Supplemental Digital Content is available in the text
Keywords: epithelial ovarian cancer, interleukin, T-helper cells, tumor necrosis factor
Abstract
This study is conducted to investigate the involvement of T-helper (Th) cells and regulatory T cells in epithelial ovarian cancer (EOC).
The percentages of Th22, Th17, Th1, and regulatory T cells in the peripheral blood of EOC patients, benign ovarian epithelial neoplasm (BOEN) patients, and healthy control (HC) were examined by flow cytometry. Enzyme-linked immunosorbent assay was used to determine serum levels of interleukin (IL)-22, IL-17, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α).
Th22 and Th17 were significantly increased in EOC patients. The plasma concentrations of IL-22 and TNF-α were significantly elevated in EOC patients compared with BOEN patients and HC. In EOC patients, there was an increased trend of Th22, IL-22, and TNF-α in stage III–IV patients compared with stage I–II patients. A positive correlation was seen among Th22, Th17, and Th1 cells in EOC patients. Similarly, positive correlations were detected between Th22 cells and IL-22 or TNF-α and between Th1 cells and interferon-γ (IFN-γ) in EOC patients. Besides, no significant difference was found in Th1 cells and regulatory T cells among EOC and BOEN patients and HC.
There is a higher circulating frequency of Th22, Th17 cells, IL-22, and TNF-α concentration in EOC patients, which may conjointly participate in the pathogenesis and growth of EOC.
1. Introduction
Ovarian cancers are the common malignancy among women and there are almost 204,000 new cases and 125,000 deaths per year worldwide.[1] The most common ovarian cancer (about 90%–95%) is epithelial ovarian cancer (EOC), which arises from the ovarian epithelium.[2] Almost 70% of patients are diagnosed at an advanced stage of tumor dissemination because of the lack of sensitive and specific biomarkers and the fact that the disease tends to develop and spread rapidly. The control of EOC at advanced-stage remains difficult.[3] Therefore, superior prognostic markers and novel therapeutic strategies are needed to improve the prognosis of EOC patients. EOC is naturally immune reactive, which is regulated by various immune cells.[4] Immunologic reaction of EOC plays a significant role on disease control, and immunotherapy has emerged as a novel treatment method for EOC.[5]
CD4 + T cells has an essential role in tumor immunology.[6] Recently, it is demonstrated that T-helper (Th) cells (such as Th1, Th2, and Th17) and regulatory T (Treg) cells participate in the pathogenesis and progression of different solid tumors with opposite antitumor or protumor effects.[7–11] Th22 cell is a newly discovered T cell subset and is identified by the production of interleukin (IL) -22, but not interferon-γ (IFN-γ) and IL-17.[12–14] The naive CD4 + cells can differentiate into Th22 subset in the presence of IL-6 and tumor necrosis factor-α (TNF-α) with the aid of plasmacytoid dendritic cells and aryl hydrocarbon receptor in human body.[12,14,15] The function of Th22 cells are mostly mediated by the cytokines of IL-22 and TNF-α.[16]
IL-22 was first described as IL-10-related-T-cell-derived inducible factor (IL-TIF).[17] IL-22 belongs to IL-10 family, which is a lineage-specific effector cytokine of Th22 cells. IL- 22 maintains its function via a transmembrane receptor complex composed of IL-22R1 chain and IL-10R2 chain, and plays a dual role in inflammatory and autoimmune diseases.[18,19] Notably, prosurvival and proliferative effects of IL-22 are confirmed by many cancers, such as lung and liver cancers.[19–21] TNF-α is another main cytokine secreted by Th22 cells, which has been proved to be overproduced in several malignancies.[22–24] Moreover, its tumor-promoting effect has been clearly proved in mouse cancer models.[24,25] However, the role of Th22 cells is not clear in human cancer. Recently, it has been demonstrated that Th22 cells participate in various tumors, including colon cancer, gastric cancer, hepatocellular carcinoma, and small- and large-cell lung cancer.[20,26–28] These studies indicate the crucial role of Th22 cells in tumorigenesis.
To the best of our knowledge, the roles of Th22 cells, IL-22 and their association with Th17 or Th1 in EOC have not been reported. In this study, we investigated the prevalence of peripheral Th22, Th17, Th1, and Treg along with plasma concentrations of IL-22, IL-17, IFN-γ, and TNF-α in EOC, benign ovarian epithelial neoplasm (BOEN) patients and healthy control (HC). The relationship among them and their associations with tumor stage were also evaluated.
2. Materials and methods
2.1. Patients and controls
A total of 34 untreated EOC patients diagnosed pathologically with a mean age of 55.6 ± 10.1 years (range: 32–79 ys) were enrolled in this study. Inclusion criteria was that all patients were diagnosed after pathologic examination. The tumor stage (stage I, II, III, or IV) was classified according to the International Federation of Gynecology and Obstetrics (FIGO) classification and protocol.[29] Tumor grade (G1, well-differentiated tumor; G2, moderate-differentiated tumor; G3, poor-differentiated tumor), histological type were assessed and classified according to FIGO and World Health Organization classifications.[29] Exclusion criteria were as follows: Patients with simultaneous active or chronic infection, autoimmune disease, diabetes, a history of other malignant tumors, or connective tissue diseases. The patients had received anticancer treatment before the study. The characteristics of the EOC patients are given in Table 1. Totally 15 BOEN patients and 15 healthy women were enrolled as control with a mean age of 54.1 ± 10.2 years and 53.7 ± 10.6 years (range: 36–73 and 33–71 ys), respectively. The peripheral blood (PB) samples from all the enrolled participants were obtained under an Institutional Review Boards-approved protocol after they admitted to hospital. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Shandong cancer Hospital, Shandong University.
Table 1.
Clinicopathological characteristics of the EOC patients in the study.
2.2. Flow cytometric analysis
Briefly, 200 μL heparinized PB with an equal volume of Roswell Park Memorial Institute 1640 medium (Sigma Chemical, St Louis, MO) was incubated for 4 hours at 37°C in the presence of 5% CO2, 25 ng/mL of phorbolmyristate acetate, 1 μg/mL of ionomycin and 1.7 μg/mL of Monensin (Alexis Biochemicals, San Diego, CA). After incubation, the cells were stained with anti-CD4-PE-Cy5 monoclonal antibodies at room temperature in the dark for 15 minutes. After fixation and permeabilization, the cells were stained with anti-IL17A-PE, anti-IL22-APC, and anti-IFN-γ-FITC monoclonal antibody at room temperature in the dark for 18 minutes. Isotype controls were used. Stained cells were analyzed by flow cytometric analysis using a FACS cytometer equipped with Cell Quest software (BD Bioscience Pharmingen, Franklin Lakes, NJ). Th22, Th17 and Th1 cells were characterized as CD4+ IFNγ− IL17− IL22+, CD4+ IL17+ IFNγ−, and CD4+ IFNγ+ cells, respectively. All antibodies mentioned above were purchased from eBioscience (San Diego, CA).
Human Regulatory T-cell Staining Kit (eBioscience, San Diego, CA) was used to evaluate CD4+ CD25+ Foxp3+ T cells (Treg cells) based on the manufacturer's protocol. Briefly, after centrifugation with Ficoll-Hypaque gradients, peripheral blood mononuclear cells were isolated. Single-cell suspension was incubated with anti-CD4-FITC monoclonal antibody and anti-CD25-APC monoclonal antibody for 30 minutes in the dark at 4°C. Then, the peripheral blood mononuclear cells were washed with 2 mL cold staining buffer and incubated with 1 mL freshly prepared Foxp3 fixation/permeabilization buffer (eBioscience, San Diego, CA) for 60 minutes at 4°C in the dark. After washing, the cells were blocked with normal rat serum for 15 minutes. Then, the cells were stained for 45 minutes in the dark at 4°C using anti-Foxp3-PE monoclonal anti-body (PCH101) or PE-conjugated rat IgG2a (used as isotype control). Flow cytometric analysis analyzed stained cells by a FACScan cytometer equipped with Cell Quest software (BD Bioscience, Franklin Lakes, NJ).
2.3. Enzyme-linked immunosorbent assay (ELISA)
Plasma was isolated from PB by centrifugation. The plasma levels of IL-22, IL-17, IFN-γ, and TNF-α were determined with a quantitative sandwich enzyme immunoassay technique according to the manufacturer's instructions (eBioscience, San Diego, CA). HRP-labeled goat anti-rabbit IgG (1:2000) were used as the secondary antibody. The absorbance value at 450 nm wavelength was read with a microplate reader (Multiskan MK-3, Finland).
2.4. Statistical analysis
Normality of our data was examined with Kolmogorov-Smirnov test. Data with normal distribution were expressed as mean ± standard deviation. Data with non-normal distribution were presented as median (range). One way ANOVA and Newman-Kuels multiple comparison tests were used for assessing the normal distribution data. Kruskal-Wallis and Nemenyi tests were used for the non-normal distribution data. In correlation analysis, the Pearson correlation test was used for normal distribution data, and the Spearman correlation test was used for the non-normal distribution data. A P < .05 was considered statistically significant. All tests were performed using SPSS 17.0 software.
3. Result
3.1. Increased Th cells in PB of EOC patients
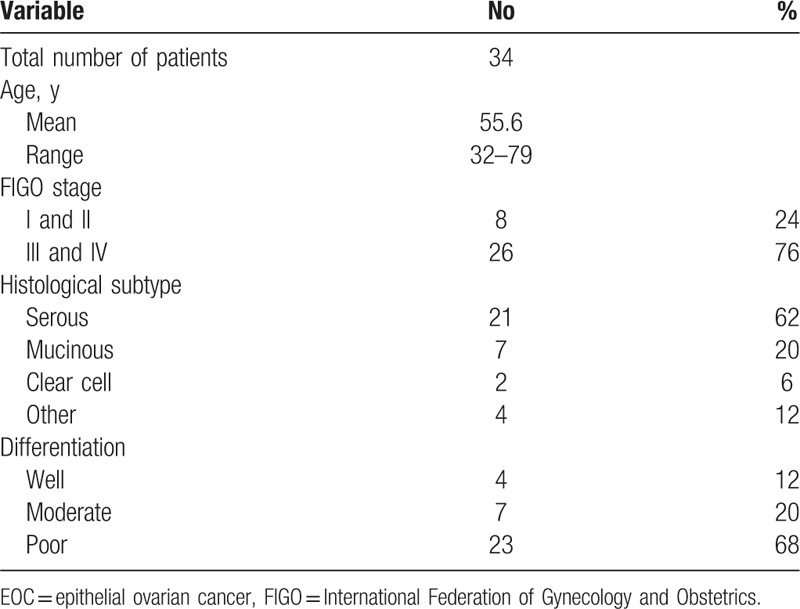
To determine the percentage of Th cells and Treg cells in PB, flow cytometry was performed. Representative flow cytometry results were shown in supplementary Figure S1. As shown in Figure 1A, significantly elevated frequencies of Th22 (CD4 + IFNγ− IL17− IL22+ T cells) were found in EOC (2.57 ± 0.88%) compared with BOEN patients (0.87 ± 0.21%, P < .001) and HC (0.82 ± 0.12%, P < .001). However, no significant difference was found in Th22 cells between BOEN patients and HC (P = .838). However, no significant difference was found in Treg (CD4+ CD25+ Foxp3+ T cells) cells and Th1 cells (CD4+ IFN-γ+ T cells) among EOC patients, BOEN patients, and HC (Fig. 1B and C). But, the percentage of Th1 in EOC patients (27.04 ± 12.21%) was higher as compared with that in BOEN patients (20.14 ± 1.49%) and HC (20.36 ± 1.45%). The percentage of Th17 (CD4+ IL17+ IFN-γ− T cells) of EOC patients (Th17: 3.15 ± 1.35%) significantly increased compared with BOEN patients (Th17: 1.06 ± 0.26%, P < .001) and HC (Th17: 1.04 ± 0.17%, P < .001). However, no significant difference was found between BOEN patients and HC (Th17: P = .961) (Fig. 1D). These results demonstrate that frequencies of Th22 and Th17 cells in EOC patients are higher than those in BOEN patients and HC.
Figure 1.
The percentages of circulating Th1 cells, Th17 cells, Th22 cells and Treg cells in EOC, BOEN patients and healthy controls. Flow cytometry was performed and quantitative results were shown. One way ANOVA and Newman-Kuels multiple comparison tests were used for assessing the normal distribution data. Kruskal-Wallis and Nemenyi tests were used for unusual data. A, Th22 (CD4 + IFNγ – IL17 – IL-22 +) cells. B, Treg (CD4 + CD25 + Foxp3 +) cells. C, Th1 (CD4 + IFNγ +) cells. D, Th17 (CD4 + IL17 + IFNγ − ) cells. ∗P < .05; n.s. = no significant difference.
3.2. Elevated IL-22 and TNF-α level in plasma of EOC patients
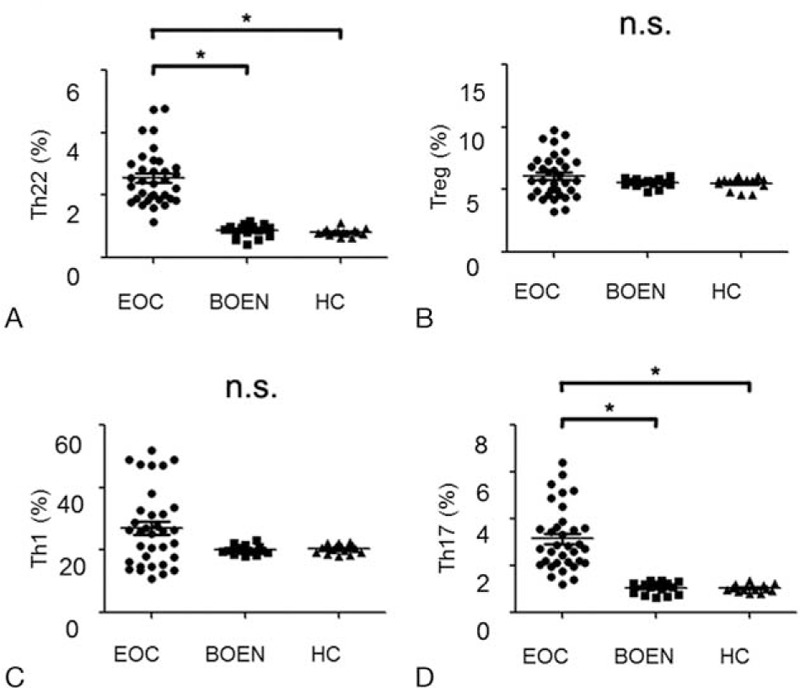
IL-22, IL-17, IFN-γ, and TNF-α in sera of EOC patients were detected with ELISA. Significantly higher levels of IL-22 were found in EOC patients (34.19 ± 4.58pg/mL) than those in BOEN patients (23.1 ± 1.78 pg/mL, P < .001) and HC (22.1 ± 1.71 pg/mL, P < .001) (Fig. 2A). Remarkable differences of TNF-α were revealed in EOC patients (median, 114; range, 5.50 – 595pg/mL,) than those in BOEN patients (median, 7.15; range, 4.31 –9.31pg/mL, P < 0.001) and HC (median, 6.32; range, 3.76 –9.31pg/mL, P < .001) (Fig. 2B). However, there was no significant difference in the IL-17 and IFN-γ levels among EOC, BOEN patients and HC (P > .05) (Fig. 2C and D). These results indicate that the levels of IL-22 and TNF-α are significantly higher in peripheral blood of EOC patients than those in BOEN patients and HC.
Figure 2.
Concentration of IL-22, IL-17, IFN-γ, and TNF-α in plasma from EOC, BOEN patients, and healthy controls. One way ANOVA and Newman-Kuels multiple comparison tests were used for assessing the normal distribution data. Kruskal-Wallis and Nemenyi tests were used for unusual data. ELISA was used. A, IL-22; (B) TNF-α; (C) IFN-γ; (D) IL-17. ∗P < .05; n.s. = no significant difference.
3.3. Correlation analysis among Th22, Th17, Treg, and Th1 Cells in EOC patients
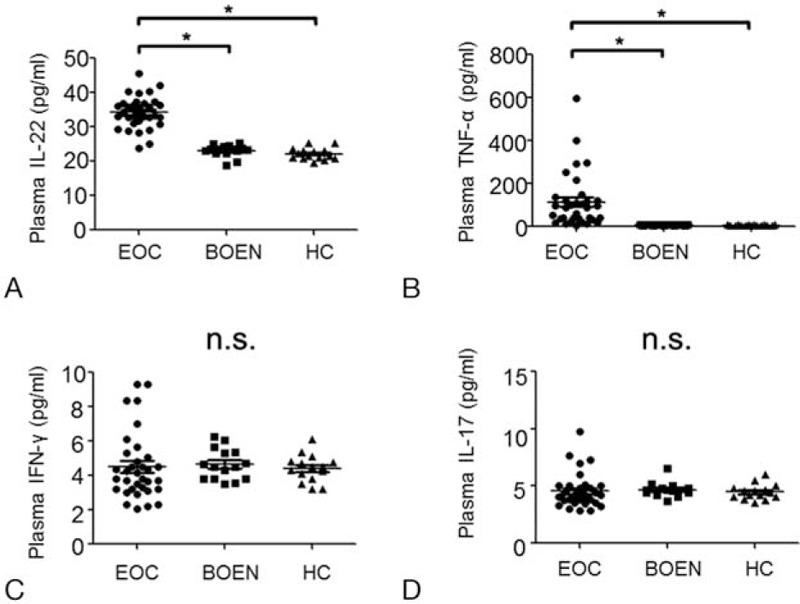
The correlations among Th22, Th17, Treg, and Th1 Cells in EOC patients were analyzed. A positive correlation was seen between Th22 cells and Th17 cells in EOC patients (r = 0.534, P = .001) (Fig. 3A). Similarly, a positive correlation was detected between Th22 and Th1 cells (r = 0.464, P = .006) or Th17 and Th1 cells (r = 0.541, P = .001) (Fig. 3B and C). However, Treg cells did not show significant correlation with Th22 (P = .760), Th17 (P = .966), and Th1 (P = .237) cells in patients with EOC. These results show that positive correlations are found between Th22 cells and Th17 cells, Th22 and Th1 cells, and Th17 and Th1 cells in EOC patients.
Figure 3.
Correlation among Th22 cells, Th17 cells, and Th1 cells in EOC patients. The Pearson or Spearman correlation test was used for correlation analysis depending on data distribution. A, Correlation between the percentages of Th22 cells and Th17 cells. B, Correlation between the percentages of Th22 cells and Th1 cells. C, Correlation between the percentages of Th17 cells and Th1 cells.
3.4. Correlation analysis among Th cells and their cytokines in EOC patients
Then, we further analyzed the correlations of Th1 cells and Th22 cells with their cytokines. Results showed that a positive correlation was found between Th1 cells and IFN-γ (r = 0.418, P = .014) (Fig. 4A). Similarly, Th22 cells were positively correlated with IL-22 (r = 0.585, P < .001) and TNF-α concentration (r = 0.495, P = .009) (Fig. 4B and C). These results demonstrate that Th22 cells have positive correlation with IL-22 or TNF-α and Th1 cells have positive correlation with IFN-γ.
Figure 4.
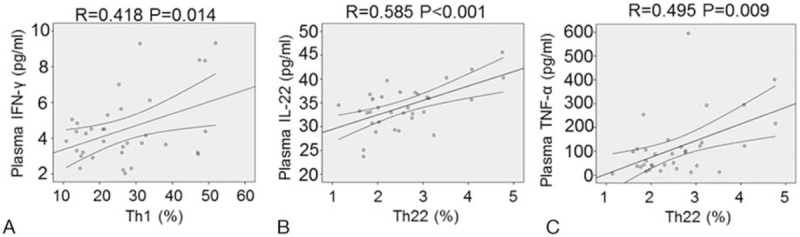
Correlation between the percentages of Th22 cells and the plasma IL-22 or TNF-α concentrations, and between the percentages of Th1 cells and the plasma IFN-γ concentrations in EOC patients. The Pearson or Spearman correlation test was used for correlation analysis depending on data distribution. A, Correlation between Th1 cells and plasma IFN-γ. B, Correlation between Th22 cells and plasma IL-22. C, Correlation between Th22 cells and plasma TNF-α.
3.5. Th22 cells and their related cytokines in different tumor stages of EOC patients
EOC patients in stage III–IV exhibited significantly higher circulating Th22 than those in stage I–II (stage I–II, 1.91 ± 0.23%; stage III–IV, 2.77 ± 0.91%, P = .012) (Fig. 5A). Furthermore, there was an increased trend of IL-22 and TNF-α in stage III–IV patients (IL-22: 35.6 ± 3.98pg/mL; TNF-α: median, 133.2, range 5.50 – 595pg/mL) compared with patients in stage I–II (IL-22: 29.8 ± 3.61 pg/mL, P = .002; TNF-α: median, 51.6, range 21.6 – 109.5pg/mL, P = .01) (Fig. 5B and C). However, no significant difference was found in Th17 cells, Th1 cells, IL-17, and IFN-γ between patients in stage I–II and stage III–IV (data not shown). Besides, no significant difference was found in Th22, Th17, Th1, IL-22, and TNF-α among 3 types of differentiation in EOC patients (data not shown). These results show that the levels of Th22, IL-22, and TNF-α are significantly higher in stage III–IV patients than those in stage I–II patients.
Figure 5.
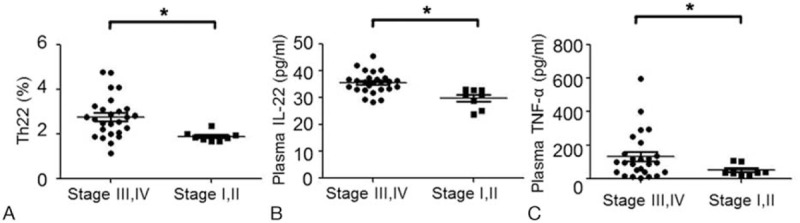
The percentages of circulating Th22 cells and concentration of plasma IL-22 and TNF-α in stage III–IV and stage I–II of EOC patients. Comparisons between 2 groups were assessed by the nonpaired t test or the Wilcoxon rank-sum test depending on data distribution. A, The percentages of circulating Th22 cells in stage III–IV and stage I–II of EOC patients. B, Concentration of IL-22 in stage III–IV and stage I–II of EOC patients. C, Concentration of TNF-α in stage III–IV and stage I–II of EOC patients. ∗P < .05.
4. Discussion
Ovarian cancers are the second (after endometrial cancer) most common of gynecologic cancers and one of the leading causes of cancer deaths among women.[30] Its malignancy and its etiology is still poorly understood. It is reported that several CD4+ T cells are involved in human cancers.[9–11,31] In our previous studies, elevated Th cells subsets (Th22, Th17, and Th1) and their related cytokines were found in autoimmune diseases, such as acute coronary syndromes and rheumatoid arthritis.[32,33] In our current study, we analyzed the percentage of Th22 cells and IL-22 level in the PB of patients with EOC for the first time, and evaluated different CD4+ Th-cell subsets (Th22, Th17, and Th1), Treg cells and their cytokines. We found that the frequencies of Th22, Th17, IL-22, and TNF-α were significantly increased in EOC patients than BOEN patients or healthy donors, suggesting that these T-cell subsets may be involved in T-cell-mediated immunity in patients with EOC.
The role of Th22 cells in different malignancy diseases is controversial. In acute myelocytic leukemia, Liu et al. showed that Th22 cells level was significantly decreased in the newly diagnosed patients and complete remission patients compared to healthy donors.[34] On the contrary, Yu et al[35] demonstrated that the frequencies of circulating Th22 cells were significantly increased in acute myelocytic leukemia patients. Moreover, Liu et al[36] found that Th22 cells in the PB were significantly elevated in gastric cancer patients and showed positive correlation between Th22 cells and tumor progression or patient survival.[28] Recently, Zhang et al[10] showed that high levels of Th22 was detected in cervical cancer and associated with lymph node metastases. In our study, we found elevated Th22 cells in the PB of EOC patients compared with BOEN and healthy donors, which suggests a potential role of Th22 cells in patients with EOC. However, several researches about Th22 cells in process of inflammation and autoimmunity indicate that Th22 cells may play a dual role depending on local microenvironment.[16] Therefore, we deduce that Th22 cells may accumulate in the local tumor tissues and participate in the development of the tumor. Further studies are necessary to clarify the role of Th22 cells in the tumor microenvironment.
IL-22, a member of the IL-10 family of cytokines, is the most important functional cytokine of Th22 cells.[12,13] IL-22 receptor is a heterodimer composed of IL-10R2 and IL-22R1 subunits. IL-22R1 is present on the membrane of several epithelial and stromal cells in various tissues, IL-22 is a key component in immune-epithelial cell cross-talk.[19,37] Considerable evidence[19,38] suggests that IL-22 plays a crucial role in the development of cancers. Increased IL-22 levels are found in a lot of malignancies including lung, liver, gastric, colon, and pancreatic cancers.[39–42] In our study, elevated IL-22 levels were detected, in accordance with increasing percentage of Th22 cells. The major T cell subsets of producing IL-22 are Th22, Th17, and Th1 cells.[12,13] Duhen's study has shown that the proportion of Th22 cells ranged from 37% to 63% in all IL-22-producing T cells, and Th22 cells are the most important T cells subset secreting IL-22.[12] This might contribute to the positive correlation between serum IL-22 and the level of Th22 cells in our current study. Thus, increased Th22 cells along with its cytokine IL-22 may play a role in the etiology and progression of EOC. Another important cytokine secreted by Th22 cells is TNF-α, which plays a key role in modulating invasion, angiogenesis, and metastasis of cancer.[43] In the last decade, previous study has detected abnormally high levels of TNF-α in the blood of EOC patients.[44] In accordance with this, we found elevated TNF-α level in plasma from EOC patients. TNF-α can induce a characteristic Th22 signature and promote Th22 differentiation.[45] Therefore, we suppose that elevated TNF-α induce more Th22 cells, which further produce more IL-22 and TNF-α. These factors together may further promote the development of EOC.
Evidence has showed that the proportion of Th17 cells within CD4+ T cell subset is significantly higher in the tumor tissues obtained from patients with ovarian cancer.[46,47] However, the proportion of circulating Th17 cells in ovarian cancer is still debated.[46] In our study, we also examined the proportion of circulating Th17 cells. A high percentage of Th17 cells were found in the PB of patients with EOC than that in BOEN patients and control groups. The difference may stem from the different types of ovarian cancer. Therefore, the circulating Th17 cells seem to positively correlate with EOC. However, no significant difference was observed in IL-17 level among EOC, BOEN patients, and HC. Therefore, further studies are needed to explain the precise mechanism of Th17 and IL-17 in the pathology of EOC. Furthermore, no significant difference was found in Th1 cells and IFN-γ level, but increased frequencies of Th1 cells were demonstrated in patients with EOC. Th1 cells have a clear antitumor effect.[48] It is suggested that Th1 adaptive immunity is essential for cancer inhibition and better outcomes.[49,50,51] These may be because of the small sample size of this study. In our further studies, we will enlarge our sample size and investigate the function of Th1 cells and IFN-γ. In this study, we observed a positive correlation between Th22 and Th17 in patients with EOC. IL-23 was required for human Th17 differentiation,[52] and IL-23 can induce the differentiation of Th22 cells from naïve T cells.[53] These might account for the positive correlation between Th22 and Th17 cells. At the same time, positive correlations between Th1 cells and Th22 or Th17 cells were found in our study. This coelevated level of Th22, Th17 and Th1 cells suggests that these T cell subsets may play a synergistic role in patients with EOC.
Our study also focused on the clinical relevance of T cell subsets and their related cytokines in EOC patients. It was demonstrated that Th22 cells increased as tumor stage advanced. Moreover, we showed an elevating level of IL-22 and TNF-α in plasma in patients with EOC progression. Hence, these results suggest that Th22 cells and related cytokines might promote progress of tumor and affect patient prognosis. Further studies are necessary to clarify the correlation between level of Th22 and the 5-year survival rate of the EOC patients.
Treg cells (CD4+ CD25+ Foxp3+ T-cells) are a specific subset of lymphocytes that have immunosuppressive effect.[30] Treg cells help to suppress inappropriate immune responses that could lead to autoimmune disorders.[54] Evidence suggests that infiltration of ovarian cancers by Treg cells suppresses the tumor-specific T cell immune response and contributes to tumor growth in the microenvironment.[55] However, circulating Treg cells have rarely been reported in EOC patients. In our study, we evaluated circulating Treg cells. However, no significant difference was observed among EOC, BOEN patients, and HC. Meanwhile, there was no significant correlation between Treg cells and other T cells subsets. Although the frequency of Treg cells in EOC do not show significant change, the function of Treg cells may have changed. In addition, precise function of Treg cells in the tumor microenvironment of EOC needs further study.
In conclusion, patients with EOC exhibited a high frequency of circulating Th22 cells and Th17 cells, and significantly increased levels of IL-22 and TNF-α. Furthermore, the increases of Th22 cells and IL-22, TNF-α levels were positively correlated with tumor progression. Therefore, we speculate that Th22 cells and related cytokines may contribute to the pathology of EOC and may provide novel therapeutic strategies and targets.
Supplementary Material
Footnotes
Abbreviations: AML = acute myelocytic leukaemia, BOEN = benign ovarian epithelial neoplasm, ELISA = enzyme-linked immuno sorbent assay, EOC = epithelial ovarian cancer, FIGO = International Federation of Gynecology and Obstetrics, HC = healthy control, IFN-γ = interferon-γ, IL = interleukin, IL-TIF = IL-10-related-T-cell-derived inducible factor, PB = peripheral blood, Th = T-helper, TNF-α = tumor necrosis factor-α, Treg = regulatory T.
T.W. and Z.Z. contributed equally to this work.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol 2006;20:207–25. [DOI] [PubMed] [Google Scholar]
- [2].Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fialova A, Partlova S, Sojka L, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer 2013;132:1070–9. [DOI] [PubMed] [Google Scholar]
- [4].Knutson KL, Maurer MJ, Preston CC, et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol Immunother 2015;64:1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mariya T, Hirohashi Y, Torigoe T, et al. Prognostic impact of human leukocyte antigen class I expression and association of platinum resistance with immunologic profiles in epithelial ovarian cancer. Cancer Immunol Res 2014;2:1220–9. [DOI] [PubMed] [Google Scholar]
- [6].Velders MP, Markiewicz MA, Eiben GL, et al. CD4+ T cell matters in tumor immunity. Int Rev Immunol 2003;22:113–40. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Y, Ma D, Tian Y, et al. The imbalance of Th17/Treg in patients with uterine cervical cancer. Clin Chim Acta 2011;412:894–900. [DOI] [PubMed] [Google Scholar]
- [8].Gu-Trantien C, Loi S, Garaud S, et al. CD4 (+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ivanova EA, Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int 2015;2015:327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang W, Tian X, Mumtahana F, et al. The existence of Th22, pure Th17 and Th1 cells in CIN and cervical cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer 2015;15:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang W, Hou F, Zhang Y, et al. Changes of Th17/Tc17 and Th17/Treg cells in endometrial carcinoma. Gynecol Oncol 2014;132:599–605. [DOI] [PubMed] [Google Scholar]
- [12].Duhen T, Geiger R, Jarrossay D, et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 2009;10:857–63. [DOI] [PubMed] [Google Scholar]
- [13].Trifari S, Kaplan CD, Tran EH, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol V 10 2009;864–71. [DOI] [PubMed] [Google Scholar]
- [14].Jia L, Wu C. The biology and functions of Th22 cells. Adv Exp Med Biol 2014;841:209–30. [DOI] [PubMed] [Google Scholar]
- [15].Hou F, Li Z, Ma D, et al. Distribution of Th17 cells and Foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin Chim Acta 2012;413:1848–54. [DOI] [PubMed] [Google Scholar]
- [16].Tian T, Yu S, Ma D. Th22 and related cytokines in inflammatory and autoimmune diseases. Expert Opin Ther Targets 2013;17:113–25. [DOI] [PubMed] [Google Scholar]
- [17].Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 2000;164:1814–9. [DOI] [PubMed] [Google Scholar]
- [18].Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109. [DOI] [PubMed] [Google Scholar]
- [19].Perusina Lanfranca M, Lin Y, Fang J, et al. Biological and pathological activities of interleukin-22. J Mol Med (Berl) 2016;94:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011;54:900–9. [DOI] [PubMed] [Google Scholar]
- [21].Ye ZJ, Zhou Q, Yin W, et al. Interleukin 22-producing CD4+ T cells in malignant pleural effusion. Cancer Lett 2012;326:23–32. [DOI] [PubMed] [Google Scholar]
- [22].Fernandez Brana M, Acero N, Anorbe L, et al. Discovering a new analogue of thalidomide which may be used as a potent modulator of TNF-alpha production. Eur J Med Chem 2009;44:3533–42. [DOI] [PubMed] [Google Scholar]
- [23].Rabinovich A, Medina L, Piura B, et al. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res 2007;27:267–72. [PubMed] [Google Scholar]
- [24].Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9:361–71. [DOI] [PubMed] [Google Scholar]
- [25].Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. [DOI] [PubMed] [Google Scholar]
- [26].Jiang R, Wang H, Deng L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC cancer 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kobold S, Volk S, Clauditz T, et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J Thorac Oncol 2013;8:1032–42. [DOI] [PubMed] [Google Scholar]
- [28].Zhuang Y, Peng LS, Zhao YL, et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother 2012;61:1965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benedet JL, Bender H, Jones H, 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209–62. [PubMed] [Google Scholar]
- [30].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [31].Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol 2011;23:159–63. [DOI] [PubMed] [Google Scholar]
- [32].Zhang L, Wang T, Wang XQ, et al. Elevated frequencies of circulating Th22 cell in addition to Th17 cell and Th17/Th1 cell in patients with acute coronary syndrome. PloS one 2013;8:e71466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang T, Li S, Yang Y, et al. T helper 17 and T helper 1 cells are increased but regulatory T cells are decreased in subchondral bone marrow microenvironment of patients with rheumatoid arthritis. Am J Transl Res 2016;8:2956–68. [PMC free article] [PubMed] [Google Scholar]
- [34].Liu LM, Zhang XX, Zhang YM. The change of peripheral blood Th22 cells in patients with acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2012;33:133–5. [PubMed] [Google Scholar]
- [35].Yu S, Liu C, Zhang L, et al. Elevated Th22 cells correlated with Th17 cells in peripheral blood of patients with acute myeloid leukemia. Int J Mol Sci 2014;15:1927–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu T, Peng L, Yu P, et al. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol 2012;32:1332–9. [DOI] [PubMed] [Google Scholar]
- [37].Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 2014;13:21–38. [DOI] [PubMed] [Google Scholar]
- [38].Ji Y, Yang X, Li J, et al. IL-22 promotes the migration and invasion of gastric cancer cells via IL-22R1/AKT/MMP-9 signaling. Int J Clin Exp Pathol 2014;7:3694–703. [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu X, Li Z, Pan W, et al. Participation of Gab1 and Gab2 in IL-22-mediated keratinocyte proliferation, migration, and differentiation. Mol Cell Biochem 2012;369:255–66. [DOI] [PubMed] [Google Scholar]
- [40].Qin S, Ma S, Huang X, et al. Th22 cells are associated with hepatocellular carcinoma development and progression. Chin J Cancer Res 2014;26:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao D, Long XD, Lu TF, et al. Metformin decreases IL-22 secretion to suppress tumor growth in an orthotopic mouse model of hepatocellular carcinoma. Int J Cancer 2015;136:2556–65. [DOI] [PubMed] [Google Scholar]
- [42].Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015;15:8–18. [DOI] [PubMed] [Google Scholar]
- [43].Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci 2008;13:5094–107. [DOI] [PubMed] [Google Scholar]
- [44].Radke J, Schmidt D, Bohme M, et al. -Cytokine level in malignant ascites and peripheral blood of patients with advanced ovarian carcinoma-. Geburtshilfe und Frauenheilkunde 1996;56:83–7. [DOI] [PubMed] [Google Scholar]
- [45].Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miyahara Y, Odunsi K, Chen W, et al. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A 2008;105:15505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Candido EB, Silva LM, Carvalho AT, et al. Immune response evaluation through determination of type 1, type 2, and type 17 patterns in patients with epithelial ovarian cancer. Reprod Sci 2013;20:828–37. [DOI] [PubMed] [Google Scholar]
- [48].Nocera NF, Lee MC, De La Cruz LM, et al. Restoring lost anti-HER-2 Th1 immunity in breast cancer: a crucial role for Th1 cytokines in therapy and prevention. Front Pharmacol 2016;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- [50].Kusuda T, Shigemasa K, Arihiro K, et al. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncology Rep 2005;13:1153–8. [PubMed] [Google Scholar]
- [51].Hao CJ, Li J, Liu P, et al. Effects of the balance between type 1 and type 2 T helper cells on ovarian cancer. Genet Mol Res 2016;15: [DOI] [PubMed] [Google Scholar]
- [52].Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 2008;9:650–7. [DOI] [PubMed] [Google Scholar]
- [53].Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007;445:648–51. [DOI] [PubMed] [Google Scholar]
- [54].Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- [55].Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


