Abstract
Background:
Dexmedetomidine showed some potential in pain control in patients undergoing knee arthroscopy. We conducted a systematic review and meta-analysis to explore the efficacy of dexmedetomidine in patients undergoing knee arthroscopy.
Methods:
We searched the randomized controlled trials (RCTs) assessing the effect of dexmedetomidine on knee arthroscopy in PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases. The primary outcome was pain scores. Meta-analysis was performed using the random-effect model.
Results:
Five RCTs were included. Overall, compared with control intervention in patients with knee arthroscopy, dexmedetomidine intervention could significantly reduce the pain scores [Std. mean difference = −0.84; 95% confidence interval (95% CI) = −1.24 to −0.44; P < .0001] and postoperative diclofenac sodium consumption (Std. mean difference = −1.76; 95% CI = −3.32 to −0.21; P = .03), improve duration of analgesic effect (Std. mean difference = 1.78; 95% CI = 0.56–3.00; P = .004), but showed no influence on hypotension [risk ratio (RR) = 0.93; 95% CI = 0.14–5.92; P = .94], bradycardia (RR = 4.93; 95% CI = 0.91–26.58; P = .06), nausea, and vomiting (RR = 1.96; 95% CI = 0.31–12.58; P = .48).
Conclusion:
Dexmedetomidine intervention was able to significantly reduce the pain scores and postoperative diclofenac sodium consumption, and improve duration of analgesic effect in patients undergoing knee arthroscopy, but had no influence on hypotension, bradycardia, nausea, and vomiting.
Keywords: dexmedetomidine, knee arthroscopy, meta-analysis, pain management, systematic review
1. Introduction
Arthroscopic surgery was known as one of the most common orthopedic surgeries.[1,2] However, irritation of free nerve endings in the synovial tissue, anterior fat pad, and joint capsule during arthroscopic excisions and resections would lead to varying levels of pain.[3,4] Early mobilization and psychological state could be affected by postoperative pain, which could result in prolonged hospital stays and affects the prognosis adversely. Adequate pain relief was very important to reduce morbidity and promote postoperative recovery.[5–7]
Intra-articular administration of drugs provided local analgesia with minimal systemic adverse effects.[8,9] These drugs mainly included local anesthetics (e.g., lidocaine and bupivacaine), opioids (e.g., morphine and fentanyl), and α2-agonists (e.g., clonidine) etc, and they were found to achieve variable durations of analgesia.[10–12] Dexmedetomidine was a highly selective, specific, and potent α2-adrenergic receptor agonist, and had sedative, anxiolytic, analgesic, anti-hypertensive, and sympatholytic properties[13–16] and showed some analgesic effect in arthroscopic surgeries.[17] Many randomized controlled trials (RCTs) reported that dexmedetomidine was able to significantly reduce pain score and postoperative diclofenac sodium consumption, as well as improve duration of analgesic effect in knee arthroscopy.[18–20]
In contrast to this promising finding, however, some relevant RCTs showed that dexmedetomidine had no influence on pain control and duration of analgesic effect in patients undergoing knee arthroscopy.[17,21] Considering these inconsistent effects, we therefore conducted a systematic review and meta-analysis of RCTs to evaluate the effectiveness of dexmedetomidine intervention on pain management in patients undergoing knee arthroscopy.
2. Materials and methods
This systematic review and meta-analysis was conducted according to the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement[22] and the Cochrane Handbook for Systematic Reviews of Interventions.[23] All analyses were based on previous published studies, and thus, no ethical approval and patient consent were required.
2.1. Literature search and selection criteria
PubMed, EMbase, Web of science, EBSCO, and the Cochrane library were systematically searched from inception to March 2017, with the following keywords
dexmedetomidine, and knee arthroscopy or knee arthroscopic surgery. No limitation was enhanced. To include additional eligible studies, the reference lists of retrieved studies and relevant reviews were also hand-searched and the process above was performed repeatedly until no further article was identified. Conference abstracts meeting the inclusion criteria were also included.
The inclusion criteria were as follows: study population, patients undergoing knee arthroscopy; intervention, dexmedetomidine intervention; control intervention; outcome measure, pain score; and study design, RCT.
2.2. Data extraction and outcome measures
The following information was extracted for the included RCTs: first author, publication year, sample size, baseline characteristics of patients, dexmedetomidine, control, study design, pain score, postoperative diclofenac sodium consumption, duration of analgesic effect, hypotension, bradycardia, nausea, and vomiting. The author would be contacted to acquire the data when necessary.
The primary outcome was pain score. Secondary outcomes included postoperative diclofenac sodium consumption, duration of analgesic effect, hypotension, bradycardia, nausea, and vomiting.
2.3. Quality assessment in individual studies
The Jadad Scale was used to evaluate the methodological quality of each RCT included in this meta-analysis.[24] This scale consisted of 3 evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). One point would be allocated to each element if they have been mentioned in article, and another 1 point would be given if the methods of randomization and/or blinding had been detailed and appropriately described. If methods of randomization and/or blinding were inappropriate, or dropouts and withdrawals had not been recorded, then 1 point was deducted. The score of Jadad Scale varied from 0 to 5 points. An article with Jadad score ≤2 was considered to be of low quality. If the Jadad score ≥3, the study was thought to be of high quality.[25]
2.4. Statistical analysis
Standard mean differences with 95% confidence intervals (95% CIs) for continuous outcomes (pain score, postoperative diclofenac sodium consumption, duration of analgesic effect), and risk ratios (RRs) with 95% CIs for dichotomous outcomes (hypotension, bradycardia, nausea, and vomiting) were used to estimate the pooled effects. All meta-analyses were performed using random-effects models with DerSimonian and Laird weights. Heterogeneity was tested using the Cochran Q statistic (P < .1) and quantified with the I2 statistic, which described the variation of effect size that was attributable to heterogeneity across studies. An I2 value greater than 50% indicated significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting 1 study in turn when necessary. Owing to the limited number (<10) of included studies, publication bias was not assessed. P < .05 in 2-tailed tests was considered statistically significant. All statistical analyses were performed with Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature search, study characteristics, and quality assessment
The flow chart for the selection process and detailed identification is presented in Fig. 1. Five hundred twenty-one publications were identified through the initial search of databases. Ultimately, 5 RCTs were included in the meta-analysis.[17–21]
Figure 1.
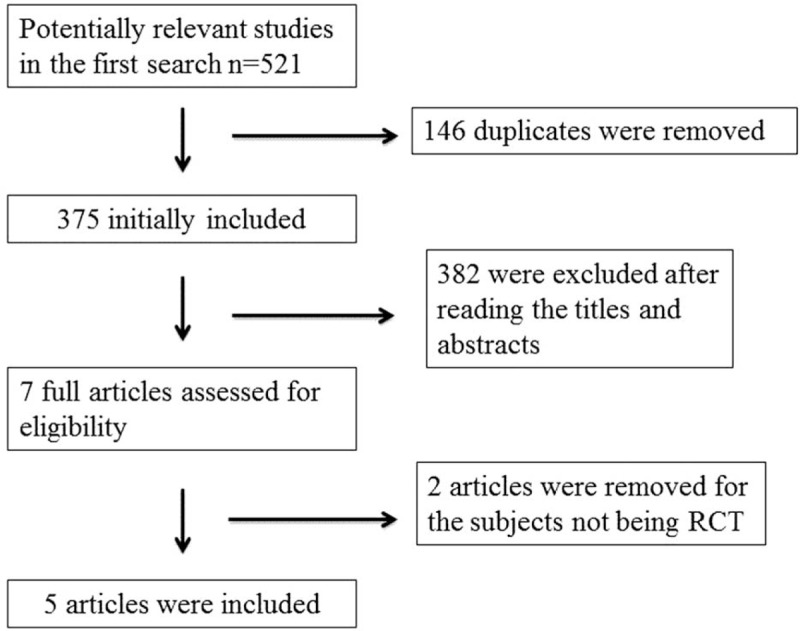
Flow diagram of study searching and selection process.
Table 1 demonstrates the baseline characteristics of the 5 eligible RCTs in the meta-analysis. The doses and methods of pregabalin were different in each RCT. They were intra-articular 18 mL ropivacaine, dexmedetomidine 2 μg/kg versus intra-articular ropivacaine (20 mL),[20] intra-articular 100 μg (1 mL) of dexmedetomidine added to 19 mL of 0.25% ropivacaine versus intra-articular 19 mL of 0.25% ropivacaine and 1 mL of isotonic saline,[19] intra-articular 1 μg/kg dexmedetomidine and isotonic saline versus intra-articular 25 mL isotonic saline,[18] dexmedetomidine 1 μg/kg intravenously, for 10 minutes followed by dexmedetomidine 0.3 μg/kg for 50 minutes versus 2 g of propacetamol,[17] and buccal dexmedetomidine 2.5 μg/kg versus buccal 0.9% NaCl 2 mL.[21]
Table 1.
Characteristics of included studies.
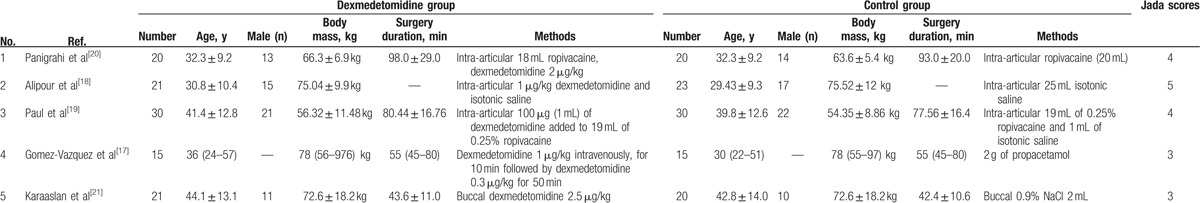
Among the 5 RCTs, 2 studies reported the pain score,[18,19] 2 studies reported the postoperative diclofenac sodium consumption,[20,21] 4 studies reported the duration of analgesic effect,[18–21] and 2 studies reported the hypotension, bradycardia nausea, and vomiting.[17,19] Jadad scores of the 5 included studies varied from 3 to 5; all 5 studies were considered to be high-quality ones according to quality assessment.
3.2. Primary outcome: pain score
These outcome data were analyzed with a random-effects model; the pooled estimate of the 2 included RCTs suggested that compared with control group, dexmedetomidine intervention was associated with a significantly decreased pain scores (Std. mean difference = −0.84; 95% CI = −1.24 to −0.44; P < .0001), with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .52) (Fig. 2).
Figure 2.
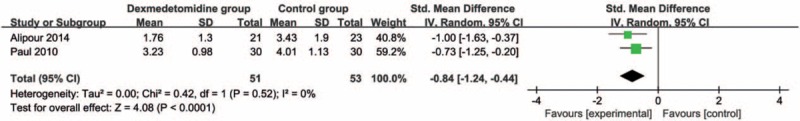
Forest plot for the meta-analysis of pain score.
3.3. Sensitivity analysis
No heterogeneity was observed among the included studies for the pain scores. Thus, we did not perform sensitivity analysis by omitting 1 study in each turn to detect the source of heterogeneity.
3.4. Secondary outcomes
Compared with control intervention, dexmedetomidine intervention showed significantly reduced postoperative diclofenac sodium consumption (Std. mean difference = −1.76; 95% CI = −3.32 to −0.21; P = .03; Fig. 3) and improved duration of analgesic effect (Std. mean difference = 1.78; 95% CI = 0.56–3.00; P = .004; Fig. 4), but had no increase in hypotension (RR = 0.93; 95% CI = 0.14–5.92; P = .94; Fig. 5), bradycardia (RR = 4.93; 95% CI = 0.91–26.58; P = .06; Fig. 6), nausea, and vomiting (RR = 1.96; 95% CI = 0.31–12.58; P = .48; Fig. 7).
Figure 3.
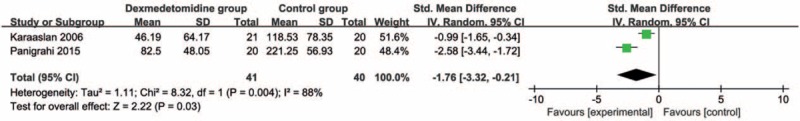
Forest plot for the meta-analysis of postoperative diclofenac sodium consumption (mg).
Figure 4.
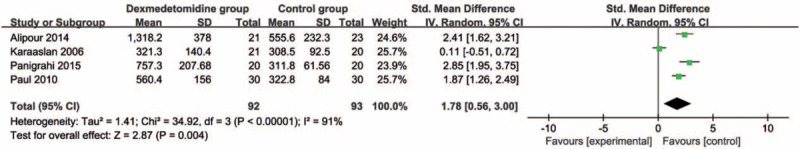
Forest plot for the meta-analysis of duration of analgesic effect (min).
Figure 5.
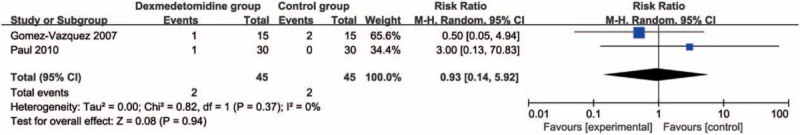
Forest plot for the meta-analysis of hypotension.
Figure 6.
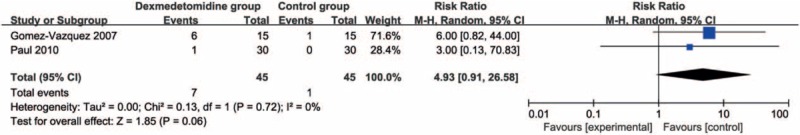
Forest plot for the meta-analysis of bradycardia.
Figure 7.
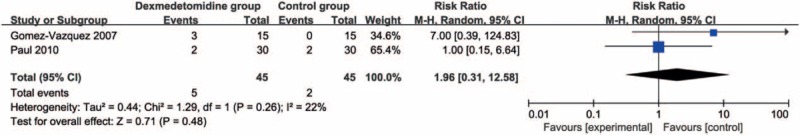
Forest plot for the meta-analysis of nausea and vomiting.
4. Discussion
Our meta-analysis clearly suggested that compared with control intervention, dexmedetomidine intervention was associated with a significantly reduced pain score and postoperative diclofenac sodium consumption, improved duration of analgesic effect, but had no effect on hypotension, bradycardia, nausea, and vomiting.
Intra-articular injection of dexmedetomidine was reported to enhance postoperative analgesia after arthroscopic knee surgery, and reduce the need for postoperative analgesia.[18] These analgesic effects relied on the direct local effect and central analgesic effect through the inhibition of transmission of painful stimuli in the posterior horn of the spinal cord. One previous study demonstrated that intra-articular dexmedetomidine 2 μg/kg and ropivacaine resulted in superior analgesic efficacy and better postoperative pain relief compared with intra-articular dexmedetomidine 1 μg/kg and ropivacaine following arthroscopic knee surgery, indicating the importance of dexmedetomidine concentration on analgesic effects.[20] Furthermore, intra-articular analgesics benefited to knee mobilization, quadriceps exercise, and walking during functional recovery.[20]
When analyzing duration of analgesic effect, there was significant heterogeneity among the 4 included RCT. After excluding 1 RCT using buccal dexmedetomidine, just low heterogeneity was found (I2 = 40%, heterogeneity P = .19).[21] Three RCTs reported that intra-articular dexmedetomidine was able to significantly improve duration of analgesic effect compared with control intervention for arthroscopic knee surgery.[18–20] However, the remaining 1 RCT showed that there was no significant difference of duration of analgesic effect between buccal dexmedetomidine and buccal 0.9% NaCl 2 mL.[21] These results indicated that the analgesia effect of intra-articular dexmedetomidine was superior to that of buccal dexmedetomidine.
In addition, dexmedetomidine intervention was found to have no increase in adverse events including hypotension, bradycardia, nausea, and vomiting when compared with control group. One RCT reported that there were no significant differences of postoperative heart rate and mean arterial pressure between intra-articular dexmedetomidine 2 μg/kg and ropivacaine versus ropivacaine.[20] However, a significant increase was noted in systolic, diastolic, and mean arterial pressures in patients receiving intravenous dexmedetomidine for several minutes compared with those patients getting propacetamol.[17] Intra-articular dexmedetomidine might be better for the control of postoperative heart rate and mean arterial pressure than intravenous dexmedetomidine for arthroscopic knee surgery.
But there were several limitations. First, only 5 RCTs were included in our meta-analysis, and 5 of them had a relatively small sample size (n < 100). The doses and methods of dexmedetomidine in the included studies were different, and might have some impact on the pooled results. The volume of injected drug may increase the intra-articular pressure and excessive pressure may induce systemic absorption after the tourniquet was released.[26] All included RCTs did not measure plasma concentrations of dexmedetomidine and correlate them with the clinical findings, which helped to confirm that the analgesia effect was local or systematic. Finally, the optimal dose and methods of dexmedetomidine for arthroscopic knee surgery remained elusive and required more clinical studies.
5. Conclusion
Dexmedetomidine showed an important ability to reduce pain and improve duration of analgesic effect in patients undergoing knee arthroscopy. Dexmedetomidine was recommended to be administrated for knee arthroscopy, but more studies should investigate its optimal dose and method.
Footnotes
Abbreviations: CIs = confidence intervals, RCTs = randomized controlled trials, RRs = risk ratios.
The authors declared no conflict of interest. And no funding was provided for this study. Research did not involve human participants and/or animals.
References
- [1].Pihl K, Englund M, Lohmander LS, et al. Signs of knee osteoarthritis common in 620 patients undergoing arthroscopic surgery for meniscal tear. Acta Orthop 2017;88:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lohmander LS, Thorlund JB, Roos EM. Routine knee arthroscopic surgery for the painful knee in middle-aged and old patients: time to abandon ship. Acta Orthop 2016;87:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dogan N, Erdem AF, Erman Z, Kizilkaya M. The effects of bupivacaine and neostigmine on articular cartilage and synovium in the rabbit knee joint. J Int Med Res 2004;32:513–9. [DOI] [PubMed] [Google Scholar]
- [4].Monk P, Garfjeld Roberts P, Palmer AJ, et al. The urgent need for evidence in arthroscopic meniscal surgery. Am J Sports Med 2017;45:965–73. [DOI] [PubMed] [Google Scholar]
- [5].Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ 2015;350:h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Imani F, Entezary S, Razi M, et al. The effect of intra-articular meperidine and bupivacaine 0.5% on postoperative pain of arthroscopic knee surgery; a randomized double blind clinical trial. Anesthesiol Pain Med 2015;5:e27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun QB, Liu SD, Meng QJ, et al. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 2015;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antoni M, Jenny JY, Noll E. Postoperative pain control by intra-articular local anesthesia versus femoral nerve block following total knee arthroplasty: impact on discharge. Orthop Traumatol Surg Res 2014;100:313–6. [DOI] [PubMed] [Google Scholar]
- [9].Gupta B, Banerjee S, Prasad A, et al. Analgesic efficacy of three different dosages of intra-articular morphine in arthroscopic knee surgeries: randomised double-blind trial. Indian J Anaesth 2015;59:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stein C, Comisel K, Haimerl E, et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med 1991;325:1123–6. [DOI] [PubMed] [Google Scholar]
- [11].Yang Y, Zeng C, Wei J, et al. Single-dose intra-articular bupivacaine plus morphine versus bupivacaine alone after arthroscopic knee surgery: a meta-analysis of randomized controlled trials. Knee Surg Sports Traumatol Arthrosc 2017;25:966–79. [DOI] [PubMed] [Google Scholar]
- [12].Wei J, Lei GH, Gao SG, et al. Single-dose intra-articular bupivacaine versus morphine after arthroscopic knee surgery: a meta-analysis of randomized-controlled studies. Clin J Pain 2014;30:630–8. [DOI] [PubMed] [Google Scholar]
- [13].Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother 2007;41:245–52. [DOI] [PubMed] [Google Scholar]
- [14].Zhang C, Li C, Pirrone M, et al. Comparison of dexmedetomidine and clonidine as adjuvants to local anesthetics for intrathecal anesthesia: a meta-analysis of randomized controlled trials. J Clin Pharmacol 2016;56:827–34. [DOI] [PubMed] [Google Scholar]
- [15].Poorzamany Nejat Kermany M, Dahi M, Yamini Sharif R, Radpay B. Comparison of the effects of dexmedetomidine and remifentanil on cognition state after cataract surgery. Anesthesiol Pain Med 2016;6:e33448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bilgi KV, Vasudevan A, Bidkar PU. Comparison of dexmedetomidine with fentanyl for maintenance of intraoperative hemodynamics in hypertensive patients undergoing major surgery: a randomized controlled trial. Anesth Essays Res 2016;10:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gomez-Vazquez ME, Hernandez-Salazar E, Hernandez-Jimenez A, et al. Clinical analgesic efficacy and side effects of dexmedetomidine in the early postoperative period after arthroscopic knee surgery. J Clin Anesth 2007;19:576–82. [DOI] [PubMed] [Google Scholar]
- [18].Alipour M, Tabari M, Faz RF, et al. Effect of dexmedetomidine on postoperative pain in knee arthroscopic surgery; a randomized controlled clinical trial. Arch Bone Joint Surg 2014;2:52–6. [PMC free article] [PubMed] [Google Scholar]
- [19].Paul S, Bhattacharjee DP, Ghosh S, et al. Efficacy of intra-articular dexmedetomidine for postoperative analgesia in arthroscopic knee surgery. Ceylon Med J 2010;55:111–5. [DOI] [PubMed] [Google Scholar]
- [20].Panigrahi R, Roy R, Mahapatra AK, et al. Intra-articular adjuvant analgesics following knee arthroscopy: comparison between single and double dose dexmedetomidine and ropivacaine a multicenter prospective double-blind trial. Orthop Surg 2015;7:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Karaaslan D, Peker TT, Alaca A, et al. Comparison of buccal and intramuscular dexmedetomidine premedication for arthroscopic knee surgery. J Clin Anesth 2006;18:589–93. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available at: http://www.cochranelibrary.com/ Accessed March 21, 2011. [Google Scholar]
- [24].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [25].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [26].Whitford A, Healy M, Joshi GP, et al. The effect of tourniquet release time on the analgesic efficacy of intraarticular morphine after arthroscopic knee surgery. Anesth Analg 1997;84:791–3. [DOI] [PubMed] [Google Scholar]


