Abstract
Objective
The aim of this study was to develop a patient-reported outcome measure specific for systemic lupus erythematosus (SLE) to assess patient satisfaction with treatment, treatment options, and medical care.
Methods
Patients with SLE were recruited from four US rheumatology practices. Concept elicitation interviews identified aspects that patients considered important and relevant regarding satisfaction with treatment and medical care. Concept elicitation interviews and clinical input were used to draft the Lupus Satisfaction Questionnaire (LSQ). A second cohort of patients with SLE participated in combined concept elicitation/cognitive debriefing interviews, after which the LSQ was revised.
Results
Fourteen patients completed concept elicitation interviews: 93% were female, 57% were white, and 85% had moderate/severe SLE. Current treatments included hydroxychloroquine (93%), steroids (79%), and belimumab (57%), and 43% were biologic naive. Patients were generally satisfied with their treatment and medical care; however, they were dissatisfied with treatment adverse effects and the number of available treatment options. Cognitive debriefing interviews (n = 8) demonstrated that the LSQ was comprehensive, clear, and relevant; therefore, only minor revisions were made to the questionnaire. The LSQ assesses satisfaction with current SLE treatments (25 items), medical care (11 items), and insurance coverage (3 items). The draft LSQ was evaluated in 195 adults with SLE. Fifty-eight percent of patients reported that they were “somewhat satisfied” with their SLE treatment.
Conclusions
The LSQ has been developed to assess treatment satisfaction among patients with SLE. Following further testing to support its validity and reliability, it will provide a useful tool to facilitate assessment of satisfaction with treatments for SLE and help inform treatment decisions.
Key Words: patient satisfaction, questionnaire design, systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic disease characterized by a wide range of clinical manifestations and fluctuating symptoms. Several classes of drugs, including glucocorticoids (referred to throughout as steroids), antimalarials, immunosuppressants, biologics, and nonsteroidal anti-inflammatory drugs, have been demonstrated to provide clinical benefit for patients with SLE, but are also associated with adverse effects.1
Treatment noncompliance and nonadherence are common problems in chronic conditions,2 such as SLE; only approximately 25% of patients with SLE take 80% of their prescribed medications or greater over a 2-year period.3 Nonadherence is associated with worse disease outcomes4,5 and higher levels of health care resource utilization.6 Many factors contribute to adherence7 and satisfaction with treatment,7,8 including number of medications,3 regimen complexity,8 and the balance between treatment efficacy and adverse effects.7
Tools to accurately assess treatment satisfaction may provide useful information in both clinical practice, when considering patients’ treatment options, and in clinical studies, when evaluating novel treatments. Although generic measures of treatment satisfaction, such as the Treatment Satisfaction Questionnaire for Medication9 and Treatment Satisfaction with Medicines Questionnaire,10 have been developed, we have been unable to identify any existing measure that is specific for patients with SLE. While general satisfaction has been assessed in SLE populations,11–13 these generic satisfaction surveys do not explore the many facets of SLE that are likely to drive patient satisfaction. SLE disease management is unique, given the array of organ manifestations and the varied treatment options available to control these symptoms. Coupled with the fact that few new SLE treatment options have been approved in the past decade and that SLE is often a debilitating and progressive disease, an SLE-specific tool to assess satisfaction with treatment, treatment options, and medical care provides a useful addition to SLE patient satisfaction research.
The aim of this study was to develop a comprehensive, SLE-specific, patient-reported outcome (PRO) measure to assess patient satisfaction with treatment, treatment options, and medical care.
MATERIALS AND METHODS
Study Design and Population
This study (HO13-13424) was designed according to best practices for the development of PRO measures.14,15 Independent review board approval (Copernicus Group, Durham, NC) was obtained.
Patients were recruited from 4 US rheumatology practices (St Clair Shores, MI; Arlington, VA; Towson, MD; and Lansing, MI). Physicians were required to be rheumatologists currently treating 5 or more patients with SLE. Patients were eligible for inclusion if they were US residents, 18 to 75 years of age, and able to speak and read English; had a clinical diagnosis of SLE; had a Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index score of 4 or greater or had SLE rated as moderate or severe (based on investigator opinion); were receiving belimumab or were naive to biological therapy; and provided written informed consent at the clinic, prior to participating in telephone interviews. Patients with a medical or psychiatric condition (including lupus-related conditions) or who were receiving treatment for a condition that results in cognitive or other (visual, hearing) impairment that in the investigator’s opinion would interfere with participating in the study were excluded. The patient sample had a range of disease severity, race/ethnicity, and organ involvement. The aim was to recruit approximately 50% of patients receiving belimumab and 50% naive to biological therapy, in order to elicit concepts of satisfaction (or dissatisfaction) relevant to both traditional and more recently introduced treatment approaches.
Study sites and participants were remunerated fair market value for their participation.
Questionnaire Development
Each clinical site completed a case report form for each patient, to record the date of diagnosis, SLE severity (as determined by the physician), the presence of comorbid conditions, and use of concomitant medications.
All patient interviews were conducted via telephone and lasted approximately 60 minutes. The first cohort of patients (n = 6) participated in concept elicitation interviews only, which were conducted using a semi-structured interview guide developed specifically for this study. Patients were asked open-ended questions to identify important concepts regarding satisfaction with treatment and medical care. Questions focused on the patients’ current treatment (including perceived effectiveness, satisfaction with frequency and mode of administration, adverse effects, and adherence) and their opinion of the health care they receive. A draft of the Lupus Satisfaction Questionnaire (LSQ) was developed based on results from these initial concept elicitation interviews and clinical input; where possible, wording provided by the patients was used.
Subsequent interviews (n = 8) involved concept elicitation and cognitive debriefing of the draft LSQ. In most cases, the concept elicitation interview was conducted prior to the patient completing the draft LSQ. Because of the length of the interviews, not all questions were asked of all patients; therefore, the number of responses provided varies and is stated throughout the results. The items perceived to be the most challenging were specifically queried, and to avoid the interviews becoming overly tedious, patients were not asked to evaluate questions that were very similar to one another. After each round of cognitive debriefing interviews, the responses were reviewed and the draft LSQ was modified accordingly. The revised draft was then used in the next round of interviews. Following the final cognitive debriefing interview, the LSQ was reviewed and refined, with input from clinicians.
Safety was not assessed in the study; however, if a patient reported a potential adverse event during his/her interview, it was reported to the study sponsor, GlaxoSmithKline.
Data Analyses
All interviews were recorded and transcribed for analysis. Patient-identifiable information was removed to ensure patient confidentiality.
Interview data were coded using the qualitative data analysis software MAXQDA (Verbi GmBH, Berlin, Germany). A coding dictionary was developed in order to organize and categorize concepts of interest. To ensure consistency, all transcripts were coded by 1 coder and then reviewed, summarized, and analyzed by a second coder.
Preliminary Evaluation of the LSQ
To conduct a preliminary evaluation of the draft version of the LSQ, it was included as part of a larger survey carried out in the United States in patients with SLE. Patients were required to have a diagnosis of SLE and be older than 18 years; patients were excluded if they worked for or had a close family member who worked for a market research or pharmaceutical company.
RESULTS
Study Population
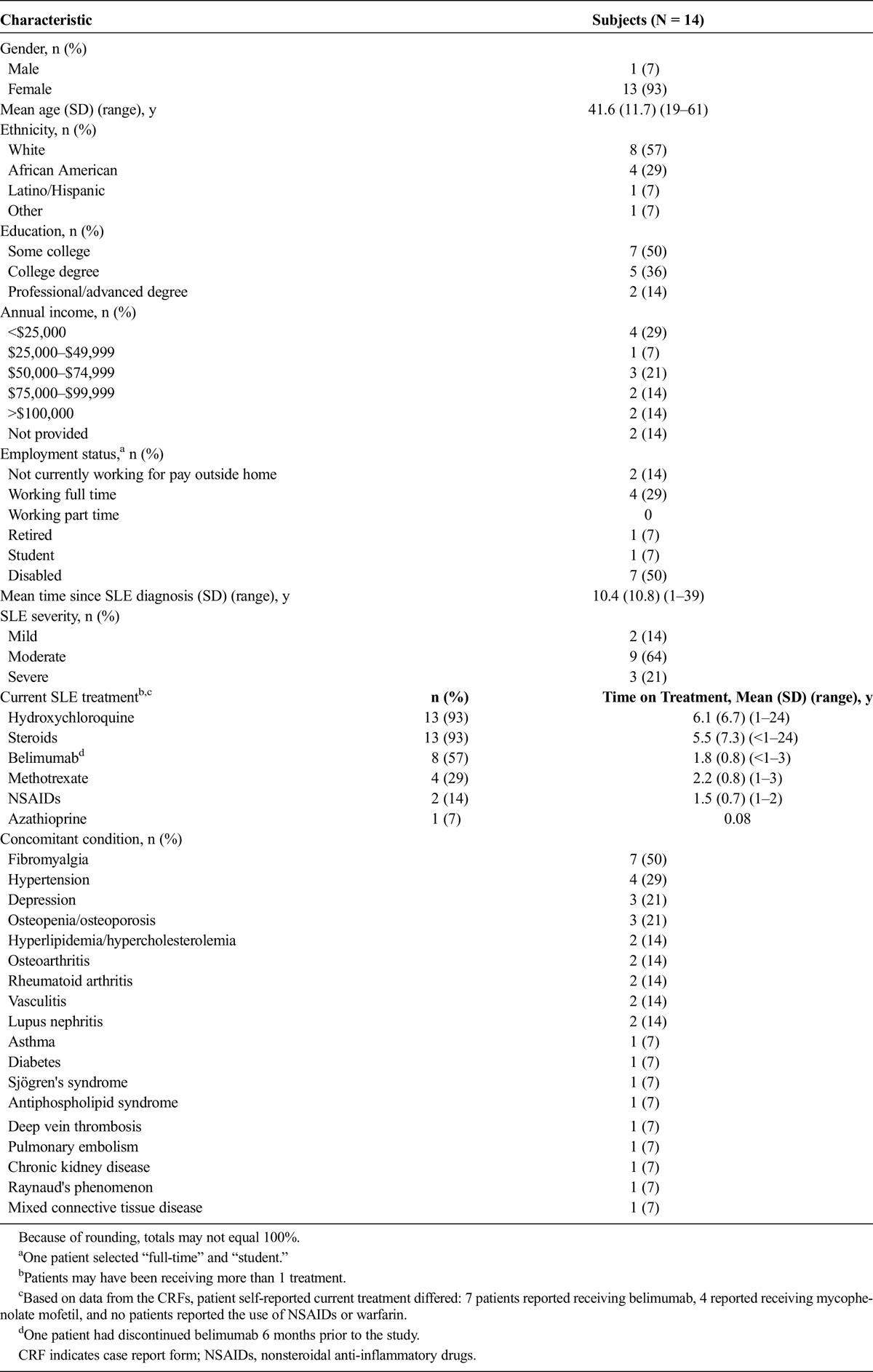
A total of 14 patients participated in the study; the majority were female (93%, 13/14), 57% (8/14) were white, and 29% (4/14) were African American (Table 1). Few patients (14%, 2/14) had professional/advanced degrees, the majority (57%, 8/14) had an annual income of less than $75,000, and one-third of patients (29%, 4/14) were working full time for pay. According to physician assessment 64% (9/14) of the patients had moderate disease, and 21% (3/14) had severe SLE. The mean time since SLE diagnosis was 10.4 (SD, 10.8) years. Based on patient responses, the most common treatments received by patients were hydroxychloroquine (93%, 13/14), steroids 93% (13/14), and belimumab (50%, 7/14). The mean treatment durations for each of these medications were 6.1 (SD, 6.7), 5.5 (SD, 7.3), and 1.8 (SD, 0.8) years, respectively.
TABLE 1.
Demographics and Clinical Characteristics of All Patients Who Completed a Concept Elicitation Interview
Concept Elicitation Interviews
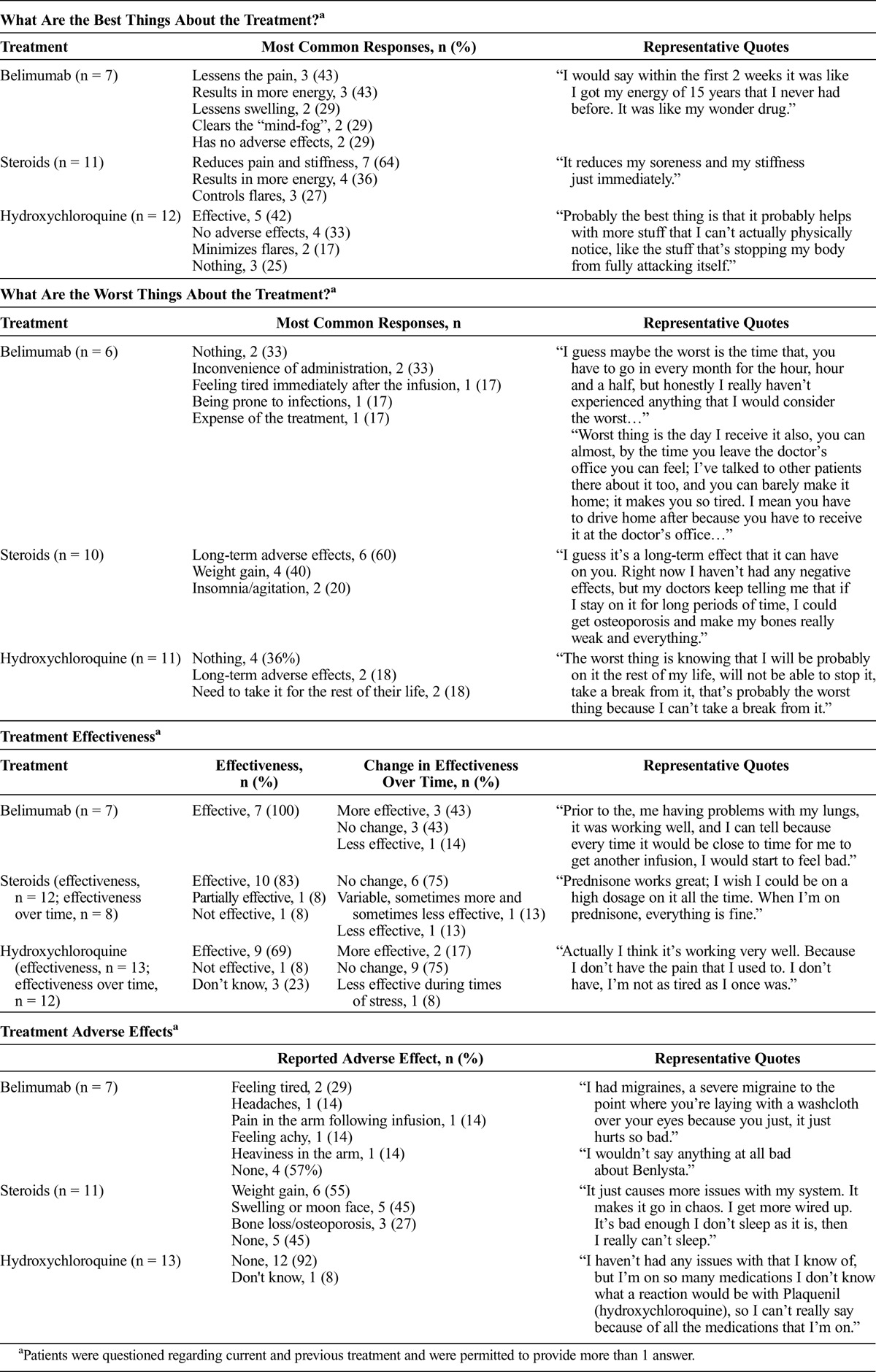
Patients (n = 9) rated overall satisfaction with their SLE treatment from 0 (not at all satisfied) to 10 (extremely satisfied); the mean overall treatment satisfaction score was 8.7 (range, 6–10). For individual SLE treatments, the mean scores were as follows: prednisone (n = 9), 7.9 (range, 7–10); hydroxychloroquine (n = 3), 7.0 (range, 5–10); and belimumab (n = 3), 9.3 (range, 8–10). When asked about the best and worst aspects of their treatment, the majority of patients remarked on factors relating to the effectiveness and adverse effects of the drugs, respectively (Table 2). When specifically asked about the effectiveness of their treatment, all patients receiving belimumab (100%, 7/7) and the majority of patients receiving steroids (85%, 11/13) and hydroxychloroquine (69%, 9/13) reported that their treatment was effective. Treatment adverse effects were common; 43% (3/7) of the patients receiving belimumab and all (11/11) patients receiving steroids reported experiencing adverse effects at some point, although 45% (5/11) of the patients stated that they were not currently experiencing adverse effects due to steroids. Most patients (92%, 12/13) reported no adverse effects from hydroxychloroquine, and 1 patient was unsure if she experienced adverse effects from hydroxychloroquine (Table 2).
TABLE 2.
Treatment Satisfaction: Responses From Concept Elicitation Interviews
All patients (n = 14) were asked if they found the frequency of administration of their medications to be acceptable. The majority of patients were receiving 1 or more medications once (n = 11) or twice (n = 7) per day or monthly (n = 7). In general, patients found the dose to be acceptable (91% for once per day [10/11], 86% for twice per day [6/7], and 100% for monthly [7/7]). (Note: Subjects could have been on more than 1 medication and responded for each medication.) However, differences were seen regarding mode of administration; compared with patients who received tablets/capsules (7%, 1/14) or an injection (0/2), a larger proportion of patients who received treatment (belimumab) by intravenous infusion reported that it was difficult (50%, 3/6). The main reasons for this were that it is inconvenient because of the need to travel to the clinic and because of the time required for the infusion.
With regard to adherence, 71% (10/14) of the patients reported that they had missed treatments; 2 patients stated that they had intentionally done so (1 patient because she was pregnant and did not think it was needed and 1 patient because she thought it was not working), and 9 patients unintentionally missed a dose (9 patients because of forgetting, and 1 patient also ran out of tablets). In addition, 56% (5/9) of the patients reported that they sometimes take their medications later in the day than prescribed.
Patients reported some dissatisfaction regarding the number of treatments available for SLE; 64% (9/14) said that there were not enough treatments available, 29% (4/14) thought that there were enough, and 7% (1/14) were uncertain. The dissatisfaction is exemplified by 1 patient who responded, “…lupus doesn’t get the attention like other areas. It’s getting better because nobody even knew what the word was when I was diagnosed. It’s becoming, they’re more aware of it now. To have only 1 drug breakthrough in 50 years to be added to the list to treat it, no, I don’t think there’s enough. I think that there should have been more that they can do for that by now.”
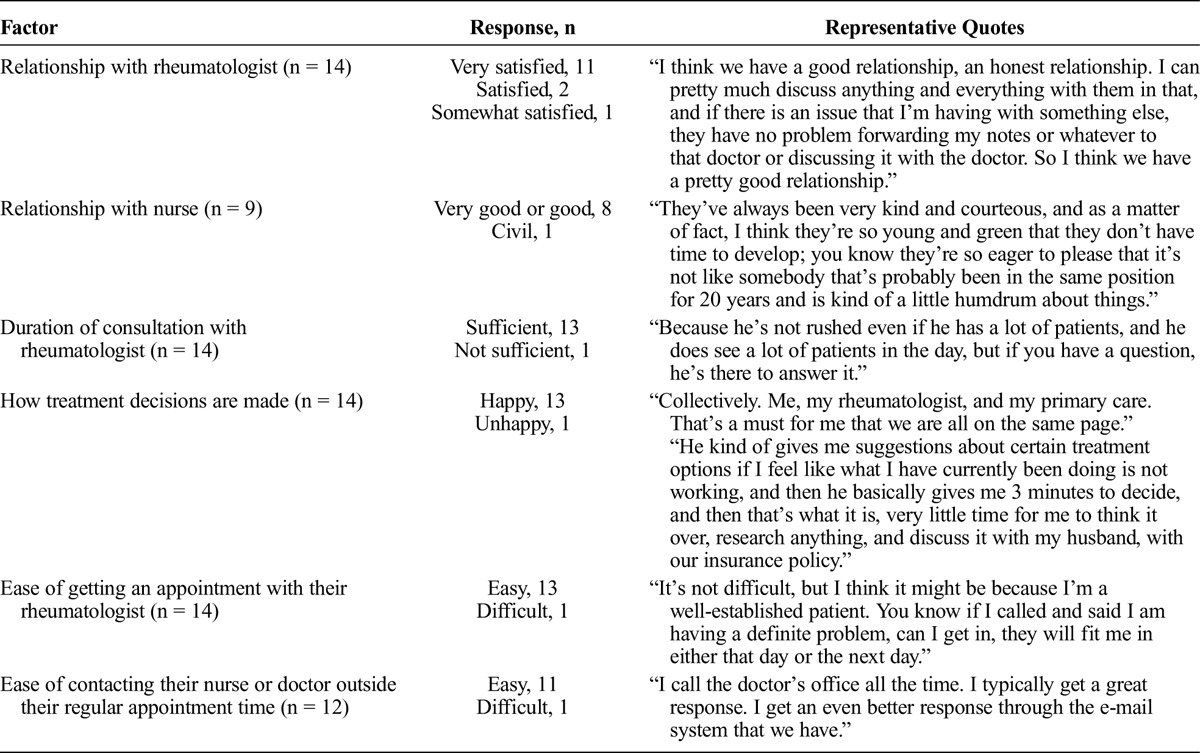
Patients were also questioned regarding their opinion of their medical care. The majority of patients were happy with their relationships with their rheumatologist and nurses and how decisions about their treatment are made (Table 3). Patients also reported a high degree of satisfaction with the ease of getting an appointment with their rheumatologist and the amount of time spent for each consultation.
TABLE 3.
Satisfaction With Health Care
Questionnaire Development and Cognitive Debriefing Interviews
An initial draft of the LSQ was developed based on the concepts identified during the first 6 concept elicitation interviews. Some revisions were then made based on clinician input. For example, the recall period was changed from 1 month to 3 months because 1 month was thought to be too short a period to assess a new treatment. Questions relating to satisfaction with health insurance, an aspect not discussed in the concept elicitation interviews, were also added based on the clinical feedback. The resulting draft of the LSQ contained 15 items assessing satisfaction with current lupus treatments, 11 items assessing satisfaction with health care providers, and 3 items assessing satisfaction with insurance coverage for lupus treatments. For the majority of questions, the response options ranged from 1 (very dissatisfied) to 7 (very satisfied).
A second patient group (n = 8) participated in a combined concept elicitation interview and cognitive debriefing of the draft LSQ. Mean age was 42.6 (11.58) years, 88% (7/8) were female, 53% (5/8) were white, 25% (2/8) were African American, 25% (2/8) had severe disease and 75% (6/8) had moderate disease. All patients had received hydroxychloroquine, 88% (7/8) had received steroids, and 53% (5/8) had received belimumab. Most patients (86%, 6/7) took approximately 5 minutes to complete the LSQ; 1 patient took approximately 10 minutes. Most patients (88%, 7/8) found it easy to respond to all questions and thought the response options were appropriate. One patient suggested revising the questions regarding treatment satisfaction so that the questions were asked separately about tablets/capsules, injections, and infusions. This revision was incorporated following the seventh cognitive debriefing interview, and the revised draft was debriefed in the final interview. During this final cognitive debriefing interview, there was little new information provided.
The majority of patients (75%, 6/8) thought the 3-month recall period was appropriate, 1 patient would have preferred a longer period (6 months), and 1 patient would have preferred a recall period of 2 weeks. While some patients said they would have answered the same if the recall period had been 1 month (86%, 6/7) or 1 year (43%, 3/7), the 3-month recall period was retained because it was endorsed by the majority of patients (75%, 6/8) and recommended by clinicians as being an appropriate period over which to assess a new treatment.
Except for the change described above, only minor revisions were made to the wording of some questions to improve clarity. For example, “different” was added to the question, “In the past 3 months, how satisfied or dissatisfied were you with how well your different health care providers worked together to treat your lupus?” to clarify that it is referring to the full team of health care providers.
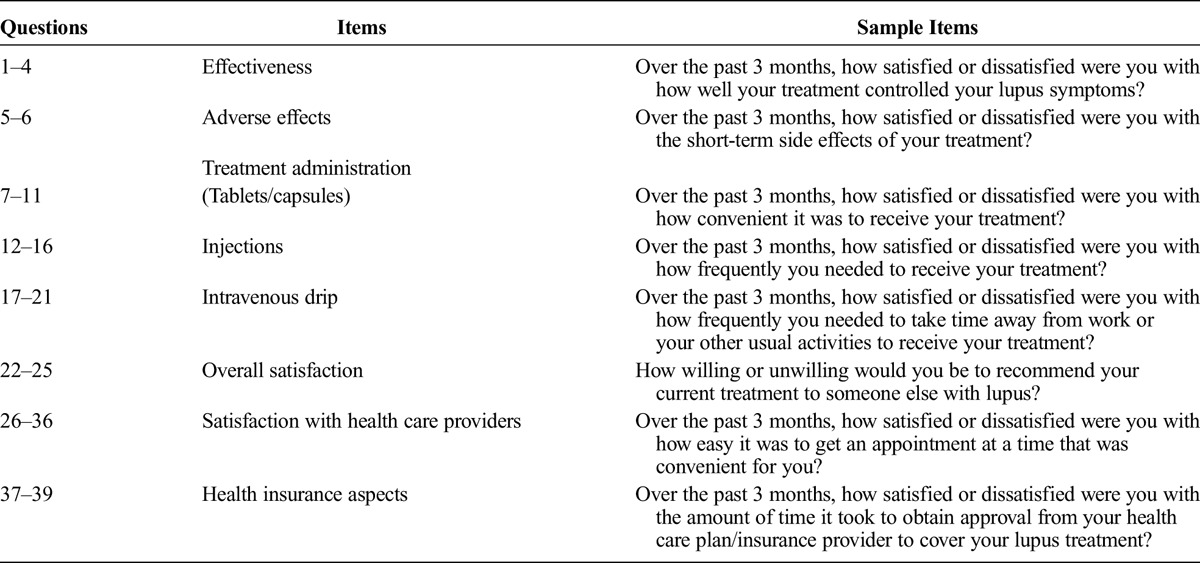
The revised LSQ contains 39 items (Table 4) and asks patients to consider all SLE treatments they have received in the past 3 months.
TABLE 4.
Summary of Items Included in the LSQ
Preliminary Evaluation of the LSQ
As a preliminarily evaluation of the LSQ, a draft version was included in a survey carried out in the United States. Of the 195 patients, 73% were female, mean age was 41.7 years, mean time since SLE diagnosis was 10.5 years, 41% were employed full time, and 53% had children. In this survey, 58% of patients reported that they were at least “somewhat satisfied” with their SLE treatment. Patients who reported a lower frequency of adverse effects and who experienced more “good days” than “bad days” were more likely to report that they were satisfied. The majority (85%) of the patients stated that they were “completely,” “very,” or “moderately” satisfied with their clinician’s management of their SLE, and 63% of patients were at least “somewhat satisfied” with the amount of time spent with their health care provider during a consultation. This initial data from the draft LSQ provide some verification of the appropriateness of the items and response options given in the measure, as patients generally provided a range of responses, and very few answered “didn’t know” or “not applicable.” As this was a cross-sectional survey, it was not possible to follow up with patients to explore responses on the LSQ in greater depth.
DISCUSSION
To the best of our knowledge, the LSQ is the first comprehensive PRO measure designed for use patients with SLE, to specifically assess satisfaction with treatment, treatment options, and medical care. The PRO was developed according to best practices and as such, used significant patient input, an iterative development process, clinical input, and cognitive debriefing of the draft questionnaire.14,15 The resulting draft of the questionnaire contains 39 items that assess treatment effectiveness, adverse effects, administration, overall treatment satisfaction, and satisfaction with health care providers and insurance. Patients are asked to consider all SLE treatments received in the last 3 months; therefore, it is important to obtain the comprehensive treatment history.
Patient input is critical to the development of PROs to ensure that all relevant concepts are identified and that the PRO is developed from the patient perspective.14,15 Although the sample size was relatively small in this study, it was demographically broad in terms of age, ethnicity, and geographic location. Although only 1 of the 14 patients was male, SLE affects more females than males (ratio of 9:1),16 such that the 13:1 ratio in the current sample seems appropriate. In addition, the majority of patients were white (57%), just under a third were African American (29%), and 7% were Hispanic. However, the LSQ will be further evaluated in a larger, more representative patient population. While the sample size in this study was small, and not all items were reviewed by all patients, we are confident that the sample size was sufficient to identify all important concepts and to confirm the content, clarity, and relevance of the PRO. Many items were conceptually similar to those reviewed by patients, and patient feedback resulted in very few, minor revisions to the questionnaire. By the last interview, there was little new information provided.
Patients should preferably not participate in both concept elicitation and cognitive debriefing interviews, whereas in this study the second cohort completed combined concept elicitation and cognitive debriefing interviews. To avoid bias, in most cases, the concept elicitation interview was performed before the patient completed the questionnaire; however, separate interviews may have enabled more detailed exploration of concepts and questionnaire feedback. Therefore, subsequent evaluation of the measure will explore whether the concepts captured in the LSQ are sufficient. Another limitation of the study was that all interviews were conducted via telephone. Although this enabled the study to be conducted over a wide geographic location, face-to-face interviews allow the interviewer to respond to any confusion suggested by patients’ facial expressions and/or body language.
Preliminary evaluation of the LSQ suggests that the items and responses are appropriate. Future validation including conducting factor analysis and evaluating internal consistency reliability, test-retest reliability, construct validity, and known group validity should be performed. To evaluate test-retest reliability, it will be necessary for a subgroup of stable patients to complete the questionnaire 7 to 10 days after their initial response. Furthermore, to enable longitudinal use of the questionnaire, a cohort will need to complete the questionnaire twice, approximately 3 months apart, so that responsiveness, sensitivity over time, the minimum clinically important change, and responder definition can be assessed. Following demonstration of these measurement properties, the LSQ will be a useful measure in clinical studies and clinical practice. In naturalistic studies, the LSQ may provide important information to fully evaluate satisfaction with novel treatments. In clinical practice, the LSQ should enhance the dialogue between physicians and patients about factors that drive satisfaction with SLE treatment. Enhanced dialogue may help align patient and clinician priorities and needs regarding treatment and may prove useful in future treatment decisions. It is of note that the concept elicitation interviews found that patients have a relatively high level of satisfaction with their treatment despite reporting high levels of adverse effects, suggesting that patients have a low level of expectation regarding the treatment of SLE. As new treatments become available, the LSQ may provide a useful tool to monitor changes in treatment expectations and satisfaction.
In summary, data from concept elicitation and cognitive debriefing interviews and clinical input were utilized to develop the LSQ, a questionnaire designed to assess patient satisfaction with SLE treatment, treatment options, and medical care. Further validation and evaluation are required to support its future use in SLE clinical trials and clinical practice.
KEY POINTS
The Lupus Satisfaction Questionnaire (LSQ) is a comprehensive patient-reported outcome (PRO) measure designed to specifically assess satisfaction with treatment, treatment options, and medical care in systemic lupus erythematosus.
This PRO was developed according to best practices, using an iterative development process with significant input from patients and clinicians.
The LSQ contains 39 items that assess treatment effectiveness, adverse effects, administration, overall treatment satisfaction, and satisfaction with health care providers and insurance.
Preliminary evaluation of the draft LSQ suggests that the items and response options are appropriate, as patients provided a range of responses and very few selected “didn’t know” or “not applicable.”
ACKNOWLEDGMENTS
Medical writing assistance was provided by Katie White, PhD, and Louisa Pettinger, PhD, of Fishawack Indicia, Ltd, and was funded by GlaxoSmithKline.
Footnotes
The authors declare no conflict of interest.
This study (HO13-13424) was conducted by Health Outcomes Solutions, which received funding from GlaxoSmithKline (GSK).
S.D.M., A.A., and H.H.C. are consultants for GSK. P.B. is a shareholder and employees of GSK. J.d.V. and D.J.C. are shareholders of GSK and were employees of GSK at the time of the study. K.P. was an employee of GSK at the time of the study.
REFERENCES
- 1.Bertsias G, Ioannidis JPA, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67:195–205. [DOI] [PubMed] [Google Scholar]
- 2.Delestras S, Roustit M, Bedouch P, et al. Comparison between two generic questionnaires to assess satisfaction with medication in chronic diseases. PLoS One. 2013;8:e56247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marengo M, Waimann C, de Achaval S, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21:1158–1165. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Perez-Gutthann S, Longenecker JC, et al. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med. 1991;91:345–353. [DOI] [PubMed] [Google Scholar]
- 5.Adler M, Chambers S, Edwards C, et al. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology. 2006;45:1144–1147. [DOI] [PubMed] [Google Scholar]
- 6.Julian LJ, Yelin E, Yazdany J, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Achaval S, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol. 2010;5:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa CD, Balp M-M, Kulich K, et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz MAPA, Rejas J, Soto J, et al. Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q). Value Health. 2008;11:913–926. [DOI] [PubMed] [Google Scholar]
- 11.Strand V, Galateanu C, Pushparajah D, et al. Limitations of current treatments for systemic lupus erythematosus: a patient and physician survey. Lupus. 2013;22:819–826. [DOI] [PubMed] [Google Scholar]
- 12.Kulczycka LL. The influence of clinical manifestations and treatment on satisfaction with life together with positive and negative emotions in systemic lupus erythematosus patients. Acta Dermatovenerol Croat. 2011;19:6–12. [PubMed] [Google Scholar]
- 13.Bennett JK, Fuertes JN, Keitel M, et al. The role of patient attachment and working alliance on patient adherence, satisfaction, and health-related quality of life in lupus treatment. Patient Educ Couns. 2011;85:53–59. [DOI] [PubMed] [Google Scholar]
- 14.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14:978–988. [DOI] [PubMed] [Google Scholar]
- 15.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;4:967–977. [DOI] [PubMed] [Google Scholar]
- 16.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum. 2008;58:15–25. [DOI] [PubMed] [Google Scholar]


