Borg Scale) interspersed with 2 minutes of active recovery (30%–40% aerobic capacity; 12.0 Borg Scale). At the end of the HI session, participants complete a 9-minute cooldown at a perceived exertion of 11.0 (Fig. 2). Throughout all exercise sessions, heart rate and power output are collected for use in post hoc analyses to assess session intensity. The protocols described previously are theoretically work matched based on an 80-kg person with a maximal aerobic capacity of 27 mL kg−1 min−1 to provide approximately 386 Met min−1 and 380 Met min−1 per week for the MI and HI conditions; respectively.
Fig. 2.
MI (left) and HI (right) training programs. Both exercise protocols have been designed to provide a similar amount of total work per session within ±0.4% over their respective time frames (MI: 386 Met min−1 and HI: 380 Met min−1; based on 80-kg male with a maximal aerobic capacity of 27 mL kg−1 min−1). Abbreviations: HI, high-intensity; MI, moderate-intensity.
Individuals assigned to the control group attend information sessions regarding the benefits of diet and exercise with respect to cognition, dementia, and brain aging; however, these individuals do not receive any instruction related to exercise prescription.
3.1. Intervention safety and compliance
Before the start of any exercise testing or training sessions, all participants are required to obtain medical clearance from their general practitioner for participation in HI exercise. Participants are provided with written documentation to provide to their general practitioner with a plain English description of the study procedures. In addition to the medical clearance, participants are required to complete the Exercise and Sports Science Australia pre-exercise screening questionnaire.
During all exercise sessions, at minimum, one staff member supervising the exercise session must hold a current senior first aid and cardiopulmonary resuscitation certification. The exercise facility has a formalized emergency plan and unrestricted access to an automated defibrillator and supplemental oxygen.
During training sessions, participant attendance is recorded for post hoc analyses. To increase compliance, participants receive encouragement during their session to meet their target intensities and are provided with session feedback at completion.
4. Outcome measures
4.1. Primary outcome measure: neuropsychological assessment
A comprehensive battery of neuropsychological tests has been selected for this study. The selected measures have demonstrated sensitivity, reliability, and validity in previous research with similar participant groups [20] and provide assessment of key areas of cognition, including verbal learning and memory, verbal attention, visuospatial function and memory, working memory, processing speed, and executive function. The neuropsychological measures include Montreal Cognitive Assessment, Wechsler Adult Intelligence Scale-III Digit Span, California Verbal Learning Test (CVLT-II), Brief Visual Memory Test, and Trail Making Test forms A and B. The unstructured task, verbal fluency, flanker, and set-shifting from the NIH EXAMINER are also administered [21]. In addition, the Cogstate battery of tests, including Groton maze learning and recall, one-back task, one-card learning, and continued paired associate learning task are completed by all participants.
The primary outcome measures (global cognitive composite score) are calculated using results from the paper and pen cognitive tasks: Montreal Cognitive Assessment, Digit Span, CVLT-II, Brief Visual Memory Test, Trails A and B, unstructured task, and verbal fluency. Adjusted (for age, gender, and APOE ε4 allele carriage) Z-scores are calculated for each measure (Z-scores for tasks with multiple measures, i.e., CVLT, short and long delay, will be averaged to provide one Z-score to ensure the composite is not heavily weighted toward one task) and are averaged to yield a composite Z-score. Further analyses will be conducted to examine the effect of the intervention on specific domains of cognitive function.
4.2. Secondary outcome measures
4.2.1. Genotyping, blood-based biomarkers, and gene expression
At baseline, 6, and 18 months, fasted (10 hours) venous blood samples are collected into serum-separating tubes (isolation of serum), tubes containing EDTA (isolation of plasma) and PAXgene tubes (for gene expression analysis). Blood samples will be analyzed for standard blood chemistry analyses, measurement of blood biomarkers and gene expression. Blood processing is conducted in accordance with the Australian Imaging Biomarkers and Lifestyle Study protocol [22]. The blood products collected for biomarker research are stored in liquid nitrogen until required for analysis.
Genotyping is completed on baseline samples only: briefly, manufacturer's instructions are followed to extract DNA from whole blood using QIAamp DNA Blood Maxi Kits (Qiagen, Hilden, Germany). TaqMan genotyping assays are used to determine APOE genotype (rs7412, assay ID: C____904973_10; rs429358, assay ID: C___3084793_20), and BDNF Val66Met single nucleotide polymorphism (rs6265, assay ID: C__11592758_10).
Gene expression profiles are assessed at baseline and 6 months on RNA extracted from PAXgene Blood RNA tubes (Becton, Dickinson and Company) using the PAXgene Blood RNA Kit (Qiagen). ThermoFisher Scientific (Life Technologies) TaqMan Human AD microfluidic array cards (Cat#4378713) are used to simultaneously assess expression of 94 AD-associated genes and 2 endogenous controls (18S and HPRT1). BDNF gene expression, not included on the array, is analyzed separately using a standalone TaqMan gene expression assay (assay ID: Hs02718934_s1) and endogenous controls (18S, assay ID: Hs99999901_s1; HPRT1, assay ID: Hs99999909_m1). TaqMan genotyping and gene expression assays are performed on a QuantStudio 12K Flex Real-Time-PCR system (Applied Biosystems, Foster City, CA) using the TaqMan GTXpress and Gene Expression Master Mix (Life Technologies), respectively.
For all blood samples, a panel of blood-based proteomic biomarkers will be measured. Blood biomarkers will be included in the panel based on current evidence available in the literature of high sensitivity and specificity in predicting conversion to dementia [23], [24], [25]. We will also quantify levels of blood biomarkers known to play an important role in the relationship between exercise and brain health, that is, growth factors such as BDNF, nerve growth factor, and vascular endothelial growth factor.
4.2.2. Magnetic resonance imaging
At baseline, 6, and 18 months, participants are positioned in a standard head coil and a brief scout T1-weighted (T1W) image obtained, followed by a magnetization-prepared rapid gradient-echo sequence and a T2 sequence: The structural magnetization-prepared rapid gradient-echo and T2-weighted images are segmented into white matter (WM), GM, and cerebrospinal fluid, and GM cortical thickness is computed [26]. Regions of interest are propagated from an atlas to the T1W images to extract various regional volumes (including hippocampal volume). The full pipeline used to segment the T1W images has been detailed previously [27]. Following the volumetric sequences, fluid-attenuated inversion recovery and diffusion tensor imaging sequences are conducted for the measurement of WM hyperintensities and WM connectivity, respectively.
Following this, two functional MRI (fMRI) blood-oxygen level dependent sequences are performed for the investigation of brain activation and connectivity while at rest and during a cognitive task. During the blood-oxygen level dependent sequences, participants are asked to (1) be at rest with their eyes open (resting-state fMRI) and then (2) participate in an n-back task (1-back and 2-back), by looking at a screen positioned above their face and by responding to buttons held in their hands (task-evoked fMRI). FMRIB Software Library Multivariate Exploratory Linear Optimized Decomposition into Independent Components is utilized to conduct independent component analyses, with the aim of identifying changes in the default mode network from preintervention to postintervention. FMRIB Software Library FMRI Expert Analysis Tool will be used to analyze patterns of brain activation during the n-back task from preintervention to postintervention [28].
4.3. Demographic, descriptive, and other potential mediating factors
4.3.1. Demographic and medical history data collection
Demographic and medical history information is collected by a staff member via questionnaire and semi-structured interview. Demographic and medical history information is collected at screening (questions pertaining to eligibility only) and baseline (more extensive detail). Comprehensive health history taken at baseline will include questions regarding prior illness/surgery and medication use (past and current, prescribed, over the counter, and supplements).
4.3.2. Physical assessments
At baseline, 3 months (mid intervention), 6 months (immediately after intervention) and 18 months (12 months after intervention completion), participants are assessed for their maximal aerobic capacity (VO2max) and peak power using a cycling-based graded exercise test. All tests use 2-minute stage durations with consistent increases in work rate at each stage until participants reach volitional fatigue. To ensure similar test durations and stage progression (i.e., no greater than 2 metabolic equivalent units), test selection is determined relative to the participants baseline body mass using the following criteria: (1) participants under 70 kg commence testing at 30 W with increases of 20 W each stage, (2) participants between 70 to 100 kg commence testing at 30 W with increases of 25 W each stage, and (3) participants over 100 kg commence testing at 40 W with increases of 35 W each stage.
During each test, heart rate is continuously recorded and expired ventilation is collected and analyzed as 15-second mean values, using a Parvo TrueOne (ParvoMedics, USA) metabolic cart, for the rate of oxygen consumption (VO2) and carbon dioxide production (VCO2). Before each test, the pneumatach is calibrated across a range of flow rates (50 to 400 L min−1), and the oxygen and carbon dioxide sensors are calibrated to a known gas mixture (16% oxygen and 4% carbon dioxide). At test completion, maximal heart rate is determined as the highest heart rate value recorded during the test, and VO2max is determined as the highest 15-second mean VO2 value obtained during the final 2 minutes of the test. Additional criteria for the assessment of VO2max involve participants reaching a maximal heart rate greater than 85% of their age predicted maximum (i.e., (220 − age) * 0.85) and a respiratory exchange ratio (VCO2/VO2) greater than 1.15. Peak power is determined using the following equation:
where PLCS is the power at the last completed stage, Fst is the fraction of the last uncompleted stage, and BMP is the body mass–specific increase in work rate per stage.
4.3.3. DXA scan
All participants undergo a DXA scan at baseline, 3-, 6-, and 18-month assessments. DXA uses very low dose radiation in a collimated beam to determine the volume of fat, muscle, and bone tissue in the whole of the body. All DXA scans are completed using the Hologic Discovery Bone Densitometer (Hologic, USA): segmental composition is determined via Hologic internal software.
4.3.4. Questionnaires
At baseline only, participants complete questionnaires regarding subjective memory complaints (Memory Assessment Clinical-Questionnaire) and personality factors (NEO Personality Inventory). Furthermore, participants complete a series of questionnaires at all time points to assess health and role function (Health Short form; SF-36), depression and anxiety (Depression, Anxiety and Stress Scale), habitual physical activity levels (international physical activity questionnaire and Community Healthy Activities Model Program for Seniors), sleep quality (Pittsburgh Sleep Quality Index), and nutrient intake (Cancer Council of Victoria Food Frequency Questionnaire).
5. Statistical analysis
Statistical analysis will be overseen by a biostatistician and will be performed using IBM SPSS Statistics, version 22, data analysis software (IBM Corporation) and the R environment, version 3.3.2 [29]. Quantitative data will be log-transformed before statistical analyses to ensure a gaussian distribution.
Each of the proteomic and gene expression biomarker panels will be represented via the top 2–3 Eigen vectors that represent the maximal variance explained by the biomarker set. To do this, singular value decomposition will be used on the log-transformed and scaled biomarker data before choosing the optimal components to represent the biomarker panel. These components will then be used in subsequent linear mixed modeling to represent overall changes in blood-based biomarkers.
To address the primary objective, intention-to-treat analyses will be conducted. Furthermore, a per-protocol analysis will be conducted using data from only those who completed the exercise intervention with at least 80% adherence. A linear mixed model will be used to examine the effect of the HI intervention, compared with the MI and control groups, on the global cognitive composite score (accounting for confounders such as gender, age, and APOE ε4 allele carriage). Post hoc analyses will be conducted for any significant group*time interactions.
To address the secondary objectives, both intention-to-treat and per-protocol analyses will again be conducted. A series of linear mixed models will be conducted to examine the effect of the HI intervention, compared with the MI group and control group, on Eigen vectors from blood biomarkers and gene expression panels, and MRI-quantified brain volume (including cortical GM volume and regions of interest such as hippocampal volume) and default mode network connectivity (accounting for confounders). Post hoc analyses will be conducted for any significant group*time interactions.
Additional mediation analyses will be conducted to evaluate the potential of particular variables (physical fitness, biomarkers) as mediators in the relationship between exercise and cognition. Moderation analyses will also be conducted to examine the moderating effect of relevant genotypes (i.e., APOE, BDNF Val66Met) on the relationship between exercise and cognition. Using the PROCESS macro in SPSS [30], ordinary least squares path analysis will be conducted to perform a series of simple mediation and moderation analyses.
6. Discussion
The IPAC study is a proof-of-principle trial that aims to compare the effects of HI cycling-based exercise on cognitive health (i.e., neuropsychological measures, MRI biomarkers, and blood-based biomarkers), with an MI cycling-based exercise intervention and a control group. The outlined protocol provides (1) robust fitness assessments and monitored training sessions, ensuring compliance and the ability to assess the contribution of changes in fitness to cognitive performance, (2) a set of validated cognitive assessments consistent with the field, allowing for comparability with the scientific literature, and (3) repeated measures of a range of brain and blood-based biomarkers associated with cognitive decline and dementia risk to help characterize possible mechanistic contributions of exercise to maintaining brain health throughout aging.
The IPAC study has the potential to provide the first evidence of its kind for the benefits of exercise intensity on cognitive function and biomarkers of brain health and may contribute to the development of “Best Practice” preventative public health strategies to enhance cognitive function and delay dementia onset in older adults. If proven effective, exercise represents a cost-effective and safe method for maintaining a healthy aging brain throughout older adulthood.
Research in Context.
-
1.
Systematic review: The authors reviewed previous published literature in the field of physical activity, cognition and dementia. Previous observational studies have reported that high-intensity physical activity is of greater benefit to cognitive function than low-intensity physical activity. Interventional research evaluating the effect of exercise on cognitive function has provided inconclusive results.
-
2.
Interpretation: There is a requirement for future studies to utilise repeated measures of fitness and monitoring of physical exertion throughout exercise interventions to ensure participants are reaching desired intensities. Furthermore, comprehensive outcome measures relating to brain health need to be measured, including cognitive tests, magnetic resonance imaging biomarkers, and blood-based biomarkers.
-
3.
Future directions: This article describes the protocol of a trial examining the effect of high-intensity exercise on cognitive function. Results from this project will be vital in the design of future large randomised controlled trials.
Acknowledgments
This IPAC study is supported by a National Health and Medical Research Council Dementia Research Development Fellowship awarded to B.M.B. (grant number: GNT1097105). In addition, a Brain Foundation research grant awarded to B.M.B., S.M.L., J.J.P., S.R.R.-S., and R.N.M. will support the gene expression analyses (no grant number).
B.M.B. prepared the manuscript and was involved in study design; S.R.R.-S. was involved in study design conception and provided critical review of the manuscript; N.C. and S.M. were involved in the administrative design and provided revisions of the manuscript; N.G. was involved in the design of the exercise intervention and fitness testing; H.R.S. and M.W. designed the neuropsychological battery; S.M.L. designed the protocol for genotyping and gene expression analysis; J.D. prepared the statistical analysis plan; K.S. prepared the MRI analysis protocol; R.N.M. was involved in study design and provided revisions of the manuscript; and J.J.P. was involved in study design conception and assisted with preparation of the manuscript.
Footnotes
There are no conflicts of interest to declare.
References
- 1.Middleton L.E., Mitnitski A., Fallah N., Kirkland S.A., Rockwood K. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS One. 2008;3:e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weuve J., Kang J.H., Manson J.E., Breteler M.M., Ware J.H., Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 3.Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., van Bockxmeer F.M., Xiao J. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K., Barnes D., Nevitt M., Lui L.Y., Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 5.Liu-Ambrose T., Nagamatsu L.S., Graf P., Beattie B.L., Ashe M.C., Handy T.C. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer A.F., Hahn S., Cohen N.J., Banich M.T., McAuley E., Harrison C.R. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 7.Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2009;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughan S., Wallis M., Polit D., Steele M., Shum D., Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014;43:623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- 9.Vidoni E.D., Johnson D.K., Morris J.K., Van Sciver A., Greer C.S., Billinger S.A. Dose-Response of Aerobic Exercise on Cognition: A Community-Based, Pilot Randomized Controlled Trial. PLoS One. 2015;10:e0131647. doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura M., Nemoto K., Kawaguchi A., Kato M., Arai T., Kakuma T. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int J Geriatr Psychiatry. 2015;30:686–694. doi: 10.1002/gps.4205. [DOI] [PubMed] [Google Scholar]
- 11.Young J., Angevaren M., Rusted J., Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015:CD005381. doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017 doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 13.Sink K.M., Espeland M.A., Castro C.M., Church T., Cohen R., Dodson J.A. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314:781–790. doi: 10.1001/jama.2015.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulin M.J., Eskes G.A., Hill M.D. Physical activity vs health education for cognition in sedentary older adults. JAMA. 2016;315:415. doi: 10.1001/jama.2015.16005. [DOI] [PubMed] [Google Scholar]
- 15.Brown B.M., Peiffer J.J., Sohrabi H.R., Mondal A., Gupta V.B., Rainey-Smith S.R. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry. 2012;2:e191. doi: 10.1038/tp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angevaren M., Vanhees L., Wendel-Vos W., Verhaar H.J., Aufdemkampe G., Aleman A. Intensity, but not duration, of physical activities is related to cognitive function. Eur J Cardiovasc Prev Rehabil. 2007;14:825–830. doi: 10.1097/HJR.0b013e3282ef995b. [DOI] [PubMed] [Google Scholar]
- 17.Borg G. Human kinetics; Champaign: 1998. Borg's Perceived Exertion and Pain Scales. [Google Scholar]
- 18.Tucker R. The anticipatory regulation of performance: the physiological basis for pacing strategies and the development of a perception-based model for exercise performance. Br J Sports Med. 2009;43:392–400. doi: 10.1136/bjsm.2008.050799. [DOI] [PubMed] [Google Scholar]
- 19.Abbiss C.R., Peiffer J.J., Meeusen R., Skorski S. Role of ratings of perceived exertion during self-paced exercise: what are we actually measuring? Sports Med. 2015;45:1235–1243. doi: 10.1007/s40279-015-0344-5. [DOI] [PubMed] [Google Scholar]
- 20.Salmon D.P., Bondi M.W. Neuropsychological assessment of dementia. Annu Rev Psychol. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer J.H., Mungas D., Possin K.L., Rankin K.P., Boxer A.L., Rosen H.J. NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc. 2014;20:11–19. doi: 10.1017/S1355617713001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lui J.K., Laws S.M., Li Q.X., Villemagne V.L., Ames D., Brown B. Plasma amyloid-beta as a biomarker in Alzheimer's disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 23.Doecke J.D., Laws S.M., Faux N.G., Wilson W., Burnham S.C., Lam C.P. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnham S.C., Rowe C.C., Baker D., Bush A.I., Doecke J.D., Faux N.G. Predicting Alzheimer disease from a blood-based biomarker profile: a 54-month follow-up. Neurology. 2016;87:1093–1101. doi: 10.1212/WNL.0000000000003094. [DOI] [PubMed] [Google Scholar]
- 25.Gupta V.B., Hone E., Pedrini S., Doecke J., O'Bryant S., James I. Altered levels of blood proteins in Alzheimer's disease longitudinal study: results from Australian Imaging Biomarkers Lifestyle Study of Ageing cohort. Alzheimers Dement (Amst) 2017;8:60–72. doi: 10.1016/j.dadm.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dore V., Villemagne V.L., Bourgeat P., Fripp J., Acosta O., Chetelat G. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70:903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeat P., Chetelat G., Villemagne V.L., Fripp J., Raniga P., Pike K. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 28.Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team . 2008. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: http://www.R-project.org. [Google Scholar]
- 30.Hayes A.F. 2012. PROCESS: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling [White Paper]http://www.afhayes.com/public/process2012.pdf Available at: [Google Scholar]