Abstract
Background:
Utilization of stereotactic radiosurgery (SRS) for treatment of high-grade gliomas (HGGs) has been slowly increasing with variable reported success rates.
Objective:
Systematic review of the available data to evaluate the efficacy of SRS as a treatment for HGG with regards to median overall survival (OS) and progression-free survival (PFS), in addition to ascertaining the rate of radiation necrosis and other SRS-related major neurological complications.
Methods:
Literature searches were performed for publications from 1992 to 2016. The pooled estimates of median PFS and median OS were calculated as a weighted estimate of population medians. Meta-analyses of published rates of radiation necrosis and other major neurological complications were also performed.
Results:
Twenty-nine studies reported the use of SRS for recurrent HGG, and 16 studies reported the use of SRS for newly diagnosed HGG. For recurrent HGG, the pooled estimates of median PFS and median OS were 5.42 months (3–16 months) and 20.19 months (9–65 months), respectively; the pooled radiation necrosis rate was 5.9% (0–44%); and the pooled estimates of major neurological complications rate was 3.3% (0–23%). For newly diagnosed HGG, the pooled estimates of median PFS and median OS were 7.89 months (5.5–11 months) and 16.87 months (9.5–33 months) respectively; the pooled radiation necrosis rate was 6.5% (0–33%); and the pooled estimates of other major neurological complications rate was 1.5% (0–25%).
Conclusion:
Our results suggest that SRS holds promise as a relatively safe treatment option for HGG. In terms of efficacy at this time, there are inadequate data to support routine utilization of SRS as the standard of care for newly diagnosed or recurrent HGG. Further studies should be pursued to define more clearly the therapeutic role of SRS.
Keywords: gamma knife, high-grade gliomas, malignant glioma, radiation necrosis, stereotactic radiosurgery
1. Introduction
The heterogeneous category of high-grade gliomas (HGGs) consists of glioblastoma multiforme (GBM), anaplastic astrocytomas (AAs), anaplastic oligodendrogliomas (AO), and the rare anaplastic oligoastrocytomas (AOAs). Almost 80% of primary central nervous system (CNS) gliomas consist of GBM and AA.[1] In the classification system by the World Health Organization, the molecular genotype is now a central component of subclassifying these tumors.[2,3] The standard treatment of newly diagnosed HGG is maximal safe resection followed by radiation therapy (RT) with concomitant and adjuvant chemotherapy.[4,5] Local RT following surgery was found to prolong median survival in GBM from 3 months without any treatments or 6 months with surgery alone to 12 months with both surgery and RT [4,6]; furthermore, surgical resection with postoperative RT has yielded an approximate median survival time of 36 months for patients with AA.[1] The addition of tumor treating fields (TTFs) appears to prolong this further.[7] Tumor recurrence occurs in almost all patients with approximately 90% of recurrences within 2 cm of the original lesion.[2,8,9]
Recurrence typically occurs within about 8 months after primary treatment.[10] A universally agreed upon treatment protocol has yet to be clearly established for recurrent HGG, but without treatment, survival is limited with a 3 to 6-month median survival without treatment.[11] Treatment of recurrence varies but can include reresection, systemic therapy, reirradiation, and TTF.[8,12] Although there is growing support for reresection of recurrent gliomas, surgery alone has been shown to be insufficient for disease control due to the infiltrative nature of the disease.[2,13] Reirradiation is a treatment modality that is being actively investigated. The primary concern from a toxicity perspective is the concern for cumulative radiation injury and the potential for radiation necrosis (RN).[8] However, reassuring evidence from recent primate studies, initial clinical series, and prospective trials appear to show considerable recovery of vital CNS structures after radiation.[8] As 90% of recurrence occurs within 2 cm of the edge of the primary tumor[14] and metastatic disease is rare, delivery of high-dose localized radiation—called stereotactic radiosurgery (SRS)—could theoretically improve local tumor control, introducing a tolerable increase in complications.[14,15]
Radiation exposure leads to both parenchymal and vascular damage, causing cell death to tumor cells along with healthy oligodendrocytes, neural progenitors, and endothelial cells; microglia and macrophages, on the contrary, tend to be more resistant to irradiation and ultimately induce an inflammatory response.[16] Song et al[17] observed the effects of SRS on mice tumor cells that initially contributed to vascular occlusion leading to hypoxia and cell death in addition to directly killing tumor cells by DNA double-strand breaks; strong antitumor immunity may also later be stimulated as a result of tumor antigens released from dying or dead tumor cells. SRS using cobalt source was first used for intracranial pathology in 1987,[18] and since then, this field has seen tremendous growth and advancement in terms of the kind of pathologies treated, dose planning, and radiation safety profiles.
SRS can be given as a single fraction radiosurgery (SFRS; single fraction of a higher radiation dose), fractionated stereotactic RT (FSRT; 2–5 fractions of a lower radiation dose), or hypofractionated stereotactic RT (HSRT; greater than 5 fractions of a higher radiation dose)—all of which improve accuracy of dose delivery with rapid reduction of dosage within critical areas.[8,19] SFRS is typically used for small tumors located in noneloquent areas, as symptomatic RN is a concern for larger tumors.[8,20] FSRT, on the contrary, has fewer severe side effects and can be used to treat larger tumors that may be located in critical areas [8,20]; however, RN is still a possible side effect.[2] HSRT allows for reduced treatment time, decreases patient discomfort, and can treat larger tumors with a smaller risk of acute toxicity in addition to reduced occurrences of symptomatic RN.[8]
There is still debate in the literature in regards to whether FSRT or SFRS is more effective with differing reports from in vitro studies.[21] However, preclinical data and clinical experience seem to support using multiple fractions over several days instead of a single large fraction.[19,22] Nahum[19] explained that the optimal fraction size and number is dependent on certain mathematical models related to the therapeutic ratio of tumor and critical tissues; the therapeutic ratio can then help guide appropriate fractionation plans for different patients. Nahum[19] also noted that SRS is rarely used alone, which can decrease the predicative value of the therapeutic ratio when considering the unknown combined effects of various multimodal treatment plans that can influence tumor killing and incidence of complications; regardless, there is still strong theoretical support for treatment to move toward larger fraction sizes.[19]
Acute toxicity from SRS treatment includes fatigue, alopecia at the entry/exit field, and radiation dermatitis.[10] Many of the side effects of localized high-dose RT have been shown to be mild, infrequent, and resolvable with symptomatic treatment with the exception of RN, which can be severe and permanent.[2] Overall, neurotoxicity from SRS has been found to be dose dependent[15] and has an estimated risk of 3% for RN based on dose–curves[10] with a reported range between 0% and 31%.[15] However, neurotoxicity directly attributed to reirradiation is difficult to determine, as most patients receive aggressive multimodal treatment including surgery, steroids, radiation, and systemic therapy, which may act as confounding factors.[10]
SRS combined with systemic therapy, such as bevacizumab (BVZ), has been shown to potentially improve median progression-free survival (PFS).[10] Concurrent chemotherapy is thought to have a radiosensitizing effect or other synergistic qualities.[10,15] Omuro et al[23] found that the addition of BVZ to the treatment plan led to fewer adverse side effect symptoms; this was attributed to the properties of BVZ, which cause decreased vascular permeability and, in turn, decreased peritumoral edema. Einstein et al[24] reported that concurrent temozolomide (TMZ) with SRS significantly prolonged median survival compared with SRS alone (20.8 vs 11 months, P = .037). Another study showed that chemotherapy with SRS was associated with increased median OS compared with SRS alone (34.5 vs 10.9 months, P = .013); median OS was also significantly increased with external brain RT (EBRT) and SRS compared with EBRT alone (25 vs 13 months, P = .0335).[25]
There are several studies reporting the use of SRS for recurrent HGG and newly diagnosed HGG. Efficacy results have been conflicting with some studies suggesting benefit and others detriment.
2. Methods
2.1. Literature review
Literature searches were performed on April 6, 2016, via PubMed for publications from 1992 to 2016. Only human studies and English-language publications were included. Key phrases used in the searches were “stereotactic radiosurgery for high grade gliomas” with 145 search results, “gamma knife surgery for high grade gliomas” with 122 search results, “stereotactic radiosurgery for recurrent gliomas’ with 222 search results, ‘stereotactic radiosurgery for primary gliomas” with 171 results, “stereotactic radiosurgery for newly diagnosed gliomas” with 43 search results, and “stereotactic radiosurgery for glioblastoma” with 291 search results. In addition, a small number of articles that were found as references listed in other articles that were obtained from the above PubMed searches were included.[23,26–28]
Ethical board approval was not necessary, as the study is a meta-analysis of already published literature. Only retrospective observational studies, prospective observational studies, and randomized clinical trials were included in this literature analysis; case reports, case series, and reviews were excluded. Studies that used SRS specifically for the treatment of HGG—classified as World Health Organization grade III and IV gliomas—were included, while the use of SRS for treatment of other disease entities was excluded. In addition, only studies that measured median OS from time of initial diagnosis were included in this meta-analysis; studies that did not report median OS from time of initial diagnosis were excluded from analysis. Specific inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria of studies using stereotactic radiosurgery for high-grade gliomas.

RN was not specifically defined in all studies that reported this adverse effect, but the majority of studies declared RN for patients with representative clinical symptomatic progression,[8,20,24,26,27,29–31] radiographic signs of progression,[20,29–36] and/or histologic confirmation.[14,15,20,23,30–33,35,37–42] Major neurological complications were defined as any neurological deficit, including cranial nerve palsy, paralysis, seizures, CNS hemorrhage, stroke and new or worsening neurological signs or symptoms, which excluded nausea, vomiting, tinnitus, dizziness, or any other transient mild symptoms. In studies that used the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) Acute and the Late Morbidity Scoring Scheme or the Common Terminology Criteria for Adverse Events grading scale, major neurological complications were classified as grades 3 and 4.[14,32,43,44]
2.2. Statistical analysis
Analyses of efficacy endpoints and toxicity including RN and other major neurological complications were carried out using STATA12 (StataCorp LP, College Station, TX). A correction of 0.5 was added to both the number of events and the number of total cases if the count of event was zero. Heterogeneity between studies was assessed using χ2 and I2 test. The inverse-variance weighted random-effects model, described by Dersimonian and Laird, was used to calculate pooled estimate of complication rates as well as 95% confidence intervals. Publication bias was assessed graphically using funnel plot (Fig. 1) and statistically using both Begg rank correlation test and Egger linear regression test. The pooled estimates of median PFS and median OS were calculated as a weighted estimate of population medians: 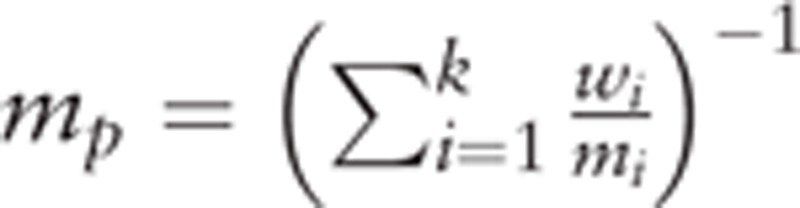 , where mi denotes the median survival within each study population (i = 1,2,…k), wi refers to the weight of each study and is equivalent to the sample size of each study divided by the total sample size. This meta-analysis was compiled according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Checklist, and its quality was assessed using the recommended checklist from Clinical Epidemiology: Practice and Methods (Table 2).[45]
, where mi denotes the median survival within each study population (i = 1,2,…k), wi refers to the weight of each study and is equivalent to the sample size of each study divided by the total sample size. This meta-analysis was compiled according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Checklist, and its quality was assessed using the recommended checklist from Clinical Epidemiology: Practice and Methods (Table 2).[45]
Figure 1.
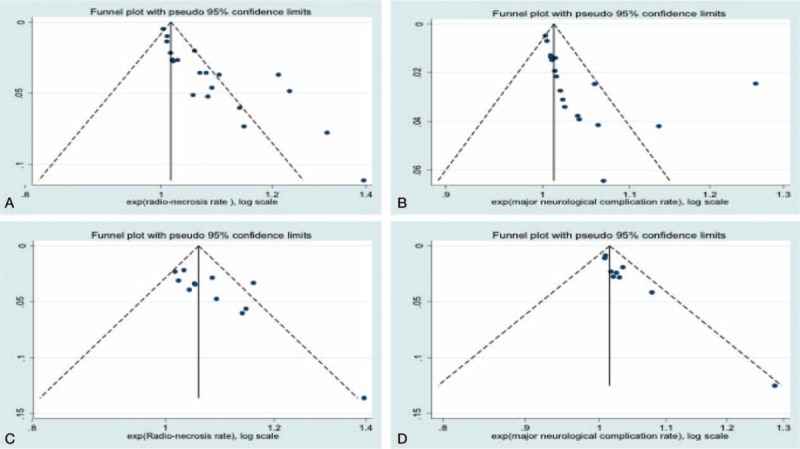
Funnel plots of (A) radiation necrosis rates for recurrent high-grade gliomas (HGGs), (B) other major neurological complications rates for recurrent HGG, (C) radiation necrosis rates for newly diagnosed HGG, and (D) other major neurological complications rates for newly diagnosed HGG.
Table 2.
Assessing the methodological quality of a systematic review.

3. Results
3.1. Literature review
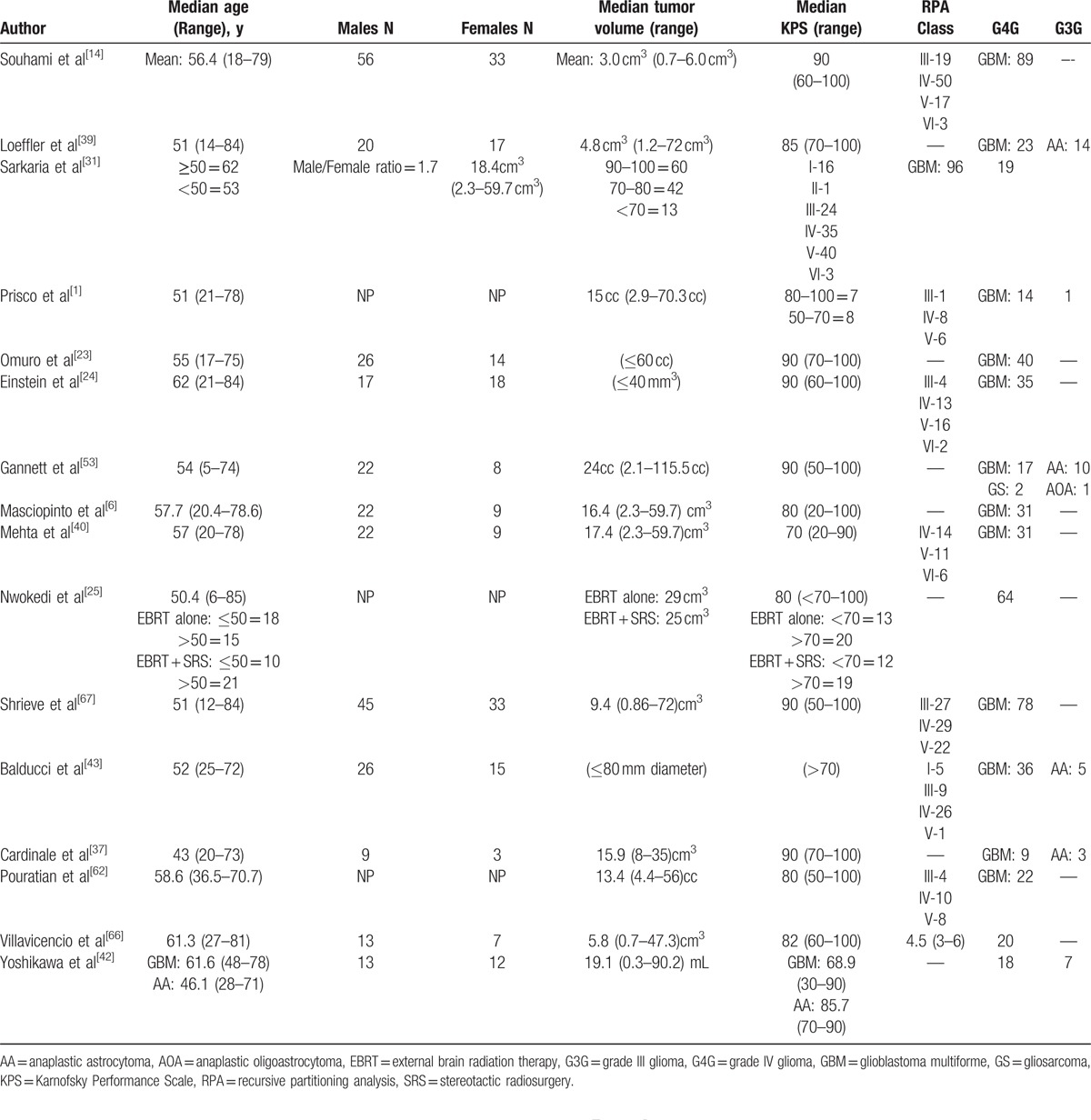
Of the 944 articles found as described in the Methods Section above, a total of 43 articles were included in this systematic review based on criteria described in Table 1 (Fig. 2). Twenty-nine studies with a total of 1686 patients reported the use of SRS for recurrent HGG, and 16 studies with a total of 685 patients reported the use of SRS for newly diagnosed HGG. This meta-analysis included mostly retrospective and prospective observational studies with only 1 randomized clinical trial that investigated the effects of SRS followed by EBRT and carmustine on median OS in the treatment of newly diagnosed HGG. Patient characteristics in studies using SRS as a treatment for recurrent HGG are listed in Table 3. Patient characteristics in studies using SRS as a treatment for newly diagnosed HGG are listed in Table 4.
Figure 2.
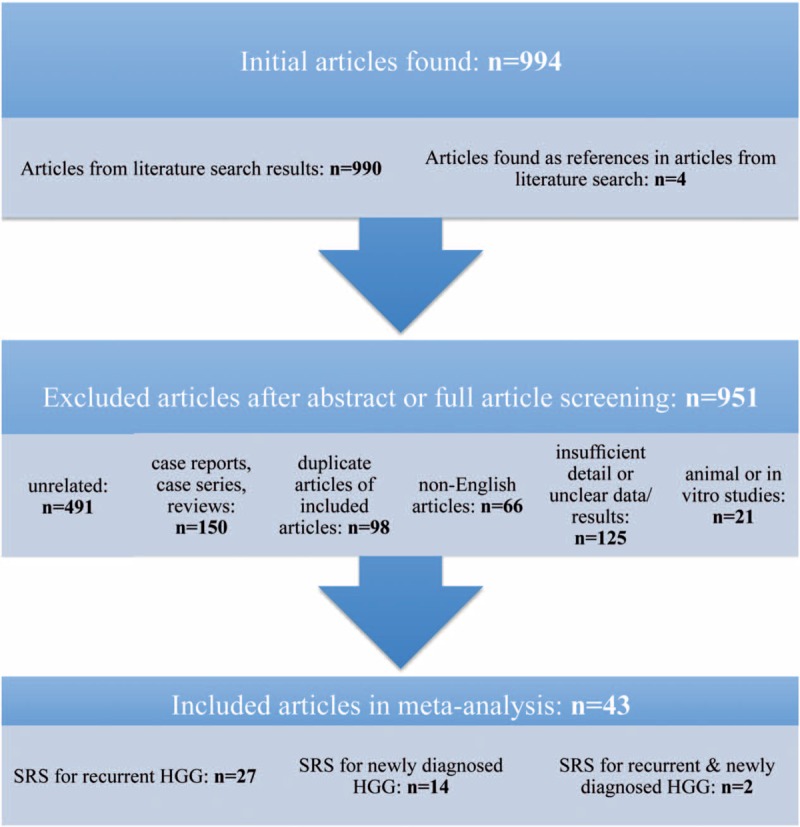
Articles evaluated for inclusion in systematic review. n = number of articles, SRS = stereotactic radio surgery, HGG = high-grade glioma.
Table 3.
Patient characteristics for recurrent or progressive high-grade gliomas.
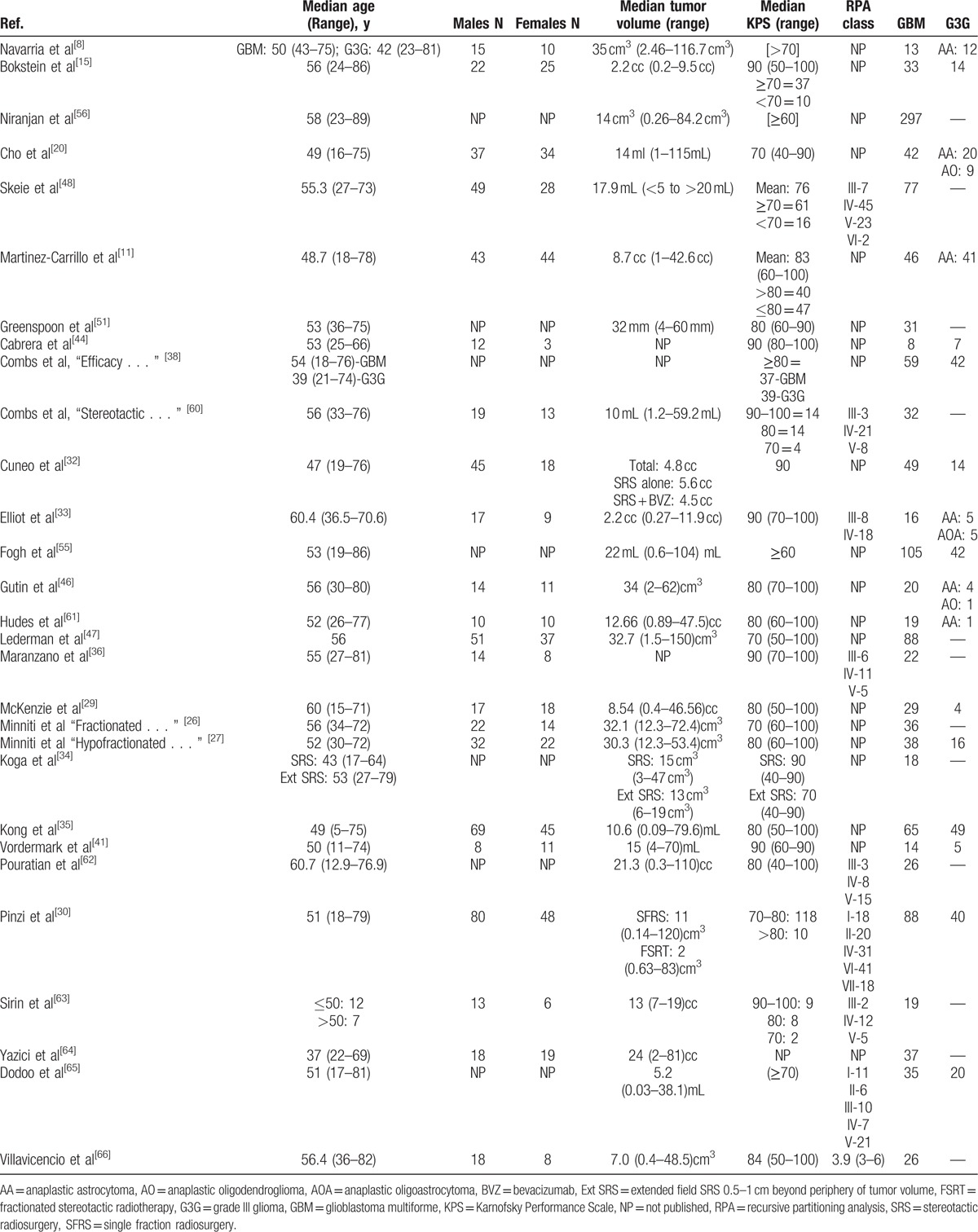
Table 4.
Patient characteristics for newly diagnosed high-grade gliomas.
3.2. Recurrent HGG
For recurrent HGG, the pooled estimates of median PFS (from the date of first SRS treatment) and median OS (from the day of diagnosis) were 5.42 and 20.19 months, respectively, based on the identified studies in Table 5. Of the 29 studies of SRS for recurrent HGG, 21 studies reported RN (Table 6) with a tally of 87 cases. Of the studies with specifically stated follow-up times, the duration of follow-up ranged from 0.5 to 141 months.[11,30] The pooled RN rate was 5.9% [3.7%, 8.1%] (Test for heterogeneity: χ2 = 89.04, df = 20, P < .001; I2 = 77.5% and test for publication bias: Egger test: P < .001; Begg test: P < .001) (Figs. 3 and 1A ). Nineteen studies reported major neurological complications associated with SRS for recurrent HGG (Table 7), accounting for a total of 88 cases, out of total 1275 cases treated. The pooled estimate of other major neurological complications rate was 3.3% [1.5%, 5.1%] (Test for heterogeneity: χ2 = 99.46, df = 18, P < .001; I2 = 81.9% and test for publication bias: Egger test: P = .021; Begg test: P < .001) (Figs. 4 and 1B). Of the studies that noted specific major neurological complications, the most commonly reported included seizures, CNS bleed, and cranial nerve palsy.[46–48]
Table 5.
Overall survival and progression-free survival for recurrent or progressive high-grade gliomas.
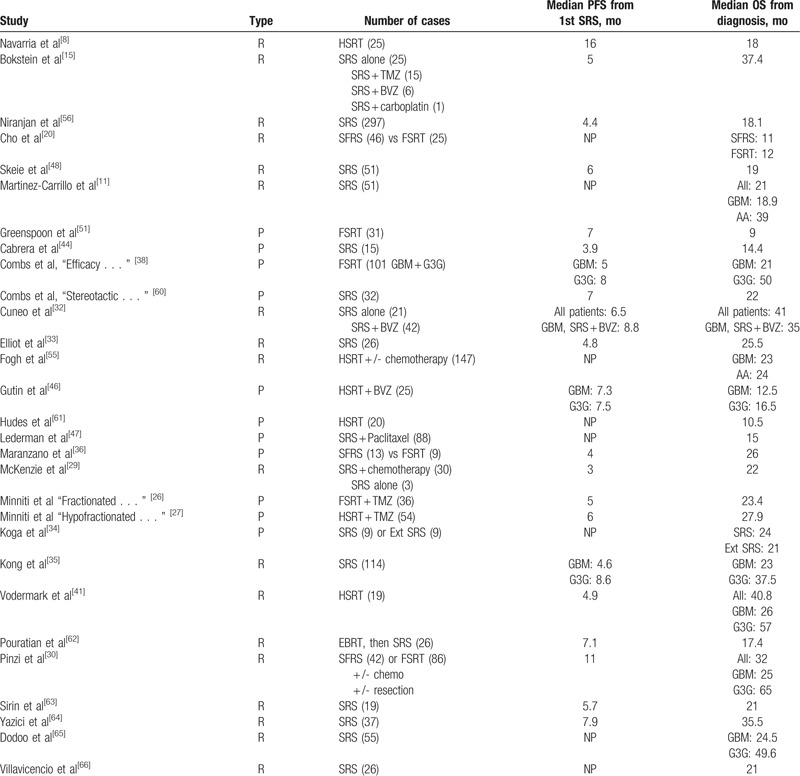
Table 6.
Radiation necrosis for recurrent or progressive high-grade gliomas.
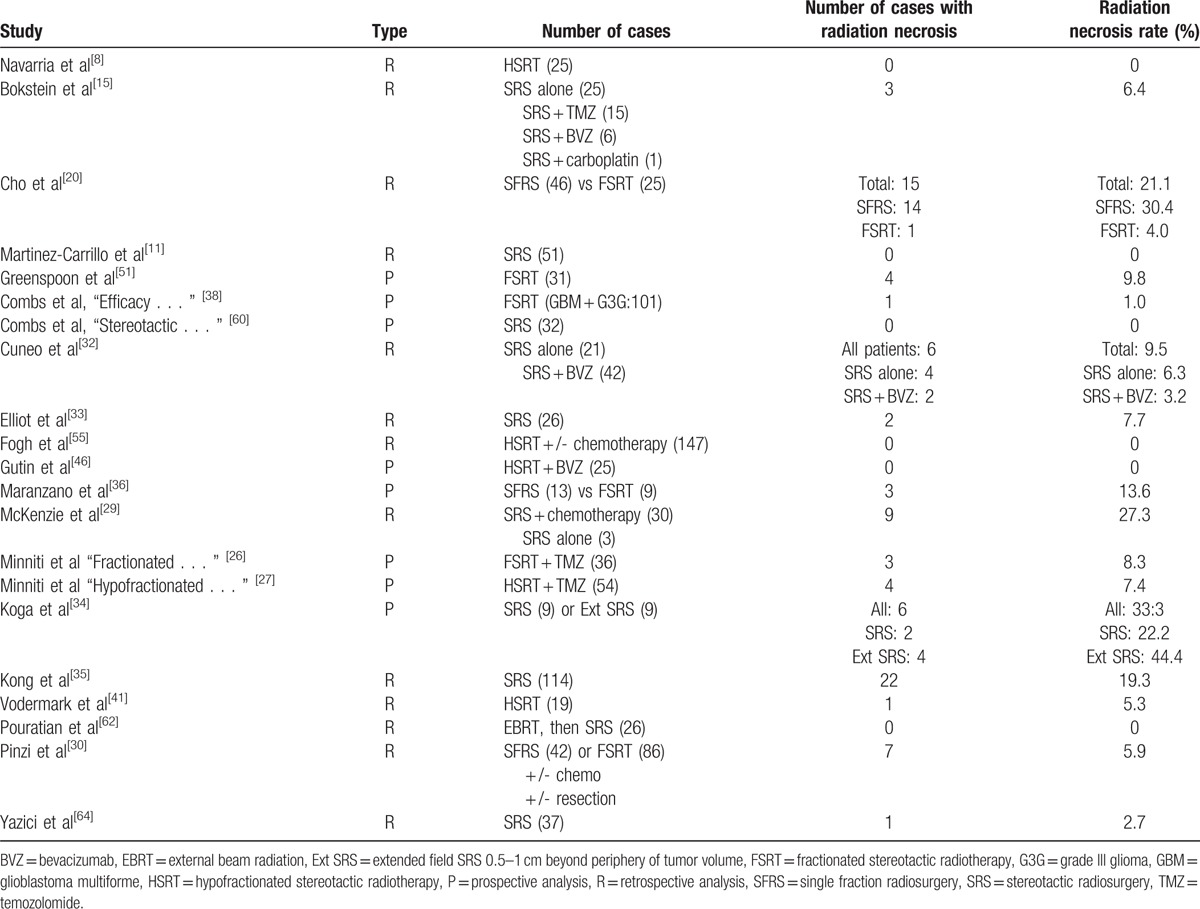
Figure 3.
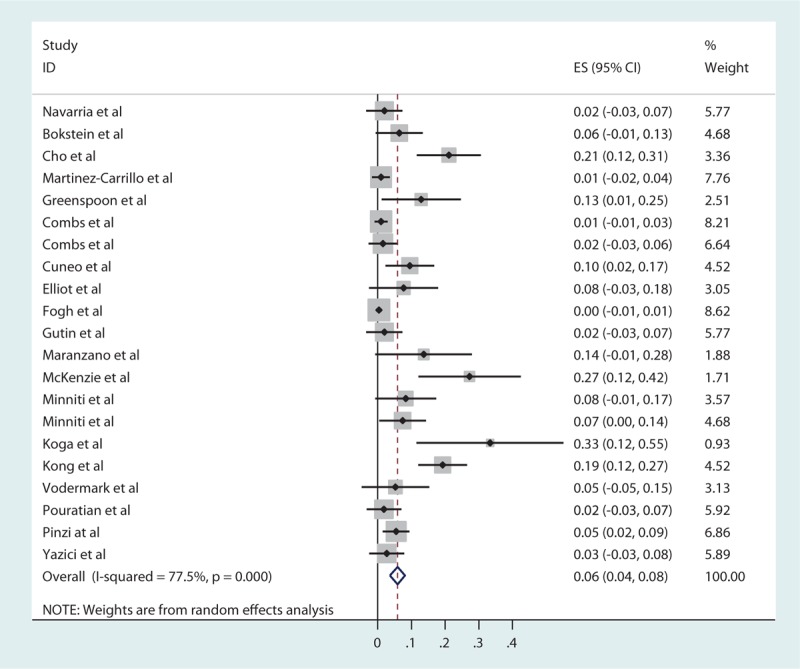
Forest plot of radiation necrosis rates for recurrent high-grade gliomas (HHGs).
Table 7.
Other major neurological complications for recurrent or progressive high-grade gliomas.
Figure 4.
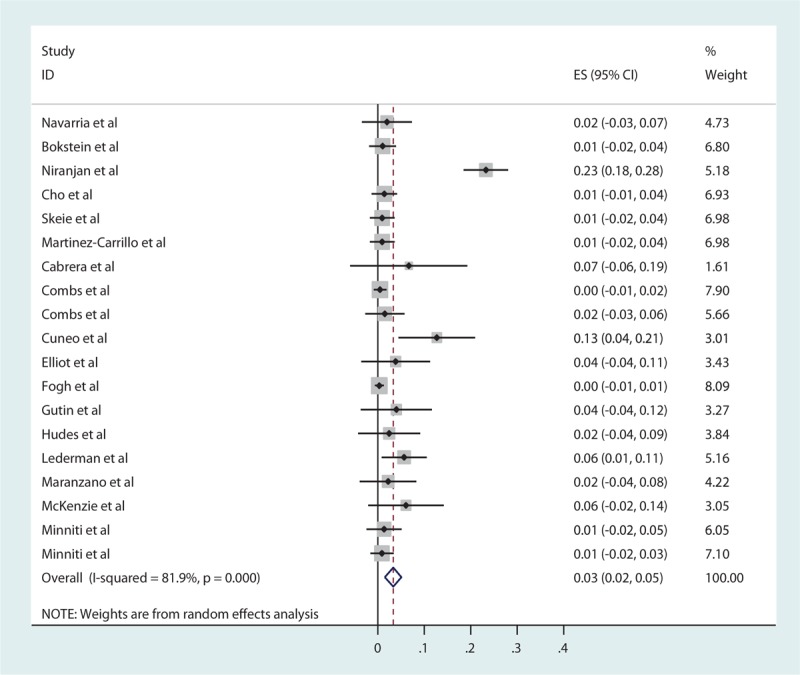
Forrest plot of other major neurological complications rates for recurrent high-grade gliomas (HHGs).
3.3. Newly diagnosed HGG
For newly diagnosed HGG, the pooled estimates of median PFS and median OS from 16 studies were 7.89 and 16.87 months, respectively, based on the identified studies in Table 8. Of the 16 studies of SRS for newly diagnosed HGG, 12 studies reported RN (Table 9) with a tally of 47 cases. Of the studies with specifically stated follow-up times, the duration of follow-up ranged from 3 to 61 months.[14,40] The pooled RN rate was 6.5% [3.6%, 9.4%] (Test for heterogeneity: χ2 = 22.02, df = 11, P = .024; I2 = 50% and test for publication bias: Egger test: P = .01; Begg test: P = .02) (Figs. 5 and 1C). Nine (2.7%) studies reported major neurological complications associated with SRS for newly diagnosed HGG (Table 10), accounting for a total of 12 cases, out of total 451 cases treated. The pooled estimate of other major neurological complications rate not associated with SRS was 1.5% [0.4%, 2.6%] (Test for heterogeneity: χ2 = 7.95, df = 8, P = .44; I2 = 0.0% and test for publication bias: Egger test: P = .001; Begg test: P = .009) (Figs. 6 and 1D). Of the studies that noted specific major neurological complications, the most commonly reported included seizures, CNS bleed, stroke, and hemiparesis.[23,31,37]
Table 8.
Overall survival and progression free survival for newly diagnosed high-grade gliomas.
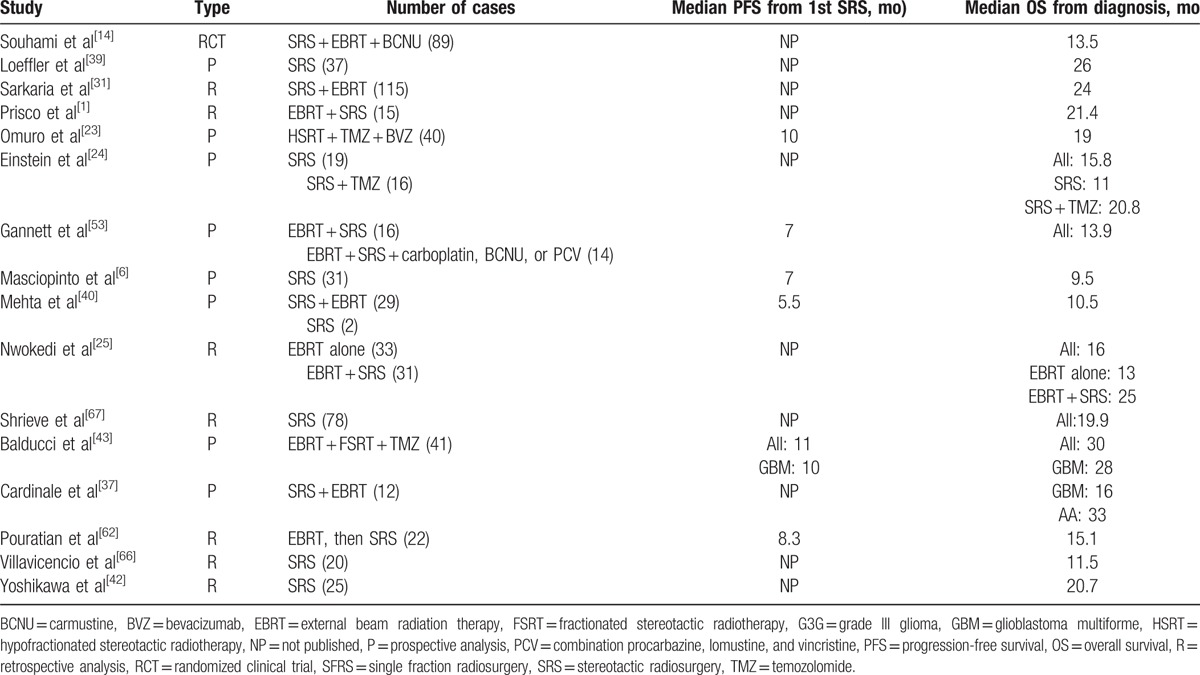
Table 9.
Radiation necrosis for newly diagnosed high-grade gliomas.
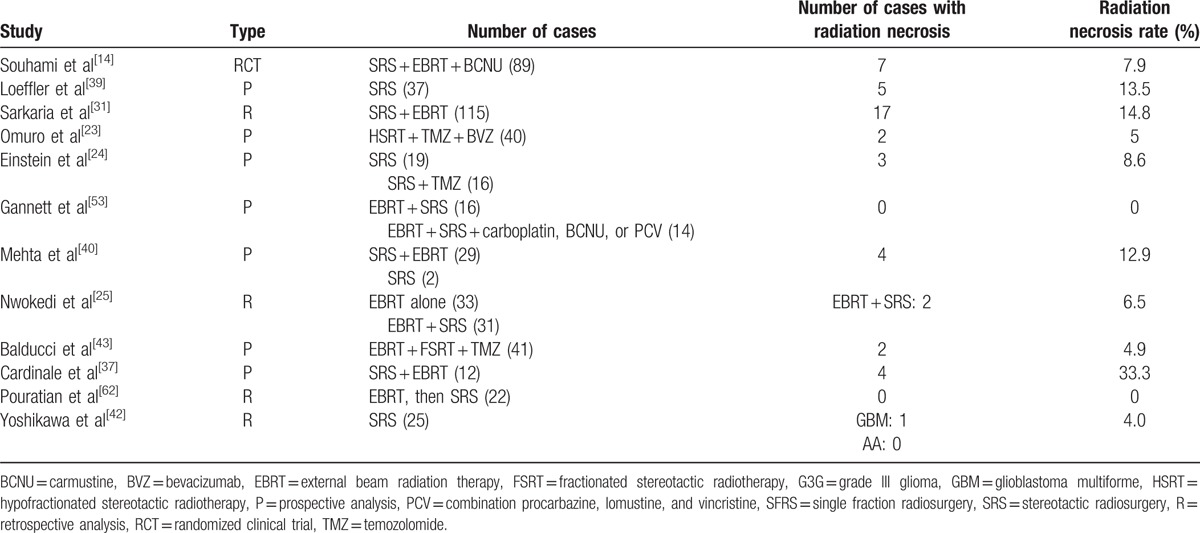
Figure 5.
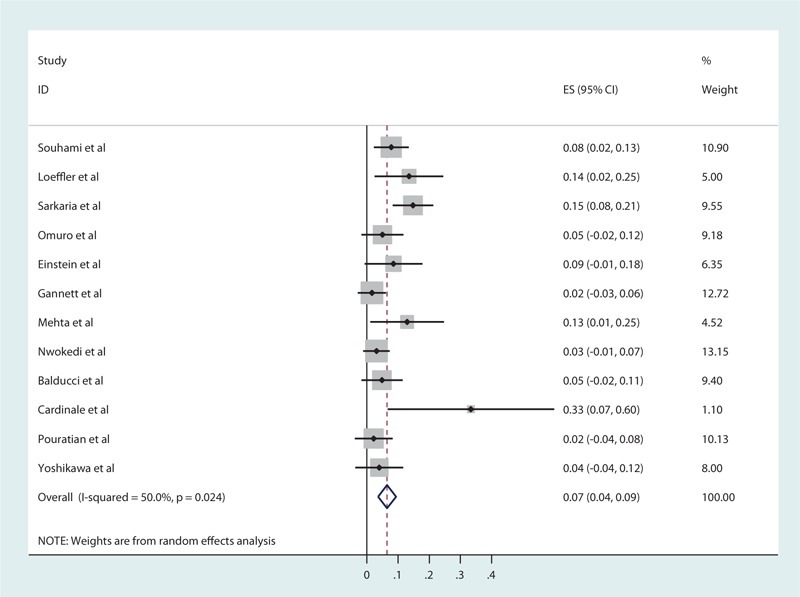
Forest plot of radiation necrosis rates for newly diagnosed high-grade gliomas (HHGs).
Table 10.
Other major neurological complications for newly diagnosed high-grade gliomas.
Figure 6.
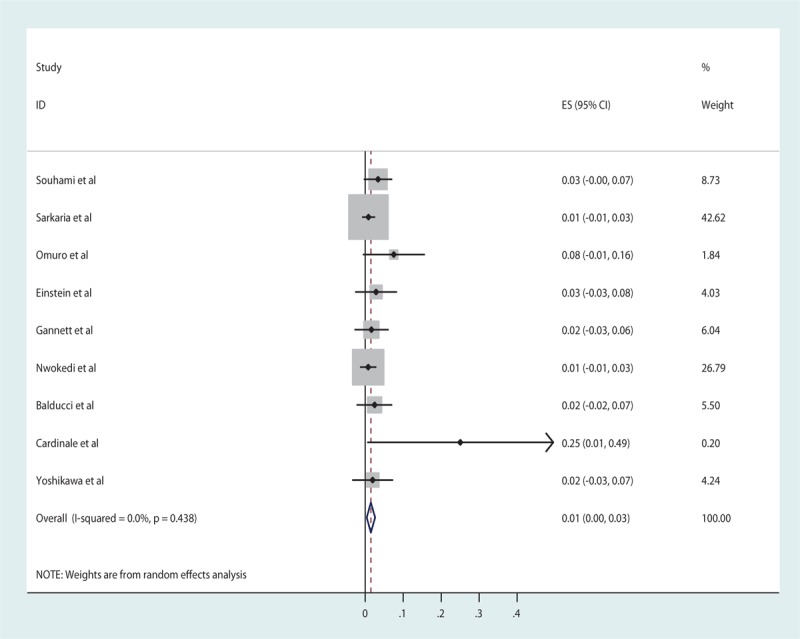
Forest plot of other major neurological complication rates for newly diagnosed high-grade gliomas (HHGs).
4. Discussion
HGG remains one of the most aggressive cancers that is almost universally fatal even with intense multimodal therapies, including surgery, radiation, and systemic therapy.[49] Various available novel treatments—SRS, brachytherapy, immunotherapy, TTF, and viral therapy—have both strengths and weaknesses along with certain side effects.[6] As this disease is characterized by aggressive local invasion but not distant metastasis, local delivery of radiation in the form of SRS has been and continues to be attempted as a treatment strategy in combination with other treatment modalities with variable reported success rates.
4.1. SRS efficacy
For newly diagnosed HGG, the survival is quite poor with a majority of patients not surviving beyond 24 months.[50] GBM in particular has a median survival of 12 to 18 months and only a 10% 5-year survival with maximal treatment.[8,51] Cairncross et al[52] found increased survival in patients with AO or AOA who had codeletions of 1p and 19q with the longest median overall survival (OS) of 14.7 years reported for those who were treated with procarbazine, lomustine, and vincristine in addition to EBRT. Our meta-analysis resulted in an estimate of 7.89 months for median PFS and 16.87 months for median OS in patients with newly diagnosed HGG. Several studies utilized a multimodal approach in the treatment of newly diagnosed HGG that likely contributed to longer survival times.
Notably, the only randomized trial (RTOG9305) included in this meta-analysis found no benefit in the treatment of newly diagnosed HGG with SRS followed by EBRT and carmustine with a median OS of 13.5 months in the SRS group and 13.6 months in the control group.[14] Souhami et al[14] explained that SRS provided no benefit even when subgroup analyses were done, questioning the efficacy of SRS on focal tumor control further when biopsies and MRI analyses found significant microscopic tumor extension outside of the contrast-enhancing tumor regions; they also acknowledged the importance of the temporal sequence of SRS in regards to outcome as other earlier reports of SRS in treatment of newly diagnosed HGG occurred after completion of EBRT rather than before.
Without any treatment, patients with recurrent HGG have a median survival of about 3 to 6 months.[11,33] More specifically, GBM patients typically do not survive beyond 13 months even with temozolomide therapy.[34] For recurrent HGG treated with SRS, this meta-analysis resulted in an estimate of 5.42 months for median PFS and 20.19 months for median OS. SRS may be most beneficial for GBM, particularly with slightly increased treatment margins. Kong et al[35] found that SRS significantly increased survival compared with a historic control group for patients with recurrent glioblastomas (23 vs 12 months, P < .001); however, this was not true for patients with grade III gliomas treated with SRS compared with their historic control counterparts (37.5 vs 26 months, P = .789). Koga et al[34] found that extended field SRS (0.5–1 cm beyond tumor volume margins) was more effective at local tumor control; yet, median OS was not statistically significant.
SRS treatment for newly diagnosed HGG appeared most beneficial for patients younger than 55 years with a Karnofsky Performance Scale (KPS) score of >70 and those with grade III gliomas compared with grade IV gliomas.[6,24,53] This was similar for patients with recurrent HGG treated with SRS with favorable prognostic factors, including younger age, higher KPS score, and smaller tumor size, and those with grade III gliomas compared with grade IV gliomas.[2,15,33–35,54,55]
According to the results of our meta-analysis with special consideration of the results from the 1 randomized clinical trial, SRS seemed to show a slight efficacy at treating recurrent HGG (pooled OS 20.19 months) compared with newly diagnosed HGG (pooled OS 16.87 months); therefore, SRS may reasonably be considered as part of treatment for recurrent HGG considering the limited treatment options for HGG, the positive safety profile of SRS, and the relatively favorable quality of life associated with SRS. However, SRS did not seem to show a benefit in treatment of newly diagnosed HGG.
The primary limitation of this meta-analysis was the selection bias present in all of the articles analyzed. This arose from the lack of randomized prospective clinical trials. This meta-analysis contained mostly retrospective and prospective observational studies with only 1 randomized clinical trial. Selection bias was noted in some studies as more favorable results for SRS in the treatment of HGG for patients with smaller tumor size, higher performance status, good response to initial chemoradiation therapy, and a prolonged time interval to recurrence [49]; therefore, these patients are not representative of the general population of patients with HGG, as they may presumably have different and perhaps better outcomes. When analyzing case selections of patients treated with external beam RT, multiple studies found that SRS-eligible patients had significantly prolonged median OS compared with SRS-ineligible patients.[14,56]
One of the biggest limitations of the current study is that the current literature on SRS treatment for HGG offers limited interpretation due to small sample sizes in studies, ranging from 15 to 147 patients, and the use of various treatment modalities, which differed both between studies and among patients within the individual studies. The robustness of a meta-analysis is strictly dependent on the quality of studies included in the meta-analysis. There were also limitations associated with the heterogeneous patient population that exhibited a median OS in patients with recurrent tumors ranging from 9 months in GBM patients[51] to 57 months in grade III glioma patients[41] and a median OS in patients with primary tumors ranging from 9.5 months in GBM patients[6] to 33 months in AA patients.[37] Not only were all types of HGGs grouped together, but also other influencing factors, such as isocitrate dehydrogenase (IDH) status and O6-methylguanine-DNA methyltransferase (MGMT) status, were not analyzed in this meta-analysis making the effects of SRS on OS and PFS not entirely clear at this time.[57] In addition, there is publication bias present in the body of literature available assessing the role of SRS in treatment of HGG. We evaluated the publication bias using best available statistical tools (Egger and Begg test); however, as these methods are based on strong and unverifiable assumptions, they do not guarantee the validity of conclusions.[58]
This meta-analysis was also statistically limited in its ability to provide more accurate results and interpretation of the current data. Many of the studies included in this analysis did not provide necessary values, such as hazard ratios (HRs), ranges, and confidence intervals, that would have facilitated in a more thorough statistical evaluation of median PFS and median OS. Median survival times or survival rates at a particular point in time are not reasonable surrogate measures for meta-analyses of survival outcomes and that, wherever possible, HRs should be calculated. Individual publications reporting on time to event outcomes, therefore, should provide more detailed statistical information, preferably log HRs and their variances, or their estimators.[59] Future clinical studies should strive to include these data in their published literature to aid in improved meta-analysis related to survival data in cancer trials.
4.2. SRS toxicity
Primary complications of concern associated with SRS are RN and other major neurological deficits. For the studies reporting on newly diagnosed HGG, our meta-analysis resulted in a pooled RN rate of 6.5% and a pooled estimate of other major neurological complications rate of 1.5%. Although documented neurotoxicity rates are low, the short life expectancy of patients with HGG makes calculating the true long-term toxicity risk of SRS challenging. However, the current data suggest that SRS is a safe treatment for newly diagnosed HGG with a small risk of RN and even smaller risk of major neurological complications. In general, according to the results of this systematic review, although SRS is safe with a very low risk of major neurological complication, SRS does not seem to provide improvement in OS for patients with newly diagnosed HGG.
For the studies reporting on recurrent HGG, this meta-analysis resulted in a pooled RN rate of 5.9% and a pooled estimate of other major neurological complications rate of 3.3%. Reporting the true toxicity risk of SRS is difficult even though documented toxicity is low because of the short life expectancy of patients with HGG. Overall, the current data suggest that SRS is a safe treatment option with a small risk of RN or any other major neurological complications; however, its efficacy in treating recurrent HGG still needs to be validated by large prospectively randomized clinical trials.
The variable definition of RN limited this study in addition to variable duration of follow-up times with short follow-up times likely resulting in lower reported toxicity rates than studies with longer follow-up times. Furthermore, the likelihood of detecting and reporting on all major neurological complications of every patient in all the retrospective studies is low.
5. Conclusion
The rapidly progressive nature of HGG adds to the difficulty in creating effective treatment plans that should focus on short duration therapy, few side effects, and limited hospitalizations in attempts to balance aggressive therapies and maintain a good quality of life.[6] SRS is a short treatment option that does not sacrifice large amounts of time precious to these patients who already have a limited life expectancy. This meta-analysis suggests that SRS may hold potential as a treatment option for recurrent HGG, especially with its low complication profile with a 5.9% rate of RN and a 3.3% rate of other major neurological complications. However, the data do not show strong enough evidence for SRS as treatment of HGG to be considered part of the standard care.
RN and other major neurological complications remain primary concerns with the use of SRS for treating HGG; however, the rates of both RN and other major neurological complications were found to be quite low for recurrent and newly diagnosed HGG treated with SRS in this meta-analysis. The results of this systematic review support that SRS is a rather safe treatment option; however, its efficacy still needs to be demonstrated by large prospective randomized controlled clinical trials. Further studies should be pursued to help define more clearly the therapeutic role that SRS plays in the treatment of HGG. With more than 40,000 people worldwide who have undergone SRS for recurrent HGG, this treatment modality is in need of additional research to determine its value in treating both recurrent and newly diagnosed HGG in order to help guide clinical practice.[56]
Footnotes
Abbreviations: AA = anaplastic astrocytoma, AOA = anaplastic oligoastrocytoma, BVZ = bevacizumab, CNS = central nervous system, EBRT = external brain radiation therapy, FSRT = fractionated stereotactic radiation therapy, GBM = glioblastoma, HGG = high-grade gliomas, HRs = hazard ratios, HSRT = hypofractionated stereotactic radiation therapy, IDH = isocitrate dehydrogenase, KPS = Karnofsky Performance Scale, MGMT = O6-methylguanine- DNA methyltransferase, OA = anaplastic oligodendroglioma, OS = overall survival, PFS = progression-free survival, RN = radiation necrosis, RT = radiation therapy, RTOG/EORTC = Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer, SFRS = single fraction radiosurgery, SRS = stereotactic radiosurgery, TMZ = temozolomide, TTF = tumor treating fields.
Funding/support: This work is supported by NIH NRCDP-K12 and NINDS K08NS092895 grant (MD).
The authors declare no conflict of interest.
References
- [1].Prisco FE, Weltman E, de Hanriot RM, et al. Radiosurgical boost for primary high-grade gliomas. J Neurooncol 2002;57:151–60. [DOI] [PubMed] [Google Scholar]
- [2].Conde-Moreno AJ, Garcia-Gomez R, Albert-Antequera M, et al. Fractionated stereotactic radiotherapy plus bevacizumab after response to bevacizumab plus irinotecan as a rescue treatment for high-grade gliomas. Rep Pract Oncol Radiother 2015;20:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- [4].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [5].Lukas RV, Mrugala MM. Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neuro Oncol Pract 2016;npw016–npw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Masciopinto JE, Levin AB, Mehta MP, et al. Stereotactic radiosurgery for glioblastoma: a final report of 31 patients. J Neurosurg 1995;82:530–5. [DOI] [PubMed] [Google Scholar]
- [7].Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 2015;314:2535–43. [DOI] [PubMed] [Google Scholar]
- [8].Navarria P, Ascolese AM, Tomatis S, et al. Hypofractionated stereotactic radiation therapy in recurrent high-grade glioma: a new challenge. Cancer Res Treat 2016;48:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology 1980;30:907–11. [DOI] [PubMed] [Google Scholar]
- [10].Taunk NK, Moraes FY, Escorcia FE, et al. External beam re-irradiation, combination chemoradiotherapy and particle therapy for the treatment of recurrent glioblastoma. Expert Rev Anticancer Ther 2016;16:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez-Carrillo M, Tovar-Martin I, Zurita-Herrera M, et al. Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int 2014;2014:657953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192–202. [DOI] [PubMed] [Google Scholar]
- [13].Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neurooncology 2016;18:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 2004;60:853–60. [DOI] [PubMed] [Google Scholar]
- [15].Bokstein F, Blumenthal DT, Corn BW, et al. Stereotactic radiosurgery (SRS) in high-grade glioma: judicious selection of small target volumes improves results. J Neurooncol 2016;126:551–7. [DOI] [PubMed] [Google Scholar]
- [16].Tabatabaei P, Visse E, Bergstrom P, et al. Radiotherapy induces an immediate inflammatory reaction in malignant glioma: a clinical microdialysis study. J Neurooncol 2017;131:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song CW, Lee YJ, Griffin RJ, et al. Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol Biol Phys 2015;93:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lunsford LD, Flickinger J, Lindner G, et al. Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery 1989;24:151–9. [DOI] [PubMed] [Google Scholar]
- [19].Nahum AE. The radiobiology of hypofractionation. Clin Oncol (R Coll Radiol) 2015;27:260–9. [DOI] [PubMed] [Google Scholar]
- [20].Cho KH, Hall WA, Gerbi BJ, et al. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys 1999;45:1133–41. [DOI] [PubMed] [Google Scholar]
- [21].Canazza A, De Grazia U, Fumagalli L, et al. In vitro effects of Cyberknife-driven intermittent irradiation on glioblastoma cell lines. Neurol Sci 2011;32:579–88. [DOI] [PubMed] [Google Scholar]
- [22].Kirkpatrick JP, Soltys SG, Lo SS, et al. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neurooncology 2017;19((Suppl 2)):ii38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 2014;20:5023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Einstein DB, Wessels B, Bangert B, et al. Phase II trial of radiosurgery to magnetic resonance spectroscopy-defined high-risk tumor volumes in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2012;84:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nwokedi EC, DiBiase SJ, Jabbour S, et al. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery 2002;50:41–6. discussion 46-47. [DOI] [PubMed] [Google Scholar]
- [26].Minniti G, Armosini V, Salvati M, et al. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol 2011;103:683–91. [DOI] [PubMed] [Google Scholar]
- [27].Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol 2013;111:187–94. [DOI] [PubMed] [Google Scholar]
- [28].Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013;310:1842–50. [DOI] [PubMed] [Google Scholar]
- [29].McKenzie JT, Guarnaschelli JN, Vagal AS, et al. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol 2013;113:403–9. [DOI] [PubMed] [Google Scholar]
- [30].Pinzi V, Orsi C, Marchetti M, et al. Radiosurgery reirradiation for high-grade glioma recurrence: a retrospective analysis. Neurol Sci 2015;36:1431–40. [DOI] [PubMed] [Google Scholar]
- [31].Sarkaria JN, Mehta MP, Loeffler JS, et al. Radiosurgery in the initial management of malignant gliomas: survival comparison with the RTOG recursive partitioning analysis. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1995;32:931–41. [DOI] [PubMed] [Google Scholar]
- [32].Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2012;82:2018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Elliott RE, Parker EC, Rush SC, et al. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg 2011;76:128–40. discussion 161–122. [DOI] [PubMed] [Google Scholar]
- [34].Koga T, Maruyama K, Tanaka M, et al. Extended field stereotactic radiosurgery for recurrent glioblastoma. Cancer 2012;118:4193–200. [DOI] [PubMed] [Google Scholar]
- [35].Kong DS, Lee JI, Park K, et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 2008;112:2046–51. [DOI] [PubMed] [Google Scholar]
- [36].Maranzano E, Anselmo P, Casale M, et al. Treatment of recurrent glioblastoma with stereotactic radiotherapy: long-term results of a mono-institutional trial. Tumori 2011;97:56–61. [DOI] [PubMed] [Google Scholar]
- [37].Cardinale RM, Schmidt-Ullrich RK, Benedict SH, et al. Accelerated radiotherapy regimen for malignant gliomas using stereotactic concomitant boosts for dose escalation. Radiat Oncol Investig 1998;6:175–81. [DOI] [PubMed] [Google Scholar]
- [38].Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 2005;23:8863–9. [DOI] [PubMed] [Google Scholar]
- [39].Loeffler JS, Alexander E, 3rd, Shea WM, et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol 1992;10:1379–85. [DOI] [PubMed] [Google Scholar]
- [40].Mehta MP, Masciopinto J, Rozental J, et al. Stereotactic radiosurgery for glioblastoma multiforme: report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys 1994;30:541–9. [DOI] [PubMed] [Google Scholar]
- [41].Vordermark D, Kolbl O, Ruprecht K, et al. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yoshikawa K, Saito K, Kajiwara K, et al. CyberKnife stereotactic radiotherapy for patients with malignant glioma. Minim Invasive Neurosurg 2006;49:110–5. [DOI] [PubMed] [Google Scholar]
- [43].Balducci M, Apicella G, Manfrida S, et al. Single-arm phase II study of conformal radiation therapy and temozolomide plus fractionated stereotactic conformal boost in high-grade gliomas: final report. Strahlenther Onkol 2010;186:558–64. [DOI] [PubMed] [Google Scholar]
- [44].Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys 2013;86:873–9. [DOI] [PubMed] [Google Scholar]
- [45].Parfrey PS, Barrett BJ. Clinical Epidemiology: Practice and Methods. Vol 1281. 2nd ed. New York, Humana Press; 2015. [Google Scholar]
- [46].Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2009;75:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lederman G, Wronski M, Arbit E, et al. Treatment of recurrent glioblastoma multiforme using fractionated stereotactic radiosurgery and concurrent paclitaxel. Am J Clin Oncol 2000;23:155–9. [DOI] [PubMed] [Google Scholar]
- [48].Skeie BS, Enger PO, Brogger J, et al. gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg 2012;78:658–69. [DOI] [PubMed] [Google Scholar]
- [49].Redmond KJ, Mehta M. Stereotactic radiosurgery for glioblastoma. Cureus 2015;7:e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Niyazi M, Ganswindt U, Schwarz SB, et al. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 2012;82:67–76. [DOI] [PubMed] [Google Scholar]
- [51].Greenspoon JN, Sharieff W, Hirte H, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther 2014;7:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013;31:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gannett D, Stea B, Lulu B, et al. Stereotactic radiosurgery as an adjunct to surgery and external beam radiotherapy in the treatment of patients with malignant gliomas. Int J Radiat Oncol Biol Phys 1995;33:461–8. [DOI] [PubMed] [Google Scholar]
- [54].Buatti JM, Friedman WA, Bova FJ, et al. Linac radiosurgery for high-grade gliomas: the University of Florida experience. Int J Radiat Oncol Biol Phys 1995;32:205–10. [DOI] [PubMed] [Google Scholar]
- [55].Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 2010;28:3048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Niranjan A, Kano H, Iyer A, et al. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J Neurosurg 2015;122:757–65. [DOI] [PubMed] [Google Scholar]
- [57].Switzeny OJ, Christmann M, Renovanz M, et al. MGMT promoter methylation determined by HRM in comparison to MSP and pyrosequencing for predicting high-grade glioma response. Clin Epigenetics 2016;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kicinski M. How does under-reporting of negative and inconclusive results affect the false-positive rate in meta-analysis? A simulation study. BMJ Open 2014;4:e004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care 2005;21:119–25. [DOI] [PubMed] [Google Scholar]
- [60].Combs SE, Widmer V, Thilmann C, et al. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer 2005;104:2168–73. [DOI] [PubMed] [Google Scholar]
- [61].Hudes RS, Corn BW, Werner-Wasik M, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys 1999;43:293–8. [DOI] [PubMed] [Google Scholar]
- [62].Pouratian N, Crowley RW, Sherman JH, et al. Gamma Knife radiosurgery after radiation therapy as an adjunctive treatment for glioblastoma. J Neurooncol 2009;94:409–18. [DOI] [PubMed] [Google Scholar]
- [63].Sirin S, Oysul K, Surenkok S, et al. Linear accelerator-based stereotactic radiosurgery in recurrent glioblastoma: a single center experience. Vojnosanit Pregl 2011;68:961–6. [DOI] [PubMed] [Google Scholar]
- [64].Yazici G, Cengiz M, Ozyigit G, et al. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol 2014;120:117–23. [DOI] [PubMed] [Google Scholar]
- [65].Dodoo E, Huffmann B, Peredo I, et al. Increased survival using delayed gamma knife radiosurgery for recurrent high-grade glioma: a feasibility study. World Neurosurg 2014;82:e623–32. [DOI] [PubMed] [Google Scholar]
- [66].Villavicencio AT, Burneikiene S, Romanelli P, et al. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: a multicenter experience. Neurosurg Rev 2009;32:417–24. [DOI] [PubMed] [Google Scholar]
- [67].Shrieve DC, Alexander E, 3rd, Black PM, et al. Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long-term outcome. J Neurosurg 1999;90:72–7. [DOI] [PubMed] [Google Scholar]


