Abstract
Background
Dehydration of airway surface liquid (ASL) disrupts normal mucociliary clearance (MCC) in sinonasal epithelium, which may lead to chronic rhinosinusitis (CRS). Abnormal chloride (Cl−) transport is one such mechanism that contributes to this disorder and can be acquired secondary to environmental perturbations, such as hypoxia at the tissue surface. The objective of this study was to assess the technological feasibility of the novel micro-optical coherence tomography (μOCT) imaging technique for investigating acquired MCC defects in cultured human sinonasal epithelial (HSNE) cells.
Methods
Primary HSNE cell cultures were subjected to a 1% oxygen environment for 12 hours to induce acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. Ion transport characteristics were assessed with pharmacologic manipulation in Ussing chambers. ASL, periciliary fluid (PCL), and ciliary beat frequency (CBF) were evaluated using μOCT.
Results
Amiloride-sensitive transport (ΔISC) was greater in cultures exposed to hypoxia (hypoxia: −13.2 ± 0.6 μA/cm2; control: −6.5 ± 0.1 μA/cm2; p < 0.01), whereas CFTR-mediated anion transport was significantly diminished (hypoxia: 28.6 ± 0.3 μA/cm2; control: 36.2 ± 1.6 μA/cm2; p < 0.01), consistent with acquired CFTR dysfunction and sodium hyperabsorption. Hypoxia diminished all markers of airway surface function microanatomy as observed with μOCT, including ASL (hypoxia: 5.0 ± 0.4 μm; control: 9.0 ± 0.9 μm; p < 0.01) and PCL depth (hypoxia: 2.5 ± 0.1 μm; control: 4.8 ± 0.3 μm; p < 0.01), and CBF (hypoxia: 8.7 ± 0.3 Hz; control: 10.2 ± 0.3 Hz; p < 0.01).
Conclusion
Hypoxia-induced defects in epithelial anion transport in HSNE led to predictable effects on markers of MCC measured with novel μOCT imaging. This imaging method represents a technological leap forward and is feasible for assessing acquired defects impacting the airway surface.
Keywords: μOCT, acquired CFTR deficiency, airway surface liquid, CFTR, chronic sinusitis, ciliary beat frequency, cystic fibrosis, mucociliary clearance, mucociliary transport, optical coherence tomography
In healthy individuals, mucociliary clearance (MCC) is part of the innate defense mechanism and functions to protect the airways by trapping inhaled ambient pathogens within the mucus layer of epithelial surfaces and propelling it out of the airways through coordinated cilia movement.1–3 In fact, MCC is the primary mechanism by which particulate matter is removed from the airways.4 Normal functioning of MCC consists of 2 equally important components: mucus production and mucus transport,4 both of which depend on coordinated ciliary beating, adequate hydration of airway surface liquid (ASL), periciliary liquid (PCL), and mucus viscosity. Ciliary beat frequency (CBF) is a quantifiable, highly regulated function of ciliated respiratory epithelium and an established indicator of well-differentiated airway epithelium.5 Similarly, both ASL and the thin layer of fluid gel surrounding the cilia, known as the PCL, serve as indices of normal MCC function. Disruption to any one of these key constituents leads to impaired MCC, regardless of whether etiology results from environmental insults (eg, smoking) or underlying pathology. When MCC is compromised, airways become vulnerable to a vicious cycle of infection and obstruction, resulting in poor tissue oxygenation with subsequent hypoxia-induced ion transport dysfunction that only further perpetuates disease progression.6,7 For example, aberrant vectorial ion transport secondary to absent or dysfunctional cystic fibrosis transmembrane conductance regulator (CFTR) in patients with cystic fibrosis (CF) results in ASL dehydration, elevated mucus viscosity, and MCC impairment.8–12 As a result, patients develop thick, inspissated secretions that cause a myriad of respiratory complications. Dysfunctional MCC is the underlying pathophysiologic process of many airway diseases characterized by inflammation and infection, such as primary ciliary dyskinesia and chronic rhinosinusitis (CRS).6,13–15
At present, there is a paucity of methods available for quantitatively assessing defects in MCC, which has greatly limited our ability to fully understand the pathogenesis of the early developmental stages of airway disease. Currently, the most common method for evaluation of MCC is through in-vivo measurement of radioactivity after inhalation of radiolabeled aerosol particles.2,16,17 Although this method has been useful in the evaluation of new drug therapies, it is limited by a number of factors,18,19 primarily with regard to its ability to provide detail at the cellular level.20 There are methods available for studying measures of functional microanatomy, such as ASL, PCL, CBF, and mucociliary transport (MCT), but they too are limited in their utility. For example, X-Z scanning confocal microscopy reliably measures ASL; however, this is not easily performed in vivo and entails the use of transient dyes.21,22 Moreover, measurement of PCL requires fixation with osmium tetroxide and preservation of ASL with perfluorocarbon, which is destructive and can only be performed on dead tissue.20 CBF can be assessed using high-frame-rate phase contrast microscopy, but this too has limited in-vivo utility.23–25 Last, all of these imaging techniques require distinct equipment, which precludes simultaneous assessment of functional measures and the dynamic study of MCC.20 Development of suitable technology capable of simultaneously evaluating functional airway microanatomy would represent a significant improvement in the study of upper airway disease.
In the airways, optical coherence tomography (OCT) creates cross-sectional images through reflectance properties inherent to the airways.26 Cross-sectional imaging facilitates simultaneous assessment of ASL, PCL, and CBF, all of which can be evaluated noninvasively without perturbations to the airway surface. In fact, OCT has become a cornerstone in biomedical imaging due to its ability to acquire relatively noninvasive, cross-sectional images that maintain a high-resolution, particularly when compared with ultrasound.27 However, its optical resolution trails that of other microscopy systems, such as confocal microscopy, due to discordance between focal depth and numerical aperture, which precludes the simultaneous acquisition of images with both high resolution and increased depth.27 More recently, an OCT system was engineered for capturing microanatomy with improved precision using micro-OCT (μOCT), which permits 1-μm resolution and unprecedented subcellular detail not possible with traditional OCT.20 Using this novel technique, functional airway parameters such as ASL and PCL, which possess natural reflectance properties, can be measured noninvasively and without exogenous contrast dyes.20
The objective of this study was to assess the technological feasibility of μOCT for investigating acquired MCC defects in cultured human sinonasal epithelial (HSNE) cells.
Materials and methods
Tissue culture
Culture of human sinonasal epithelial cells
Institutional review board approval was obtained from the University of Alabama at Birmingham prior to initiation of the study. Human nasal turbinate tissue was collected and cultured at an air-liquid interface (ALI) according to our previously described methods.28–33 Maximal differentiation with cilia production occurs at 3 weeks.29 Contingent upon contamination of nonepithelial cells, approximately 80% of the surface could be expected to exhibit well-differentiated cilia by 2 weeks.29 Cultures were incubated in a hypoxia chamber (1% oxygen) for 12 hours and compared with control filters for all studies.
Bioelectric measurements
Chemicals and solutions
All chemicals were acquired from Sigma-Aldrich (St. Louis, MO). Bath solution contents consisted of (in mmol/L): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose, with a pH of 7.3 or 7.4 in a gassed mixture of 95% O2 and 5% CO2, at 37°C. All studies were conducted in low Cl− (6 mmol/L) mucosal baths. Pharmacologic manipulations consisted of amiloride (100 μmol/L), forskolin (20 μmol/L), and CFTRInhibitor-172 (10 μmol/L), as described elsewhere.30,31,34 Amiloride, which inhibits epithelial sodium channels (ENaC), was administered to assure that any changes in short-circuit current (ISC) were independent of effects upon ENaC activity. Forskolin, an indirect CFTR activator that operates via a cAMP-dependent pathway, was administered to assess CFTR function indirectly. CFTRInh-172 is a highly specific CFTR inhibitor and blocks CFTR-dependent Cl− current.
Measurement of ISC and conductance
Transwell inserts were placed in Ussing chambers for monitoring of ion transport and pharmacologic blockade as described elsewhere.30 Apical monolayers were analyzed in short-circuit conditions following compensation of fluid resistance with automatic voltage clamps (VCC 600; Physiologic Instruments, San Diego, CA). Inserts were subsequently mounted in the aforementioned bath solution at 37°C. Short-circuit measurements were recorded at 1 sample/s and, by convention, positive deflections indicated net anion movement from the serosal to mucosal surface.
Analysis of CBF
HSNE cultures were visualized on an inverted microscope (Fisher Scientific, Pittsburgh, PA) using a 20× objective lens. A high-speed monochromatic digital video camera (Model A602f-2; Basler AG, Ahrensburg, Germany) was used to record data at a sampling rate of 100 frames/s. Images were analyzed with Sisson-Ammons Video Analysis (SAVA, version 2.0.8 W) system.5,32,33,35–37 Using the inverted microscope, large regions of beating cilia were identified within HSNE ALI cultures for each experiment. Captured images were then analyzed for CBF with virtual instrumentation software. Prior to apical fluid application to cell monolayers (addition of apical fluid stimulates CBF), baseline recordings of CBF were performed in both control and hypoxia-exposed cultures for 5 minutes. After baseline CBF measurement, a solution consisting of 100 μL of phosphate-buffered saline (PBS) and 20 μmol/L forskolin was added and stimulated CBF was recorded for another 15 minutes and compared with vehicle control.
Reported CBFs represent the arithmetic means of all data points. All experiments were carried out at room temperature (23°C).
μOCT
Image acquisition
μOCT imaging of HSNE cultures was performed with incident illumination of the apical cell surface to assess microanatomic parameters of ASL, PCL, and CBF in vitro. To reduce errors in geometric measurements, the imaging optics axis was placed within 10 degrees of normal to the cell plane as previously described.20 All imaging was performed at 4 regions of interest per each well (2 points at 1 mm from the center and another at 1 mm from the edge for 2 different locations).
Image analysis
ASL and PCL were quantitatively evaluated by directly measuring the visible thickness of the respective layers in the image. To account for refractory properties of the liquid, layer thickness measurements were corrected for the index of refraction of the liquid (n = 1.33). Alternatively, CBF was evaluated using a time series of images and quantitatively measured by identifying peak amplitude frequency in the temporal Fourier transform of areas demonstrating oscillatory behavior.20 All parameters were assessed at 5 uniformly distributed areas of the image. All images were analyzed using ImageJ version 1.50i (National Institutes of Health, Bethesda, MD) and MATLAB® R2016a (The MathWorks, Natick, MA).
Statistical analysis
Statistical analyses were performed using Excel (Microsoft, Redmond, WA) using 2-tailed, unpaired t tests for all Ussing chamber, CBF, and μOCT studies of HSNE cultures. All values are reported as the mean ± standard error of the mean (SEM). p < 0.05 was considered statistically significant.
Results
Ion transport phenotype of hypoxia-induced HSNE cultures reflects acquired CFTR deficiency
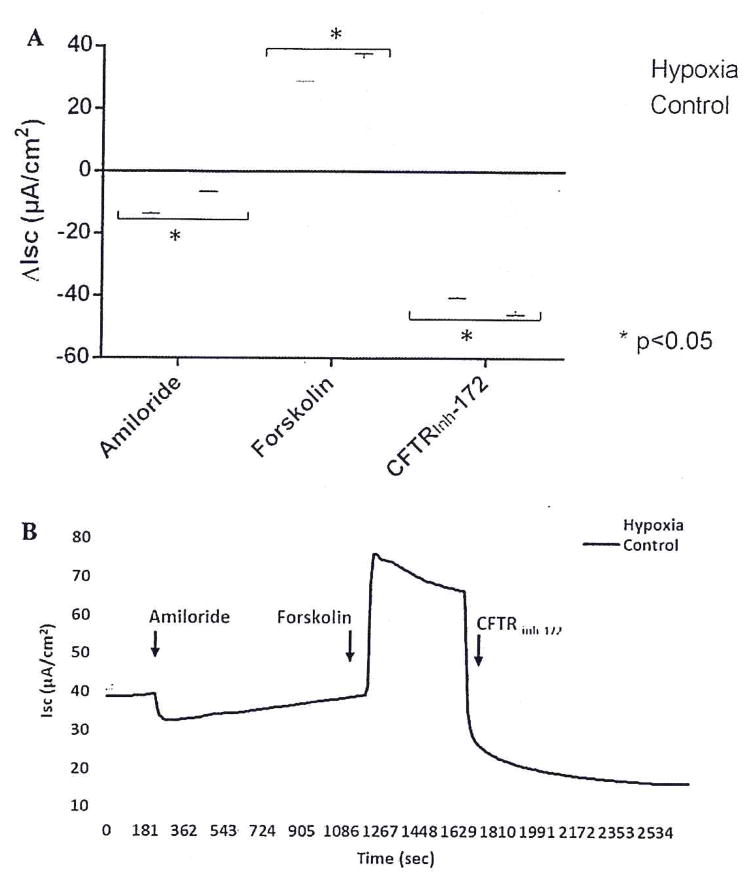
To establish an acquired CFTR dysfunction phenotype, cells were subjected to 12 hours of hypoxia, as described elsewhere.6,14 Ussing chamber analysis (Fig. 1) was performed on both hypoxia-induced and control HSNE cultures after pharmacologic manipulation with amiloride, forskolin, and CFTRInh-172. Amiloride-sensitive ΔISC was significantly more robust in hypoxia-induced HSNE when compared with the control group (−13.2 ± 0.6 μA/cm2 vs −6.5 ± 0.1 μA/cm2; p < 0.01), suggesting that epithelial Na+ transport is enhanced under hypoxic conditions, similar to what is observed in HSNE from CF patients.14,30–33 Forskolin-stimulated anion transport was significantly greater in the control group compared with the hypoxia-induced HSNE cultures (36.2 ± 1.6 μA/cm2 vs 28.6 ± 0.3 μA/cm2; p < 0.01), which suggests that hypoxia induced a state of acquired (partial) CFTR deficiency in human sinonasal epithelium. Addition of the CFTR-specific inhibitor, CFTRInh-172, led to a significantly larger reduction in CFTR-mediated Cl− transport in the control group (−44.5 ± 1.4 μA/cm2 vs −40.1 ± 0.5 μA/cm2; p < 0.05). This supports the observation that diminished forskolin-stimulated Cl− secretion is secondary to CFTR deficiency.
FIGURE 1.
(A) Representative Ussing chamber current tracings and (B) summary of current measurements from hypoxia-induced and control HSNE cultures after administration of amiloride, forskolin, and CFTRInh-172. Error bars represent standard error of the mean. Significant findings are indicated with a bracketed asterisk. CFTRInh-172 = cystic fibrosis transmembrane conductance regulator inhibitor 172; HSNE = human sinonasal epithelial (cells).
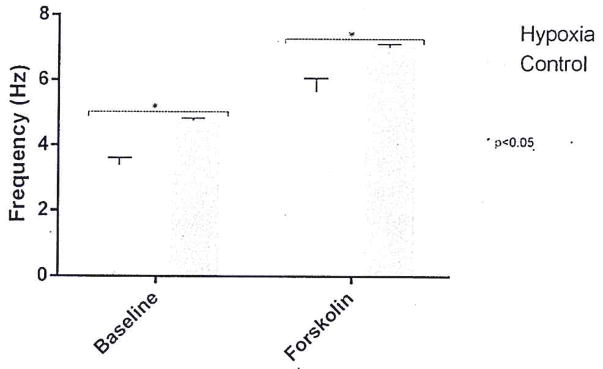
Hypoxia reduces CBF in HSNE cultures
Both baseline and forskolin-stimulated CBF were evaluated using traditional measures (SAVA imaging) to identify whether 12-hour hypoxia would diminish ciliary function (Fig. 2). Baseline CBF was significantly greater in control (n = 6) than in hypoxia-induced (n = 6) HSNE cultures (4.7 ± 0.08 Hz vs 3.4 ± 0.2 Hz; p < 0.01). Similarly, forskolin-stimulated CBF was significantly faster in the control group (7.0 ± 0.09 Hz vs 5.6 ± 0.4 Hz; p < 0.01).
FIGURE 2.
Summary of CBF measurements from hypoxia-induced and control HSNE cultures at baseline and after pharmacologic manipulation with forskolin. Error bars represent standard error of the mean. Significant findings are bracketed with an asterisk. HSNE = human sinonasal epithelial (cells).
Measurement of functional airway microanatomy using μOCT
With diminished markers of mucociliary function confirmed in our model of acquired CFTR deficiency in HSNE, μOCT was used to assess ASL, PCL, and CBF in hypoxia-induced (n = 10) and control (n = 10) HSNE cultures (Fig. 3). Mean ASL and PCL depth thickness were significantly greater in the control group when compared with hypoxia-induced HSNE cultures (ASL: 9.0 ± 0.9 μm vs 5.0 ± 0.4 μm; p < 0.01; PCL: 4.8 ± 0.3 μm vs 2.5 ± 0.1 μm; p < 0.01) (Fig. 4). Mean CBF was also significantly faster in the controls compared with the hypoxic cultures (10.2 ± 0.3 Hz vs 8.7 ± 0.3 Hz; p < 0.01), consistent with Hoffman contrast microscopy. MCT could not be measured during analysis due to a thin layer of thick mucus overlying cell cultures, which prevented the measurement of quantifiable movement.
FIGURE 3.
μOCT images demonstrating ASL and PCL thickness in control (A) and hypoxia-induced (B) HSNE cultures. ASL = airway surface liquid; μOCT = microoptical coherence tomography; HSNE = human sinonasal epithelial (cells); PCL = periciliary fluid.
FIGURE 4.
Summary of ASL, PCL, and CBF measurements in hypoxia-induced and control HSNE cultures at baseline. Left y-axis corresponds to ASL and PCL thickness (μm); right y-axis corresponds to CBF (Hz). Error bars represent SEM. Significant findings are bracketed with an asterisk. ASL = airway surface liquid; CBF = ciliary beat frequency; PCL = periciliary fluid.
Discussion
Hypoxia-induced reductions in CFTR-mediated Cl− transport in HSNE cultures lead to predictable changes in ion transport phenotype, as previously described.6 In this study, we confirmed that both the ion transport phenotype and measurements of airway functional microanatomy in hypoxia-induced HSNE cultures mimic those observed in mild cases of CF, as demonstrated by significantly reduced forskolin-sensitive current and increased amiloride-sensitive current with a corresponding diminishment of ASL, PCL, and CBF. In fact, the enhanced amiloride response indicated a state of Na+ hyperabsorption at baseline while the reduced activation with forskolin, in combination with the reduction of CFTR-mediated Isc to similar levels, reflected a state consistent with acquired (partial) CFTR deficiency. These findings confirm that hypoxia-induced HSNE cultures represent a suitable model for studying acquired CFTR defects in vitro.
Until recently, assessment of these functional parameters has been rather cumbersome because multiple devices were required for gathering these measures. However, the recent development of the μOCT system has permitted simultaneous acquisition of ASL, PCL, CBF, and MCT parameters with resolution comparable with “gold standard” imaging techniques. In contrast to images acquired with the SAVA system and traditional OCT imaging, which has an axial resolution of approximately 3 μm, μOCT systems can achieve axial resolution of 1 μm in tissue.20 This permits more precise PCL measurement, which requires axial resolution that is a fraction of normal physiologic conditions for conditions in which PCL is significantly reduced, such as in patients with CF where PCL is only ~3 μm.20 In the current study, we were able to assess ASL, PCL, and CBF using μOCT. However, due to a thick, stationary mucus layer overlying HSNE cultures, we were unable to measure MCT. For validation, we compared our findings to those recorded with the SAVA system. Although both systems clearly demonstrated significantly reduced CBF in hypoxia-induced HSNE cultures, direct comparisons were not possible due to the differing temperatures and environmental conditions. μOCT is performed at a physiologic temperature in 100% humidity conditions, so it is closer to what would be expected physiologically, whereas Hoffman contrast microscopy is taken at room temperature. μOCT also demonstrated significantly reduced ASL and PCL thickness in the hypoxia-induced cultures, which is anticipated with reduced function or levels of CFTR. Furthermore, the ability of μOCT to accurately measure both ASL and PCL represents a significant advantage and supports the use of this technology to assess these parameters, particularly when they are reduced due to underlying pathology.
The development of an imaging system capable of simultaneously capturing measurements of functional microanatomy with improved precision using μOCT represents a significant advancement for both in-vivo and in-vitro studies of the upper airway. μOCT can be used to measure these functional parameters under a variety of conditions in multiple airway models. This model of acquired CFTR deficiency will be invaluable for understanding the mechanism and severity of CFTR dysfunction in CRS and will lead to new insights into disease pathogenesis. Importantly, this technology can rapidly measure effects of CFTR potentiators that provide a possible new avenue of therapy for ameliorating acquired forms of CFTR deficiency in diseases such as CRS.
Conclusion
Our findings suggest that hypoxia-induced HSNE cultures are a suitable model for the in-vitro study of acquired CFTR defects. μOCT permits high-resolution imaging and facilitates simultaneous measurement of functional microanatomy parameters, such as ASL, PCL, and CBF. In the upper airways, this technology represents a feasible option for investigating in-vitro MCC dysfunction and its role in the development sinus disease.
Acknowledgments
Funding sources for the study: National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-01 and R35HL135816 to B.A.W.), National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award to B.A.W. and Core Center to S.M.R.), and NIH (T32CA091078 to K.E.T.).
Footnotes
Potential conflict of interest: B.A.W.: Cook Medical, Olympus, and Smith and Nephew, consultant.
This study is winner of the Spring ARS Basic Science Research Award. The findings were presented at the 2017 American Rhinologic Society’s Spring Meeting, on April 27, 2017, in San Diego, CA.
References
- 1.Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Robinson M, Bye PT. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33:293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 3.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 4.Hariri BM, Cohen NA. New insights into upper airway innate immunity. Am J Rhinol Allergy. 2016;30:319–323. doi: 10.2500/ajra.2016.30.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 6.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope. 2011;121:1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller W, Haussinger K, Ziegler-Heitbrock L, Heyder J. Mucociliary and long-term particle clearance in airways of patients with immotile cilia. Respir Res. 2006;7:10. doi: 10.1186/1465-9921-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27:387–395. doi: 10.2500/ajra.2013.27.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014;20:623–631. doi: 10.1097/MCP.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:70–75. doi: 10.2500/ajra.2012.26.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tipirneni KE, Woodworth BA. Medical and surgical advancements in the management of cystic fibrosis chronic rhinosinusitis. Curr Otorbinolaryngol Rep. 5:24–34. doi: 10.1007/s40136-017-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122:1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. Laryngoscope. 2015;125(suppl 7):S1–S13. doi: 10.1002/lary.25335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho DY, Woodworth BA. Acquired cystic fibrosis transmembrane conductance regulator deficiency. Adv Otorbinolaryngol. 2016;79:78–85. doi: 10.1159/000445134. [DOI] [PubMed] [Google Scholar]
- 16.McShane D, Davies JC, Wodehouse T, Bush A, Geddes D, Alton EW. Normal nasal mucociliary clearance in CF children: evidence against a CFTR-related defect. Eur Respir J. 2004;24:95–100. doi: 10.1183/09031936.04.00097503. [DOI] [PubMed] [Google Scholar]
- 17.Regnis JA, Robinson M, Bailey DL, et al. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med. 1994;150:66–71. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 18.Bennett WD, Almond MA, Zeman KL, Johnson JG, Donohue JF. Effect of salmeterol on mucociliary and cough clearance in chronic bronchitis. Pulm Pharmacol Ther. 2006;19:96–100. doi: 10.1016/j.pupt.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Laube BL, Karmazyn YJ, Orens JB, Mogayzel PJ., Jr Albuterol improves impaired mucociliary clearance after lung transplantation. J Heart Lung Transplant. 2007;26:138–144. doi: 10.1016/j.healun.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One. 2013;8:e54473. doi: 10.1371/journal.pone.0054473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worthington EN, Tarran R. Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol Biol. 2011;742:77–92. doi: 10.1007/978-1-61779-120-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175–181. doi: 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- 24.Di Benedetto G, Magnus CJ, Gray PT, Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J Physiol. 1991;439:103–113. doi: 10.1113/jphysiol.1991.sp018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Gardecki JA, Nadkarni SK, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011;17:1010–1014. doi: 10.1038/nm.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu KK, Unglert C, Ford TN, et al. In vivo imaging of airway cilia and mucus clearance with micro-optical coherence tomography. Biomed Optical Express. 2016;7:2494–2505. doi: 10.1364/BOE.7.002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson DJ, Gray MA, Kilanowski FM, et al. Murine epithelial cells: isolation and culture. J Cyst Fibros. 2004;3(suppl 2):59–62. doi: 10.1016/j.jcf.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–196. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 30.Dean N, Ranganath NK, Jones B, et al. Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis. Int Forum Allergy Rhinol. 2014;4:565–570. doi: 10.1002/alr.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Illing EA, Cho DY, Zhang S, et al. Chlorogenic acid activates CFTR-Mediated Cl− secretion in mice and humans: therapeutic implications for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2015;153:291–297. doi: 10.1177/0194599815586720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho DY, Skinner D, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator activation by the solvent ethanol: implications for topical drug delivery. Int Forum Allergy Rhinol. 2016;6:178–184. doi: 10.1002/alr.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:el04090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipirneni KE, Cho DY, Skinner D, et al. Characterization of primary rat nasal epithelial cultures in CFTR knockout rats as a model for CF sinus disease. Laryngoscope. doi: 10.1002/lary.26720. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:307–312. doi: 10.2500/ajra.2011.25.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 37.Conger BT, Zhang S, Skinner D, et al. Comparison of cystic fibrosis transmembrane conductance regulator (CFTR) and ciliary beat frequency activation by the CFTR Modulators Genistein, VRT-532, and UCCF-152 in primary sinonasal epithelial cultures. JAMA Otolaryngol Head Neck Surg. 2013;139:822–827. doi: 10.1001/jamaoto.2013.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]