Abstract
The purpose of this study was to evaluate the prescription trend and pattern of oral antidiabetic (OAD) medications, which are extensively used worldwide for treating type 2 diabetes, in 2 age groups.
In this population-based study, data obtained from the National Health Insurance Research Database, Taiwan, were analyzed to investigate the prescription trend of all types of OAD medications during 2005 to 2012. We used descriptive statistics to demonstrate the trend of prescription patterns stratified by age (aged 65 years and above or younger than 65).
Sulfonylurea (SU) was once the most commonly used drug, but the proportion of its prescription had declined gradually (76.83% in 2005 to 63.70% in 2012). Consequently, biguanide (BG) became the most commonly used drug since 2010 (64.31% in 2005 to 74.41% in 2012). In addition, the prescriptions of thiazolidinedione decreased significantly (9.20% in 2005 to 2.86% in 2012), whereas the usage of DPP-4 inhibitor increased with time (3.73% in 2009 to 19.64% in 2012). The treatment choice of SU and α-glucosidase inhibitor (AGI) was higher in elderly patients compared with the younger population (SU: 62.70% in 2012, AGI: 12.78% in 2012). Two-drug combination therapies were the prevalent treatment choices for patients with type 2 diabetes (44.77% in 2012), particularly in the elderly group; however, ≥3 drug combination therapies increased gradually during the study period, particularly in the younger group.
This descriptive study presents the change in the prescription of OAD medication for different age groups during 2005 to 2012.
Keywords: antidiabetic medication, glycemic control, prescription trends, type 2 diabetes
1. Introduction
Diabetes is one of the most critical epidemic diseases in the world. According to the International Diabetes Federation, the global prevalence of diabetes was 8.3% in 2013, which will increase to 10.1% by 2035, and this is equivalent to approximately 3 new cases every 10 seconds.[1] In Taiwan, diabetes is the fifth leading cause of death for 25 years. Moreover, the Nutrition and Health Surveys in Taiwan conducted during 1993 to 1996 and 2005 to 2008 revealed a significant increase in the crude prevalence of diabetes from 5.33% to 9.05%.[2]
In contrast to type 1 diabetes, which is treated only by insulin, different mechanisms of drugs were developed for type 2 diabetes including sulfonylureas (SU), meglinides (MG), biguanides (BG), α-glucosidase inhibitors (AGI), thiazolidinediones (TZD), dipeptidyl peptidase-4 inhibitors (DPP4-I), glucagon-like peptide-1 (GLP-1) agonists, and sodium/glucose cotransporter-2 inhibitors.
Because only 6% of patients with type 2 diabetes in Taiwan use insulin alone for treatment,[3] analyzing oral antidiabetic (OAD) medication usage is imperative for understanding its current prescription trend and economic burden. Several drug utilization studies on glucose-lowering drugs (GLDs) have been previously conducted in the United States,[4] United Kingdom,[5–7] and other European countries.[8–10] Most of such studies have reported a substantial increase in the prescription of antidiabetic medications as well as a decreased SU usage and an increased BG and DPP4I usage. In Taiwan, 2 studies separately conducted during 1997 to 2003 and 2000 to 2009 have revealed similar results and a prescription trend toward combination therapy.[3,11]
Because of the increasing number of OAD medication choices, we can be able to evaluate considerably more specific circumstances that may influence clinical OAD use, such as changing treatment guidelines, aging population, new adverse effects of drugs, and changing health insurance payment system.
The New England Journal of Medicine (NEJM) published a meta-analysis in 2007 that reported an increased risk of acute myocardial infarction (AMI) and cardiovascular-related deaths associated with rosiglitazone usage,[12] which caused a 50% decline in its prescription after warning.[13] In addition, a study examining pioglitazone usage and the associated risk of bladder cancer was published in the British Medical Journal and received extensive attention.[14–16]
Treatment goal setting for type 2 diabetes have undergone a major shift since 2006,[17] the target HbA1c was 7.0 since then, however many issues of intensive care came out later, including intensive glycemic control may increase mortality and fails to reduce cardiovascular events.[18] In 2010, the American Diabetes Association published standard medical care in diabetes, emphasizing on individualized goal setting based on life expectancy, comorbidities, hypoglycemia awareness, and duration of diabetes.[19]
Only one study investigated the prescription trend of antidiabetic medication as well as examining new GLDs, such as DPP4-I in Taiwan. Ou et al found that healthcare costs spent on DPP4-I increased significantly during 2008 to 2013 and DPP4-I was the most commonly used as 2nd and 3rd line antidiabetic treatment.[20] The result showed that every category of antidiabetic medication was used more among elderly. However, this study did not analyze the prescription trend according to different age category. Moreover, the difference in antidiabetic prescription between elderly and younger Asian populations has not been researched yet.
Age is another crucial factor for consideration. Because aging has become an inevitable trend in developed countries, prescribing medication for elderly patients with type 2 diabetes is highly common. However, many factors influence drug prescription for elderly patients including age, renal function, drug compliance, comorbidities, and adverse effects. Two past studies on the trend of antidiabetic prescriptions reported inconsistent patterns for the elderly.[21,22]
The aim of the current study was to analyzing the prescription data by the type of therapy and by the age group of patients from Taiwan's National Health Insurance Research Database (NHIRD) and try to have more understanding of the prescribing trends of OAD medication for type 2 diabetes. We included current drugs, such as DPP4-I, in our analysis as long as fixed-dose combination treatment and compared the difference in prescription between older and younger patients.
2. Subjects, materials, and methods
2.1. Data sources
In this population-based study, data from the NHIRD were analyzed to understand the trend of OAD prescription for type 2 diabetes. The National Health Insurance (NHI) program in Taiwan was launched by the Bureau of National Health Insurance (BNHI) in 1995, and this program covers all healthcare services for >95% of the population of Taiwan. The National Health Research Institutes (NHRI) maintains the Longitudinal Health Insurance Database (LHID) in coordination with the BNHI; the LHID is a representative database of 1,000,000 subjects who were randomly sampled from the 2000 registry of all NHI enrollees through a systematic sampling method for research purposes. No statistically significant differences exist in age, sex, or healthcare costs between the sample group and the enrollees, as reported by the NHRI. These databases have been used for epidemiological research, and the information provided regarding prescription, diagnoses, and hospitalizations is of high quality.[23,24] All patients’ records/information were anonymized and de-identified before the analysis. The Institutional Review Board of the Taichung Armed Forces General Hospital, Taiwan, approved this study.
2.2. Identification of the study group
In this study, we analyzed the registration files and original claims data of patients during 2005 to 2012. All patients with type 2 diabetes were identified according to International Classification of Diseases, Ninth Revision codes 250.XX, either thrice during a study year or once with the prescription of antidiabetic medication. All patients claimed at least 1 prescription of an OAD. All antidiabetic prescriptions were identified after confirmation of the diagnosis of type 2 diabetes. Our unit of observation was the antidiabetic prescription which may include >1 OAD. Several classes of OAD medications were identified using specific drug codes for each drug in the NHIRD, namely 1, SU; 2, MG; 3, BG; 4, AGI; 5, TZD; and 6, DPP-4I. In 2013, the Food and Drug Administration (FDA) approved a new class of OAD medication, sodium/glucose cotransporter-2 inhibitor, which increases renal glucose excretion and inhibits renal glucose reabsorption. However, we excluded it from our analysis because it was approved by the Taiwan FDA only in 2014.
We recorded the prescription rates and patterns for each antidiabetic medication from every antidiabetic prescription each year during the study. The prescription rates were defined as the number of each antidiabetic prescription divided by the total number of antidiabetic prescriptions. The prescription patterns were classified into 4 categories: monotherapy, 2-drug combination therapy, 3-drug combination therapy, and ≥4 drug therapy. The prescription pattern was defined as how many antidiabetic medication among 1 single prescription. We tried to observe the prescription trend and the prescribing behavior behind each single prescription. For example, if a patient initiates a sulfonylurea but switches to DPP-4 inhibitors, he or she used 1 antidiabetic medication at each time, thus his/her treatment would be categorized into monotherapy in both situation. For fixed-dose combination medication, the prescription rates for each component of the drug were added to its original category. The definition of fixed-dose combination is that ≥2 drugs contained in a single dosage form, such as a capsule or tablet.
2.3. Statistical analysis
To understand the prescription trend of OAD medication, we used descriptive statistics to demonstrate the trend of OAD prescription, stratified by age (aged 65 years and above or younger than 65). All statistical analyses were conducted using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
3. Results
Our study population comprised 39,878 diabetic patients having claimed at least 1 OAD in 2005, which increased to 60,219 in 2012. Table 1 shows the number of patients enrolled each year and the number of their OAD prescriptions. The number of antidiabetic prescriptions in 2005 was 33,7004, which increased to 439,720 in 2012. Figure 1 shows the prescription rates of OAD medication in each study year. SU was once the most commonly used drug, but its prescription proportion declined gradually (76.83% in 2005 to 63.7% in 2012). However, there was no statistical difference for the changing trend of SU. BG became the most commonly used drug since 2010 (64.31% in 2005 to 74.41% in 2012). The prescriptions of TZD dropped significantly (9.2% in 2005 to 2.86% in 2012), whereas the usage of DPP-4I (3.73% in 2009 to 19.64% in 2012) increased with time. The prescription trends of MG and AGI were similar, demonstrating a slight increase initially and then decreasing thereafter.
Table 1.
The type 2 diabetes patient and prescription numbers in each year.
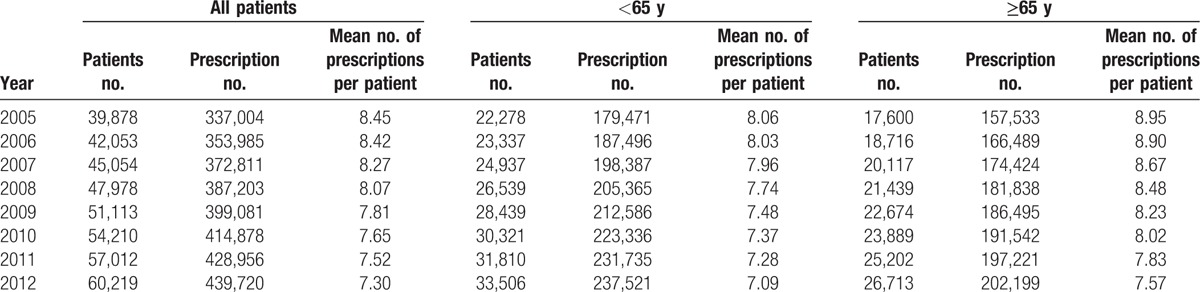
Figure 1.
Prescribing rates of oral antidiabetic medication in Taiwan, 2005 to 2012.
Compared with patients aged younger than 65 years, elderly patients received more MG (8.17% and 5.03% for elderly and younger patients in 2012, respectively) and AGI (12.78% and 11.07% for elderly and younger patients in 2012, respectively) prescriptions and lower TZD (2.45% and 3.31% for elderly and younger patients in 2012, respectively) and BG (68.16% and 79.73% for elderly and younger patients in 2012, respectively) prescriptions. In the elderly group, the usage rate of DPP4-I was 18.9% in 2012, which was slightly lower than that of the younger group (20.26% in 2012).
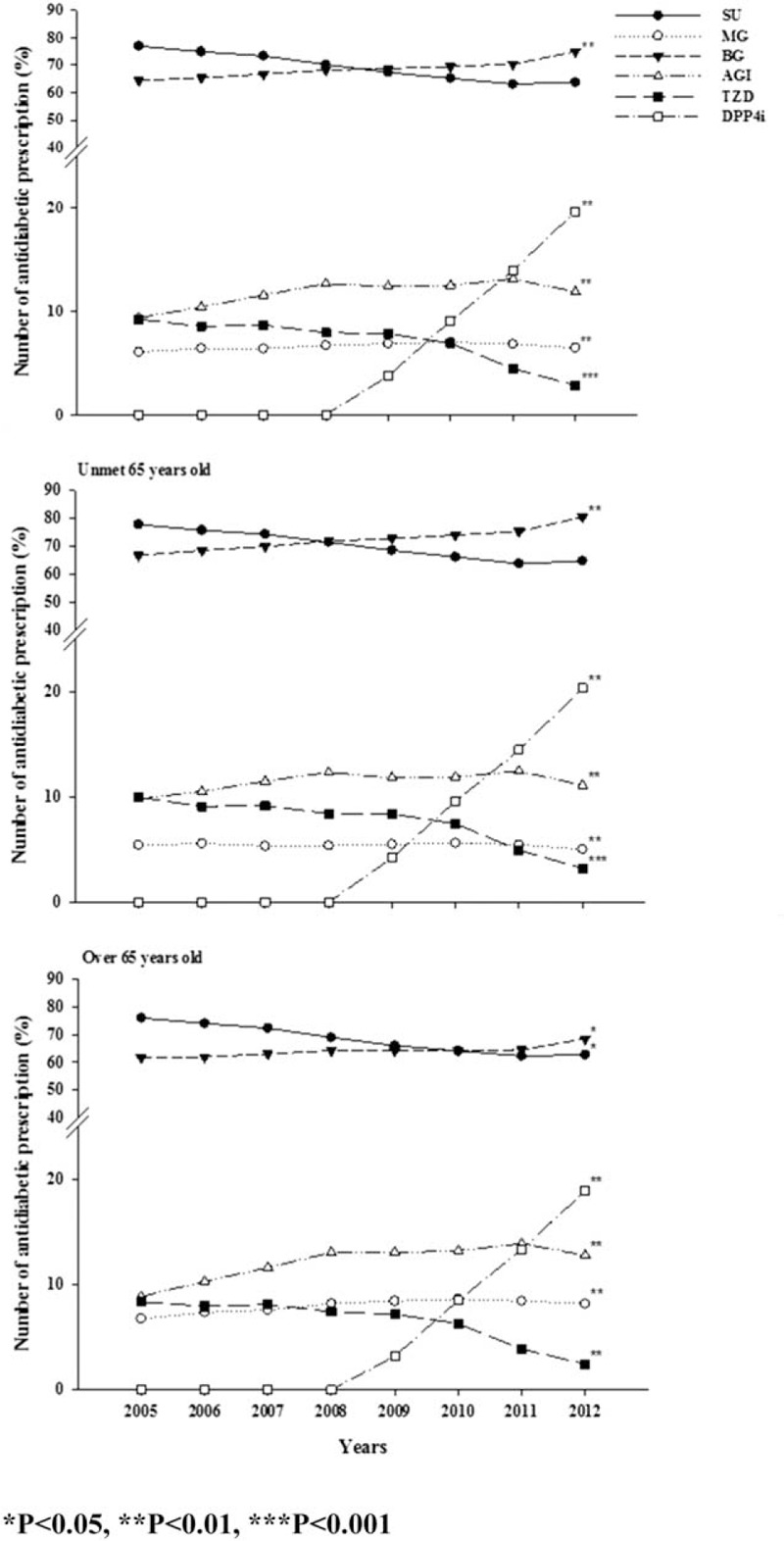
Figure 2 illustrates a comparison of prescription patterns, including monotherapy and combination therapy, between the groups. The proportion of combination therapy accounted for 60% of all prescriptions, and the most frequently used regimen in both groups was the 2-drug combination therapy. Regarding the prescription trend, the 3-drug combination therapy and ≥4 drug combination therapies were used increasingly in both groups during the study period. Regarding the monotherapy category, BG became the most commonly used drug since 2010 instead of SU. In the 2-drug combination therapy, SU combined with BG remained the most commonly used regimen. P value for trend was also shown in the figure.
Figure 2.
Prescribing patterns of oral antidiabetic medication in Taiwan, 2005 to 2012.
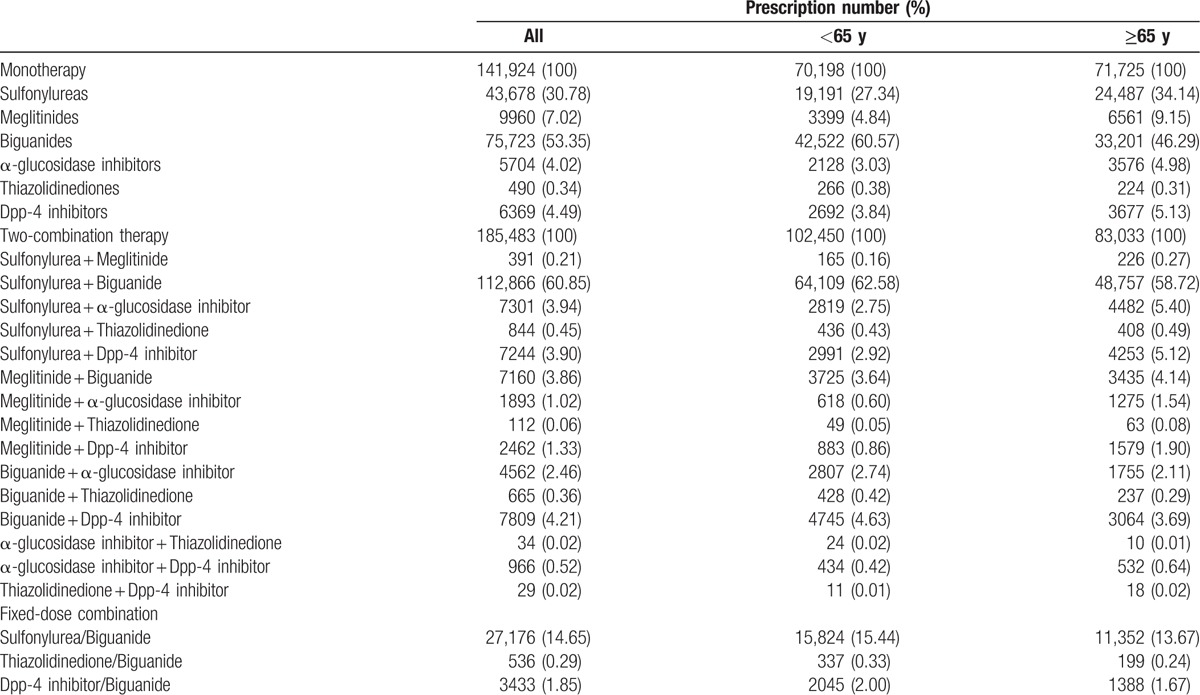
Table 2 shows the difference in the prescription trend and pattern of monotherapy and 2-drug combination therapy between both groups in 2012. For the monotherapy category, elderly patients used SU, AGI, and DPP-4I most frequently (SU, 34.14%; AGI, 4.98%; and DPP-4I, 5.13% in 2012). Two-drug combination therapy was still the prevalent treatment for elderly patients. Elderly patients more frequently used SU-based and DPP-4I-based combination therapies, whereas the younger group used more of BG-based combination therapy. Moreover, the prescription of fixed-dose combination therapies increased with time for elderly patients; however, not as much as for younger patients in 2012.
Table 2.
Oral anti-diabetic medication used alone or in 2 combination in Taiwan, 2012.
4. Discussion
We examined time trends in the prescription patterns of OAD medication for type 2 diabetes during 2005 to 2012 by using data from the LHID, a representative database of 1,000,000 subjects randomly sampled from the 2000 registry of all NHI enrollees by using a systematic sampling method for research purposes. The results revealed a constantly changing OAD prescription trend and a significant difference in this trend between both age groups during 2005 to 2012.
Treatment goal setting for type 2 diabetes has undergone a major shift since 2006,[17] the target HbA1c was set 7.0 since then. In 2008, the American Diabetes Association (ADA) published standard medical care in diabetes and change the preprandial glucose target to 70 to 130 mg/dL.[25] In 2010, the ADA reset the preprandial glucose target back to 80 to 130 mg/dL, while emphasizing on individualized goal setting based on life expectancy, comorbidities, hypoglycemia awareness, and duration of diabetes.[19] These changes had influences on prescribing behavior of physician and thus the prescription trend was changing.
Among the OAD medications, BG became the most widely used medication since 2010. By contrast, the usage rate of SU dropped gradually. Because SU was introduced for managing type 2 diabetes mellitus in Taiwan in the 1970s, drugs of this class are considered the core oral treatment for patients with this disease. However, clinical physicians raised concerns about the side effects of such drugs such as hypoglycemia and weight gain; this may explain the decline in the prescription of such drugs.[11] Moreover, additional advantages of BG were discovered such as facilitating weight loss, improving insulin resistance, reducing cardiovascular mortality among obese patients with diabetes, and reducing cancer risk.[26,27] The American Diabetic Association regarded metformin as the first line antidiabetic drug as did other guidelines.[28,29]
TZD, introduced in Taiwan in 2001, activates peroxisome proliferator-activated receptors (PPARs) and increases insulin sensitivity by acting on adipose tissues, muscles, and the liver to increase glucose utilization and reduce glucose production. This drug is widely used because of its antihypoglycemic effect. In 2007, the NEJM reported that an increased risk of AMI and cardiovascular-related death was associated with rosiglitazone.[12] Moreover, the Journal of American Medical Association reported that rosiglitazone was associated with an increased risk of congestive heart failure, acute myocardial infarction, and mortality compared with other combination oral hypoglycemic agent treatments.[30] This medication was suspended in European countries in 2010, and the FDA placed a warning on the medication package. The Taiwan FDA has strictly restricted the use of rosiglitazone since 2011; therefore, pioglitazone became the predominantly used TZD drug. This may explain the significant decrease in the use of TZD during past decade. Nevertheless, few reports and studies have mentioned the possible association of pioglitazone with bladder cancer, and this topic warrants further examination.[14–16]
Our results revealed that old and new drugs for diabetes are currently combined for optimal treatment. Acarbose, a type of AGI, which lowers postprandial blood glucose, is still used in combination therapies for type 2 diabetes in Taiwan. This is probably because of its relatively cheap price and minimal effect on the fasting blood glucose, leading to a lesser possibility of hypoglycemia, particularly in elderly patients. However, the gastrointestinal disorders and symptoms caused by AGI should not be ignored.[31,32]
Regarding the new generation of antidiabetic medication, DPP-4I is one of the most widely used drugs. Results from previous studies have revealed that the prescription trend for this drug is increasing rapidly, and this trend is consistent with that observed in our study. Rafaniello et al[33] determined an overall increase in the proportion of monotherapy treatment, particularly for DPP4-I, in recent years. Oishi et al[22] discovered that combination therapy is the prevalent treatment of choice in addition to the increased use of BG and DPP4-I. DPP-4I has several advantages such as an appropriate effect on blood glucose lowering, a neutral effect on the body weight, and relatively lesser hypoglycemia rate; however, its higher cost might impose an economic burden on the healthcare system. Moreover, long-term safety data,[34] such as the association of DPP4-I with the risk of arthritis,[35] or DPP4-I with the risk of heart failure, are still incomplete.[36,37]
In the analysis of the prescription patterns, the proportion of combined therapy constituted 60% of all prescriptions. Although metformin is the major prescription for monotherapy, metformin combined with SU was the preferred choice of 2-drug combination treatment, which is consistent with the current treatment guidelines. In recent years, early combination therapy has become increasingly crucial in diabetes treatment. Current guidelines also encourage the early use of combination therapy involving submaximal doses of each drug to lower blood glucose more effectively with less adverse outcomes.[38] According to Yu et al[39] the overall control of diabetes in Taiwan improved considerably after the introduction of new healthcare models and initiatives during 2006 to 2011, which could be the evidence of early combination treatment.
A previous meta-analysis revealed that fixed-dose combination therapy could lower the inadherence rate to 26%.[40] Another systematic review indicated that fixed-dose combination therapy increased patient adherence and satisfaction in addition to lowering treatment costs.[41] In our study, the trend of prescribing a fixed-dose combination therapy increased over the past years.
Our results showed an increasing trend of prescribing ≥4 drug combination therapies. Although clinical guidelines recommend using insulin after >3 OAD medications are prescribed, patients in Taiwan are still reluctant to use insulin injection. Therefore, physicians tend to prescribe more types of oral medications.
The antidiabetic prescription for elderly patients with type 2 diabetes slightly varied from that for younger patients. Elderly patients tend to receive more MG and AGI, but less TZD and BG. TZD would cause heart failure problem in elderly,[30] while BG could cause lactic acidosis especially when elderly have renal impairment.[42] Though there is no specific difference regarding drug choice in current treatment guideline, elderly with multiple morbidity, and decreased heart and renal function still have limits of drug choice. This result is consistent with that in a study conducted in Japan.[22]
Among the elderly patients, 51.9% were undergoing SU monotherapy. SU is a widely used medication that causes severe hypoglycemia in elderly patients, leading to fatal consequences. The use of SU increases the risk of hypoglycemia,[43] emergency department admissions,[44] and hip fracture in elderly patients.[45] Therefore, the current prescription behavior of SU and its adverse effects toward elderly patients should be evaluated more carefully.
The percentage of DPP-4I use in elderly patients in our study was extremely low compared with that in Japan, which was 34.6%. This is probably because of the high costs of DPP-4I, which may affect the choice of physicians who are under Taiwan's NHI system. Therefore, because of its advantages, we consider that the use of DPP-4I will constantly increase in the near future; however, physicians should be aware of its adverse effects on elderly patients.
In our study, more monotherapy was prescribed to elderly patients compared with younger patients; this may be because of the multiple factors that must be considered for treating elderly patients including adjusted treatment goal, daily life activities, comorbidities, life expectancy, adverse drug effects (such as hypoglycemia), potential benefits, and individualized preference.[46,47]
Some limitations exist in our study. First, DPP4-I was introduced for the first time in Taiwan since 2011; hence, only 2 years of data were available for analysis. Therefore, it was difficult to evaluate the prescription trend from a limited study. Second, no HbA1c data of NHIRD were available; therefore, we could not calculate the individual severity of type 2 diabetes with the treatment outcome, whereas the change of disease severity overtime is another factor that might be associated with the use of medication. Third, patients may not take all the medication from prescription claimed due to altered drug adherence. However, Under the National Health Insurance in Taiwan, most patients can have their medication within the same hospital or clinic which physician belong to. Fourth, since we used prescription claimed to observe the prescription trend of antidiabetic medication, some of the diabetic patients could be missed because they may not use any medication. Also, we cannot tell which prescription was for incident patients or prevalent users, owing to the NHIRD was started since 2005. However, this study aimed to evaluate the overall prescription trend, so the result still reflected clinical usage of each type of antidiabetic medication.
5. Conclusion
This descriptive study describes the change in the prescription of OAD medication in Taiwan. The prescription trend has changed gradually over the past decade. Monotherapy, as the conventional treatment, was used less during the study period, while 2-combination therapy and 3-combination therapy was used more during study period. More new antidiabetic medication was made each year, and physicians now have more choices for treating type 2 diabetes. Further research is required for exploring the effect of each factor associated with the prescription trend as well as the effect of the prescription trend on medical cost. Also, future study aims to see how the characteristics of either patients or physicians affect the prescribing trend of antidiabetic medication.
The results of this study can serve as a reference for clinical physicians and central health authorities in prescribing medication and implementing health policies for enhancing the care quality for patients with diabetes.
Footnotes
Abbreviations: ADA = American Diabetes Association, AGI = α-glucosidase inhibitor, AMI = acute myocardial infarction, BG = biguanide, BNHI = Bureau of National Health Insurance, DPP4-I = dipeptidyl peptidase-4 inhibitors, FDA = Food and Drug Administration, GLDs = glucagon-like peptide-1, GLP-1 = glucagon-like peptide-1 agonist, LHID = Longitudinal Health Insurance Database, MG = meglinides, NEJM = New England Journal of Medicine, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OAD = oral antidiabetic medication, PPARs = peroxisome proliferator-activated receptors, SU = sulfonylurea, TZD = thiazolidinediones.
Each author's individual contributions: Y-CL conceived of the study and supervised all aspects of its implementation. W-MC completed the analyses and drafted the content. H-EH, YSL, Y-HW assisted with the statistical analysis and revised the content. K-HH and M-CL assisted with the statistical analysis and revised the content. All authors helped to conceptualize ideas, interpret findings, and review drafts of the manuscript. The authors stated that they had no interests which might be perceived as posing a conflict or bias.
The authors report no conflicts of interest.
References
- [1].Federation ID. IDF Diabetes Atlas, 6th ed.; 2013. [Google Scholar]
- [2].Chang HY, Hsu CC, Pan WH, et al. Gender differences in trends in diabetes prevalence from 1993 to 2008 in Taiwan. Diabetes Res Clin Pract 2010;90:358–64. [DOI] [PubMed] [Google Scholar]
- [3].Chang CH, Jiang YD, Chung CH, et al. National trends in anti-diabetic treatment in Taiwan, 2000–2009. J Formos Med Assoc 2012;111:617–24. [DOI] [PubMed] [Google Scholar]
- [4].Cohen FJ, Neslusan CA, Conklin JE, et al. Recent antihyperglycemic prescribing trends for US privately insured patients with type 2 diabetes. Diabetes Care 2003;26:1847–51. [DOI] [PubMed] [Google Scholar]
- [5].Patel H, Srishanmuganathan J, Car J, et al. Trends in the prescription and cost of diabetic medications and monitoring equipment in England 1991–2004. J Public Health (Oxf) 2007;29:48–52. [DOI] [PubMed] [Google Scholar]
- [6].Filion KB, Joseph L, Boivin JF, et al. Trends in the prescription of anti-diabetic medications in the United Kingdom: a population-based analysis. Pharmacoepidemiol Drug Saf 2009;18:973–6. [DOI] [PubMed] [Google Scholar]
- [7].Leal I, Romio SA, Schuemie M, et al. Prescribing pattern of glucose lowering drugs in the United Kingdom in the last decade: a focus on the effects of safety warnings about rosiglitazone. Br J Clin Pharmacol 2013;75:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boyc KS, Yurgin N, Lage MJ. Trends in the prescription of antidiabetic medications in France: evidence from primary care physicians. Adv Ther 2007;24:803–13. [DOI] [PubMed] [Google Scholar]
- [9].Baviera M, Monesi L, Marzona I, et al. Trends in drug prescriptions to diabetic patients from 2000 to 2008 in Italy's Lombardy Region: a large population-based study. Diabetes Res Clin Pract 2011;93:123–30. [DOI] [PubMed] [Google Scholar]
- [10].Mazzaglia G, Yurgin N, Boye KS, et al. Prevalence and antihyperglycemic prescribing trends for patients with type 2 diabetes in Italy: a 4-year retrospective study from national primary care data. Pharmacol Res 2008;57:358–63. [DOI] [PubMed] [Google Scholar]
- [11].Chiang CW, Chiu HF, Chen CY, et al. Trends in the use of oral antidiabetic drugs by outpatients in Taiwan: 1997–2003. J Clin Pharm Ther 2006;31:73–82. [DOI] [PubMed] [Google Scholar]
- [12].Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. [DOI] [PubMed] [Google Scholar]
- [13].Stewart KA, Natzke BM, Williams T, et al. Temporal trends in anti-diabetes drug use in TRICARE following safety warnings in 2007 about rosiglitazone. Pharmacoepidemiol Drug Saf 2009;18:1048–52. [DOI] [PubMed] [Google Scholar]
- [14].Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012;344:e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsiao FY, Hsieh PH, Huang WF, et al. Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control study. Drug Saf 2013;36:643–9. [DOI] [PubMed] [Google Scholar]
- [16].Hsu JC, Cheng CL, Ross-Degnan D, et al. Effects of safety warnings and risk management plan for thiazolidinediones in Taiwan. Pharmacoepidemiol Drug Saf 2015;24:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006;29:1963–72. [DOI] [PubMed] [Google Scholar]
- [18].Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes care 2010;33Suppl:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ou HT, Chang KC, Liu YM, et al. Recent trends in the use of antidiabetic medications from 2008 to 2013: a nation-wide population-based study from Taiwan. J Diabetes 2017;9:256–66. [DOI] [PubMed] [Google Scholar]
- [21].Orlando V, Guerriero F, Putignano D, et al. Prescription patterns of antidiabetic treatment in the elderly. Results from Southern Italy. Curr Diabetes Rev 2016;12:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oishi M, Yamazaki K, Okuguchi F, et al. Changes in oral antidiabetic prescriptions and improved glycemic control during the years 2002–2011 in Japan (JDDM32). J Diabetes Investig 2014;5:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tsan YT, Lee CH, Ho WC, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013;31:1514–21. [DOI] [PubMed] [Google Scholar]
- [24].Tsan YT, Lee CH, Wang JD, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–30. [DOI] [PubMed] [Google Scholar]
- [25].American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008;31Suppl:S12–54. [DOI] [PubMed] [Google Scholar]
- [26].Lamanna C, Monami M, Marchionni N, et al. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2011;13:221–8. [DOI] [PubMed] [Google Scholar]
- [27].Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 2010;3:1451–61. [DOI] [PubMed] [Google Scholar]
- [28].American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013;36Suppl:S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians of London; 2008. [PubMed] [Google Scholar]
- [30].Lipscombe LL, Gomes T, Levesque LE, et al. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 2007;298:2634–43. [DOI] [PubMed] [Google Scholar]
- [31].Glucose-lowering treatment of type 2 diabetes. Part II—glucose-lowering drugs after metformin: a choice based largely on adverse effects. Prescrire Int 2015;24:130–5. [PubMed] [Google Scholar]
- [32].Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015;49:540–56. [DOI] [PubMed] [Google Scholar]
- [33].Rafaniello C, Arcoraci V, Ferrajolo C, et al. Trends in the prescription of antidiabetic medications from 2009 to 2012 in a general practice of Southern Italy: a population-based study. Diabetes Res Clin Pract 2015;108:157–63. [DOI] [PubMed] [Google Scholar]
- [34].Karagiannis T, Paschos P, Paletas K, et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369. [DOI] [PubMed] [Google Scholar]
- [35].Crickx E, Marroun I, Veyrie C, et al. DPP4 inhibitor-induced polyarthritis: a report of three cases. Rheumatol Int 2014;34:291–2. [DOI] [PubMed] [Google Scholar]
- [36].Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015;385:2107–17. [DOI] [PubMed] [Google Scholar]
- [37].dos Santos L, Salles TA, Arruda-Junior DF, et al. Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circ Heart Fail 2013;6:1029–38. [DOI] [PubMed] [Google Scholar]
- [38].Del Prato S, Felton AM, Munro N, et al. Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract 2005;59:1345–55. [DOI] [PubMed] [Google Scholar]
- [39].Yu NC, Su HY, Tsai ST, et al. ABC control of diabetes: survey data from National Diabetes Health Promotion Centers in Taiwan. Diabetes Res Clin Pract 2009;84:194–200. [DOI] [PubMed] [Google Scholar]
- [40].Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9. [DOI] [PubMed] [Google Scholar]
- [41].Hutchins V, Zhang B, Fleurence RL, et al. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin 2011;27:1157–68. [DOI] [PubMed] [Google Scholar]
- [42].DeFronzo R, Fleming GA, Chen K, et al. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 2016;65:20–9. [DOI] [PubMed] [Google Scholar]
- [43].Braatvedt GD, Sykes AJ, Panossian Z, et al. The clinical course of patients with type 2 diabetes presenting to the hospital with sulfonylurea-induced hypoglycemia. Diabetes Technol Ther 2014;16:661–6. [DOI] [PubMed] [Google Scholar]
- [44].Rajpathak SN, Fu C, Brodovicz K, et al. Sulfonylurea monotherapy and emergency room utilization among elderly patients with type 2 diabetes. Diabetes Res Clin Pract 2015;109:507–12. [DOI] [PubMed] [Google Scholar]
- [45].Rajpathak SN, Fu C, Brodovicz KG, et al. Sulfonylurea use and risk of hip fractures among elderly men and women with type 2 diabetes. Drugs Aging 2015;32:321–7. [DOI] [PubMed] [Google Scholar]
- [46].Cayea D, Boyd C, Durso SC. Individualising therapy for older adults with diabetes mellitus. Drugs Aging 2007;24:851–63. [DOI] [PubMed] [Google Scholar]
- [47].Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006;295:1935–40. [DOI] [PubMed] [Google Scholar]


