Abstract
The aim of the study is to systematically review the evidence on post parathyroidectomy (PTX) changes as measured by echocardiogram (ECHO) in patients with primary hyperparathyroidism (PHPT).
PHPT may increase risk of cardiovascular morbidity/mortality. Conclusions of studies assessing ECHO changes, pre versus post PTX, are inconsistent.
A systematic literature search was conducted to locate published and unpublished studies. Randomized control trials, nonrandomized control trials, and observational studies were included. Variables were reported as means and standard deviations. An inverse variance statistical method, with random-effects analysis model, was applied to continuous data. The effect measure was standardized mean difference, confidence interval of 95%. Primary outcome measure was left ventricular ejection fraction (LVEF). Secondary outcome measures were left ventricular mass index (LVMI), peak early over peak late diastolic velocity ratio (E/A ratio), isovolumetric relaxation time (IVRT), intraventricular septal thickness (IVST), and posterior wall thickness (PWT).
Fourteen studies were included. Follow-up time ranged 3 to 67 months. No significant differences (P > .05) in primary outcome measure LVEF (SMD = −0.03, CI = −0.24, 0.19), or secondary outcome measures E/A Ratio (SMD = −0.05, CI = −0.24, 0.14), IVST (SMD = 0, CI = 0.31, 0.32), PWT (SMD = 0.01, CI = −0.38, 0.39), LVMI (SMD = −0.18, CI = −0.74, 0.38), and IVRT (SMD = −0.84, CI = −1.83, 0.14) were observed.
There was no significant difference in LVEF pre to post PTX. Due to heterogeneity of current literature, we were unable to determine if other outcome measures of cardiac function are affected after PTX in patients with PHPT. We recommend a randomized control trial be conducted to make concrete conclusions.
Keywords: cardiac morbidity, echocardiogram changes, parathyroidectomy, primary hyperparathyroidism
1. Introduction
Primary hyperparathyroidism (PHPT) is the third most common endocrine disorder following diabetes and hypothyroidism, with a prevalence of 1% in the adult population. It increases with age in both sexes and is most prevalent in postmenopausal women.[1,2] The impact of the disease is significant with symptomatic patients suffering from nephrolithiasis, osteoporosis, and overall reduction in overall quality of life.[3] Recent evidence suggests it may also be associated with increased cardiovascular morbidity and mortality.[4,5]
Surgery in the form of parathyroidectomy (PTX) is the only known treatment for PHPT, and is recommended in patients with symptomatic disease (nephrolithiasis, osteoporosis, overt skeletal disease) and in patients who are asymptomatic with significant hypercalcemia (>1.0 mg/dL/0.25 mmol/L).[6] Current guidelines suggest observation for asymptomatic patients over 50 years of age.[6] The impact of PHPT on overall cardiovascular morbidity and mortality, especially among asymptomatic patients, is unclear. Therefore, the safety of observation for patients with asymptomatic PHPT has not been established. Echocardiographic data has shown that left ventricular hypertrophy is common in PHPT patients and that a reduction in left ventricular mass often takes place after PTX, however, the literature is conflicting on whether cardiac function improves post-PTX.[5,7,8] A recent meta-analysis found an improvement in 1 cardiac index, left ventricular mass in studies with a short duration of follow-up only (<6 months).[9]
There is sufficient literature to warrant a systematic review and meta-analysis examining multiple echocardiogram (ECHO) measures of cardiac function following PTX. The purpose of this review was to synthesize the evidence on PTX-induced ECHO changes in patients with PHPT. The authors hypothesized that PTX may have an effect on ECHO changes. The primary outcome measure was left ventricular ejection fraction (LVEF) (%). Secondary outcome measures included peak early over peak late diastolic velocity ratio (E/A ratio), interventricular septal thickness (IVST) (mm), posterior wall thickness (PWT) (mm), left ventricular mass index (LVMI) (g/m2), and isovolumetric relaxation time (IVRT) (ms). To our knowledge, this is the first systematic review and meta-analysis done on the aforementioned cardiac outcomes.
2. Methods
2.1. Search strategy
A search methodology was used to assist in locating both published and unpublished studies. An experienced librarian and one of the principal investigators completed 2 independent searches. Research databases and conference meeting abstracts were searched for articles published from 1970 to current and included PubMed, Cochrane Library (Wiley), BIOSIS (Thomson-Reuters), CINAHL (EBSCO), Web of Science (Thompson-Reuters), CINAHL, and EMBASE (OVID). The grey literature search was explored by searching clinical trials databases (clinicaltrials.gov, clincialtrialsregister.eu, www.who.int/trialsearch, www.ukctg.nihr.ac.uk). The National Institute of Health database and google scholar were searched using terms “primary hyperparathyroidism,” “parathyroidectomy,” and “cardiovascular risk.” We also searched Open grey, Grey matters and Grey Literature Report. Dissertations and Theses (Proquest), the Canadian Health Research Collection (Ebrary), as well as the annual meeting abstracts of the endocrine society 2001–14 which include the following journals (Journal of Clinical Endocrinology, Endocrinology, Endocrine Reviews, Meeting Abstracts, Book Series), and The Society for Endocrinology 2013–15, which include the following journals (Endocrinology Diabetes and Metabolism Case Reports, Endocrine Connections, Journal of Endocrinology, Journal of Molecular Endocrinology, Endocrine-related Cancer, Clinical Endocrinology, and Endocrine Abstracts). We also searched the conference proceedings for the International Journal of Cardiovascular Medicine, Surgery, Pathology and Pharmacology, International Academy of Cardiology, and Cardiology. The conference proceedings for Annals of Surgery and JAMA-Surgery were searched for surgical journals.
The search strategies employed database-specific subject headings and keywords for “parathyroidectomy,” “surgical procedures, operative,” “treatment outcome,” “parathyroid surgery,” “surgical treatment,” “cardiovascular disease,” “cardiovascular risk,” “heart disease,” “hyperparathyroidism, primary,” “echocardiogram.” Alerts were set up for each database to receive publication notifications for new related articles.
2.2. Study selection strategy
Articles included were from any country, all in English, and were research articles. Only full-text articles were included. The articles included randomized and nonrandomized trials and observational studies. We only included adults (≥18 years of age), nonpregnant, with a diagnosis of PHPT. Articles that studied patients who underwent PTX in which there was a comparison made between pre- and post-PTX ECHO changes were included. A minimum of 10 patients per group was required for inclusion in the study. Our primary outcome measure was LVEF. Secondary outcome measures included LVMI, E/A ratio, IVRT, IVST, and PWT.
Articles excluded were those published prior to 1970 as measures of parathyroid hormone (PTH) were in routine use after 1970. We excluded studies that compared post PTX results to a control group that did not undergo PTX, studies that re-reported results on a previously published cohort, studies that did not use echocardiography, studies that examined patients with secondary hyperparathyroidism or tertiary hyperparathyroidism, and studies in which patients did not undergo PTX. Additionally, non-research articles such as editorials, review articles, commentaries, letters, and systematic review were excluded.
A total of 674 articles were retrieved by searching various databases and an additional 675 were retrieved from hand searching and grey literature search which was then imported into EPPI Reviewer 4.0 reference manager.[11] Based on the inclusion and exclusion criteria, 2 reviewers independently reviewed all articles. After removing duplicate articles, 981 articles were included for screening. Articles were screened by title, abstract, and full text in level 1, 2, and 3 screening, respectively. After each level of screening kappa statistics was calculated to measure reviewer's agreement. The agreement between the 2 reviewers was substantial for level 3 screening (kappa 0.688).[12] The PRISMA diagram demonstrating the selection process is displayed in Figure 1.[13]
Figure 1.
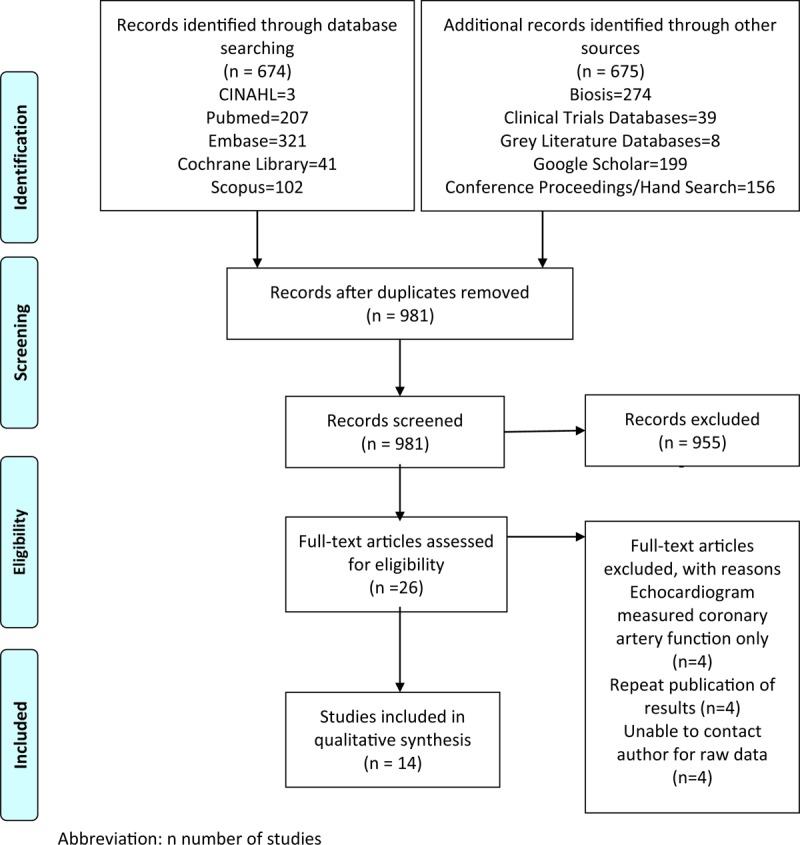
Prisma diagram. n = number of students.
2.3. Data extraction strategy
Qualitative and quantitative data necessary for analysis was obtained from each article. One reviewer extracted the data using an excel template which was then reviewed by a second reviewer. Information on study location, design, patient demographics, and effect measures were collected. Demographic details were extracted on the patients included in the studies. Data on the following outcome measures were extracted: LVEF (%), E/A ratio, IVST (mm), PWT (mm), LVMI (g/m2), and IVRT (ms). All data values not reported in SI units were converted to SI units using Google calculator when entered into excel template. Authors were contacted via email in attempt to obtain missing relevant information.
2.4. Quality assessment strategy
Randomized control before-and-after studies were assessed using The Cochrane Collaboration's tool for assessing risk of bias.[14] Studies were ranked as low risk, unclear, or high risk. Before-and-after studies with no control group were analyzed using the Quality Assessment Tool from the NIH Institute for Before-and-After Studies with No Control Group.[15] Studies were ranked as good, fair, or poor. Despite the quality of evidence, all articles were included in the analysis.
2.5. Statistical analysis
All variables were reported as means and standard deviation. Data was analyzed as continuous data as we were assessing outcomes before and after surgery in the same patients. The statistical method applied was inverse variance with a random-effects analysis model to account for variations in measurement tools. The effect measure was standardized mean difference. Confidence interval was 95%. Heterogeneity of the effect across studies was assessed by means of Cochrane Q χ2 statistic and I2 statistic. Lack of homogeneity was considered for Cochrane Q χ2 test P ≤.10 and/or for I2 statistics ≥50%. The z-statistics was computed for each clinical outcome, and results were considered statistically significant at a P <.05. A funnel plot analysis was conducted on the primary outcome measure to assess for publication bias. Statistical analysis and graphs were made using the Review Manager (RevMan) software package (version 5.3 for OSX, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
2.6. Ethics approval
Ethics approval was not required to conduct this review as the data was collected and synthesized from previously conducted trails, and all data is anonymous. There are no ethical considerations.
3. Results
Of the 981 citations identified and retrieved, we reviewed 26 potentially relevant articles. Twelve studies did not fit our inclusion criteria and were therefore discarded (Fig. 1). We finally included 14 studies. The included studies were published between 1990 and 2013. The baseline characteristics of the included studies are summarized in Table 1.
Table 1.
Study characteristics.
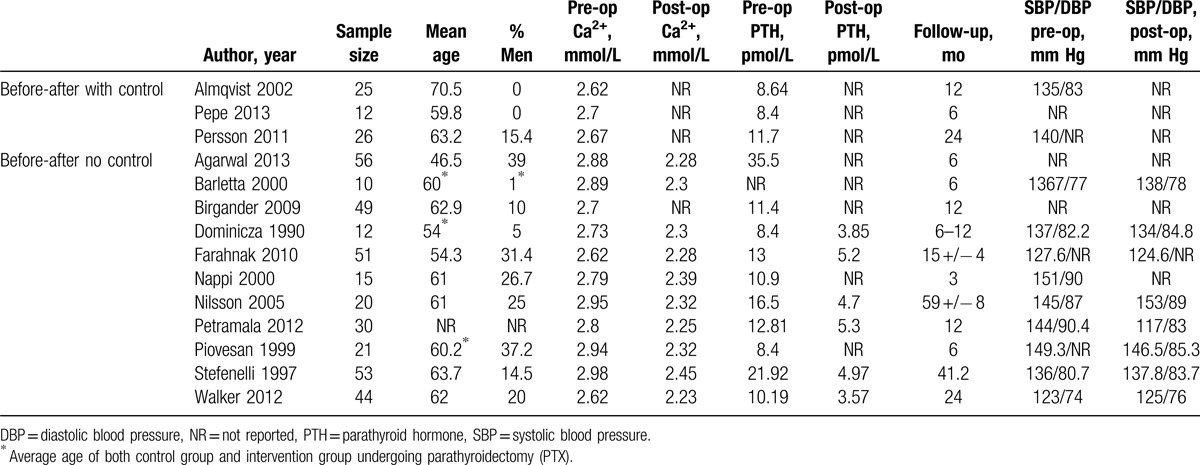
3.1. Study quality assessment
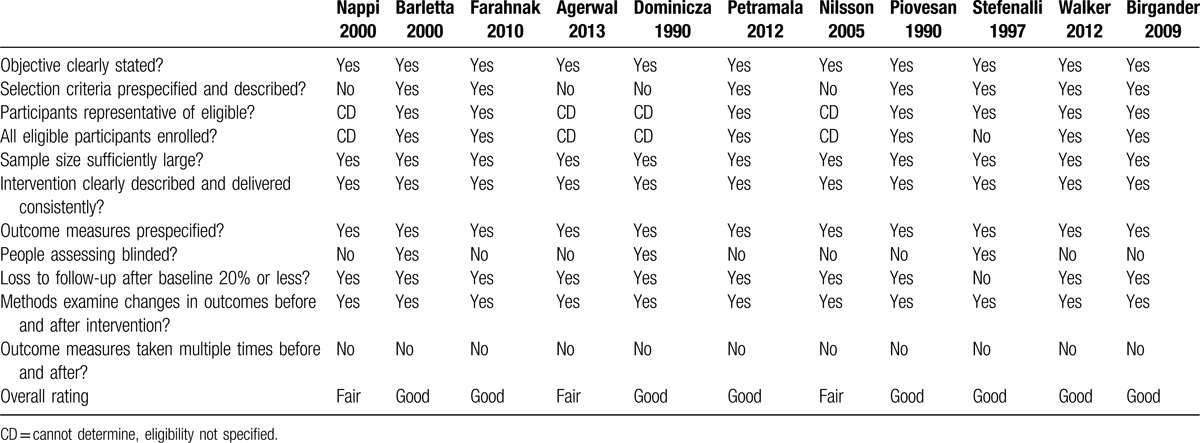
Quality assessment for bias detection was completed on all 14 studies. Quality of the 3 randomized control before-and-after studies ranged from low risk to high risk (Table 2). Quality of the 11 before-and-after studies with no control group ranged from fair to good (Table 3).
Table 2.
Quality assessment of randomized control before-and-after studies using The Cochrane Collaboration Tool.
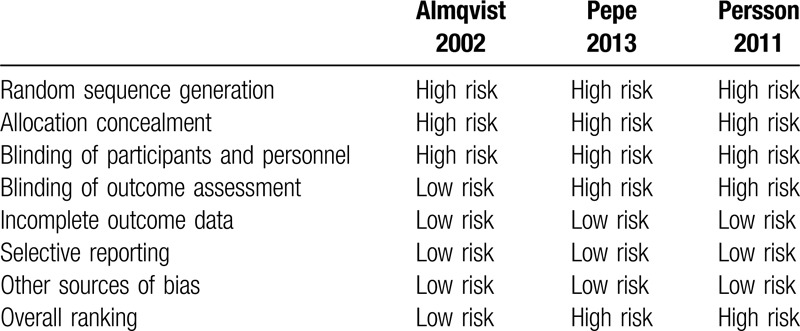
Table 3.
Quality assessment of before-and-after studies with no control group using the quality assessment tool from the NIH Institute.
3.2. Primary outcome measure: LVEF
Outcomes were analyzed using a randomized effects variance model. There was no significant difference (P > .05) in the primary outcome measure LVEF (SMD = −0.03, CI = −0.24, 0.19) (Fig. 2A). The degree of relative heterogeneity was I2 = 35%. A subgroup analysis on LVEF was completed. Studies with short-term follow-up (6 months or less) did not show any significant difference (SMD: −0.20, CI = −0.59, 0.28), I2 = 41% (Fig. 2B). Studies with long-term follow-up (>6 months) ranged in follow-up times from over 6 months to 5 years, and did not show any significant difference (SMD = 0.21, CI = 0.01, 0.40), I2 = 0 (Fig. 2C). A secondary subgroup analysis was done to include only the 3 randomized control before-and-after studies, and did not show any significant difference (SMD: 0.14, CI = −0.21, 0.49, I2 = 0%) (Fig. 2D).
Figure 2.
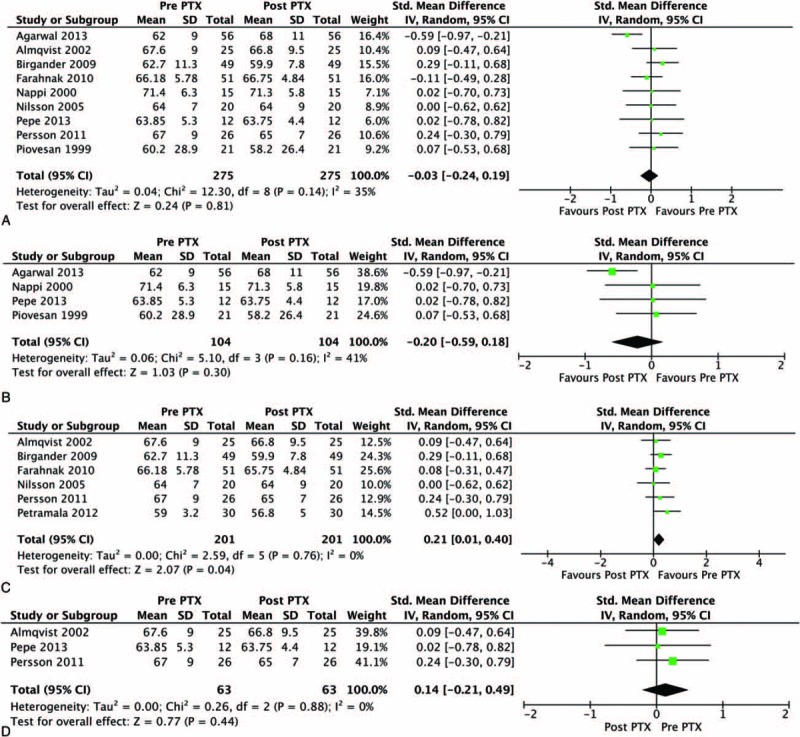
Forest plot for left ventricular ejection fraction (LVEF). (A) All studies. (B) Studies with short-term follow-up (6 mo or less). (C) Studies with long-term follow-up (>6 mo). (D) Randomized control before and after studies only.
3.3. Secondary outcome measures
There was no significant difference with a low degree of heterogeneity for E/A ratio (SMD = −0.05, CI = −0.24, 0.14), I2 = 0 (Fig. 3A). There were no significant differences with a moderate degree of heterogeneity for IVST (SMD = 0, CI = 0.31, 0.32), I2 = 50% (Fig. 3B) and PWT (SMD = 0.01, CI = −0.38, 0.39), I2 = 54% (Fig. 3C). There were no significant differences and a high degree of heterogeneity for LVMI (SMD = −0.18, CI = −0.74, 0.38), I2 = 88% (Fig. 3D), and IVRT (SMD = −0.84, CI = −1.83, 0.14), I2 = 95% (Fig. 3E).
Figure 3.
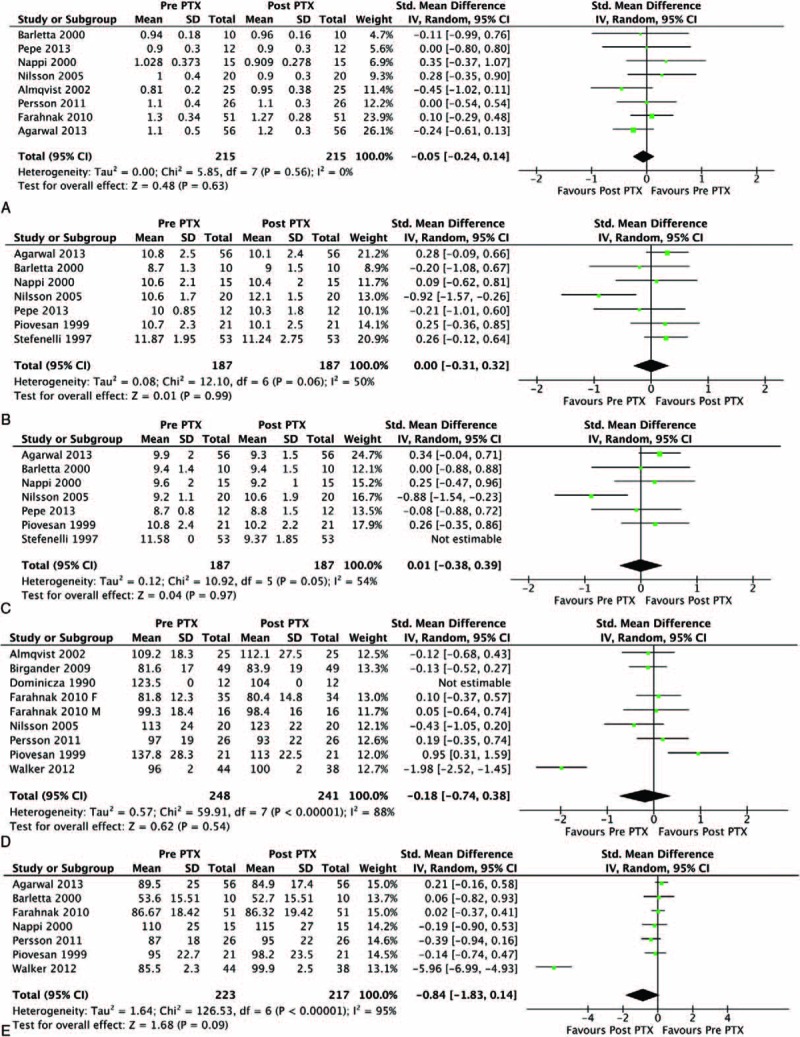
Funnel plots for secondary outcome measures. (A) E/A ratio (peak early/peak late diastolic velocity). (B) Interventricular septal thickness (IVST) (ms). (C) Posterior wall thickness (PWT) (mm). (D) Left ventricular mass index (LVMI) (g/m2). (E) Isovolumetric relaxation time (IVRT) (ms).
4. Discussion
This manuscript reviews 14 studies published within the last 26 years provides an up-to-date analysis of the data on ECHO changes following PTX in patients with PHPT.
4.1. Quality of evidence
The quality of included randomized control before-and-after studies ranged from low risk to high risk. All studies receive a high-risk assessment for the areas of random sequence generation, allocation concealment, and blinding of personnel and participants.[16–18] Two out of 3 studies also received a high-risk assessment for blinding of assessment, as it was not addressed within their manuscript.[17,18]
The quality of included observational before-and-after studies with no control group ranged from fair to good. All studies included clearly stated the study objectives.[19–29] All studies included had a sufficient sample size, as defined as at least 10 participants at the outset of the study.[19–29] All but one study had <20% loss to follow-up from baseline.[28] Studies rated fair did not clearly outline prespecified inclusion criteria, so we were unable to assess if participants were representative of those eligible for the study, or if all eligible participants were enrolled.[19,22,24,25] Many studies did not report to have blinded assessors interpreting the ECHO findings.[17–19,21,23–27]
There was evidence of publication bias as assessed by funnel plot for the primary outcome measure (Fig. 4).
Figure 4.
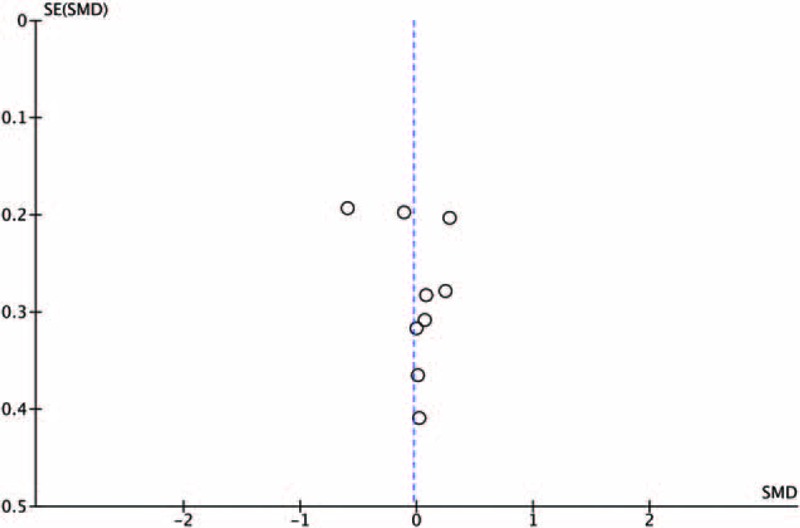
Funnel plot for left ventricular ejection fraction (LVEF) all studies.
4.2. Justification for exclusion
Only full-text articles were included so all data points could be accessed and the quality of study could be assessed thoroughly. We did not include studies with participants under the age of 18 as our study question focused on adults with PHPT and the cardiac effects the disease has over time. We did not include studies that included pregnant women, as we wanted to reduce the number of potential confounding factors on cardiac function pre and post PTX. We did not include studies that assessed secondary or tertiary hyperparathyroidism, as the nature of these diseases differ, and the current body of literature best supported an analysis on PHPT.
4.3. Findings
The meta-analysis conducted on the 3 randomized control before-and-after studies revealed no significant difference in LVEF pre to post PTX, with a low degree of heterogeneity. It is possible there are no differences in the secondary outcomes measures; however, due to the paucity of evidence, we are unable to make a strong conclusion whether or not PTX has any effect on the trajectory of cardiovascular changes.
Observational studies describe increased cardiovascular morbidity and mortality in patients with PHPT.[30–35] PTH and Ca2+ may have independent effects on cardiovascular function. PTH may act as a hypertrophic factor on myocardial muscle cells, inducing left ventricular hypertrophy, as well as inotropic effects on the heart itself via its ability to increase heart rate and coronary blood flow.[36,37] Another proposed mechanism is related to arterial stiffness. Increased vascular stiffness has been demonstrated in PHPT in multiple studies.[7,28] PTH-induced vascular stiffness could increase cardiac afterload, thereby contributing to the development of LVH. A recent meta-analysis reported higher preoperative serum PTH was associated with a greater decrease in LVM, whereas higher calcium level was not.[9] Calcium has been suggested to be an independent mediator of LVH, as high serum calcium can impair cardiac relaxation.[28] Calcium itself has a positive inotropic effect.[38]
The analysis completed on the secondary outcome measures is more hypotheses generating than hypothesis proving or disproving. Due to the paucity of current evidence, we are unable to make a strong conclusion whether PTX has the potential to slow or reverse disease, as interpreted by ECHO. The difficulty in assessing this question may lie in the natural history of the disease. It is difficult to know how long subjects within studies have been living with PHPT and hypercalcemia before diagnosis, and therefore before intervention. At this point, it is unknown how high PTH or calcium levels have to be, and for what duration they have to be elevated before significant changes occur. It is unknown at what stage of disease, if any, surgical intervention can be beneficial. Most published studies include subjects with symptomatic disease. Perhaps intervention at a presymptomatic stage and a lower level of hypercalcemia could alter disease trajectory.
4.4. Strengths and limitations
This marks the first paper to thoroughly examine the impact of PTX on ECHO changes in patients with PHPT. A major strength of this review is the thorough literature search performed to include all possible studies done on this topic in the last 10 years. We followed a strict methodology. Our study has internal validity as all outcome measures were reported consistently across studies. This paper may serve as an important benchmark on which future studies may be modeled. Using a rigid systematic review approach, a sample of only 3 randomized control trials were identified. The lack of high-level evidence on this topic to date is a concern in such a prominent endocrine disease. In unveiling current gaps in the literature, direction for future research can be identified, and a concrete consensus on the cardiac effect of PHPT may be attained.
The limitations occur more at an individual study level than at a systematic level. The variation in study type is a limitation. All but 3 studies published on this topic were observational studies, using a before-and-after with no control group design. Only 3 studies conducted randomization. The inconsistent quality of the included studies is a second unfavorable variable. The range in the follow-up times in studies (6 months to 5 years), and sample sizes of the studies (n = 12 to n = 56), adds another source of limitation. There is inherent limitation in the fact that we are measuring surrogate outcomes, objective ECHO measures, in place of clinical outcomes. There is no direct translation that can be made from ECHO findings to patient functioning, survival, and quality of life. Therefore, we cannot conclude that PTX does not have a direct effect on ECHO measurements and therefore does not have a direct effect on overall cardiac functioning and overall patient success.
5. Conclusions and future recommendations
The meta-analysis conducted on the 3 randomized control before-and-after studies revealed no significant difference in LVEF pre to post PTX. There was a low degree of heterogeneity between these studies. The analysis of other secondary outcome measures is hypothesis generating. There were no significant differences in E/A ratio, IVST, PWT, LVMI, and IVRT, and the heterogeneity ranged from low to high with these outcome measures. The analysis included all study types and there was a large degree of variation in study quality.
The gold standard next step would be to complete a large, randomized control trial on the topic. Significant limitations may include time and financial expense. A study that screens for early hypercalcemia and implements surgical intervention at a presymptomatic stage may assess if early intervention has any effect on disease trajectory, and therefore long-term cardiovascular morbidity and mortality in patients with PHPT.
Acknowledgments
The authors would like to thank John Costella, Associate Librarian, Western University, London, Ontario, Canada.
Footnotes
Abbreviations: E/A ratio = peak early over peak late diastolic velocity ratio, ECHO = echocardiogram, IVRT = isovolumetric relaxation time, IVST = intraventricular septal thickness, LVEF = left ventricular ejection fraction, LVMI = left ventricular mass index, PHPT = primary hyperparathyroidism, PTX = parathyroidectomy, PWT = posterior wall thickness.
The authors report no conflicts of interest.
References
- [1].Lundgren E, Rastad J, Thrufjell E, et al. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery 1997;121:287–94. [DOI] [PubMed] [Google Scholar]
- [2].Palmér M, Jakobsson S, Akerström G, et al. Prevalence of hypercalcemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest 1998;18:39–46. [DOI] [PubMed] [Google Scholar]
- [3].Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med 2000;343:1863–75. [DOI] [PubMed] [Google Scholar]
- [4].Hedbäck G, Odén A, Tisell LE. The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg 1991;15:399–405. [DOI] [PubMed] [Google Scholar]
- [5].Piovesan A, Molineri A, Casasso F, et al. Left ventricular hypertrophy in primary hyperparathyroidism: effects of successful parathyroidectomy. Clin Endocrinol 2009;50:321–8. [DOI] [PubMed] [Google Scholar]
- [6].Bilezikian JP, Khan AA, Potts JT. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 2009;94:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohara N, Hiramatsu K, Shigematsu S, et al. Effect of parathyroid hormone on left ventricular diastolic function in patients with primary hyperparathyroidism. Miner Electrolyte Metab 1995;21:63–6. [PubMed] [Google Scholar]
- [8].Nilsson IL, Aberg J, Rastad J, et al. Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism: effects of parathyroidectomy. Surgery 2000;128:895–902. [DOI] [PubMed] [Google Scholar]
- [9].McMahon DJ, Carrelli A, Palmeri N, et al. Effect of parathyroidectomy upon left ventricular mass in primary hyperparathyroidism: a meta-analysis. J Clin Endocrinol Metab 2015;100:4399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rao DS, Phillips ER, Divine GW, et al. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 2004;89:5415–22. [DOI] [PubMed] [Google Scholar]
- [11].EPPI-Reviewer [software computer program]. Version 4.0. London: Social Science Research Unit, Institute of Education, University of London; 2010. [Google Scholar]
- [12].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [14].Ryan R. The Cochrane Collaboration's Tool for Assessing Risk of Bias; Adapted Using EPOC's Criteria for Studies Other than RCTs (Control Before-and-After Studies). In Study Quality Guide. Cochrane Consumers and Communication Review Group; 2013:28. [Google Scholar]
- [15].National Heart, Lung, and Blood Institute. Quality Assessment Tool for Before-After (Pre-Post) studies with no control group; 2014. [Google Scholar]
- [16].Almqvist EG, Bondeson AG, Bondeson L, et al. Cardiac dysfunction in mild primary hyperparathyroidism assessed by radionuclide angiography and echocardiography before and after parathyroidectomy. Surgery 2002;132:1126–32. [DOI] [PubMed] [Google Scholar]
- [17].Pepe J, Curione M, Morelli S, et al. Parathyroidectomy eliminates arrhythmic risk in primary hyperparathyroidism, as evaluated by exercise test. Eur J Endocrinol 2013;169:255–61. [DOI] [PubMed] [Google Scholar]
- [18].Persson A, Bollerslev J, Rosen T, et al. Effect of surgery on cardiac structure and function in mild primary hyperparathyroidism. Clin Endocrinol (Oxf) 2011;74:174–80. [DOI] [PubMed] [Google Scholar]
- [19].Agarwal G, Nanda G, Kapoor A, et al. Cardiovascular dysfunction in symptomatic primary hyperparathyroidism and its reversal after curative parathyroidectomy: results of a prospective case control study. Surgery 2013;154:1394–403. [DOI] [PubMed] [Google Scholar]
- [20].Barletta G, De Feo ML, Del Bene R, et al. Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab 2000;85:1815–21. [DOI] [PubMed] [Google Scholar]
- [21].Birgander M, Bondeson A, Bondeson L, et al. Cardiac structure and function before and after parathyroidectomy in patients with asymptomatic primary hyperparathyroidism. Endocrinologist 2009;19:154. [Google Scholar]
- [22].Dominiczak AF, Lyall F, Morton JJ, et al. Blood pressure, left ventricular mass and intracellular calcium in primary hyperparathyroidism. Clin Sci (Lond) 1990;78:127–32. [DOI] [PubMed] [Google Scholar]
- [23].Farahnak P, Ring M, Caidahl K, et al. Cardiac function in mild primary hyperparathyroidism and the outcome after parathyroidectomy. Eur J Endocrinol 2010;163:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nappi S, Saha H, Virtanen V, et al. Left ventricular structure and function in primary hyperparathyroidism before and after parathyroidectomy. Cardiology 2000;93:229–33. [DOI] [PubMed] [Google Scholar]
- [25].Nilsson IL, Aberg J, Rastad J, et al. Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism-effects of parathyroidectomy. Surgery 2000;128:895–902. [DOI] [PubMed] [Google Scholar]
- [26].Luigi P, Chiara FM, Laura Z, et al. Arterial hypertension, metabolic syndrome and subclinical organ damage in patients with asymptomatic primary hyperparathyroidism: preliminary results. Int J Endocrinol 2012;2012:408295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Piovesan A, Molineri N, Casasso F, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999;50:321–8. [DOI] [PubMed] [Google Scholar]
- [28].Stefenelli T, Mayr H, Bergler-Klein J, et al. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med 1993;95:197–202. [DOI] [PubMed] [Google Scholar]
- [29].Walker MD, Fleischer J, Rundek T, et al. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab 2009;94:3849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Palmér M, Adami HO, Bergström R, et al. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 1987;102:1–7. [PubMed] [Google Scholar]
- [31].Farr HW, Fahey TJ, Jr, Nash AG, et al. Primary hyperparathyroidism and cancer. Am J Surg 1973;126:539–43. [DOI] [PubMed] [Google Scholar]
- [32].Kaplan L, Katz AD, Ben-Isaac C, et al. Malignant neoplasms and parathyroid adenoma. Cancer 1971;28:401–7. [DOI] [PubMed] [Google Scholar]
- [33].Wajngot A, Werner S, Grandberg P, et al. Occurrence of pituitary adenomas and other neoplastic diseases in primary hyperparathyroidism. Surg Gynecol Obstet 1980;151:401–3. [PubMed] [Google Scholar]
- [34].Hedback G, Tisell LE, Bengtsson BA, et al. Premature death in patients operated on for primary hyperparathyroidism. World J Surg 1990;14:829–35. [DOI] [PubMed] [Google Scholar]
- [35].Ronni-Sivula H. Causes of death in patients previously operated on for primary hyperparathyroidism. Ann Chir Gynaecol 1985;74:13–8. [PubMed] [Google Scholar]
- [36].Schlüter KD, Piper HM. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol 1992;263(part 2):H1739–46. [DOI] [PubMed] [Google Scholar]
- [37].Ogino K, Burkhoff D, Bilezikian JP. The hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related protein. Endocrinology 1995;136:3024–30. [DOI] [PubMed] [Google Scholar]
- [38].Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest 1981;67:1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


