Abstract
The aim of the study is to describe the clinical characteristics and prognosis of malignant transformation of adenomyosis in patients with endometrial cancer.
In this retrospective descriptive study, the clinical data of patients with endometrial cancer (n = 127) who were admitted at our hospital between January 2006 and December 2013 were evaluated.
Among the 127 patients with endometrial cancer, 24 patients had endometrial cancer concurrently with adenomyosis. Among these 24 patients, 3 were diagnosed with malignant transformation of adenomyosis. Postoperative pathological investigations in the cancer+adenomyosis group revealed endometrial adenocarcinoma of Grade I (n = 21) and II (n = 3). The patients with malignant transformation of adenomyosis were relatively younger than the other patients. In those 3 patients, both the estrogen and progesterone receptors were strongly expressed in eutopic endometrium and were weakly positive in ectopic endometrium.
Although adenomyosis is usually benign, it might also be a precursor of malignant disease. As the incidence of adenomyosis malignant transformation is low, and its clinical manifestations are nonspecific, it may only be confirmed by postoperative pathological examination. Further investigations on larger sample size may provide additional data about prognosis of adenomyosis malignant transformation.
Keywords: adenomyosis, endometrial carcinoma, estrogen, malignancy, uterine fibroids
1. Introduction
Endometrial cancer is the sixth most common cancer in women worldwide, with 320,000 new cases diagnosed in 2012.[1] The mortality rate is 1.7 to 2.4 per 100,000 women. In the United States, uterine cancer is the most common gynecologic malignancy.[2] Endometrial adenocarcinoma, adenomyosis, and uterine fibroids often occur or exist concurrently. Estrogens play an extensive role in the development of female reproductive organs and abnormality in estrogen levels result in the concurrent manifestation of these diseases.
Adenomyosis is a benign lesion in which endometrial glands and endometrial stroma invade uterine myometrium and smooth muscles with varying degree of proliferation. It is a special endometriosis that occurs inside the uterine muscle wall. In 1925, Sampson firstly reported a case of ovarian endometriosis with malignant transformation.[3] Later, many similar cases were reported in China and other countries.[4–7] The malignant transformation rate of endometriosis is 1.5% in China, whereas it is 0.7% to 1.0% in other countries.[8,9] Rolly[10] reported the first case of malignant transformation of adenomyosis. Later, many similar cases were reported, and most of them were endometrioid adenocarcinomas.[11–18]
Due to limited clinical data, the clinical and pathological features of endometrial adenocarcinoma originating from adenomyosis are still not clear. The present descriptive study aimed to evaluate the pathological changes in adenomyotic foci in hysterectomy specimens, and to point out a possible mechanism of carcinogenesis in adenomyotic foci inside the myometrium.
2. Methods
2.1. Patients
Patients with endometrial carcinoma (n = 127) who were hospitalized and received treatment between January 2006 and December 2013 at the Central Hospital of Lishui city, Zhejiang province, China, were included in the study.
The inclusion criteria were as follows: indications for surgery and treatment were confirmed; chemotherapy and endocrine therapy not received before surgery; and postoperative histological examination confirmed the diagnosis of endometrial carcinoma, with uterine fibroids or adenomyosis. The exclusion criteria were as follows: severe cardiopulmonary dysfunction, kidney disease, liver and kidney dysfunction, serious blood diseases, or other surgical contraindications; or primary tumors on other parts of the body.
This study was approved by the Medical Ethics Committee of the Central Hospital of Lishui City, Zhejiang Province, China.
According to the results of intraoperative pathological examination, the patients were divided into 2 groups: patients diagnosed with endometrial carcinoma combined with adenomyosis or without adenomyosis. Then, the group of patients with adenomyosis was subdivided into 2 on the basis of with or without malignant transformation.
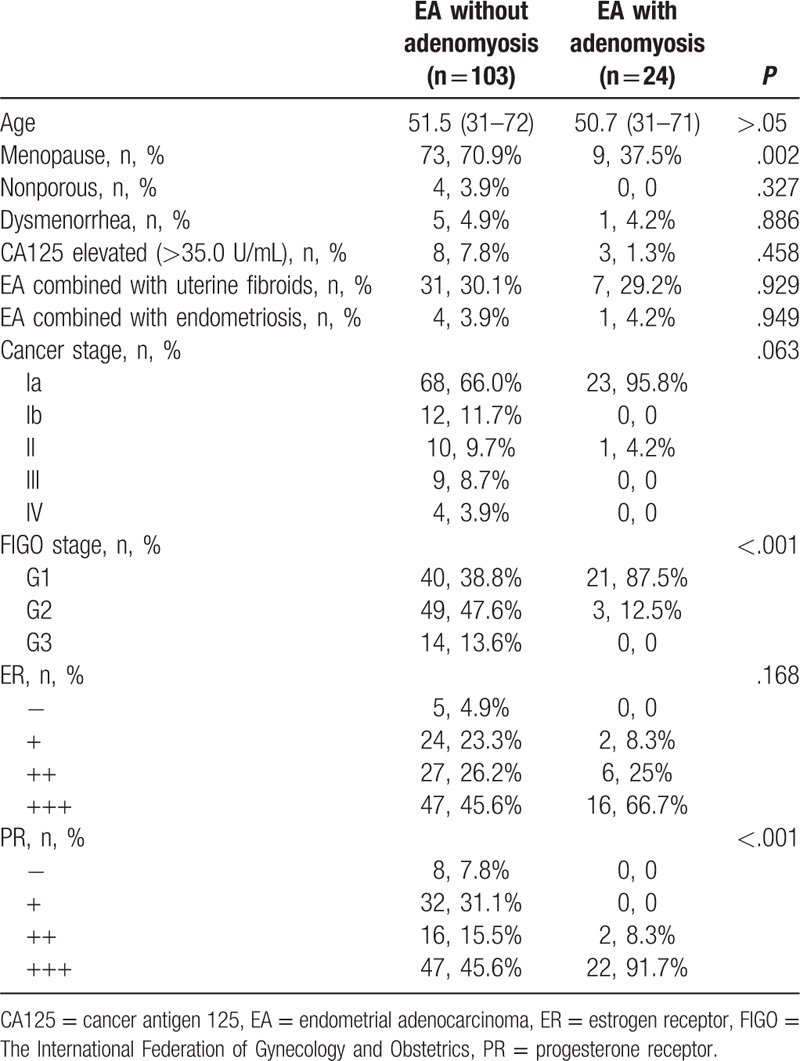
All patients underwent B-mode ultrasound and pathological examination (curettage). The diagnosis of uterine fibroids and adenomyosis were confirmed by histopathological examination after surgery. The pathological differentiation and staging of endometrial carcinoma was performed according to the criteria provided by the International Federation of Gynecology and Obstetrics (Table 1).[19]
Table 1.
Characteristics of patients with EA.
2.2. Treatment
All patients underwent standard staging operation. Intraoperative frozen biopsy samples were sent to laboratory immediately. The peritoneal washing fluid was collected. The patients with high risk were required to take postoperative adjuvant chemotherapy and radiotherapy.
2.3. Statistical analysis
This was a descriptive study. The data were analyzed using SPSS 17.0 (IBM, Armonk, NY). Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Non-normally distributed data were expressed as median (range), and analyzed using the Mann-Whitney U test. Categorical data were presented as counts and percentages, and chi-square or Fisher exact tests were used to compare the data among the groups. A P value <.05 was considered statistically significant.
3. Results
3.1. General characteristics of patients
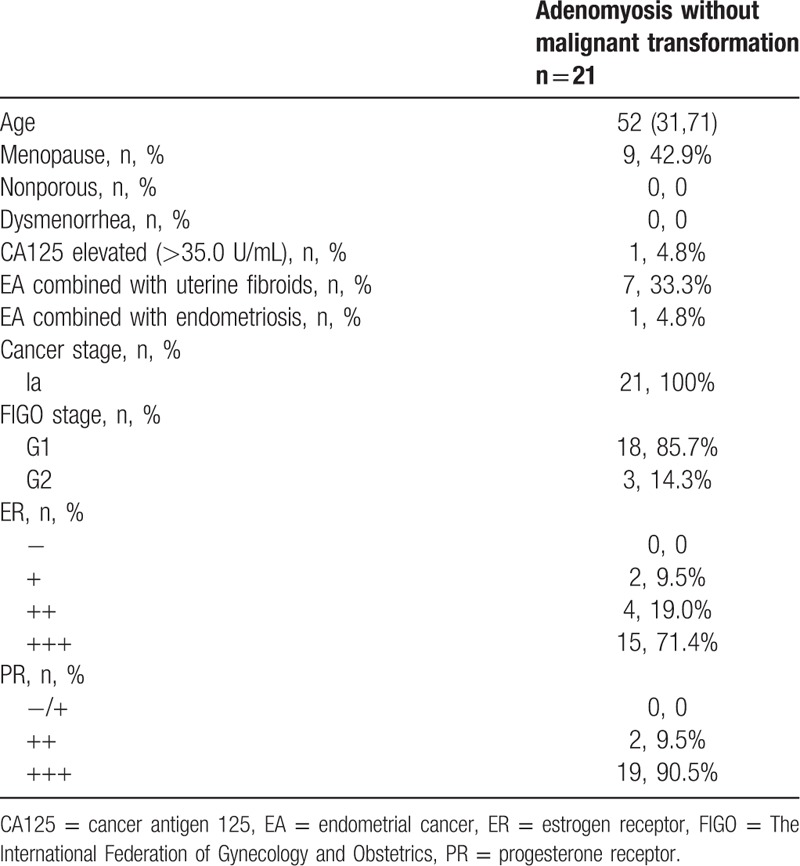
For the group of patients without adenomyosis, 103 patients had endometrial carcinoma without adenomyosis (81.1%); their mean age was 51.5 years (range: 31–72 years). For the group of patients with adenomyosis, 24 patients had endometrial carcinoma combined with adenomyosis (18.9%); their mean age was 50.7 years (range: 31–71 years) (Table 1). Among those 24 patients, 3 were diagnosed with malignant transformation of adenomyosis (2.4%); their mean age was 41 years (range: 31–48 years) (Table 2). Among those 24 patients with adenomyosis, the remaining 21 patients were not found to have malignant transformation of adenomyosis (16.5%); their mean age was 52 years (range: 33–71 years) (Table 3).
Table 2.
Patients with malignant adenomyosis transformation.
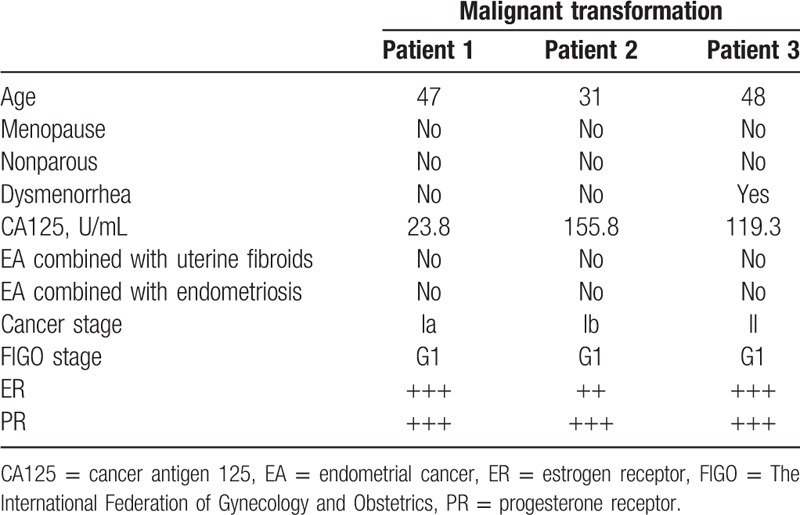
Table 3.
Patients without malignant adenomyosis transformation.
Among patients without adenomyosis, 4 patients were without reproductive history, and 73 patients (70.9%) were menopausal. Among patients with adenomyosis, all patients had childbearing history, and 9 (37.5%) were menopausal. Endometrial carcinoma was commonly found in patients during perimenopause, and there was no significant difference in the onset age between the 2 groups. Nevertheless, the patients who were diagnosed with malignant transformation of adenomyosis appeared to be younger (P < .05).
3.2. Clinical manifestations
Most of the patients were admitted to the hospital for the treatment of irregular bleeding (postmenopausal) or menstrual disorders. Five patients among those without adenomyosis and 1 patient among those with adenomyosis showed dysmenorrhea. Eight patients among those without adenomyosis were found to have increased cancer antigen 125 (CA125) levels, with the highest value of 97.40 U/L. Similarly, the 3 patients with malignant transformation of adenomyosis were also found to have increased CA125 levels and 2 of them had values of >100 U/L.
Among those without adenomyosis, 31 patients (30.1%) had endometrial carcinoma along with uterine fibroids and 4 patients (3.9%) had endometrial carcinoma combined with endometriosis; all of them were without malignant transformation.
3.3. Diagnosis
Among patients without adenomyosis, B-mode ultrasound suggested that the endometrium was thickened with changes in ultrasound signal in 73 cases, whereas masses were observed in the remaining 30 cases. Twenty-one patients were diagnosed with atypical hyperplasia and 82 patients with adenocarcinoma by pathological examination of preoperative curettage.
Among patients with adenomyosis, preoperative B-mode ultrasound suggested endometrial thickening in 17 cases (71.8%), myometrium echo change was found in 7 cases, the possibility of uterine fibroid was found in 8 cases (33.3%), and the adenomyosis was found in 2 cases (8.3%). Eleven patients (45.8%) were diagnosed with complex hyperplasia and atypical hyperplasia and 12 patients (50.0%) with adenocarcinoma by pathological examination of preoperative curettage. The remaining patient was initially diagnosed with endometrial hyperplasia and underwent hysterectomy for the treatment of uterine fibroid; however, the postoperative pathology results showed highly differentiated endometrial adenocarcinoma.
3.4. Comparison of tumor staging between the 2 groups
Among patients with adenomyosis, 1 patient was diagnosed with Stage II carcinoma, whereas all the other patients were diagnosed with Stage Ia carcinoma. The malignant transformation of adenomyosis did not seem to influence the postoperative pathology stage. Among patients without adenomyosis, more than half of the patients were diagnosed with Stage Ia (66%), followed by Stages Ib (11.7%), II (9.7%), III (8.7%), and IV (3.9%).
Compared with patients without adenomyosis, the differentiation degree of tumor tissue was obviously better in patients with adenomyosis (P < .05). Moreover, the differentiation degree of tumor tissue in patients with malignant transformation of adenomyosis seemed to be better than in patients without transformation.
3.5. Comparison of immunohistochemistry results between the groups
Some data were missing because of the large time span of the study. PR expression was significantly higher in patients with adenomyosis than that in patients without adenomyosis (P < .05); but malignant transformation of adenomyosis did not seem to influence estrogen receptor (ER) expression.
3.6. Comparison of immunohistochemistry results between eutopic and ectopic endometrium
Among patients with malignant transformation of adenomyosis, the progesterone receptor (PR) expression of eutopic endometrium was strongly positive (Fig. 1A), whereas the PR expression of ectopic endometrium was weakly positive (Fig. 1B). Among patients with malignant transformation of adenomyosis, the PR expression of eutopic endometrium in patients with malignant transformation and in normal ectopic endometrium was strongly positive (Figs. 2 and 3).
Figure 1.
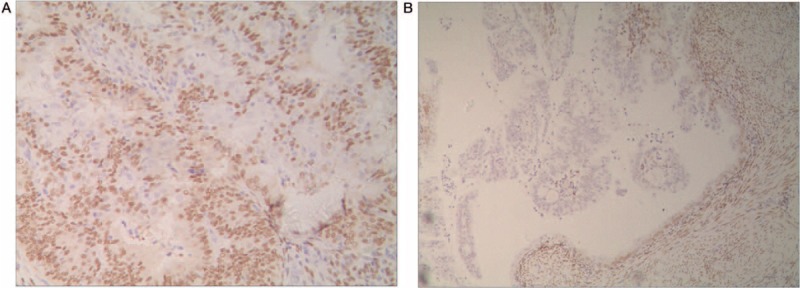
The expression of progesterone receptor (PR) in patients with malignant transformation of adenomyosis. (A) The PR expression of eutopic endometrium with malignant transformation was strongly positive (EnVision, ×200). (B) The PR expression of ectopic endometrium with malignant transformation was weakly positive (EnVision, ×100).
Figure 2.
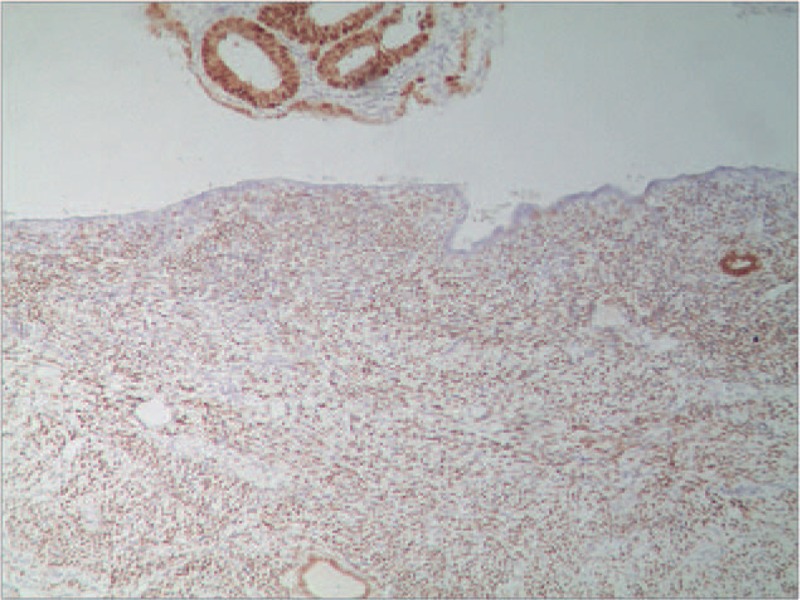
The expression of progesterone receptor (PR) in patient with malignant transformation of adenomyosis. The PR expression of eutopic endometrium in patients with malignant transformation and in normal ectopic endometrium were all strongly positive (EnVision, ×100).
Figure 3.
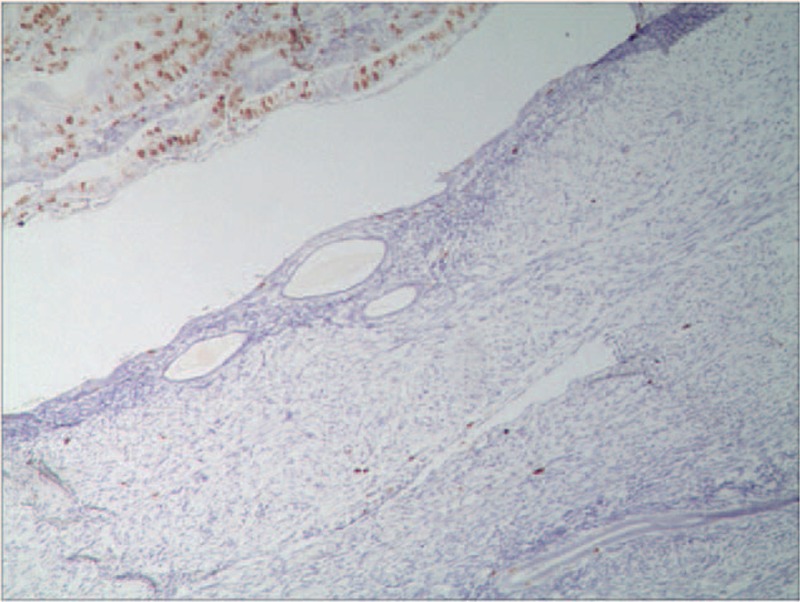
The expression of Ki67 in patient with malignant transformation of adenomyosis. The Ki67 expression was positive in ectopic endometrium with malignant transformation and negative in normal ectopic endometrium (EnVision, ×100).
4. Discussion
Among 127 patients with endometrial carcinomas, 24 patients (18.9%) were diagnosed with endometrial carcinomas combined with adenomyosis, of which, 3 patients were diagnosed with malignant transformation of adenomyosis. The malignant transformation rate of adenomyosis was 2.4% in this case series. In 1980, Hernandez and Woodruff et al[20] collected the case reports and reviewed the literatures, and 16 cases with malignant transformation of adenomyosis were analyzed. Among them, the malignant transformation concurrently originated from lesions of endometrium or adenomyosis in 8 cases, whereas the malignant transformation originated from the lesions of adenomyosis in the other 8 cases. In the present study, all 3 patients with adenomyosis malignant transformation had concurrent endometrial carcinoma, and the rate of malignant transformation was in accordance with the results of Kucera et al,[21] wherein the rate of endometrioid adenocarcinoma was about 2.9% in patients with adenomyosis malignant transformation.
Malignant transformation of adenomyosis lacks specific clinical symptoms and physical signs, with no specific laboratory investigations and no clinical symptoms at early stage. Preoperative diagnosis is difficult due to lack of malignant adenomyosis-related physical symptoms and signs. Moreover, serum CA125 is not always elevated. Hence, postoperative pathological diagnosis is the gold standard for adenomyosis malignant transformation, which is based on both Sampson's[1] standard and Scott's supplementary criteria[22]: no cancerous foci in endometrium and other parts of pelvis; ensure that the malignant lesion is originating from the epithelial or mesenchymal part of adenomyosis rather than the malignant invasion or metastasis from other parts; there are endometrial glands or stromal cells around the malignant lesions, or there is evidence for the presence of adenomyosis; and there is evidence of gland structure transformed from benign to malignant.
In the present study, the sensitivity of B-mode ultrasound echo changes for myometrium was higher (P < .05), but attention was not paid to those aforementioned factors because the presence of malignancy was only confirmed after postoperative pathology. It is required to expand the sample size of future studies to ensure whether these patients have typical clinical manifestations. On examining the tissues of malignant adenomyosis by microscopy, it was found that the malignant foci were originating from the mesenchymal part of adenomyosis.
The incidence of malignant transformation of adenomyosis is rare, and the possible reasons. Currently, there is a lack of epidemiological survey with large sample size of malignant transformation of adenomyosis. Pathologic sampling is limited, and it is difficult to make early diagnosis. In addition, the pathologists’ inadequate understanding about invasion of normal endometrium by malignant adenomyosis during the late period of malignant transformation, and myometrial invasion of endometrial carcinoma further pose difficulty in analyzing the morbidity rate of adenomyosis malignant transformation.
Pan Xiao Yu[23] described some of the risk factors of malignant adenomyosis, which include age between 40 and 50 years, early menarche, short menstrual cycle, first delivery at young age, fertility, curettage during early trimester of pregnancy, obesity, and history of tamoxifen intake. In the present study, the mean ages of patients with malignant adenomyosis ranged from 31 to 48 years old, and they were in line with the high-risk population. Previous studies indicated that the malignant transformation of adenomyosis and the occurrence of endometrial adenocarcinoma have some similarities with the malignant transformation of ovarian endometrial implantation, and they are closely related with estrogen stimulation. Lavery et al[24] found that unopposed estrogen hormone replacement therapy could result in malignant transformation of residual lesions in patients with endometriosis, suggesting that excessive estrogen stimulation could induce the malignant transformation of ectopic endometrium. In the present study, ER and PR expression of the patients with endometrial carcinoma with adenomyosis was obviously higher than in patients with endometrial carcinoma alone and patients with endometrial carcinoma and uterine fibroid. In those 2 groups, the majority of patients were postmenopausal women of older age, and it also might be related with the decline of hormone levels. Previous studies have shown that the ER and PR expression of endometrial carcinoma were associated with its histological grade, pathological type, and the biological behavior of myometrial invasion. The present study was in accordance with the fact that the patients with adenomyosis had good pathological differentiation and early clinical staging. Moreover, the 3 patients with malignant transformation of adenomyosis seem to have similar pathological differentiation and clinical stage.
The relapse, invasion, implantation, and biological behavior of metastasis of adenomyosis are very similar with malignant tumors, and they are also related with oncogenes and tumor-suppressor genes. Sasaki et al[25] reported 7 cases of malignant transformation of adenomyosis in which carcinoma tissue-related p53 protein expression was positive in 5 cases, and its expressions in all the normal glands were negative. Wu et al[26] reviewed clinical and pathological data of 39 cases with adenomyosis malignant transformation, with focus on ER and PR as well as p53. Among 39 patients with malignant transformation, 13 patients underwent ER investigations, and 8 were negative (62%). Similarly, 13 patients underwent PR investigations, and 7 were negative (54%). p53 expression was detected in 9 cases, for a positive rate of 100%.
The present study indicated that the expression of both PR and ER were positive in patients with endometrial carcinoma combined with adenomyosis or endometrial carcinoma combined with uterine fibroids. The expression of p53 and Ki67 was positive in eutopic malignant endometrium and negative in normal ectopic endometrium. In the 3 patients with malignant transformation of adenomyosis, both the ER and PR expressions were positive in eutopic endometrium and were weakly positive in ectopic endometrium. The changes explained the loss of ER and PR expression during the malignant transformation process with the same hormone level in a certain extent. It also resulted in the no expression of ER and PR antibodies, suggesting that the regulation of receptor levels plays a non-negligible role in the process of malignant transformation.
Because the incidence of adenomyosis with malignant transformation is low, the related researches are limited. As the locations of the lesion of adenomyosis malignant transformation and endometrial carcinoma are close to each other and the tissue origins are same, the prognosis and treatment are also similar for both the conditions. It is recommended that the treatment and postoperative follow-up suggestions should be based on corresponding cell type of endometrial malignant transformation. Finally, statistical analyses were performed in the present study, it must be pointed out that these analyses are unreliable because of the small sample size of some groups and heterogeneity in the size of the groups. Therefore, those analyses have to be taken as descriptive and not as formal analyses.
In summary, although adenomyosis lesion is usually benign, it might also be a precursor of malignant disease. As the incidence of adenomyosis malignant transformation is low, and its clinical manifestations are nonspecific, it may only be confirmed by postoperative pathological examination. Further investigations on larger sample size may provide additional data about prognosis of adenomyosis malignant transformation.
Footnotes
Abbreviations: CA125 = cancer antigen 125, ER = estrogen receptor, PR = progesterone receptor.
The authors report no funding and conflicts of interest.
References
- [1].World Cancer Research Fund International. Cancer facts and figures: Endometrial cancer rates. Available at: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/endometrial-cancer-cancer-lining-womb-statistics Accessed December 15, 2015. [Google Scholar]
- [2].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [3].Sampson JA. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Am J Obstet Gynecol 1925;9:111–4. [Google Scholar]
- [4].Boyraz G, Selcuk I, Yazicioğlu A, et al. Ovarian carcinoma associated with endometriosis. Eur J Obstet Gynecol Reprod Biol 2013;170:211–3. [DOI] [PubMed] [Google Scholar]
- [5].Del Carmen MG, Smith Sehdev AE, Fader AN, et al. Endometriosis-associated ovarian carcinoma: differential expression of vascular endothelial growth factor and estrogen/progesterone receptors. Cancer 2003;98:1658–63. [DOI] [PubMed] [Google Scholar]
- [6].Erzen M, Rakar S, Klancnik B, et al. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol 2001;83:100–8. [DOI] [PubMed] [Google Scholar]
- [7].Lee WL, Chang WH, Wang KC, et al. The risk of epithelial ovarian cancer of women with endometriosis may be varied greatly if diagnostic criteria are different: a nationwide population-based cohort study. Medicine 2015;94:e1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nishida M, Watanabe K, Sato N, et al. Malignant transformation of ovarian endometriosis. Gynecol Obstet Invest 2000;50suppl 1:18–25. [DOI] [PubMed] [Google Scholar]
- [9].Corner GW, Jr, Hu CY, Hertig AT. Ovarian carcinoma arising in endometriosis. Am J Obstet Gynecol 1950;59:760–74. [Google Scholar]
- [10].Rolly F. Über einen Fall von Adenomyoma Uteri, Übergang in Karzinom und Metastasenbildung. Virchows Arch 1897;150:555. [Google Scholar]
- [11].Motohara K, Tashiro H, Ohtake H, et al. Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol 2008;13:266–70. [DOI] [PubMed] [Google Scholar]
- [12].Ismiil ND, Rasty G, Ghorab Z, et al. Adenomyosis is associated with myometrial invasion by FIGO 1 endometrial adenocarcinoma. Int J Gynecol Pathol 2007;26:278–83. [DOI] [PubMed] [Google Scholar]
- [13].Kazandi M, Zeybek B, Terek MC, et al. Grade 2 endometrioid adenocarcinoma arising from adenomyosis of the uterus: report of a case. Eur J Gynaecol Oncol 2010;31:719–21. [PubMed] [Google Scholar]
- [14].Boes AS, Tousseyn T, Vandenput I, et al. Pitfall in the diagnosis of endometrial cancer: case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol Oncol 2011;32:431–4. [PubMed] [Google Scholar]
- [15].Hirabayashi K, Yasuda M, Kajiwara H, et al. Clear cell adenocarcinoma arising from adenomyosis. Int J Gynecol Pathol 2009;28:262–6. [DOI] [PubMed] [Google Scholar]
- [16].Jha P, Ansari C, Coakley FV, et al. Case report: Imaging of Mullerian adenosarcoma arising in adenomyosis. Clin Radiol 2009;64:645–8. [DOI] [PubMed] [Google Scholar]
- [17].Mori M, Furusawa A, Kino N, et al. Rare case of endometrioid adenocarcinoma arising from cystic adenomyosis. J Obstet Gynaecol Res 2015;41:324–8. [DOI] [PubMed] [Google Scholar]
- [18].Taga S, Sawada M, Nagai A, et al. A case of endometrioid adenocarcinoma arising from adenomyosis. Case Rep Obstet Gynecol 2014;2014:569295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- [20].Hernandez E, Woodruff JD. Endometrial adenocarcinoma arising in adenomyosis. Am J Obstet Gynecol 1980;138(part 1):827–32. [DOI] [PubMed] [Google Scholar]
- [21].Kucera E, Hejda V, Dankovcik R, et al. Malignant changes in adenomyosis in patients with endometrioid adenocarcinoma. Eur J Gynaecol Oncol 2011;32:182–4. [PubMed] [Google Scholar]
- [22].Scott RB. Malignant changes in endometriosis. Obstet Gynecol 1953;2:283–9. [PubMed] [Google Scholar]
- [23].Pan XY, Cai YL, Zhuang YH, et al. Malignant adenomyosis: a case report and literature review. Modern Med 2015;43:611–3. [Google Scholar]
- [24].Lavery S, Gillmer M. Malignant transformation of residual endometriosis in women on unopposed oestrogen hormone replacement therapy. BJOG 2001;108:1106–7. [DOI] [PubMed] [Google Scholar]
- [25].Sasaki T, Sugiyama T, Nanjo H, et al. Endometrioid adenocarcinoma arising from adenomyosis: report and immunohistochemical analysis of an unusual case. Pathol Int 2001;51:308–13. [DOI] [PubMed] [Google Scholar]
- [26].Wu YX, Liu Q, Li QR, et al. Clinicopathological analysis of 3 cases of malignant adenomyosis. J Modern Oncol 2011;19:1834–8. [Google Scholar]


