Abstract
BACKGROUND
Cocaine-induced neuroplastic changes may result in a heightened propensity for relapse. Using regional cerebral blood flow (rCBF) as a marker of basal neuronal activity, this study assessed alterations in rCBF and related resting state functional connectivity (rsFC) to prospectively predict relapse in patients following treatment for cocaine use disorder (CUD).
METHODS
Pseudocontinuous arterial spin labeling functional magnetic resonance imaging and resting blood oxygen level-dependent functional magnetic resonance imaging data were acquired in the same scan session in abstinent participants with CUD before residential treatment discharge and in 20 healthy matched control subjects. Substance use was assessed twice weekly following discharge. Relapsed participants were defined as those who used stimulants within 30 days following treatment discharge (n = 22); early remission participants (n = 18) did not.
RESULTS
Voxel-wise, whole-brain analysis revealed enhanced rCBF only in the left posterior hippocampus (pHp) in the relapsed group compared with the early remission and control groups. Using this pHp as a seed, increased rsFC strength with the posterior cingulate cortex (PCC)/precuneus was seen in the relapsed versus early remission subgroups. Together, both increased pHp rCBF and strengthened pHp-PCC rsFC predicted relapse with 75% accuracy at 30, 60, and 90 days following treatment.
CONCLUSIONS
In CUD participants at risk of early relapse, increased pHp basal activity and pHp-PCC circuit strength may reflect the propensity for heightened reactivity to cocaine cues and persistent cocaine-related ruminations. Mechanisms to mute hyperactivated brain regions and delink dysregulated neural circuits may prove useful to prevent relapse in patients with CUD.
Keywords: Cocaine dependence, Functional connectivity, Functional MRI, Hippocampus, Limbic system, Regional cerebral blood flow
The associations formed during repeated drug self-administration strengthen specific neural pathways (synaptic plasticity) that are thought to be critical in the development and persistence of substance use disorders (1–3). Substance-induced changes in long-term potentiation and long-term depression underlie these alterations in synaptic transmission and may include various forms of neuronal reorganization, including the recruitment of new pathways, the reinforcement of existing connections, and dendritic arborization (4). One prominent hypothesis of recidivism is that these neuroadaptations produce conditional responses based on complex environmental stimuli; the presentation of these stimuli in the absence of drug leads to cue-induced drug cravings and subsequent drug-seeking behaviors (1). This human situation has been modeled preclinically using the conditioned reinstatement paradigm (5).
The learning of drug-cue associations induces synaptic changes in the mesocorticolimbic and corticostriatal pathways thought to induce the persistent vulnerability to relapse (6–8). Patients with cocaine use disorder (CUD) are at particularly high risk for relapse, with more than 70% of individuals treated for cocaine addiction relapsing within a few weeks following treatment completion (9). Despite this, few studies to date have addressed neural predictors of cocaine relapse risk. While research using task-related functional magnetic resonance imaging (MRI) (10–12) has offered some utility in identifying relapse predictors, it has been difficult to formulate generalized principles linking brain processes and treatment outcome predictions. In recent studies, we have considered whether using the strength of addiction-relevant brain circuits in the absence of specific task demands (i.e., resting state functional connectivity [rsFC]) would provide a useful window into the neuroplastic alterations that heighten relapse risk. rsFC is measured by the strength of the temporal correlation of low-frequency blood oxygen level-dependent (BOLD) fluctuations between discrete anatomical regions when an individual is not engaged in a specific task requiring attentional, emotional, or executive processing (13). Using this technique, we identified striatal-amygdalar circuits that contribute to relapse risk in cocaine-addicted populations (14). These findings support the role of brain regions involved in the consolidation and reconsolidation of cocaine-cue and cocaine-context associations in driving relapse (15,16).
While functional connectivity is thought to reflect coherent changes in neuronal activity between brain regions (or networks), alterations in neuronal firing within a region also offer a relevant measure of and window into drug-induced neuroplasticity (17). One of the best means to noninvasively measure such basal firing rates is the use of regional cerebral blood flow (rCBF), which, due to the tight coupling between metabolism and neuronal activity, provides a physiologically relevant measure of activity (18). The present study, therefore, utilized rCBF to identify local neuroplastic alterations as a function of relapse status. We then used identified regional alterations in neuronal activity as a seed region in a functional connectivity analysis to identify circuits modulating or modulated by this local change in neuronal activity. Using a population of participants with CUD scanned just before discharge from a residential treatment program, we predicted that participants who relapsed soon after discharge (i.e., within 30 days), relative to those who remained abstinent and healthy control subjects, would show rCBF differences in brain regions most closely associated with neuroplastic changes in preclinical models of addiction (e.g., ventral and dorsal striatum, amygdala, hippocampus, orbitofrontal cortex, insula) (6). In turn, circuit strength between these regions would be more disrupted in the relapsed group relative to the other two groups and further predict treatment outcome.
METHODS AND MATERIALS
Participants
Participants were 40 individuals with CUD who met criteria for cocaine dependence on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Participants were administered a comprehensive medical history and physical examination, a general laboratory panel, urine drug screen (UDS), and a guided interview of lifetime substance use history during the first and second week of inpatient treatment. Participants were excluded if they had any history of major illness, had an estimated IQ below 70 (per the Wechsler Test of Adult Reading), met criteria for any neurological or active Axis I disorder (other than substance use disorders), or were on psychotropic medications. Other drug use among CUD individuals was not a condition for exclusion, as long as cocaine dependence was the primary diagnosis. CUD participants were recruited following admission to one of three residential cocaine-dependence treatment programs in Dallas, Texas, and were admitted as soon as possible after their last reported use of cocaine. All three programs utilized the Minnesota Model psychosocial treatment approach. UDS were conducted throughout residential treatment to verify abstinence. Healthy matched control subjects (n = 21) had no lifetime history of substance use disorder. Other psychiatric, medical, and cognitive exclusion criteria were similar to that of the cocaine-dependent participants.
All aspects of the research protocol were reviewed and approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and the Veterans Administration North Texas Health Care System. Participants signed an informed consent before study participation and were compensated for their participation. Following 2 to 4 weeks of residential treatment and just before discharge, participants underwent MRI scanning.
Assessment Measures
Cocaine use history was assessed with the Timeline Followback (19). The Timeline Followback uses a calendar and other memory aids to gather retrospective estimates of an individual’s cocaine use (days used and dollars spent) across the lifetime (from age of first use) and during the 90 days before abstinence. Cocaine craving was assessed with the Cocaine Craving Questionnaire-Brief (20) and Obsessive Compulsive Cocaine Scale (21) (Supplement 1). Lifetime cocaine dependence severity was assessed with the Inventory of Drug Use Consequences (22).
CUD participants were followed for 24 weeks (168 days) posttreatment or until relapse to stimulant use, whichever came first. Follow-up sessions occurred twice weekly (once by phone) and included a structured interview assessing substance use since their previous visit and a UDS during the in-person visit. Relapse was defined a priori as any use of cocaine or amphetamine (either by self-report or UDS) after discharge and marked as the day of first use or the day of their first missed appointment if participants missed two consecutive appointments and all attempts to contact participant (e.g., phone, mail, collaterals) were unsuccessful. Participants who used a stimulant (or stopped participating in the study, as defined above) before 30 days following discharge from their residential program were considered as relapsed; other participants were considered to be in early remission; group assignments were based on DSM-IV criteria for early remission 1 month after discharge from a controlled environment.
MRI Data Acquisition
Participants completed a 5-minute high-resolution anatomical scan, a 4-minute pseudocontinuous arterial spin labeling scan, and a 6-minute resting BOLD scan during which they were instructed to lie as still as possible with their eyes open. CUD individuals completed scans during their final week of a 2- to 4-week residential treatment program. One control subject was excluded from all imaging analysis due to a technical error during acquisition of the resting data.
MRI acquisition was performed on a Philips 3T magnetic resonance scanner (Philips Medical Systems, Best, The Netherlands). rCBF was measured using a balanced pseudocontinuous arterial spin labeling single-shot echo-planar imaging sequence (23): in-plane resolution = 3 × 3 mm2, 27 slices (thickness/gap = 5/0 mm), labeling duration = 1650 milliseconds, post labeling delay = 1525 milliseconds, repetition time/echo time = 4150/14 milliseconds, interval between consecutive slice acquisitions = 35.5 milliseconds, and number of controls/labels = 30 pairs. Resting BOLD functional magnetic resonance imaging data were acquired using a single-shot echo-planar imaging sequence: in-plane resolution = 3.25 × 3.25 mm2, 36 slices (thickness/gap = 3/0 mm), repetition time/echo time = 1700/25 milliseconds, flip angle = 70°, and 212 volumes. For spatial normalization purposes, high-resolution T1-weighted images were acquired with a spatial resolution of 1 × 1 × 1 mm3.
Perfusion Data Processing and Analysis
The control and label images were co-registered and then motion corrected separately using AFNI (National Institute of Mental Health, Bethesda, Maryland) (24). The CBF-weighted images were obtained by surround subtraction of sincinterpolated control and label images (25), which were then averaged and spatially smoothed with a Gaussian kernel (full-width at half maximum = 6 mm). Quantitative rCBF was estimated based on a previously described model (23,26). For group analysis, the CBF images were spatial normalized to standard Talairach space and resampled at 3 × 3 × 3 mm3 and then normalized to the whole-brain average CBF (rCBFn) to improve the sensitivity in detecting group differences (27) (see Supplement 1 for additional details).
Group effects on baseline rCBFn were assessed within the gray matter region (masks obtained by segmentation of structure images in SPM8, Wellcome Trust Centre for Neuroimaging, London, United Kingdom) using a voxel-wise general linear mixed-effects model with gender and years of education as covariates. Effects were considered significant at pcorrected < .05 (uncorrected p < .005 combined with a cluster threshold of 27 voxels), based on familywise multiple comparison correction (24).
Resting Data Processing and Analysis
Resting BOLD data were preprocessed with slice-timing correction, motion correction, quadratic detrending, and temporal band-pass filtering (.01–.1 Hz). Nuisance covariates, including six-motion parameters and signals in white matter and cerebrospinal fluid, were regressed out, followed by Gaussian spatial smoothing (full-width at half maximum = 6 mm), spatial normalization, and nonlinear registration (28).
Brain regions showing significant group effects in rCBFn, if any, were used as seed regions of interest (ROI) to inspect rsFC changes among the relapsed, early remission, and control groups. A correlation coefficient map for each seed ROI was obtained by correlating the average time course from the seed with every voxel’s time course and then was transformed to Z-score using Fisher’s z transformation. Similar to the rCBFn data analysis, group effects in the rsFC strength were evaluated and effects were considered significant at pcorrected < .05 (uncorrected p < .025 combined with a cluster threshold of 70 voxels). Regression analysis was conducted on the seed region rCBFn against the relevant rsFC strength to investigate the relationship between these two functional indices.
Functional and Structural Indices
To determine whether group differences in resting rCBFn could be accounted for by underlying structural differences, regression analysis was conducted between rCBFn and gray matter volume in regions with significant rCBFn group effects. Gray matter volumes were extracted based on segmentation in FreeSurfer (Massachusetts General Hospital, Boston, Massachusetts) and were normalized to individual cortical/subcortical gray matter volume to account for individual differences in brain size (29).
Predictive Models of Relapse
A hierarchical survival analysis was performed to predict the days to relapse posttreatment using the average rCBFn value and the average rsFC strength extracted from those brain regions showing group effects as predictors in a Cox regression model (α = .05) (SPSS 20.0, IBM Corporation, Armonk, New York). Time-dependent receiver operating characteristic (ROC) curves were plotted (at 30-day intervals) to estimate the predictive power of the relapse model over the 168-day follow-up period (30) (Supplement 1).
RESULTS
Demographic and Clinical Characteristics
Twenty-two CUD participants relapsed within 30 days following treatment discharge; 18 remained in early remission. Only one participant relapsed using amphetamine. There were no statistically significant differences in age or race between the relapsed, early remission, and control (n = 20) groups (Table 1). The two CUD subgroups did not differ in gender, cocaine craving, 90-day or lifetime cocaine use, lifetime Inventory of Drug Use Consequences, cigarettes smoked per day, other substance use disorders (abuse/dependence), or time from admission to treatment and the scanning session. However, there were more female subjects, a greater number of cigarettes smoked per day, and higher IQ and education in the healthy control group relative to the cocaine-dependent participants.
Table 1.
Demographic and Clinical Characteristics of Healthy Control Subjects and Cocaine-Dependent Participants in Relapse and Early Remission Participants (means ± SD)
| Control Subjects (n = 20) | Relapsed (n = 22) | Early Remission (n = 18) | |
|---|---|---|---|
| Age (Years) | 42.2 ± 8.9 | 44.7 ± 6.4 | 44.3 ± 6.7 |
| Gendera | |||
| Male | 65.0% | 86.4% | 94.4% |
| Female | 35.0% | 13.6% | 5.6% |
| Race | |||
| African-American | 47.6% | 66.7% | 83.3% |
| Caucasian | 42.9% | 28.6% | 11.1% |
| Hispanic | 4.8% | 4.8% | 5.6% |
| Other | 4.8% | 0% | 0% |
| Education (Years)b,c | 13.8 ± 1.0 | 11.5 ± 1.6 | 12.8 ± 1.8 |
| Estimated FSIQb,d | 97.9 ± 10.7 | 87.8 ± 9.4 | 87.7 ± 7.6 |
| Nicotine Use | |||
| Packs/dayb,e | .05 ± .2 | 1.0 ± .9 | .9 ± .9 |
| % smokers b,e | 5% | 68% | 67% |
| Secondary Substance Use Disorders | |||
| Alcohol | – | 8 | 7 |
| Amphetamine | – | 0 | 1 |
| Cannabis | – | 3 | 3 |
| Opioid | – | 2 | 1 |
| Other | – | 2 | 0 |
| Time from Admission to MRI Scan (Days) | – | 21.5 ± 4.2 | 21.0 ± 4.6 |
| InDUC | |||
| Recentb,e | 1.2 ± 2.9 | 75.5 ± 23.1 | 85.2 ± 16.4 |
| Lifetimeb,e | 5.2 ± 6.9 | 37.9 ± 7.2 | 40.7 ± 2.5 |
| Craving | |||
| CCQ-Brief | – | 2.6 ± .9 | 2.3 ± .8 |
| OCCS | – | 23.9 ± 9.0 | 25.0 ± 6.4 |
Comparisons between groups were by t test or chi-squared.
CCQ-Brief, Cocaine Craving Questionnaire-Brief; FSIQ, full-scale IQ; InDUC, Inventory of Drug Use Consequences; MRI, magnetic resonance imaging; OCCS, Obsessive-Compulsive Cocaine Scale.
Difference between control and early remission group, p < .05.
Difference between control and relapsed group, p < .001.
Difference between early remission and relapsed group, p < .05.
Difference between control and early remission group, p < .01.
Difference between control and early remission group, p < .001.
Regional Cerebral Blood Flow
Whole-brain, voxel-wise analysis of variance revealed group rCBFn differences only in the left posterior hippocampus (pHp) (−29, −38, −4; 729 mm3; peak F2,55 = 11.5) (Figure 1A). These differences were driven by higher left pHp rCBFn in the relapsed group relative to the other two groups (Figure 1B). Post hoc voxel-wise analysis showed left pHp rCBFn was significantly higher in the relapsed group relative to control subjects (p < .05). Differences between the relapsed and early remission groups did not reach whole-brain corrected statistical significance.
Figure 1.
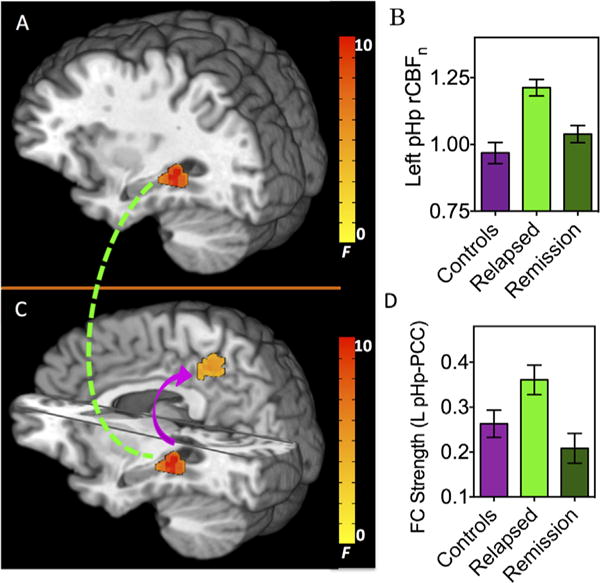
Basal neuronal activity and connectivity alterations as a function of treatment outcome (relapsed vs. early remission) in cocaine-dependent and healthy control individuals. Top panel: (A) Regional cerebral blood in the left posterior hippocampus (pHp) significantly differed between healthy control subjects and cocaine-dependent individuals who relapsed 30 days before and after completion of residential treatment (pHp: −29, −38, −4; 729 mm3; peak F2,55 = 11.5). (B) Left pHp normalized regional cerebral blood flow (rCBFn) group means and standard deviations. Post hoc voxel-wise analysis showed left pHp rCBFn was significantly higher in the relapsed group relative to control subjects (p < .05). (C) Using the left pHp in (A) as a seed to assess group differences in resting state functional connectivity (FC), pHp-posterior cingulate cortex (PCC) connectivity strength significantly differed between the three groups (PCC: −5, −41, 45; 2052 mm3; peak F2,55 = 6.8). (D) Left (L) pHp-PCC connectivity strength group means and standard deviations. Post hoc voxel-wise contrasts revealed significantly increased circuit strength in the relapsed group relative to the early remission participants (p < .05).
Posterior Hippocampal Functional Connectivity
Using the left pHp cluster identified above as a seed ROI, whole-brain rsFC was computed from the resting BOLD acquisition to investigate circuit connectivity alterations between the left pHp and its interconnected brain regions. Significant group differences in connectivity strength were observed only between the pHp and posterior cingulate cortex (PCC)/precuneus (also denoted as posteromedial cortex but subsequently referred to simply as PCC) (−5, −41, 45; 2052 mm3; peak F2,55 = 6.8) (Figure 1C), again driven by higher connectivity strength in the relapsed group relative to the other two groups (Figure 1D). Post hoc voxel-wise contrasts revealed significantly (p < .05) increased circuit strength in the relapsed group relative to the early remission participants. Differences between the relapsed and control groups did not reach whole-brain corrected statistical significance.
There were no significant relationships (controlling for gender and years of education) between left pHp rCBFn and pHp-PCC circuit strength in the relapsed (r = .11, p = .97), early remission (r = .29, p = .23), or control (r = .41, p = .07) group. Neither pHp rCBF nor pHp-PCC functional connectivity significantly correlated with craving as assessed by either the Cocaine Craving Questionnaire-Brief or Obsessive Compulsive Cocaine Scale. However, craving measures were obtained several days before MRI data acquisition.
Relationship Between Functional and Structural Indices
Left hippocampal gray matter volume (normalized to individual subcortical gray matter volume) did not significantly differ between groups. Nevertheless, left hippocampal gray matter volume was positively correlated with left pHp rCBFn (r = .62, p = .004, controlling for gender and years of education) in the control group but not in either the relapsed (r = −.20, p = .36) or early remission (r = .03, p = .90) groups (Figure 2).
Figure 2.
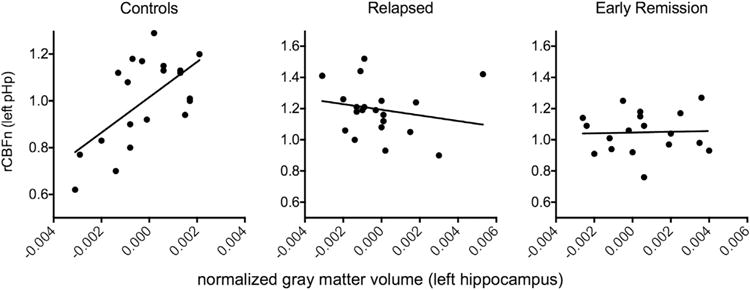
Relationship between left hippocampal gray matter volume and left posterior hippocampal (pHp) neuronal activity (regional cerebral blood flow). The relationship between left posterior hippocampal normalized regional cerebral blood flow (rCBFn) and hippocampal gray matter volume was significant only in the healthy control group (r = .62, p = .004, left panel) but not the relapsed (r = −.20, p = .36, middle panel) or early remission (r = .03, p = .90, right panel) groups.
rCBF and rsFC Strength Predict Days to Relapse
Using participant-specific days to relapse (rather than the categorical division of relapse and early remission), we conducted a post hoc hierarchical Cox regression to examine the predictive power of connectivity within the pHp-PCC circuit beyond that already accounted for by left pHp rCBFn in the left pHp. As illustrated in Table 2, relapse prediction was significantly improved after connectivity strength of the pHp-PCC circuit was added to the model (significance of changes from model 1: Δp = .012; model 2 in Table 2). In addition, the importance of the rCBFn in the left pHp in the prediction decreased when controlling for connectivity strength (Table 3).
Table 2.
Models to Predict Time to Relapse with rCBFn and rsFC Strength
| Model | Model χ2 | Model p | Δχ2 | Δp |
|---|---|---|---|---|
| 1 | 10.01 | .002 | 10.09 | .001 |
| 2 | 16.16 | .0003 | 6.27 | .012 |
Dependent variable: Time to relapse up to 168 days.
Model 1: Predictors: (Constant), rCBFn (regions: L pHp).
Model 2: Predictors: (Constant), rCBFn (regions: L pHp), rsFC strength (L pHp-PCC).
L, left; PCC, posterior cingulate cortex; pHp, posterior hippocampal; rCBFn, normalized regional cerebral blood flow; rsFC, resting state functional connectivity.
Table 3.
Coefficients of Cox Regression Models
| Model | β (SE) | Exp (β) | Wald | p | |
|---|---|---|---|---|---|
| 1 | rCBFn (L pHp) | 3.69 (1.18) | 40.16 | 9.75 | .002 |
| 2 | rCBFn (L pHp) | 2.98 (1.26) | 19.62 | 5.55 | .018 |
| rsFC (L pHp-PCC) | 2.81 (1.11) | 16.57 | 6.43 | .011 |
Dependent variable: Time to relapse up to 168 days.
Model 1: Predictors: (Constant), rCBFn (regions: L pHp).
Model 2: Predictors: (Constant), rCBFn (regions: L pHp), rsFC strength (L pHp-PCC).
β (SE), coefficient (standard error); Exp(β), eβ (the natural exponential function of the coefficient); L, left; PCC, posterior cingulate cortex; pHp, posterior hippocampal; rCBFn, normalized regional cerebral blood flow; rsFC, resting state functional connectivity.
To estimate the predictive power of the relapse model using both pHp rCBFn and pHp-PCC rsFC (model 2) over time (e.g., days to relapse), we used time-dependent ROC analysis to calculate time-dependent area under the curve, AUC(t), at t = 30, 60, 90, 120, and 150 days. AUC(t) of model 2 (Figure 3) shows time-dependent ROC curves with time-varying sensitivity and specificity of the model’s predictability (adjusted for gender and years of education). Overall, the model predictability remained relatively stable at approximately 75% accuracy from 30 to 90 days, with a subsequent decrease to 67% at the later time points (AUC(t): 30 [74.4%], 60 [75.8%], 90 [75.3%], 120 [67.5%], and 150 [66.7% days).
Figure 3.
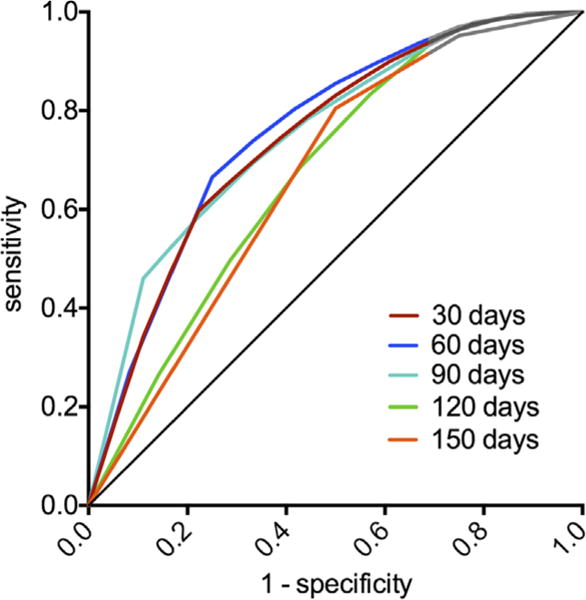
Receiver operator curves of posterior hippocampal (pHp) regional cerebral blood flow and pHp-posterior cingulate cortex functional connectivity strength in predicting cocaine relapse. The receiver operator curves illustrate the sensitivity and specificity of pHp regional cerebral blood flow and pHp-posterior cingulate cortex functional connectivity strength in predicting relapse at 30-day intervals following completion of residential treatment.
DISCUSSION
CUD participants who relapsed within 30 days following treatment discharge were distinguished by increased resting rCBF in the left pHp and increased functional connectivity between that pHp ROI and the PCC. While both findings significantly predicted days to relapse over a 6-month period, the prediction power was enhanced when the two measures were considered concurrently. The relevance of the hippocampus and PCC to contextual-related cravings and self-referential thinking (e.g., obsessive thoughts of drug use) support these findings as clinically meaningful. Unexpectedly and in contrast to our a priori hypotheses, brain regions showing neuroplastic changes following chronic cocaine self-administration (6) or associated with animal models of addictive behaviors (31) (e.g., striatum, orbitofrontal cortex, amygdala, insula) did not significantly differ as a function of treatment group.
Relevance of pHp to and Cocaine-Related Neuroplasticity and Relapse
Long-term potentiation was first demonstrated in the hippocampus (32), a region essential for developing, processing, and retaining contextual information (33). Direct glutamatergic and cholinergic input (34,35), coupled with dopaminergic innervation (36,37), modulates synaptic plasticity within the hippocampus. An increasingly robust literature suggests that the hippocampus interacts with the corticostriatal circuits to impact memory and decision making (38). Processes within the hippocampus, in concert with other striatal-limbic regions, underlie the reinstatement of cocaine-seeking behaviors (39–42), and integrated inputs from the hippocampus, amygdala, and prefrontal cortex generate motivational signals that modulate repeated drug self-administration (43).
The hippocampus can be conceptualized into two distinct subregions with distinct functions (44): a rostral/dorsal zone that serves cold cognitive functions (e.g., learning, memory, navigation, exploration) and a caudal/ventral zone that corresponds to hot/affective functions (e.g., stress, emotion, affect). The dorsal part of the hippocampus (dHp) in rodents, which is considered homologous to the primate pHp (45), has been particularly implicated in addiction-related contextual memories. Lesions of the dHp disrupt both the acquisition and expression of cocaine conditional place preference (46), and inactivation of the dHp and basolateral amygdala selectively decreases cocaine-seeking behavior in rats (15). The basolateral amygdala and dHp also interact to regulate cocaine-related memory reconsolidation (47), while stimulation of type 1 metabotropic glutamate receptors in the dHp are necessary for context-induced cocaine-seeking behavior (48). In clinical studies, we have observed decreased left pHp rCBF in response to the administration of cholinergic receptor modulators (49) and reduced rsFC strength between pHp and dorsomedial prefrontal cortex (50) in cocaine-dependent individuals (although neither study assessed whether these differences predicted subsequent cocaine relapse). Luo et al. (51) posited a transsynaptic link from the dHp to the ventral tegmental area using the lateral septum as a relay nuclei, by which environmental context regulates goal-directed behavior. This linkage is supported by human studies demonstrating convergent resting connectivity between the hippocampus, ventral tegmental area, and nucleus accumbens (52).
The increased pHp rCBF observed in our relapsers is taken to reflect increased basal Hp neural activity. As task-related increases in neuronal metabolism only account for a small portion (<5%) of brain activity, basal neuronal activity yields highly relevant information regarding brain functioning (53). Resting measures during mind wandering also assess a significant phase of our waking hours (up to 15%) (54). Although resting-state brain measures of neuronal activity, to our knowledge, have not been reported as relapse predictors, greater medial temporal lobe BOLD activity during rest predicts heightened performance on declarative memory tasks in young adults (55). Baseline cortical electroencephalogram activity, as measured by electroencephalography, also predicts risk-taking behavior (56). We propose that enhanced basal pHp brain activity in CUD with enhanced basal pHp brain activity and subsequent environmental or emotional drug cues postdischarge may provoke greater activation of hippocampal-corticostriatal pathways resulting in greater drug cravings, drug seeking, and relapse (46,57,58). Thus, even in the absence of heightened basal subjective craving, potentially persistent neurobiological cocaine-induced alterations may produce increased vulnerability to the elicitation of cocaine-related memories and subsequent use.
A strong positive correlation was observed only in the control group between pHp rCBF and Hp gray matter volume. This interdependent relationship between resting neuronal activity and gray matter has previously been reported in subcortical and default mode network (DMN)-related regions (59), as well as in the frontal cortical regions of heroin-dependent participants during the administration of both placebo and heroin (60). The absence of this structure/function association in both cocaine-dependent subgroups suggests disease-associated processes may be driving the differences in pHp neural activity. Further, in contrast to a recent report by Xu et al. (61), we did not observe a difference in hippocampal volume between relapsed and early remission individuals.
Relationship between pHp-PCC Circuitry to Cocaine Relapse
The PCC is a primary hub of the DMN, a network of associated brain regions that demonstrate stronger coherence during rest as one relates oneself to the present environment (62,63). This self-referential thought involves two key processes: the experiential (the momentary awareness of the present) and the narrative (the personalization of past experiences) (64). Brain activation during the elicitation of autobiographical memories, a type of narrative, shows significant overlap with DMN regions, involving both the hippocampus and PCC (65). Recursive self-focused thinking (i.e., rumination), in particular, elicits activation of the PCC, as well as limbic and prefrontal regions (66). The heightened rumination in depressed individuals, for example, is associated with increased PCC activity during a rumination task (67) and PCC-anterior cingulate connectivity at rest (68), and in cocaine-dependent individuals, PCC activation is associated with cue-induced craving (69). Of particular relevance to our findings, stronger intrinsic connectivity between the hippocampus and PCC during rest predicts superior performance on measures of episodic memory (70) and disruption of this connection is associated with age-related memory decline (71). In our CUD individuals who relapsed, heightened pHp-PCC rsFC may reflect strengthened episodic memories associated with cocaine use (72,73). This strengthened memory circuitry may underlie the persistent obsessive thoughts, or rumination, commonly observed in addicted individuals (74,75).
As both pHp rCBF and pHp-PCC connectivity strength significantly, and independently, predicted days to relapse, the conjectured pHp-related cocaine use contextual cues and the pHp-PCC related cocaine-focused ruminations may index two complementary processes contributing to relapse. Future research using paradigms to explore these different constructs are needed to confirm these suppositions.
Relevance to Previous Neuroimaging Studies of Relapse
To our knowledge, resting rCBF has not previously been explored as a predictor of relapse in individuals with substance use disorders, nor does the extant literature report differences in hippocampal activity as a predictor of relapse. While connectivity studies have identified alterations in striatal-insular (14) and cortical-amygdalar (76) circuit strength in cocaine-dependent individuals (in the same population used in the present study) and midbrain-amygdala midbrain-orbitofrontal cortex rsFC strength in alcohol-dependent individuals (77), functional connections between the pHp and PCC have not been previously identified as relapse predictors [although the circuit is well described within the DMN (65)]. On the other hand, a variety of approaches have implicated alterations in PCC reactivity in the relapse process: CUD participants exhibiting a greater PCC response to cocaine cues (78) or a disinhibition task (10) showing more severe or longer times to relapse, respectively, and in methamphetamine-dependent individuals, PCC activation, in concert with other regions, during a two-choice prediction (79) and decision-making (80) task predicting drug use relapse.
Strengths and Limitations
As the demographics and characterization of the relapsed and early remission groups were well matched for age, race, gender, education, IQ, baseline craving, and most cocaine and nicotine use variables, together with the relatively large cohort of both treatment completers and control subjects, we were able to isolate our findings to relapse status only. Although education and gender in the control group differed from the patient groups, these variables were considered as covariates in the imaging analysis. Further, the CUD participants were at least 2 weeks drug abstinent, precluding any acute drug and/or withdrawal effects of cocaine that confound imaging studies conducted in the first several days of abstinence. Participants were also without other active DSM-IV nonsubstance use disorders and were not taking psychotropic medications. Finally, participants were contacted twice weekly and UDS was obtained weekly for follow-up. Close follow-up over the 6-month posttreatment period further allowed days to relapse to be considered as a regressor. Technically, changes in perfusion revealed by ASL are more localized to the parenchyma, whereas BOLD changes are more representative of changes in the veins and venules (18), offering a more physiologically direct measure of brain activity.
As UDS must be obtained at least every other day to assure detection of cocaine use, we could not verify exact time to last cocaine use based on the weekly UDS. Second, we were unable to directly test the clinical significance of the observed changes in resting pHp CBF and the pHp-PCC functional connectivity strength, as the study design did not examine constructs specifically related to the function of these regions. Thus, the relationship of resting pHp CBF and pHp-PCC functional connectivity strength to drug-related cravings and ruminations remains unproven. Nevertheless, the identification of both pHp activity and pHp-PCC circuit strength as independent predictors of relapse, coupled with the extant literature, supports further explorations of the interaction between personalized, contextual cravings and the DMN upon relapse. Confirmation of these findings may yield new targets for both pharmacologic and behavioral interventions in the treatment of relapse prevention.
Supplementary Material
Acknowledgments
This study was supported by the National Institute on Drug Abuse Grant DA023203, the National Institute on Drug Abuse Intramural Research Program, and the University of Texas Southwestern Center for Translational Medicine UL1TR000451.
We are grateful to Rani Varghese for her skilled assistance in magnetic resonance imaging scanning and the assistance of the staff on the Substance Abuse Team at the Veterans Affairs North Texas Health Care System, Homeward Bound Inc., and Nexus Recovery Center for their support in the screening and recruitment of study participants.
BA is funded by the National Institute on Drug Abuse and the National Institute on Arthritis and Musculoskeletal and Skin Disorders, and has received honoraria from the American Academy of Addiction Psychiatry. HL is funded by the National Institute of Mental Health.
Footnotes
DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Clinicaltrials.gov: Impulsivity, Neural Deficits and Cocaine Addiction; http://clinicaltrials.gov/ct2/show/NCT00744601?term=adinoff&rank=4;#NCT00744601.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.12.027.
References
- 1.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 3.Racz I. Neuroplastic changes in addiction. Front Mol Neurosci. 2014;6:56. doi: 10.3389/fnmol.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 6.Madsen HB, Brown RM, Lawrence AJ. Neuroplasticity in addiction: Cellular and transcriptional perspectives. Front Mol Neurosci. 2012;5:99. doi: 10.3389/fnmol.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking [published online ahead of print September 6] Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 10.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worhunsky PD, Stevens MC, Carroll KM, Rounsaville BJ, Calhoun VD, Pearlson GD, Potenza MN. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 14.McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: Implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 16.Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: Implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 17.Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: Implications for addiction. Neurosci Biobehav Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Vaughan JT, et al. High-resolution, spin-echo BOLD, and CBF fMRI at 4 and 7 T. Magn Reson Med. 2002;48:589–593. doi: 10.1002/mrm.10252. [DOI] [PubMed] [Google Scholar]
- 19.Sobell LC, Sobell MB. A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychological and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 20.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 21.Hormes JM, Coffey SF, Drobes DJ, Saladin ME. The Obsessive Compulsive Cocaine Use Scale: Development and initial validation of a self-rated instrument for the quantification of thoughts about cocaine use. Drug Alcohol Depend. 2012;120:250–254. doi: 10.1016/j.drugalcdep.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonigan JS, Miller WR. The inventory of drug use consequences (InDUC): Test-retest stability and sensitivity to detect change. Psychol Addict Behav. 2002;16:165–168. [PubMed] [Google Scholar]
- 23.Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63:765–771. doi: 10.1002/mrm.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 25.Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 26.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Aslan S, Lu H. On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn Reson Imaging. 2010;28:928–935. doi: 10.1016/j.mri.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng X, Christensen GE, Gu H, Ross TJ, Yang Y. Implicit reference-based group-wise image registration and its application to structural and functional MRI. Neuroimage. 2009;47:1341–1351. doi: 10.1016/j.neuroimage.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 30.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 31.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamsler A, McHugh TJ, Gerber D, Huang SY, Tonegawa S. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc Natl Acad Sci U S A. 2010;107:1618–1623. doi: 10.1073/pnas.0912540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado MR, Dickerson KC. Reward-related learning via multiple memory systems. Biol Psychiatry. 2012;72:134–141. doi: 10.1016/j.biopsych.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 41.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 42.Cooper DC, Klipec WD, Fowler MA, Ozkan ED. A role for the subiculum in the brain motivation/reward circuitry. Behav Brain Res. 2006;174:225–231. doi: 10.1016/j.bbr.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci. 2011;34:548–559. doi: 10.1016/j.tins.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Fanselow MS, Dong HW. Are the dorsal and ventral hippo-campus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki M, Tohyama K, Matsunaga S, Nakamura M, Tomizawa N, Inoue T, et al. MRI identification of dorsal hippocampus homologue in human brain. Neuroreport. 2004;15:2173–2176. doi: 10.1097/00001756-200410050-00005. [DOI] [PubMed] [Google Scholar]
- 46.Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- 47.Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, et al. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology. 2010;35:1485–1499. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahn I, Shohamy D. Intrinsic connectivity between the hippo-campus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23:187–192. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 54.Sayette MA, Reichle ED, Schooler JW. Lost in the sauce: The effects of alcohol on mind wandering. Psychol Sci. 2009;20:747–752. doi: 10.1111/j.1467-9280.2009.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM. Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci U S A. 2008;105:18555–18560. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gianotti LR, Knoch D, Faber PL, Lehmann D, Pascual-Marqui RD, Diezi C, et al. Tonic activity level in the right prefrontal cortex predicts individuals’ risk taking. Psychol Sci. 2009;20:33–38. doi: 10.1111/j.1467-9280.2008.02260.x. [DOI] [PubMed] [Google Scholar]
- 57.Collier TJ, Routtenberg A. Electrical self-stimulation of dentate gyrus granule cells. Behav Neural Biol. 1984;42:85–90. doi: 10.1016/s0163-1047(84)90472-2. [DOI] [PubMed] [Google Scholar]
- 58.Ursin R, Ursin H, Olds J. Self-stimulation of hippocampus in rats. J Comp Physiol Psychol. 1966;61:353–359. doi: 10.1037/h0023253. [DOI] [PubMed] [Google Scholar]
- 59.Varkuti B, Cavusoglu M, Kullik A, Schiffler B, Veit R, Yilmaz O, et al. Quantifying the link between anatomical connectivity, gray matter volume and regional cerebral blood flow: An integrative MRI study. PLoS One. 2011;6:e14801. doi: 10.1371/journal.pone.0014801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denier N, Schmidt A, Gerber H, Schmid O, Riecher-Rossler A, Wiesbeck GA, et al. Association of frontal gray matter volume and cerebral perfusion in heroin addiction: A multimodal neuroimaging study. Front Psychiatry. 2013;4:135. doi: 10.3389/fpsyt.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Kober H, Wang X, DeVito EE, Carroll KM, Potenza MN. Hippocampal volume mediates the relationship between measures of pre-treatment cocaine use and within-treatment cocaine abstinence. Drug Alcohol Depend. 2014;143:74–80. doi: 10.1016/j.drugalcdep.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 65.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 66.Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, et al. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 73.Muller CP. Episodic memories and their relevance for psychoactive drug use and addiction. Front Behav Neurosci. 2013;7:34. doi: 10.3389/fnbeh.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 75.Modell JG, Glaser FB, Cyr L, Mountz JM. Obsessive and compulsive characteristics of craving for alcohol in alcohol abuse and dependence. Alcohol Clin Exp Res. 1992;16:272–274. doi: 10.1111/j.1530-0277.1992.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 76.McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 78.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 79.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 80.Gowin JL, Harle KM, Stewart JL, Wittmann M, Tapert SF, Paulus MP. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014;39:1379–1387. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


