Abstract
It is still debatable whether complete mediastinal lymph node dissection (MLND) is associated with better survival than mediastinal lymph node sampling (MLNS) in surgical treatment of nonsmall cell lung cancer (NSCLC). We aimed to assess the impact of lymph node dissection on long-term survival among stage I NSCLC patients.
In this cohort study, 317 stage I NSCLC Chinese patients in Shanghai Chest Hospital were followed up for at least 10 years to evaluate the impact of different lymph node dissection modes on their survival. Among them, 161 patients were in the MLND group and 156 in the MLNS group. Overall survival and median survival times were calculated for the 2 groups. The association between lymph node dissection and the survival of NSCLC patients was assessed using Cox proportional-hazard models.
Patients in the MLND group presented better survival (median survival time = 154.67 months) than those in the MLNS group (median survival time = 124.67 months). The MLNS had higher mortality than the MLND group, with the crude hazard ratio of the MLNS group relative to the MLND group as 1.32 (95% confidence interval [CI] 0.97, 1.78). After adjusting for age and sex, the association between lymph node dissection and mortality (hazard ratio 1.36, 95% CI 1.00, 1.84) was statistically significant (P = .047). Further adjusting for baseline clinical characteristics, the association (hazard ratio 1.40, 95% CI 1.02, 1.92) remained statistically significant (P = .036). The association between lymph node dissection mode and mortality was strong among patients with tumor size between 2.0 and 3.0 cm (hazard ratio 2.79, 95% CI 1.45, 5.37).
We found that the MLND was associated with better survival for patients with early-stage NSCLC, compared with the MLNS. The effects of MLND on survival may depend on tumor size. Our findings have important implications in the treatment of early-stage NSCLC. Further prospective studies with a large sample size are needed to confirm our findings.
Keywords: cohort study, lymph node dissection, mortality, nonsmall cell lung cancer, survival
1. Introduction
Surgical resection has been proved to be the single curative treatment for patients with early stage nonsmall cell lung cancer (NSCLC).[1–3] Lobectomy (or pneumonectomy) with lymph node dissection has become a common treatment procedure. However, it is still debatable whether the complete mediastinal lymph node dissection (MLND) is superior to the mediastinal lymph node sampling (MLNS) in the survival of NSCLC patients with surgical treatment. In a randomized trial of systematic lymph node dissection in Chinese patients with resectable NSCLC, Wu et al[4] reported that lobectomy (pneumonectomy) combined with systematic lymph node dissection improved survival in NSCLC patients. However, their findings are questionable due to the imbalanced distribution of early-stage patients between 2 groups. Globally, about 40% to 50% NSCLC diagnosed are in stage I.[5] Several studies evaluated the association between lymph node dissection mode and survival in patients with stage I NSCLC who underwent curative-intent surgery, and concluded that the dissection mode did not significantly influence either disease-free survival or overall survival.[6,7] In a randomized multi-institutional trial, Darling et al[8] reported that MLND does not improve survival compared to MLNS in patients undergoing resection for N0 or nonhilar N1, T1 or T2 NSCLC. On the contrary, in a recent study of 15,195 patients of stage I to III NSCLC treated with surgical resection, David et al[9] concluded that the number of lymph nodes sampled influenced both overall survival and cancer-specific survival, but the optimal number of lymph nodes sampled remains unclear.
China has experienced a rapid economic growth in recent decades. The prevalence of NSCLC has increased over time.[10] As a large number of patients with early stages of NSCLC are detected in China[11]; it is critically important to understand that which lymph node dissection mode has better survival among early-stage NSCLC patients. The previous research in this area in China mainly focused on 3-year or 5-year survival rates.[4,12] In this retrospective cohort study, we analyzed the 10-year survival rates according to lymph node dissection modes in Chinese patients with stage I NSCLC.
2. Methods
2.1. Approaches for lymph node dissection
There is no consensus on the approaches for lymph node dissection currently. To standardize and improve preoperative and intraoperative lymph node staging and pathologic evaluation of NSCLC, the European Society of Thoracic Surgeons (ESTS) guidelines suggest that systematic lymph node dissection is recommended in all cases to ensure complete resection.[13] Two lymph node dissection modes were compared in this study: the MLNS with a predetermined selection of the lymph node stations specified by the surgeon; and MLND with all the lymph nodes dissected and removed systematically within anatomical landmarks.
2.2. Participants
All NSCLC cases in this cohort study were recruited from Shanghai Chest Hospital from January 1998 to January 2005. During this period, 4240 patients with diagnosed lung cancer were operated with either pneumonectomy or lobectomy. Eligible patients for this study met the following criteria: without preoperation chemotherapy; with complete lesion removal via lobectomy; consistent with the ESTS MLND or MLNS; postoperation staging was stage I (T1a-2aN0M0); without postoperation chemotherapy; and with at least 10-year follow-up. A total of 317 patients met the above criteria according to the 2005 international definition of complete resection in lung cancer surgery[14] and 2009 lung cancer staging.[15] Among 317 participants, 161 patients were in the MLND group and 156 in the MLNS group.
2.3. Follow-up
All participants were followed up to the date of death or April 30, 2015 if surviving. The data on mortality status during the follow-up period were collected through the death records in the Shanghai Centre for Disease Control and Prevention. The postoperative survival time was calculated as the difference between the surgery date and the date of death. Patients surviving beyond April 30, 2005 were censored on that day and their survival time was determined as the difference between April 30, 2015 and the surgery date.
2.4. Data analysis
The Kaplan–Meier method was used for calculating median survival time. To adjust for potential confounding factors, Cox proportional-hazard models were used to calculate adjusted hazard ratios (HRs) with the MLND group as the reference. A P value of less than .05 was considered significant. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL). This project was approved by the Ethics Review Committee of Shanghai Chest Hospital, and the need for individual consent was waived by the ethics committee due to the retrospective nature.
3. Results
3.1. Characteristics of different lymph node dissection groups
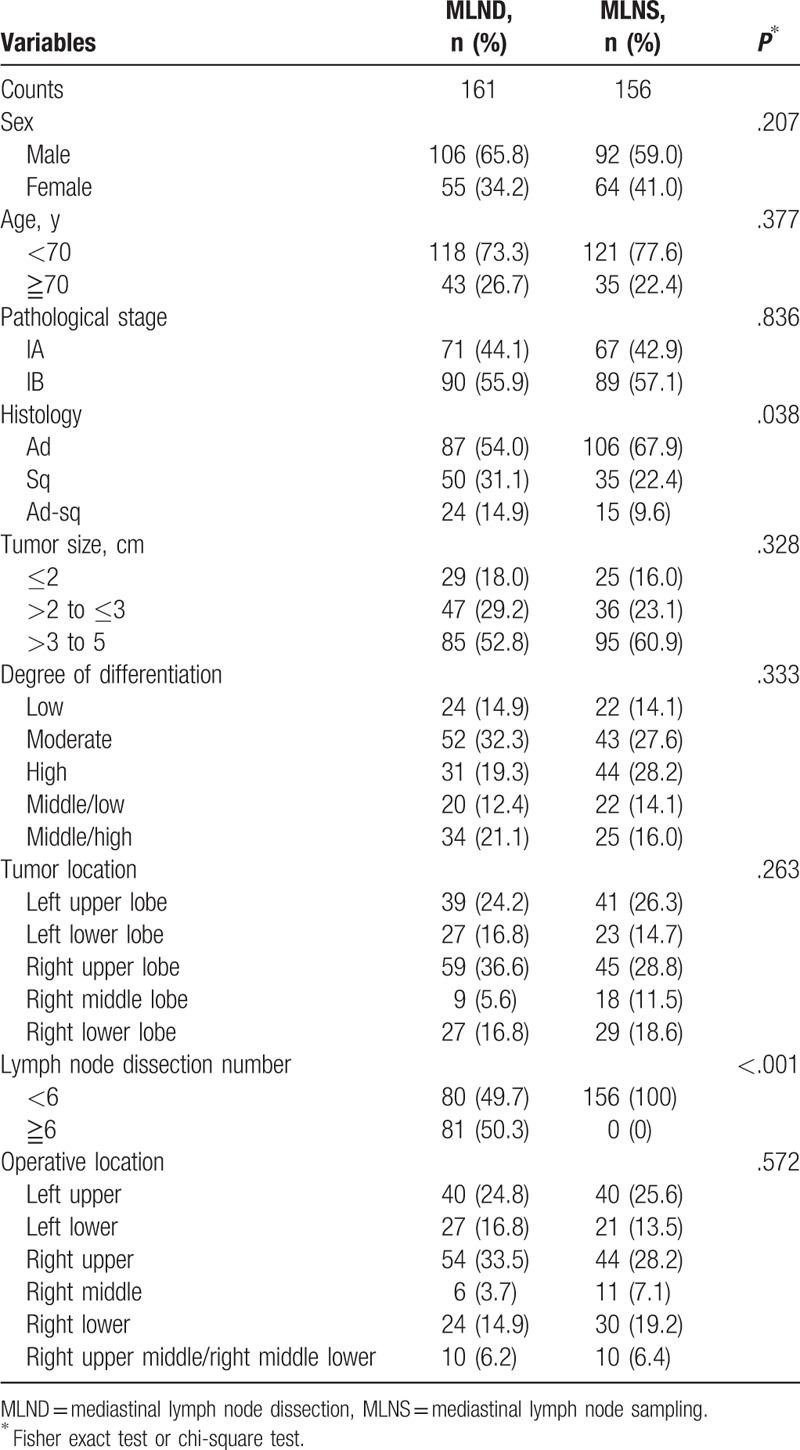
Table 1 shows clinical characteristics of the 2 groups. As expected, the MLNS group had a significantly less number of total dissected lymph nodes than in the MLND group (P < .001). The 2 groups were also significantly different in histological types (P = .038). However, there were no significant differences between MLND and MLNS groups in other clinical characteristics.
Table 1.
Clinical characteristics of different lymph node dissection groups.
3.2. Survival time and cumulative survival rates of different lymph node dissection groups
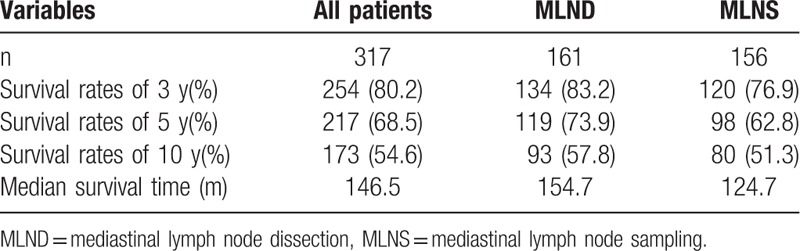
Among the 317 patients, the median survival time was 146.47 months. The 3, 5, and 10-year cumulative survival rates were 80.1%, 68.5%, and 54.6%, respectively. The median survival time was 124.67 months for the MLNS group and 154.67 for the MLND groups, as shown in Fig. 1. The MLND group survived longer than the MLNS group. The 3, 5, and 10-year cumulative survival rates were 76.9%, 62.8%, and 51.3% for the MLNS group, and 83.2%, 73.9%, and 57.8% for the MLND group, respectively (Table 2).
Figure 1.
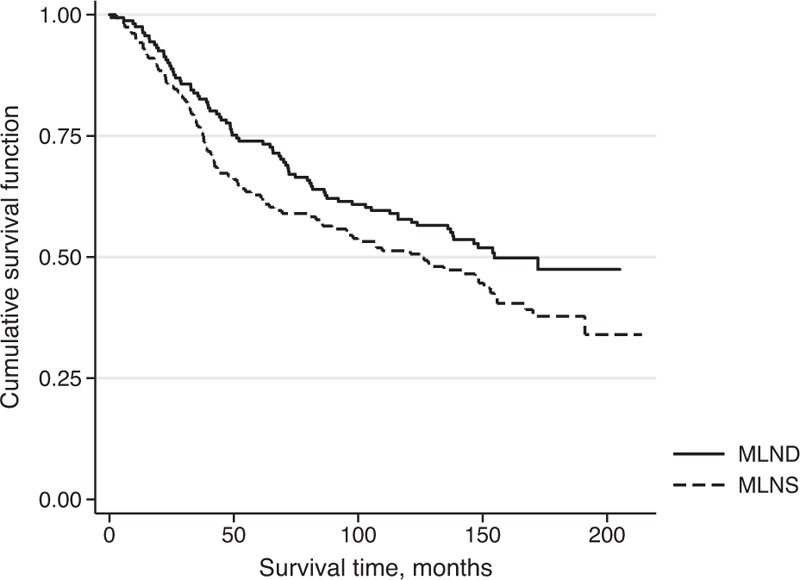
Survival time (month) by lymph node dissection groups: mediastinal lymph node dissection (MLND) and mediastinal lymph node sampling (MLNS) in stage I nonsmall cell lung cancer patients.
Table 2.
Survival rates and median survival time of different lymph node dissection groups.
3.3. Association between lymph node dissection and mortality after adjusting for baseline characteristics
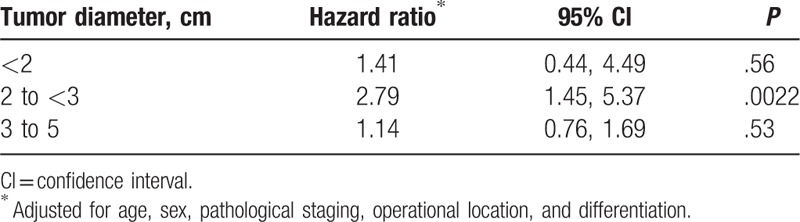
In univariate analysis, age, sex, pathological staging, operative location, tumor size, tumor location, and differentiation were significantly associated with survival, but pathological types and differentiation were not significantly associated with survival (Table 3). As a result, it is important to adjust for potential confounding effects of those baseline characteristics when assessing the association between different lymph node dissection modes and survival. Table 4 shows the crude and adjusted HRs and their 95% confidence intervals (CIs). The crude HR was 1.32 (95% CI 0.97, 1.78) between lymph node dissection and mortality, indicating that the MLNS group had a higher mortality than the MLND group, although the difference is not significant (P = .073). However, after adjustment for age and sex, the association (HR 1.36, 95% CI 1.00, 1.84) became statistically significant (P = .047). With further adjustment for age, sex, pathological staging, operative location, tumor size, tumor location, and differentiation, the HR (1.40, 95% CI 1.02, 1.92) remained statistically significant (P = .036). Table 5 shows the adjusted HRs stratified by tumor size. The association was only strong and statistically significant for patients with tumor sized 2 to <3 cm (HR 2.79, 95% CI 1.45, 5.37).
Table 3.
Univariate analysis of postoperative survival in the 317 patients with stage I NSCLC according to baseline characteristics.
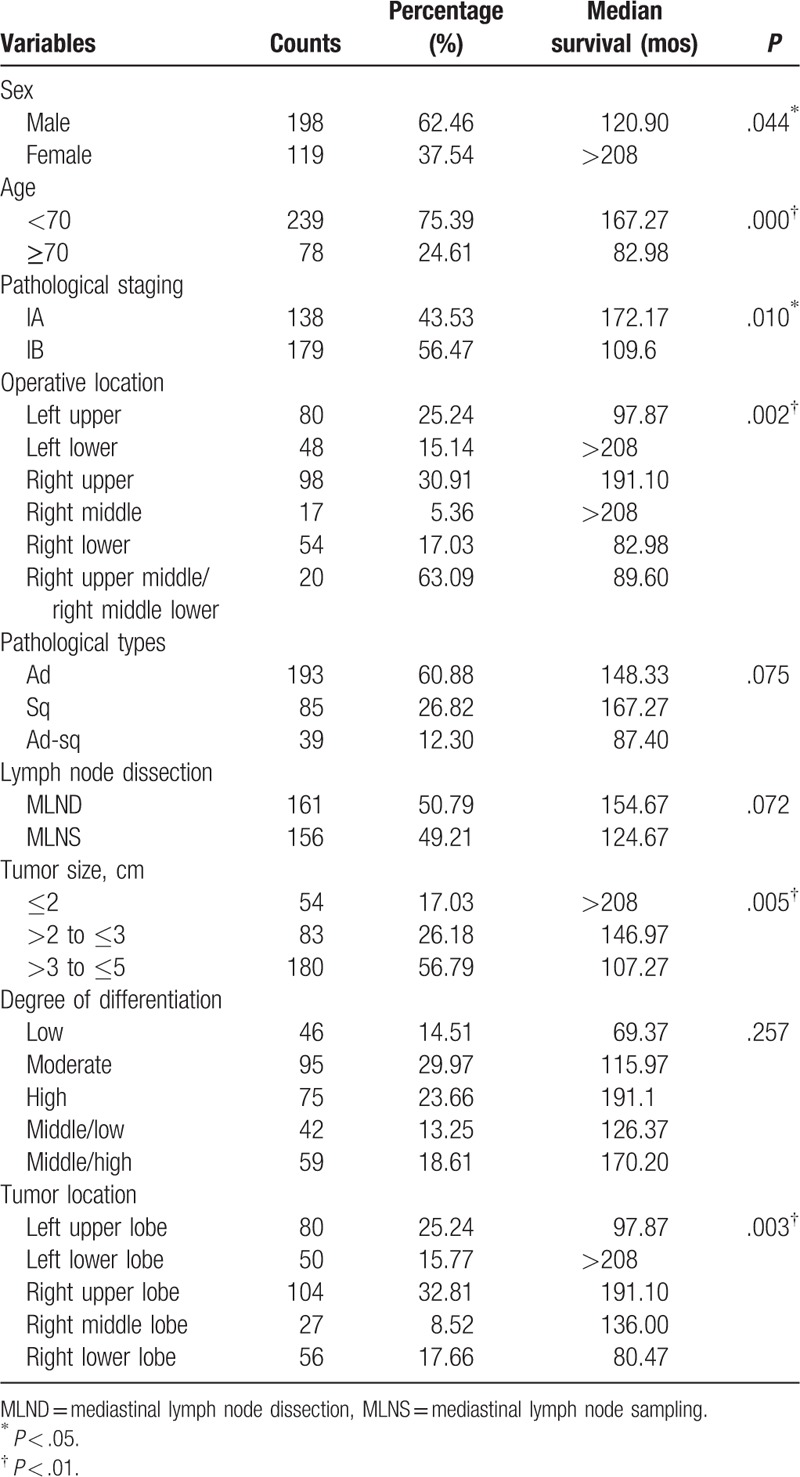
Table 4.
Association between lymph node dissection and survival in 317 stage I NSCLC patients.
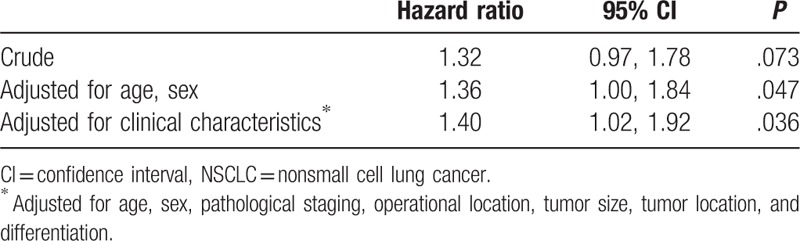
Table 5.
Association between lymph node dissection and survival stratified by tumor size.
4. Discussion
In this retrospective study of 317 NSCLC patients, we found that the MLND was associated with longer survival time and lower mortality compared with the MLNS group. A number of baseline clinical characteristics were also found to be relevant with patients’ survival rates. After adjustment for those baseline characteristics, the association between different lymph node dissection modes and survival was statistically significant. The focus of the recent debate is whether the MLND is associated with improved survival rates of early-stage NSCLC. With our long-term follow-up data from China, our findings support the use of compete lymph node dissection for early-stage NSCLC patients. These findings, if further confirmed in more prospective studies, would have important implications in the treatment of Chinese patients with early-stage NSCLC.
With recent improvement in diagnostic techniques such as radiologic imaging, more patients are being detected in early stage of NSCLC in China. It has been considered that tumor cells would rarely metastasize through the pathway of lymph drainage to the regional lymph nodes[16,17] at this stage. Therefore, is it really necessary to execute complete mediastinal lymph node dissection for stage I NSCLC patients? In our study, we found that MLND was associated with higher survival time and lower mortality in stage I NSCLC Chinese patients. In another study of 526 patients with 268 in the MLND group and 264 in the MLNS, Wu et al[4] found that the MLND group had a significantly higher survival rate than the MLNS group. These findings support assumption that NSCLC patients who have undergone MLND would have better prognosis. However, some studies reported contradictory results. Okada et al[6] divided patients with stage I NSCLC into 2 groups: selective mediastinal dissection (n = 377) and systematic lymph node dissection groups (n = 358). They found there was no significant difference in disease-free survival or overall survival, and the rates of distant metastasis and local recurrence were also similar in these 2 groups. They concluded that selective mediastinal dissection and systematic lymph node dissection groups had similar clinical outcomes. A systematic review and meta-analysis by Huang et al[18] also revealed that results for overall survival, local recurrence rates, and distant metastasis rates were similar between MLND and MLNS groups in early-stage NSCLC patients. In another meta-analysis, Wright et al[19] suggested that MLND is associated with improved survival compared with MLNS group in patients with stage I to IIIA NSCLC undergoing resection. In a randomized trial, Darling et al[8] reported that the MLND did not improve survival compared with the MLNS. However, in their study, the MLND group had a higher survival rate that the MLNS group, although the difference was not statistically significant. Although we do not know the reasons for the inconsistent findings among different studies, 1 possible explanation is the differences in the clinical characteristics such as tumor size.
Generally, the MLND is considered to have the benefit of identifying the metastatic state of lymph nodes for accurate pathological staging. Several studies reported the impact of systematic lymph node dissection on the accuracy of staging, and these findings, nevertheless, are still inconclusive due to the lack of controls or a limited number of controls.[12,20] Furthermore, some researchers had raised the question that the complete mediastinal lymph node dissection did not have significant advantage in staging, and reported that the 2 dissection groups had similar proportions of N1 and N2.[21,22] We could not assess this aspect since our study only included patients with N0.
In this study, we found that tumor size was a key factor associated with survival, which is consistent with some other studies.[9,23,24] Okada et al[23] found that tumor size was associated with the prognosis in patients receiving complete resection of NSCLC. Christian et al[24] reported that the risk of death would increase 58% and 118% once the tumor size reached 2 and 5 cm thresholds, respectively. Our study further demonstrated that the association between dissection mode and mortality depended on tumor size, and there appeared to be a stronger association in those with tumor diameter 2 to <3 cm. Ma et al[12] also reported an obvious association in those with tumor diameter 2 to <3 cm, but there was no association in those with tumor size <2 cm. These findings, if confirmed, suggest that the dissection modes should be determined according to tumor size. Those with tumor diameter 2 to 3 cm would benefit most from the MLND.
Most of the previous studies in China evaluated the impact of lymph node dissection on the survival of patients with NSCLC by comparing 3 or 5-year survival outcomes. The strength of this study was that we followed the participants for 10 years or longer for assessing the impact of nodal dissection on long-term survival. The major limitation of this study was the relatively small sample size. Although we found that systematic dissection might be associated with better survival and lower mortality, the accuracy of this association needed to be further assessed. As shown in Table 4, after adjustment for age and sex, the association (HR 1.36, 95% CI 1.00, 1.84) was statistically significant (P = .047). With further adjusted for age, sex, pathological staging, operative location, tumor size, tumor location, and differentiation; the hazard ratio (1.40, 95% CI 1.02, 1.92) remained statistically significant (P = .036). Our study suggests that the MLNS group potentially has 2% to 92% higher mortality risk than the MLND group, and prospective research with larges samples would further indicate the impacts of dissection modes on mortality.
5. Conclusions
For stage I NSCLC patients, complete mediastinal lymph node dissection is associated with better survival and lower mortality compared with the mediastinal lymph node sampling. The effects of complete mediastinal lymph lode dissection on survival may depend on tumor size. Our findings have important implications in the treatment of early-stage NSCLC, and further prospective studies with large samples to confirm our findings are warranted.
Acknowledgments
We thank all patients for their participation, staff members in the Department of Anaesthesia, Department of Radiology, and Department of Radiotherapy, the Shanghai Chest Hospital for their cooperation and assistance in data collection.
Footnotes
Abbreviations: CI = confidence interval, ESTS = European Society of Thoracic Surgeons, MLND = mediastinal lymph node dissection, MLNS = mediastinal lymph node sampling, NSCLC = nonsmall cell lung cancer.
YS-T and FM contributed equally to this work.
Funding: Funding for this study was provided by a project grant from the Science and Technology Commission of Shanghai Municipality (15DZ1930702) and the Shanghai Pharmaceutical Association (2014-YY-01).
Conflicts of interest: ZW was supported by a Research fellowship from the National Health and Medical Research Council (NHMRC) of Australia (APP1042343).
References
- [1].Reif MS, Socinski MA, Rivera MP. Evidence-based medicine in the treatment of non-small-cell lung cancer. Clin Chest Med 2000;21:107–20. ix. [DOI] [PubMed] [Google Scholar]
- [2].Scott WJ, Howington J, Movsas B. American College of Chest P. Treatment of stage II non-small cell lung cancer. Chest 2003;123(1 suppl):188S–201S. [DOI] [PubMed] [Google Scholar]
- [3].Smythe WR. American College of Chest Physicians. Treatment of stage I non-small cell lung carcinoma. Chest 2003;123(1 suppl):181S–7S. [PubMed] [Google Scholar]
- [4].Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1–6. [DOI] [PubMed] [Google Scholar]
- [5].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [6].Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028–32. [DOI] [PubMed] [Google Scholar]
- [7].Adachi H, Sakamaki K, Nishii T, et al. Lobe-specific lymph node dissection as a standard procedure in surgery for non-small cell lung cancer: a propensity score matching study. J Thorac Oncol 2017;12:85–93. [DOI] [PubMed] [Google Scholar]
- [8].Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].David EA, Cooke DT, Chen Y, et al. Does lymph node count influence survival in surgically resected non-small cell lung cancer? Ann Thorac Surg 2017;103:226–35. [DOI] [PubMed] [Google Scholar]
- [10].Chen W, Zheng R, Zeng H, et al. Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luo X, Zheng S, Liu Q, et al. Should nonsmokers be excluded from early lung cancer screening with low-dose spiral computed tomography? Community-based practice in Shanghai. Transl Oncol 2017;10:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma K, Chang D, He B, et al. Radical systematic mediastinal lymphadenectomy versus mediastinal lymph node sampling in patients with clinical stage IA and pathological stage T1 non-small cell lung cancer. J Cancer Res Clin Oncol 2008;134:1289–95. [DOI] [PubMed] [Google Scholar]
- [13].Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787–92. [DOI] [PubMed] [Google Scholar]
- [14].Rami-Porta R, Wittekind C, Goldstraw P. International Association for the Study of Lung Cancer (IASLC) Staging Committee. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- [15].Milroy R. Staging of lung cancer. Chest 2008;133:593–5. [DOI] [PubMed] [Google Scholar]
- [16].Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005;50:227–34. [DOI] [PubMed] [Google Scholar]
- [17].Kim AW. Lymph node drainage patterns and micrometastasis in lung cancer. Semin Thorac Cardiovasc Surg 2009;21:298–308. [DOI] [PubMed] [Google Scholar]
- [18].Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e109979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290–4. discussion 294–5. [DOI] [PubMed] [Google Scholar]
- [21].Izbicki JR, Passlick B, Karg O, et al. Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg 1995;59:209–14. [DOI] [PubMed] [Google Scholar]
- [22].Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358–65. [discussion 365–356]. [DOI] [PubMed] [Google Scholar]
- [23].Okada M, Nishio W, Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg 2004;77:1926–30. [discussion 1931]. [DOI] [PubMed] [Google Scholar]
- [24].Christian C, Erica S, Morandi U. The prognostic impact of tumor size in resected stage I non-small cell lung cancer: evidence for a two thresholds tumor diameters classification. Lung Cancer 2006;54:185–91. [DOI] [PubMed] [Google Scholar]


