Abstract
Intralesional steroid injections are the standard treatment for hypertrophic scars and keloids. The procedure is, however, quite painful and is unpopular with patients because of this. Topical application of anesthetic creams, such as Ametop gel (tetracaine) and EMLA cream (lidocaine and prilocaine), has limited efficacy because of poor drug penetration. The onset of the analgesic effect is also slow, which means that the use of topical anesthetics is time-consuming in clinical practice.
We hypothesized that a commercially available cryotip could be used to provide fast-acting topical cryoanesthesia that would reduce the pain associated with steroid injections.
Thirty patients with hypertrophic scars or keloids were enrolled in the study. Scars were injected with the steroid, triamcinolone acetonide, with or without prior application of the cryotip (−10 °C) for 15 seconds. The degree of pain was evaluated in each case using the visual analogue scale (VAS) and the verbal descriptor scale (VDS), together with any side-effects caused by application of the cryotip.
The VAS pain scores showed a statistically significant (P < .01) difference between the pretreated and the control scars (pain scores 7.87 ± 1.31 and 2.7 ± 1.37, respectively). The VDS pain scores also showed a statistically significant (P < .01) difference between the pretreated and the control scars. And its average scores were 7.89 ± 0.32 and 2.68 ± 0.25, respectively.
Application of the cryotip before injection could provide a rapid and effective means of reducing the pain associated with steroid injections. Painless would result in better therapeutic effect.
Keywords: cryoanesthesia, hypertrophic scars, injection pain, keloids
1. Introduction
Pain is a common clinical phenomenon associated even with procedures intended to reduce pain, such as anesthetic injections before dental surgery.[1] Hypertrophic scars and keloids, which are characterized by a red, rigid, and raised appearance, are benign fibrous overgrowths of scar tissue that result from abnormal wound healing after trauma.[2,3] Keloids extend beyond the borders of the original wound, do not usually regress spontaneously and tend to recur after excision. In addition to the adverse cosmetic effect, they often lead to pruritus, pain, and contractures. There is no consensus on the ideal treatment for hypertrophic and keloid scars. A multitude of therapies are used without definitive superior outcome.[4] Intralesional steroid injections play a major role in the management of keloids and, following repeated administration, the scars become flatter and softer and eventually a good result can be achieved in the majority of cases.[5] The injections are, however, painful and this, together with the frequency of the injections, means that the treatment is unpopular with patients, especially children and older people.[1] Lack of compliance and missed injections reduce the effectiveness of this treatment. Topical anaesthetics, such as Ametop gel (tetracaine) and EMLA cream (lidocaine and prilocaine), are widely used to reduce the pain of injections, but have limited efficacy because of poor drug penetration. The slow onset of analgesic effect is also a problem,[6] and there is a real need for an effective topical anesthetic procedure that can be used in a busy clinical setting to reduce the pain of local steroid injections.
Cryotherapy is a convenient and relatively noninvasive technique that is used for pain management in trauma and disease,[7,8] and to reduce postoperative pain. Since cryotherapy is comparatively free from side effects, it has been used in sports medicine to relieve pain caused by soft tissue injuries and also to reduce the discomfort of laser treatment.[9,10] Given there is no effective topical anesthetic methods in clinic and the cryotip favorable efficacy and safety profile, we decided to evaluate whether cryotherapy can reduce the pain associated with intralesional steroid injections used to treat pathological scars.
2. Patients and methods
2.1. Patients
Thirty outpatients (19 females and 11 males), with hypertrophic scars or keloids, were enrolled in the study randomly. Patients were excluded from the study if they had ever received any treatment to treat their scars. The study protocol was approved by the Ethics Committee of Shanghai 9th People's Hospital, Shanghai Jiao Tong University School of Medicine and all participants provided written informed consent prior to the study. Neither patients nor doctors were informed about the patients’ benefits of the cryotip.
2.2. Cryotip device
The cryotip device (shown in Fig. 1) was purchased from JL Biotech Development Limited (Beijing, China). The device has a receptacle body for cooled liquid and is equipped with a temperature indicator and a metal tip for skin contact. The receptacle body was filled with 70% alcohol and other detergent and the device was the placed in a freezer at –10 °C for at least 1 hour before use to achieve an operating temperature of –10 °C. After sterilization, the metal tip was applied to the injection site for 15 seconds to induce analgesia. Contact with normal skin was avoided to prevent frostbite.
Figure 1.

Cryotip device.
2.3. Study design
Two arms were used in this experimental protocol. In the 1st arm, 2 scars on the same part of the same patient were randomly designated as control or experimental scars. Control scars were injected without cryotherapy (Fig. 2A). The cryotip was placed on the experimental scars for 15 seconds (Fig. 2B) and these scars were then injected (Fig. 2C). In the 2nd arm, the scar to be injected was divided into 2 parts down a center line. The control side was injected first, without cryotherapy (Fig. 2D). The cryotip was then placed on the experimental side for 15 seconds (Fig. 2E) and then this side was injected (Fig. 2F). Patients were used as their own control since different individuals have different experiences of pain. Patients scars were injected once per month and pain intensity scores were recorded after each injection.
Figure 2.
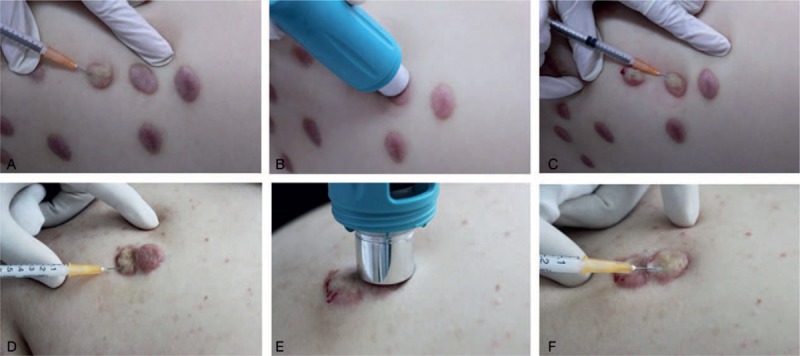
Injection sites used in 1st (A–C) and 2nd (D–E) protocols. In the 1st protocol, 2 adjacent scars were randomly designated as control or experimental. The control scar was injected without cryoanethesia (A). The cryotip was placed on the experimental scar for 15 seconds (B) before injection (C). In the 2nd protocol, the scar to be treated was divided into 2 parts down a center line. The control side was injected without cryoanethesia (D). The cryotip was then placed on the experimental side for 15 seconds (E) before injection (F).
2.4. Pain evaluation
Pain was evaluated using 2 pain rating scales, the visual analogue scale (VAS) and the verbal descriptor scale (VDS), as previously described.[11,12]
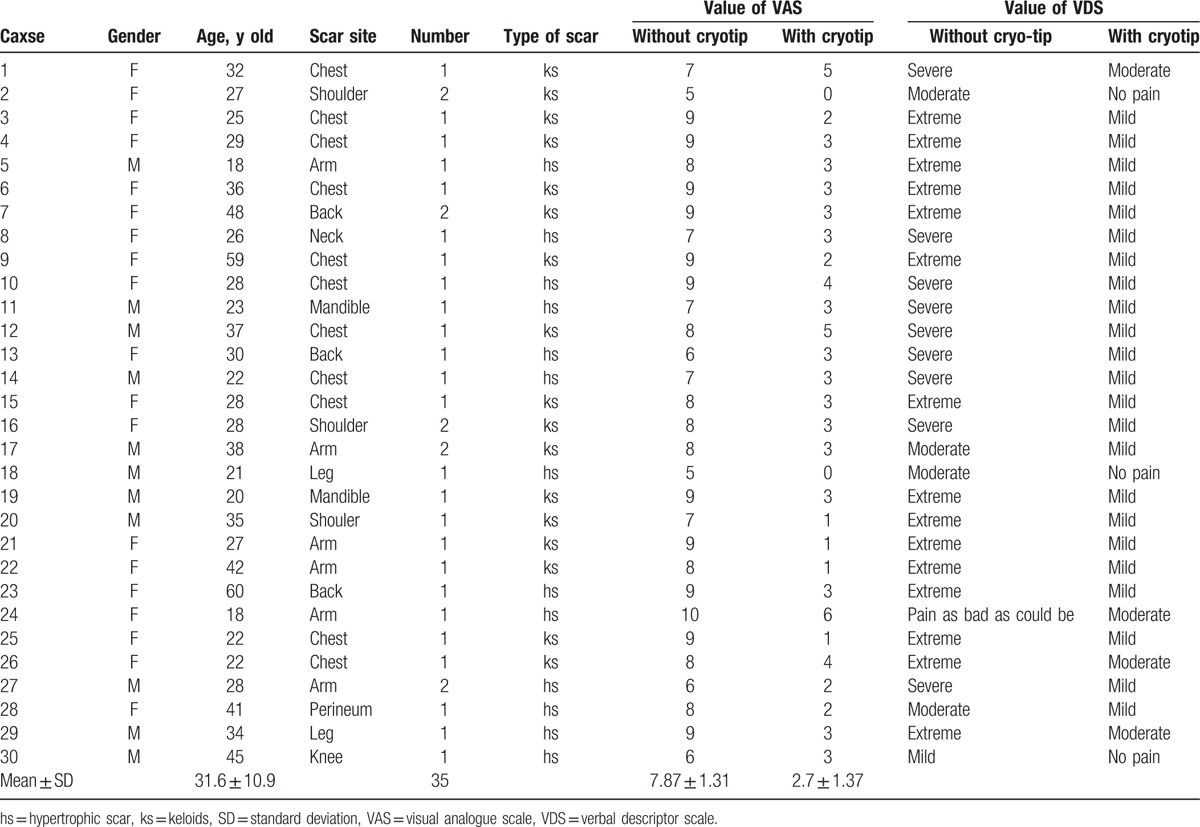
The VAS (Fig. 3A) consists of a straight line, with the endpoints defining extreme limits (no pain and very severe pain). The patient is asked to mark his or her pain level on the line between the 2 endpoints. The distance between “no pain” and the mark then defines the patient's pain level. The VAS has a 10-point scale; a score of zero indicates that the injection is painless and a score of 10 indicates that the injection causes the worst possible pain. In the VDS (Fig. 3B), words are used to describe different levels of pain. Using the VDS pain thermometer, the patient is asked to choose from “no pain,” “mild pain,” “moderate pain,” “severe pain,” “extreme pain,” and “pain as bad as could be” to describe his or her pain. Treatments and assessments were carried out by 2 therapists who had previously received training in scar management. Pain scores were recorded for both the control group (without cryotip) and experimental group (with cryotip) (Table 1).
Figure 3.
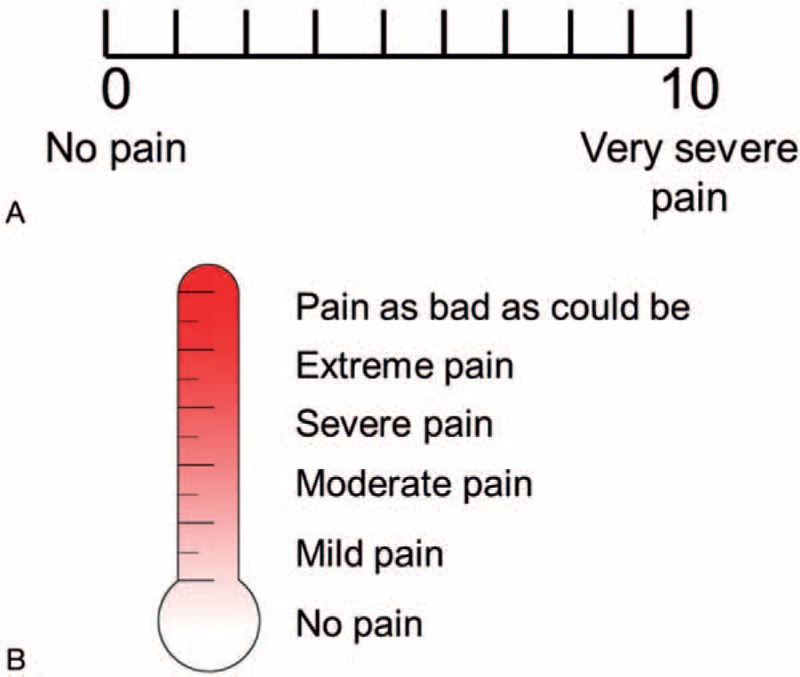
(A) Visual analogue scale and (B) verbal descriptor scale.
Table 1.
Patient demographics and pain scores.
2.5. Statistical analysis
The data were analyzed used a paired t test.
3. Results
3.1. Patient demographics
A total of 30 patients, 19 females (63.3%) and 11 males (36.7%), were included in the study. Since some scars were divided into 2 parts (a control part and an intervention part), the total number of scars included in the study was 35. Hypertrophic scars or keloids on the chest, shoulders, arms, and back accounted for 80% of the total lesions (Table 1).
The patients, who were all Asian, were between 18 and 60 years old, with an average age old 31.6 years. They had received no other treatment before the cryotherapy injection.
3.2. VAS pain scores
VAS pain scores indicated a statistically significant (P < .01) difference in injection pain between pretreated with cryotherapy and nonpretreated areas (2.7 ± 1.37 and 7.87 ± 1.31, respectively) (Table 1). The data are presented graphically in Fig. 4. A total of 100% of the subjects experience less pain following cryotherapy and, on average, pain was reduced by 66.04%.
Figure 4.
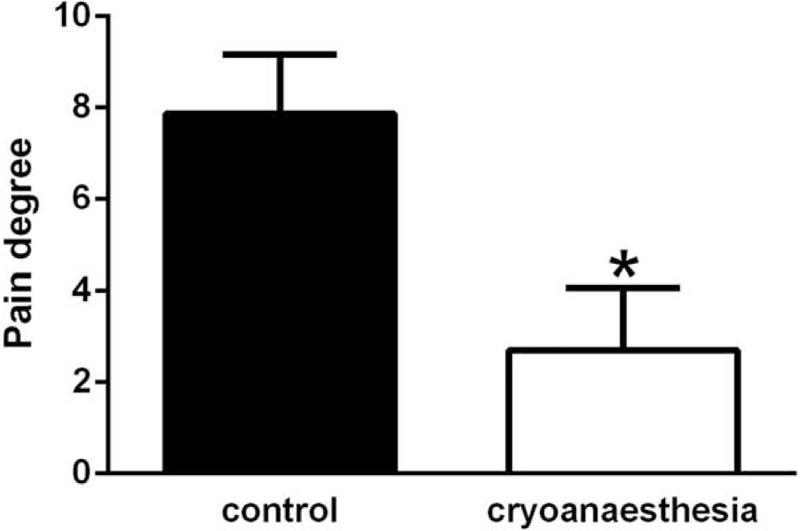
Pain intensity scores determined using visual analogue scale (VAS). There is a significant difference between the control group and the group that received cryoanesthesia (P < .01).
3.3. VDS pain scores
VDS scores also indicated a significant difference (P < .01) in injection pain between pretreated with cryotherapy and nonpretreated areas (Table 1 and Fig. 5). A total of 93.3% of the patients considered cryotherapy to be very effective for pain relief and 6.7% of the patients considered the treatment to have modest effects. All of the patients wanted to use the cryotip for their next course of injections.
Figure 5.
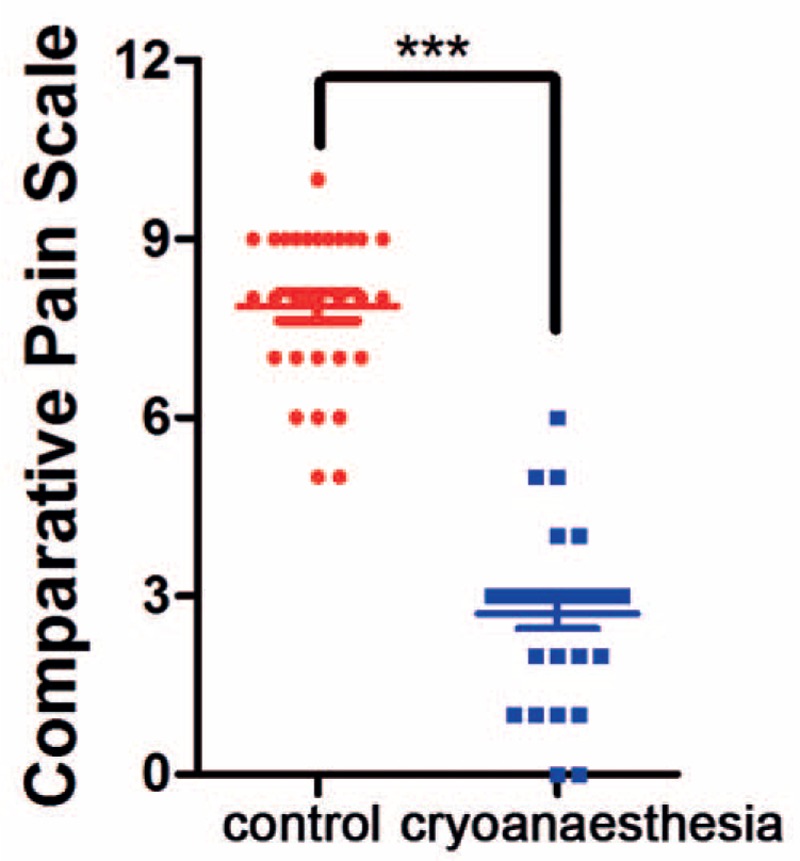
Pain intensity scores determined using verbal descriptor scale (VDS). There is a significant difference between the control group and the group that received cryoanesthesia (P < .01).
3.4. Side-effects
One patient developed skin pigmentation when the cryotip was placed on normal skin for 15 seconds. After 3 months, the pigmentation resolved spontaneously.
4. Discussion
Although a variety of surgical and nonsurgical methods have been used to treat hypertrophic scars and keloids,[13–15] intralesional steroid injections remain the standard treatment.[16] Mean scar volumes were reduced from baseline values of 0.73 ± 0.701 to 0.14 ± 0.302 mL after repeated monthly intralesional injections of triamcinolone acetonide.[17] Pain, however, which affects patient compliance, inevitably has an impact on the effectiveness of the treatment. The last decade has witnessed major improvements in techniques for injecting local anaesthetics that have allowed surgeons to carry out more procedure under local anesthesia, with minimal or no pain during the injection of the local anesthetic.[18]
To our knowledge, this is the first study to explore the use of a cryotip to decrease injection pain. We have shown that the cryotip effectively reduced pain intensity scores, in 2 cases to 0. Without the cryotip, almost all patients were afraid of injections into hypertrophic scars or keloids. VAS pain scores without the cryotip were 5 or higher, with 1 patient recording a score of 10 (Table 1). After applying the cryotip for only 15 seconds, pain intensity scores decreased markedly. Average VAS pain scores were significantly lower (P < .01) in the treated group than in the control group (2.7 ± 1.37 and 7.87 ± 1.31, respectively). Use of the cryotip did not cause any frost bite and delayed pain, and all patients wanted to use the device for future injections. This simple but effective method for alleviating injection pain is likely to increase patient compliance and could provide a good option for both patients and plastic surgeons.
Compared with other local anesthetic agents and techniques, the cryotip is both time-saving and cost effective. The ability to inject local anesthetics with minimal pain will decrease or eliminate the need for sedation in many plastic surgery procedures. The use of these techniques is likely to increase because of improved patient safty, convenience, and satisfaction, as well as decreased cost.
Pain is an unpleasant response evoked by many different stimuli and injection pain is very common and the cryotip can be applied in other clinical phenomenon. Since its discovery in 1921, for example, insulin has been the cornerstone of diabetes treatment[19] but needle fear prevents many patients from self-injecting or finger-pricking.[20] Although many strategies have been used to improve insulin administration, including subcutaneous infusion, inhalation, and even oral delivery, no one has better effects than subcutaneous injection. Hence, we can apply the idea of cryoanesthetic to alleviate the insulin injection pain, and pain-free administration would significantly improve both patient compliance and disease management.[21]
5. Conclusion
Our aim is to found an effective, safe, and convenient way to reduce the pain associated with steroid injections and significantly improve patient compliance and disease management. Cryoanesthesia using a cryotip substantially reduced pain during intralesional steroid injections to treat hypertrophic scars and keloids. The result is in accordance with our hypothesis and the cryotip can play a great role in clinical injection pain relief.
Acknowledgments
The authors thank grants from surface project of National Natural Science Foundation of China (NO. 81272109) for the support.
Footnotes
Abbreviations: VAS = visual analogue scale, VDS = verbal descriptor scale.
XW and XW are recognized as co-first authors.
Funding/support: This research was supported by grants from surface project of National Natural Science Foundation of China (NO. 81272109).
The authors have no conflicts of interest to disclose.
References
- [1].van Wijk A, Lindeboom JA, de Jongh A, et al. Pain related to mandibular block injections and its relationship with anxiety and previous experiences with dental anesthetics. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:S114–9. [DOI] [PubMed] [Google Scholar]
- [2].Mundy LR, Miller HC, Klassen AF, et al. Patient-reported outcome instruments for surgical and traumatic scars: a systematic review of their development, content, and psychometric validation. Aesthetic Plast Surg 2016;40:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aschoff R. Therapy of hypertrophic scars and keloids. Hautarzt 2014;65:1067–77. [DOI] [PubMed] [Google Scholar]
- [4].Klinger M, Marazzi M, Vigo D, et al. Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthetic Plast Surg 2008;32:465–9. [DOI] [PubMed] [Google Scholar]
- [5].Trisliana Perdanasari A, Lazzeri D, Su W, et al. Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg 2014;41:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lalonde D, Wong A. Local anesthetics: what's new in minimal pain injection and best evidence in pain control. Plast Reconstr Surg 2014;134:40S–9S. [DOI] [PubMed] [Google Scholar]
- [7].Nadler SF, Weingand K, Kruse RJ. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 2004;7:395–9. [PubMed] [Google Scholar]
- [8].Evans PJ. Cryoanalgesia. The application of low temperatures to nerves to produce anaesthesia or analgesia. Anaesthesia 1981;36:1003–13. [DOI] [PubMed] [Google Scholar]
- [9].Hubbard TJ, Denegar CR. Does cryotherapy improve outcomes with soft tissue injury? J Athl Train 2004;39:278–9. [PMC free article] [PubMed] [Google Scholar]
- [10].Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med 2004;32:251–61. [DOI] [PubMed] [Google Scholar]
- [11].Nyrén O, Adami HO, Bates S, et al. Self-rating of pain in nonulcer dyspepsia. A methodological study comparing a new fixed-point scale and the visual analogue scale. J Clin Gastroenterol 1987;9:408–14. [PubMed] [Google Scholar]
- [12].Yazici Sayin Y, Akyolcu N. Comparison of pain scale preferences and pain intensity according to pain scales among Turkish Patients: a descriptive study. Pain Manag Nurs 2014;15:156–64. [DOI] [PubMed] [Google Scholar]
- [13].Li Z, Jin Z. Comparative effect and safety of verapamil in keloid and hypertrophic scar treatment: a meta-analysis. Ther Clin Risk Manag 2016;12:1635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guadanhim LR, Gonçalves RG, Bagatin E. Observational retrospective study evaluating the effects of oral isotretinoin in keloids and hypertrophic scars. Int J Dermatol 2016;55:1255–8. [DOI] [PubMed] [Google Scholar]
- [15].Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg 2017;43:S3–18. [DOI] [PubMed] [Google Scholar]
- [16].Meymandi SS, Moosazadeh M, Rezazadeh A. Comparing two methods of cryotherapy and intense pulsed light with triamcinolone injection in the treatment of keloid and hypertrophic scars: a clinical trial. Osong Public Health Res Perspect 2016;7:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Muneuchi G, Suzuki S, Onodera M, et al. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconstr Surg Hand Surg 2006;40:111–6. [DOI] [PubMed] [Google Scholar]
- [18].Boyce RA, Kirpalani T, Mohan N. Updates of topical and local anesthesia agents. Dent Clin North Am 2016;60:445–71. [DOI] [PubMed] [Google Scholar]
- [19].Best CH. The internal secretion of the pancreas. Can Med Assoc J 1962;87:1046–51. [PMC free article] [PubMed] [Google Scholar]
- [20].Simmons JH, McFann KK, Brown AC, et al. Reliability of the diabetes fear of injecting and self-testing questionnaire in pediatric patients with type 1 diabetes. Diabetes Care 2007;30:987–8. [DOI] [PubMed] [Google Scholar]
- [21].Wong CY, Martinez J, Dass CR. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol 2016;68:1093–108. [DOI] [PubMed] [Google Scholar]


