Abstract
The aim of this study was to identify clinical signs and symptoms of ovarian torsion (OT) in children and to develop a simple predictive score.
A chart review of patients with acute adnexal pathologies treated at the University Children's Hospital Basel, Switzerland, between March 2006 and June 2015 was performed. Medical records were screened for demographic and clinical data. These included clinical symptoms, laboratory studies, imaging, and type of treatment. The diagnosis OT was defined as intraoperative visualization of the torsed ovary around its pedicle at least 360 degrees. Variables predictive for OT were identified and the following score for the likelihood of having OT was developed: age (points = number of years) minus 3 points (if vomitus = “yes”) and plus 1 point (if “pain duration >12 hours”).
A total of 80 patients with acute adnexal pathologies were identified. OT was recorded in 17 (21%) cases and ovarian cysts (OC) only in 63 (79%) cases. Patients who presented with OT were significantly younger than patients with OC only (P = .001). Correspondingly, 11 (65%) of the patients with OT had no menarche compared to 3 (5%) patients with OC only (P = .001). Vomiting (P = .001), a shorter pain duration (P = .01), and an elevated C-reactive protein (CRP) (P = .01) were observed significantly more often in patients with OT. The sensitivity of a positive OT score was 0.81 and increased to 1.00 if restricted to girls between 2 to 12 years of age.
The presence of vomiting, short duration of abdominal pain, and elevated CRP level have a predictive value for the diagnosis of OT. In these patients, an exploratory laparoscopy should be conducted without delay. The presented OT score appears to be a helpful tool in diagnosing OT in children.
Keywords: acute adnexal pathologies, children, ovarian torsion, predictive score
1. Introduction
The incidence of ovarian torsion (OT) among females between 1 and 20 years of age is estimated to be 4.9 of 100000.[1] OT accounts for approximately 3% of all cases of children with acute abdominal pain and requires immediate surgical intervention.[2] Other common acute adnexal pathologies in the pediatric population include simple ovarian cysts (OCs) with or without rupture.[3,4] Owing to the nonspecific clinical presentation and poor specificity of radiologic tests, the diagnosis of OT in girls remains challenging.[5–7] The aim of this study was to identify clinical signs and symptoms predictive for OT in children and to develop a simple predictive score.
2. Patients and methods
2.1. Study design
This study was approved by the Swiss Ethics Committee (EKNZ 2015–00202). Girls aged 2 to 18 years presenting with acute adnexal pathologies to the Department of Pediatric Surgery of the University Children's Hospital Basel between January 2006 and June 2015 were included. Patients younger than 1 year were excluded because of the high prevalence of fetal OCs in this age group.[8] Medical records were examined for demographic and clinical data. These included nausea, vomiting, tachycardia defined as the “attendant vegetative symptoms”, menarche, fever, the quality, localization and duration of pain, C-reactive protein (CRP) level, leucocyte count, radiologic imaging studies, initial working diagnosis at presentation, type of treatment, and postoperative diagnosis. Treatment included immediate surgical intervention or in-house observation. A CRP level >5 mg/L was defined as elevated, a rectal temperature >38.5°C as fever, and a leukocyte count ≥10 (109/L) as leukocytosis.[9]
Normal values for heart rate were defined as previously described.[10] Finally, the diagnosis OT was defined as intraoperative visualization of the torsion of the ovary around its pedicle at least 360 degrees.
2.2. Statistics
The association between OT versus OC only and any “attendant vegetative symptom” (nausea, vomiting, tachycardia), CRP level, and leukocyte count was analyzed in a logistic model. Categorical data were compared using a χ2 test. In variables with low cell count (<5), a Fisher exact test was used. For continuous variables, the mean and the standard deviation were compared using a t-test. In variables with non-normal distribution, a nonparametric Mann-Whitney U test was used. Statistical analysis was performed with R Core Team (2016) (R: A language and environment for statistical computing, version 3.3.2. R Foundation for Statistical Computing, Vienna, Austria). A P value of .05 (P = .05) was considered significant. The predictive OT score was based on a logistic regression model with final diagnosis as outcome and CRP level, age, nausea, vomitus, tachycardia, fever, location of pain, duration of pain, muscular guarding, tenderness on palpation, leukocytosis, and enlarged ovaries as potential predictors. A LASSO (least absolute shrinkage and selection operator) analysis was used to select the variables which showed the best predictive power for OT. To assess the regulation parameter (lambda), and thereby the strength of the shrinkage and number of variables chosen for the final model, 10-fold cross-validation was used. To this end, the data were randomly split into 10 approximately equally sized groups and the LASSO performed on 9 of the groups. The remaining group, randomly chosen, served as a (internal) validation set. The in-sample as well as the cross-validation missclassification rate was estimated. Based on the cross-validation missclassification rate, the lambda was chosen restricting the number of variables used in the predictive model to 3 variables to avoid overfitting. The predictive score was built by simplifying the coefficients to integers. For the resulting score, the receiver operator characteristics (ROC) curve was estimated and the optimal cut-off was defined according to the Youden-Index.
3. Results
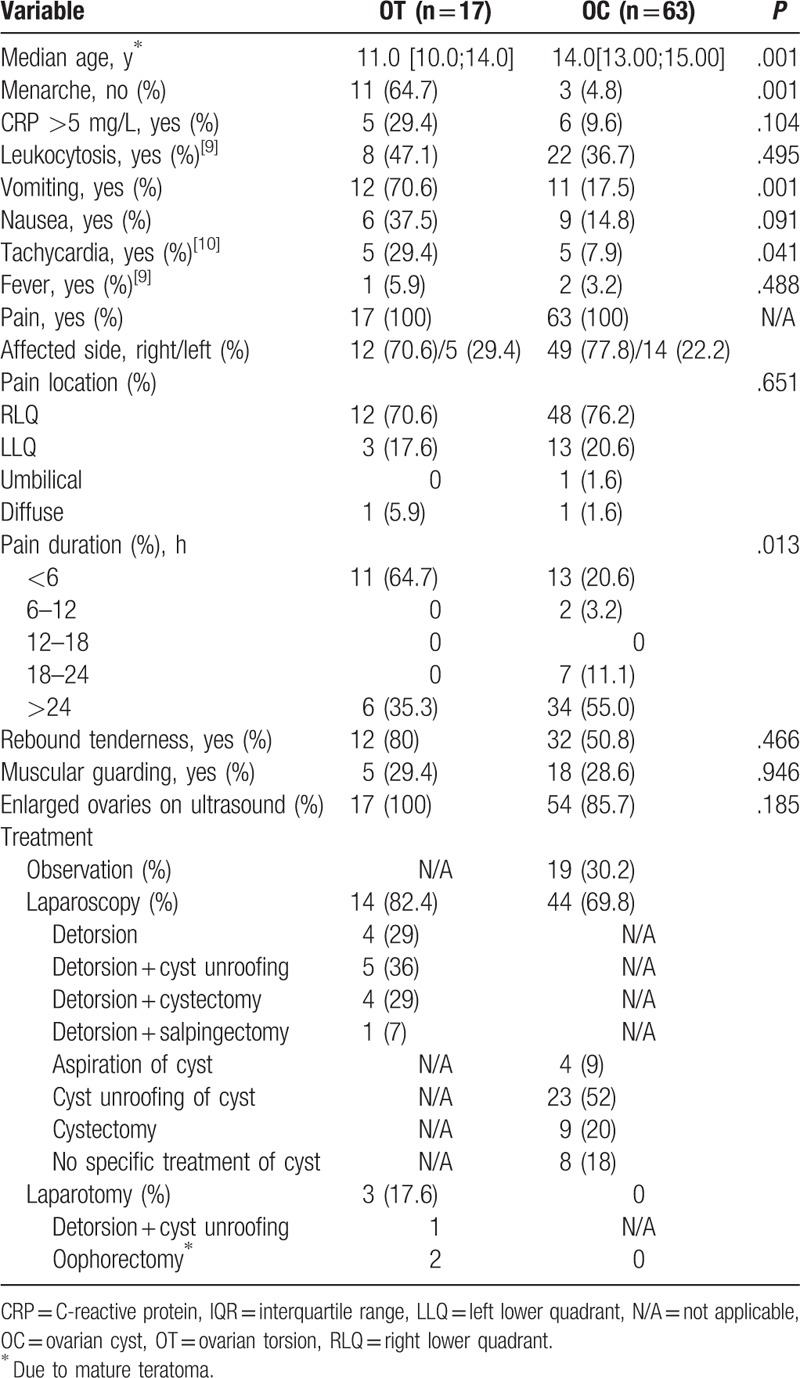
Table 1 summarizes the demographic variables, the symptoms, and treatment by final diagnosis of OT versus OC only. A total of 80 patients with acute adnexal pathologies were identified. OT was registered in 17 (21%) cases and OC only in 63 (79%) cases. The median age of the girls with OT (median age 11.0 years, interquartile range [IQR] 10.0–14.0 years) was significantly less compared to girls with OC only (median age 14.0 years, IQR 13.0–15.0 years) (P = .001). Correspondingly, 11 (65%) of the patients with OT had no menarche compared to 3 (5%) of the children with OC only (P = .001). Twelve of the girls (71%) with OT presented with vomiting compared to 11 (18%) with OC only (P = .001). Also, nausea (OT: 6 [38%] vs. OC only: 9 [15%]; P = .091) and tachycardia (OT: 5 [30%] vs. OC only: 5 [8%]; P = .041) were more frequent in patients with OT. Nineteen children (24%) of our study population were subjected to inpatient observation. Sixty-one patients (76%) underwent immediate surgical intervention. During surgery, OT was confirmed in 17 cases (28%) and OC only in 44 cases (70%). Fourteen girls (82%) with OT were treated laparoscopically. Four of these (29%) underwent simple laparoscopic detorsion, 5 (36%) had laparoscopic detorsion and unroofing of a cyst, 4 (29%) had laparoscopic detorsion and cystectomy, and in 1 patient (7%) a laparoscopic salpingectomy was carried out because of necrosis of the ovary. In 3 patients (18%) with OT, an ovarian teratoma was preoperatively suspected by ultrasound and therefore primary laparotomy was done. Mature teratoma was confirmed in 2 of these cases and oophorectomy was performed. In the third patient, a simple OC with internal hemorrhage was identified after detorsion of the ovary followed by unroofing of the cyst. All 44 patients with OC only were treated laparoscopically. Laparoscopic unroofing of a cyst was performed in 23 patients (52%), 9 (20%) had laparoscopic cystectomy, and in 4 cases (9%) a simple laparoscopic aspiration of the cyst was done. Eight patients (18%) underwent diagnostic laparoscopy only. Of the 19 (24%) patients who were subjected to in-house observation, 9 patients (47%) were diagnosed by ultrasound as having a simple OC, 7 patients (37%) a simple OC with internal hemorrhage, and 3 girls (16%) as having a ruptured simple OC.
Table 1.
Patient characteristics and clinical data of girl with ovarian torsion versus ovarian cyst only.
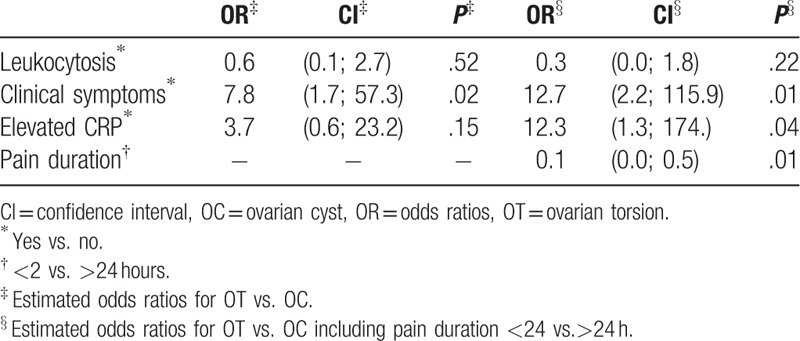
The estimated odds ratios for OT versus OC only are displayed in Table 2. OT was associated with the existence of at least “attendant vegetative symptom” (nausea, vomiting, tachycardia) (P = .02). The odds for OT increased 7.8-fold when patients had at least one of these symptoms. Pain duration in patients with OT was shorter than in patients with OC only (P = .01). Including the variable “pain duration,” the odds for OT increased up to 12.7-fold when at least “attendant vegetative symptom” was present. Furthermore, the variable “elevated CRP” became significant (P = .01). Of note, “enlarged ovaries” on abdominal ultrasound and “leukocytosis” showed no association with OT.
Table 2.
Estimated odds ratios for ovarian torsion versus ovarian cyst only.
The following OT score was developed: age (points = number of years) minus 3 points (if vomitus = “yes”) and plus 1 point (if “pain duration >12 hour”). The less points the more likely the patient suffers OT. The optimal cut-off to classify patients to OT or OC only was 11.5 points. Applied to the entire study population, the area under the ROC curve (AUC) was 0.88 and the sensitivity of a positive OT score was 0.81. Three patients with OT were not identified. The unifying characteristic of these patients was their age of 14 years. In a subgroup analysis of the OT score in patients aged 2 to 12 years, the AUC was 0.94 and the sensitivity of a positive OT increased to 1.00.
4. Discussion
In this study we set out to identify clinical signs and symptoms predictive for OT in children and to develop a simple predictive score. By combining the clinical presentation and the results of the diagnostic tests of patients with OT and other adnexal pathologies without OT (simple OC, simple OC with internal hemorrhage, ruptured simple OC), we identified 4 variables, which were associated with OT. In a secondary analysis, we developed a simple predictive score for the diagnosis of OT in children.
At the time of presentation, 65% of the girls with OT were prior to menarche compared to only 6% in the group of patients with OC only (P = .001). This explains the fact that patients with OT were younger than patients with OC only. In a series of 49 girls with OT within a similar age group by Chang et al, only one-third of the patients presented before menarche.[11,12] Variations in age at menarche among different ethnic groups and associations between early puberty and socioeconomic factors like lower parental education and lower household income were reported.[12–14] As the educational level and the household income in Switzerland are high, this might be an influencing factor for later occurrence of menarche in our study population.
In the study by Chang et al, the mean age was 12.5 years (range 8.8–16.2 years) and Servaes et al published a study on 74 girls with a median age of 11 years (IQR 7.0–14.5 years).[6,11] In our study, the median age was 11.0 years (IQR 10.0–14.0). Although this range is quite narrow, we feel what our study is analyzing is a representative cohort of adolescents within an age group, which corresponds to the ones reported in the literature. Patient’ age is a very crucial factor. In our study population, there were no younger girls suspicious for acute adnexal pathologies. However, very young children also represent a typical age group for adnexal pathology, which has 2 peaks, one in early infancy and the other around puberty. In our study, patients younger than 1 year were excluded because of the high prevalence of fetal OCs in this age group.[8]
In the present study, the variables“vomiting” and “pain duration” were predictive for OT. Twelve of the 17 patients with OT (71%) presented with vomiting, which was significantly more frequent than in patients with OC only (P = .001). This finding is well in line with data from other studies.[15–18] Nausea and tachycardia also occurred less frequently in the OC only group (Table 1). Vomiting is usually observed with the onset of abdominal pain and may be triggered by the vagal reflex secondary to pain.[16] Pain duration in girls with OT was significantly shorter than in patients with OC only (P = .01), which corresponds to a literature review by Huchon et al.[19]
Another observation of our study was a higher CRP level in patients with OT compared to OC only (P = .01). CRP levels rise in response to inflammation and tissue necrosis.[20] The higher CRP concentration could be explained by the process of tissue degeneration in OT compared to acute adnexal pathologies without impaired blood flow to the ovary. Wander et al[21] reported alternating CRP concentrations during the menstrual cycle. However, these hormone-dependent CRP concentrations always remain clearly <5 mg/L.[21] In the present study, only a CRP concentration of >5 mg/L was considered elevated. Therefore, the reported hormone-dependent alternations seem to have minor influence on the observed elevated CRP concentration in case of OT.
All patients of our study population were subjected to an abdominal ultrasound examination by a pediatric radiologist. In 17 patients with OT (100%) and 54 patients with OC, only (86%) the affected ovary appeared enlarged (Table 1). We did not analyze specific sonographic characteristics like echotexture, presence of peripheral cysts, and evidence of flow on Doppler sonography. Servaes et al reported that the most common sonographic finding of OT was an enlarged and heterogeneous adnexal mass. In contrast, blood flow assessed by Doppler sonography was normal in >60% of surgically proven children with OT.[6] Therefore, we focused on the size of the ovary as the diagnostic parameter for ultrasound. However, in this study, “enlarged ovaries” on abdominal ultrasound showed no association with OT and were not predictive for OT.
Two patients with OT (12%) had a mature teratoma and oophorectomy was performed in both cases because of the surgeon's preference. In general, ovary-sparing surgery is considered the first-line treatment.[22–25] Unfortunately, the decision-making process against ovarian-sparing surgery was not documented in the medical records.
There is an ongoing controversy about the ideal treatment of cystic adnexal pathologies in children and adolescents. Mayer et al[26] suggested unroofing of simple OCs and ovary-sparing excision of complex OCs. According to Rousseau et al,[16] “simple puncture or unroofing of the cysts should be avoided and cystectomy should be done to avoid recurrence.” In the present study, in case of OT, unroofing of a cyst after detorsion of the ovary was the preferred treatment in favor to maximize ovarian preservation despite the risk of recurrence (Table 1). Regardless of the surgical treatment strategy, awareness for possible malignancy in adnexal pathologies must be of major importance to every surgeon.[27,28] In case of cystic teratoma, there is a risk for dissemination of malignant cells by unroofing of a cyst.[29] A careful preoperative workup with radiologic imaging and laboratory tests including tumor markers is strongly recommended.[30]
The sensitivity of our predictive OT score was 0.81 as 3 patients with OT were not identified. Each of these 3 patients was 14 years of age. Based on the reduced value of our OT score in adolescent girls, we analyzed the subgroup of patients aged 2 to 12 years. After this, the sensitivity increased to 1.00, indicating a good performance of the OT score in this age group. Nevertheless, our OT score should be applied with caution and has to be evaluated in an independent cohort before considering it a reliable diagnostic test.
We are aware of some limitations of the study including its retrospective nature, the single center design, and the corresponding small patient population. However, up to now there is no prospective study on the predictive value of clinical, laboratory, or imaging parameters regarding the likelihood of OT. As stated by a recent review by Rey-Bellet Gasser et al[31] a multicenter prospective study would be required considering the low incidence of OT. Moreover, only surgically proven cases of OT were included. Therefore, we cannot exclude that there might have been patients with spontaneous detorsion of the ovary in the OC only group, who were not classified as OT.
5. Conclusion
The presence of vomiting, short duration of abdominal pain, and elevated CRP level has a predictive value for the diagnosis of OT. In these patients, an exploratory laparoscopy should be conducted without delay. The presented OT score appears to be a helpful tool in diagnosing OT in children aged 2 to 12 years. Patients with acute adnexal pathologies other than OT may be observed closely by repeated clinical examination and imaging studies if needed.
Footnotes
Abbreviations: AUC = area under curve, CRP = C-reactive protein, EKNZ = Ethikkommission Nordwest- und Zentralschweiz (Swiss Ethics Committee), IQR = interquartile range, LASSO = Least absolute shrinkage and selection operator, OC = ovarian cyst, OT = ovarian torsion, ROC = receiver operator characteristics.
We give explicit assurance that each of the listed authors meets each of the authorship requirements as stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (www.icmje.org).
The authors report no conflicts of interest.
References
- [1].Guthrie BD, Adler MD, Powell EC. Incidence and trends of pediatric ovarian torsion hospitalizations in the United States, 2000–2006. Pediatrics 2010;125:532–8. [DOI] [PubMed] [Google Scholar]
- [2].Breech LL, Hillard PJ. Adnexal torsion in pediatric and adolescent girls. Curr Opin Obstet Gynecol 2005;17:483–9. [DOI] [PubMed] [Google Scholar]
- [3].Anthony EY, Caserta MP, Singh J, et al. Adnexal masses in female pediatric patients. AJR Am J Roentgenol 2012;198:426–31. [DOI] [PubMed] [Google Scholar]
- [4].Kelleher CM, Goldstein AM. Adnexal masses in children and adolescents. Clin Obstet Gynecol 2015;58:76–92. [DOI] [PubMed] [Google Scholar]
- [5].Appelbaum H, Abraham C, Choi-Rosen J, et al. Key clinical predictors in the early diagnosis of adnexal torsion in children. J Pediatr Adolesc Gynecol 2013;26:167–70. [DOI] [PubMed] [Google Scholar]
- [6].Servaes S, Zurakowski D, Laufer MR, et al. Sonographic findings of ovarian torsion in children. Pediatr Radiol 2007;37:446–51. [DOI] [PubMed] [Google Scholar]
- [7].Rossi BV, Ference EH, Zurakowski D, et al. The clinical presentation and surgical management of adnexal torsion in the pediatric and adolescent population. J Pediatr Adolesc Gynecol 2012;25:109–13. [DOI] [PubMed] [Google Scholar]
- [8].Galinier P, Carfagna L, Juricic M, et al. Fetal ovarian cysts management and ovarian prognosis: a report of 82 cases. J Pediatr Surg 2008;43:2004–9. [DOI] [PubMed] [Google Scholar]
- [9].Lo SF, Kliegman RM. Reference Intervals for Laboratory Tests and Procedures. In: Kliegman RM, ed. Nelson textbook of pediatrics. Philadelphia, PA: Elsevier, 2016; Chapter 727. [Google Scholar]
- [10].Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011;377:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang YJ, Yan DC, Kong MS, et al. Adnexal torsion in children. Pediatr Emerg Care 2008;24:534–7. [DOI] [PubMed] [Google Scholar]
- [12].Kelly Y, Zilanawala A, Sacker A, et al. Early puberty in 11-year-old girls: millennium cohort study findings. Arch Dis Child 2017;102:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song Y, Ma J, Agardh A, et al. Secular trends in age at menarche among Chinese girls from 24 ethnic minorities, 1985 to 2010. Glob Health Action 2015;27:26929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Braithwaite D, Moore DH, Lustig RH, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control 2009;20:713–20. [DOI] [PubMed] [Google Scholar]
- [15].Houry D, Abbott JT. Ovarian torsion: a fifteen-year review. Ann Emerg Med 2001;38:156–9. [DOI] [PubMed] [Google Scholar]
- [16].Rousseau V, Massicot R, Darwish AA, et al. Emergency management and conservative surgery of ovarian torsion in children: a report of 40 cases. J Pediatr Adolesc Gynecol 2008;21:201–6. [DOI] [PubMed] [Google Scholar]
- [17].Descargues G, Tinlot-Mauger F, Gravier A, et al. Adnexal torsion: a report on forty-five cases. Eur J Obstet Gynecol Reprod Biol 2001;98:91–6. [DOI] [PubMed] [Google Scholar]
- [18].Cass DL. Ovarian Torsion. Semin Pediatr Surg 2005;14:86–92. [DOI] [PubMed] [Google Scholar]
- [19].Huchon C, Fauconnier A. Adnexal torsion: a literature review. Eur J Obstet Gynecol Reprod Biol 2010;150:8–12. [DOI] [PubMed] [Google Scholar]
- [20].Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol 2008;136:138–46. [DOI] [PubMed] [Google Scholar]
- [22].Oltmann SC, Fischer A, Barber R, et al. Pediatric ovarian malignancy presenting as ovarian torsion: incidence and relevance. J Pediatr Surg 2010;45:135–9. [DOI] [PubMed] [Google Scholar]
- [23].Oltmann SC, Garcia NM, Barber R, et al. Pediatric ovarian malignancies: how efficacious are current staging practices? J Pediatr Surg 2010;45:1096–102. [DOI] [PubMed] [Google Scholar]
- [24].Özcan R, Kuruoğlu S, Dervişoğlu S, et al. Ovary-sparing surgery for teratomas in children. Pediatr Surg Int 2013;29:233–7. [DOI] [PubMed] [Google Scholar]
- [25].Oue T, Uehara S, Sasaki T, et al. Treatment and ovarian preservation in children with ovarian tumors. J Pediatr Surg 2015;50:2116–8. [DOI] [PubMed] [Google Scholar]
- [26].Mayer JP, Bettolli M, Kolberg-Schwerdt A, et al. Laparoscopic approach to ovarian mass in children and adolescents: already a standard in therapy. J Laparoendosc Adv Surg Tech A 2009;19suppl 1:111–5. [DOI] [PubMed] [Google Scholar]
- [27].Brandt ML, Helmrath MA. Ovarian cysts in infants and children. Semin Pediatr Surg 2005;14:78–85. [DOI] [PubMed] [Google Scholar]
- [28].Celik A, Ergün O, Aldemir H, et al. Long-term results of conservative management of adnexal torsion in children. J Pediatr Surg 2005;40:704–8. [DOI] [PubMed] [Google Scholar]
- [29].Watanabe E, Tanaka K, Takeda N, et al. Surgical technique to prevent spillage of cyst fluid during operation for cystic ovarian tumors. Pediatr Surg Int 2013;29:645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lacher M, Kuebler JF, Yannam GR, et al. Single-incision pediatric endosurgery for ovarian pathology. J Laparoendosc Adv Surg Tech A 2013;23:291–6. [DOI] [PubMed] [Google Scholar]
- [31].Rey-Bellet Gasser C, Gehri M, Joseph JM, et al. Is it ovarian torsion? A systematic literature review and evaluation of prediction signs. Pediatr Emerg Care 2016;32:256–61. [DOI] [PubMed] [Google Scholar]


