Abstract
Due to the lack of an accurate preoperative diagnostic method of central lymph node metastasis (CLNM) of papillary thyroid cancer (PTC), the prophylaxis of central lymph node dissection remains controversial. The present study investigated the clinicopathological features of PTC patients and the risk factors of CLNM. The clinicopathological features of PTC patients with respect to sex, age, initial symptoms, observation, tumor diameter, multifocality, extrathyroidal invasion, and pathological data combined with other thyroid diseases, were analyzed retrospectively. The risk factors of CLNM were analyzed by Chi-squared test and multivariate logistic regression model. The CLNM rate of PTC was 40.6% (1331/3273). On average, 7.0 (4.0, 12.0) central lymph nodes were dissected, and 3.70 (±3.8) lymph nodes were proved to be metastatic. Univariate analysis showed that sex (P < .001), age (P < .001), tumor diameter (P < .001), extrathyroid invasion (P < .001), multifocality (P = .001), concurrent nodular goiter (P < .001), initial symptoms (P < .001), and observation or not (P < .001) were related to CLNM. The observation time was neither related to CLNM (P = .469) nor extrathyroidal invasion (P = .137). Tumors localized in the lower part of the thyroid were the risk factors for CLNM (P < .001) while multifocality was unrelated (P = .68). The metastasis rate of bilateral multiple regions > unilateral multiple regions > single region (P = .003). Multivariate logistic regression analysis showed that sex, age, tumor diameter, extrathyroidal invasion, and observation were independent risk factors of CLNM. Male, younger age, large tumor size, and extrathyroidal invasion were independent risk factors for CLNM. CLNM was related to multiple regions occupied by tumors in the thyroid but unrelated to multifocality. The tumor occupying a single region and localized in the lower part of thyroid could be used as a predictive factor for CLNM. For tumors that could not be diagnosed as benign or malignant, observation may be an option, since no evidence of disease progression was presented during observation.
Keywords: central lymph node, central lymph node metastasis, thyroid papillary carcinoma
1. Introduction
During the past 3 decades, the incidence of thyroid cancer has continually increased.[1,2] In 2012, the new cases of thyroid cancer were approximately 2,98,000 worldwide, and the number of deaths was 40,000. The number of new cases of thyroid cancer in China accounted for 15.6% of that worldwide, and the number of deaths accounted for 13.8%.[2] In South Korea, the incidence of thyroid cancer was higher than any other cancers.[2] In Italy, thyroid cancer was the second common cancer in women ≤45-year-old.[3] In the USA, thyroid cancer has been the fifth most common cancer in women since 2010.[4] Papillary thyroid cancer (PTC) accounted for >90% of all the new thyroid cancers.[5] From 1973 to 2006, the incidence of PTC has increased 3.2-fold.[6] Despite the high incidence, the prognosis of PTC is commonly favorable, with the mortality rate of 5/1 million. The 5-year survival of PTC is >97%, and the 10-year survival is >90%. Only a few patients were deceased due to recurrence or distant metastasis.[7,8] Due to the indolent feature of most PTCs, the American Thyroid Association (ATA) guidelines (2015 edition) for surgical indications and central lymph node dissection (CLND) also showed a tendency of conservation.[9]
Lymph node metastasis (LNM) often occurs at an early stage of PTC, and initially appears in the central region.[10] LNM causes an advanced Tumor Node Metastasis (TNM) stage in PTC patients and affects the local recurrence rate,[11,12] which can be a major predictor of poor prognosis.[13] Therefore, therapeutic CLND for patients with CLNM has been chosen with a consensus. Some studies showed that the detection specificity of the lymph node by ultrasound (US) was 87% to 97% while the sensitivity was only 23% to 38%,[14–16] and it is challenging to assess the retropharyngeal and mediastinal deep central lymph nodes.[17] Due to the high false negative rate, surgeons could not accurately assess the metastatic conditions of cervical lymph nodes and rationally choose therapeutic CLND. CLND was known to be the risk factor of permanent complications for patients, and therefore, routine prophylactic CLND has not been accepted by all surgeons. How to avoid over and under treatment when choosing surgical procedures is yet controversial. Therapeutic CLND should be encouraged, and the prophylactic CLND should be reduced in order to decrease the surgical risk, and improve potential benefits. Therefore, finding sensitive predicting factors of CLNM is the focus of current research. The purpose of this study was to review and analyze the correlation between clinical features and CLNM in 3273 PTC patients, retrospectively, and discuss the predictive value of these features.
2. Methods
2.1. General information
2.1.1. Inclusion criteria
The patients who fulfilled the following criteria were included in the study. Patients who underwent initial thyroid surgery; patients who did not display any history of the head and neck surgery or radiation; patients who had no history of other malignant tumors; patients who revealed PTC in postoperative paraffin-based pathological diagnosis; other types of thyroid or metastatic carcinomas were absent in patients; patients who underwent ultrasound (US) examination of thyroid and cervical lymph nodes at the initial consultation, and their data and images were preserved; patients whose personal information, surgical records, incidence, diagnosis, and treatment process were recorded; patients who underwent surgical procedures including lobectomy, isthmus resection, or total/near-total thyroidectomy + CLND.
2.1.2. Data collection
The data with respect to the age and sex of patients, initial diagnosis, and observation time, were collected. The US reports and images were provided by the sonographer. The surgeons completed the diagnosis by re-reading the image. In the event of a disagreement, the US data were reviewed by experienced chief US physicians. In the case of continued inability to diagnose, the US-guided fine-needle aspiration biopsy (FNAB) was examined, or follow-up observations were recorded. A total of 168 patients underwent the FNA examination and were confirmed to be malignant. Tumor diameter, multifocality, extrathyroidal invasion, complicated with other thyroid diseases, and other data were assimilated by pathologists’ reports. The maximum size of the cancer nodules in multifocal and irregular carcinomas was measured. The tumor locations were recorded according to the positions of the thyroid tumors found in preoperative US examination and surgical records, classified as the upper part (upper 1/3), the middle part (middle 1/3), the lower part (lower 1/3), and the isthmus. The tumors occupying multiple regions were recorded independently. Single or multiple tumors located in 2 or more areas of unilateral glandular lobe were labeled as single focal multiple regions and multiple multifocal regions. Multiple tumor foci occupying only a single region were marked as the single focus. Surgery and repeated reading of the US images were performed by experienced thyroid surgeons whose annual surgical load was >100 operations.[18]
2.2. Baseline data
Three thousand six hundred four cases encompassing thyroid carcinoma patients underwent an initial operation in the Yunnan Province Thyroid Diagnosis and Treatment Center of the First Affiliated Hospital of Kunming Medical University from January 2007 to July 2016. These included: 3553 cases of PTC, accounting for 98.5% higher than that reported in previous literature, which may be related partially to the unoperated patients of advanced medullary carcinoma and all patients of undifferentiated carcinoma; 261 cases of patients with negative intraoperative frozen results, patients’ willingness, and other factors; 2 cases of elderly patients with short life expectancy and who did not receive CLND. Seven cases who received palliative operation and 10 cases with incomplete information were excluded. Thus, a total of 3273 patients were included in the study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (2016 ethical review No. 40).
2.3. Surgical program
Preoperative suspicious malignancies were treated by surgery, and the final operation was determined according to the frozen section examination results. The surgeon routinely informed patients and their families about the risk of complications and recurrence at different surgical ranges, and was inconsistent with both. All cases were 1 stage surgery, 2848 cases underwent total or near total thyroidectomy, and 425 patients with unilateral single focus carcinoma were operated on with ipsilateral lobe and isthmus resection. The range of CLND was according to the Revision of Cervical Lymph Nodes Division of American Society for Head and Neck Surgery (2002) and multidisciplinary consensus in the United States of America (2009).[18,19] The upper margin was subhyoid edge, the lower margin was suprasternal fossae, the posterior margin was vertebral anterior fascia, and the external edge was the interior of the carotid sheath, including all paratracheal, pretracheal, and prelaryngeal lymph nodes and adipose tissues. In this study, 2866 patients completed the surgery, and 407 cases only received CLND of ipsilateral lesions, including all ipsilateral, pretracheal, and prelaryngeal lymph nodes and adipose tissues. The inner edge was the nourishing vein of the lymph nodes above the contralateral thymus.
2.4. Statistical methods
SPSS20.0 statistical software package (IBM, NY, USA) was used for statistical analysis. Mean ± standard deviation described the normal distribution of the measurement data. The median described the skew distribution. Rate and composition ratio presented the count data. Chi-squared test was used to analyze the count data of the hypothesis test. Two independent samples t test was used for the measurement data of normal distribution. Two classification and multiclassification logistic regression analyses were used for multivariate analysis. P < .05 indicated that the difference was statistically significant.
3. Results
3.1. Clinical data and pathological features
The male/female ratio in 3273 patients was 1:4, and the average age was 43.4 (±11.39) years. Tumors were divided into 4 groups based on the diameter: ≤1 cm (T1a), 1 to 2 cm (T1b), 2 to 4 cm (T2), and 4 cm. Totally, 1097 cases (33.5%) were multifocal, including 871 cases of bilateral lesions and 226 of unilateral multifocal lesions. Two hundred seventy seven cases showed extrathyroidal invasions. One thousand thirty six cases were complicated with Hashimoto disease, and 1079 cases were complicated with nodular goiter (Table 1). The median of the dissection number of CLNs was 7.0 (4.0, 12.0). A total of 1331 cases presented CLNM, the metastasis rate was 40.6% (1331/3273), and the average number of metastatic lymph nodes was 3.70 (±3.84).
Table 1.
General information and pathological data of patients.
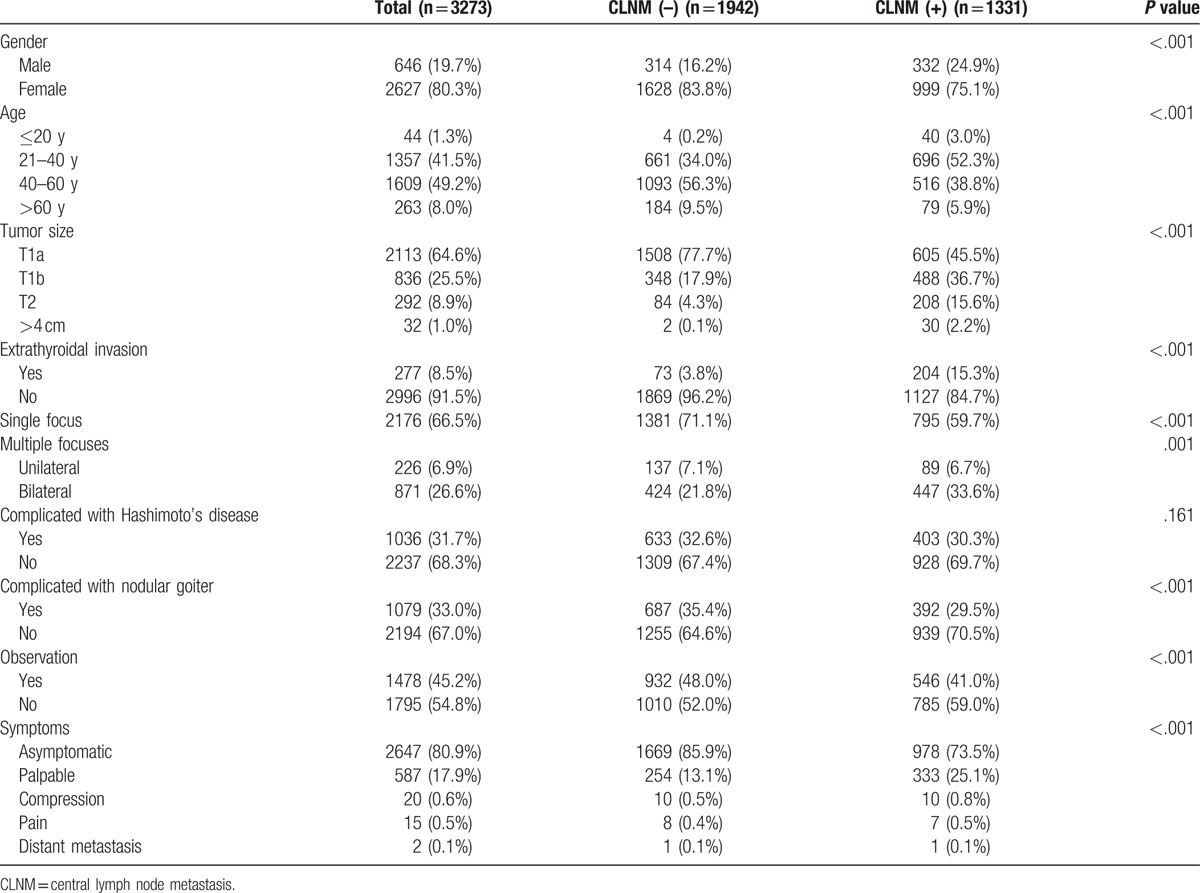
Two thousand six hundred forty seven cases were detected by US examination without clinical symptoms, 587 cases showed neck palpable mass or thickening, 20 cases presented compression symptoms (such as hoarseness, dyspnea, and dysphagia), 17 cases had pain, and 2 cases manifested distant metastasis. The average course ratio of the disease with or without clinical symptoms was 20.34:9.82 months and the average ratio of the diameter was 1.75:0.92 cm. One thousand seven hundred ninety five cases of the first diagnosis patients were suspected to be malignant and received direct surgical treatment. Patients initially diagnosed with benign nodules were closely observed and annually monitored by US examination. Patients who could not be diagnosed as benign or malignant would be under close observation and reviewed by US examination within a period of 3 to 6 months. As soon as malignancy was suspected by US or FNAB, surgical treatment was undertaken directly. A total of 1478 patients in the observation period showed malignancy and underwent an operation immediately. The median time of the observation group from finding the nodules to pathologically confirming the malignancy was 12 (7.0, 24.0) months. The average ratio of the tumor diameter of the direct surgery group and observation group was 1.09:1.06 cm. The metastasis rates of the 2 groups were 43.7% (785/1795) and 36.9% (546/1478), respectively. Two independent samples t test was used in 1478 observation patients, which revealed that the observation time was not related to CLNM (t = 0.724, P = .469) or extrathyroidal invasion (t = 1.499, P = .137), and the difference was not statistically significant.
3.2. Tumor locations
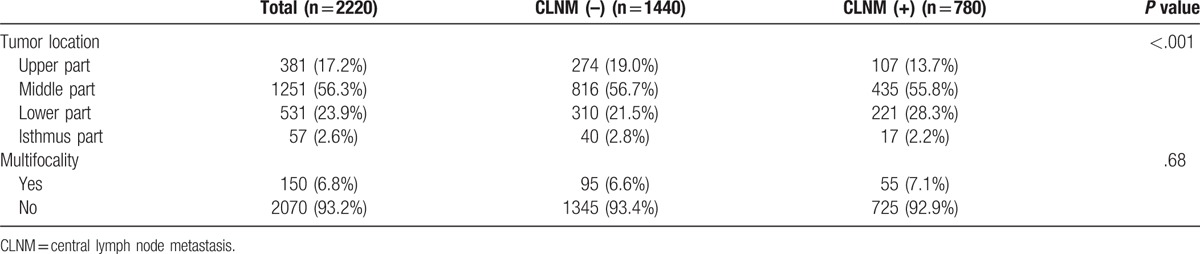
The thyroid tumors of PTC patients confined to a single region were divided into upper, middle, lower, and isthmus parts according to the different locations. However, tumors occupying multiple regions of the thyroid were excluded. 2220/3273 patients fulfilled the inclusion criteria. According to the tumor locations, the regional CLNM rates were as follows: lower part (41.6%) > middle part (34.8%) > isthmus part (29.8%) > upper part (28.1%), and the difference was statistically significant (P < .001). This cohort of patients was further analyzed based on the presence or absence of multifocality, and the difference was not statistically significant (P = .68) (Table 2).
Table 2.
The correlation between tumor location and CLNM.
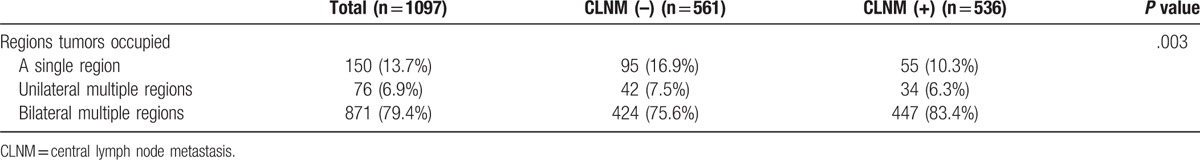
We hypothesized that the lymphatic drainage pathway of PTC lymph node metastasis was correlated to the tumor location in the thyroid. In the present study, a total of 1097 patients with multiple tumors were divided into 3 groups according to the different regions of the thyroid occupied by the tumor: a single region, multiple unilateral regions, and multiple bilateral regions. The study showed that the metastasis rate was 55/150 (36.7%) when multifocal carcinoma occupied a single region of the thyroid, 34/76 (44.7%) when the carcinoma occupied >2 regions of the unilateral thyroid, and the highest up to 447/871 (51.3%) when the carcinoma occupied multiple areas of the bilateral thyroid; the difference was statistically significant (P = .003) (Table 3).
Table 3.
The correlation between tumors occupying different regions of thyroid and CLNM.
3.3. Multivariate analysis of factors affecting the CLNM
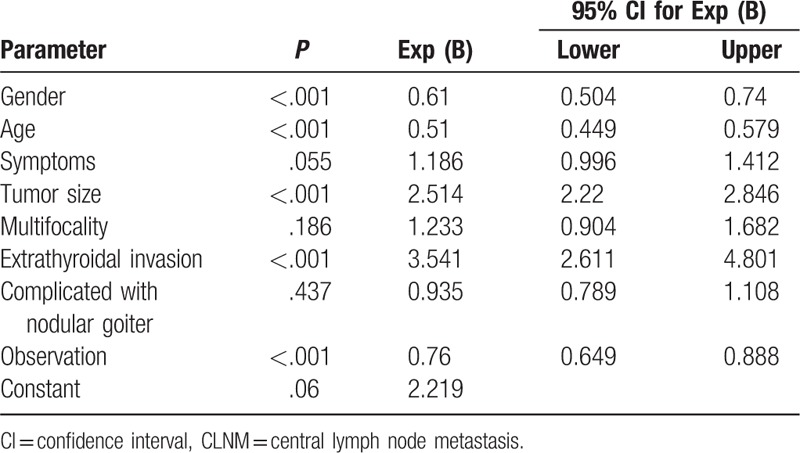
Sex, age, signs (with or without symptoms), tumor size, multifocality, extrathyroidal invasion, complications with nodular goiter, observation, and other factors were included in the logistic regression model analysis. The results were shown in Table 4. Sex, age, tumor size, and extrathyroidal invasion were found to be independent factors affecting CLNM.
Table 4.
Multivariate logistic regression analysis of CLNM.
4. Discussion
The primary metastasis route of PTC is through LNM. According to previous reports, the median metastasis rate was 20 to 50.[19–21] In this study, the rate of CLNM was 40.6%, which was consistent with the previous reports. Some studies suggested that although CLNM could increase the recurrence rate, it did not affect the disease-specific survival,[22] whereas CLND could increase the risk of permanent complications and might decrease the patients’ quality of life. Thus, prophylactic CLND might be objected. In the present study, even though all the operations were performed by expert surgeons, 351 patients’ parathyroid glands were removed inadvertently during surgery, accounting for 10.7% of the total (included partial tissue, capsule or entire parathyroid gland). In the pathological sections, the incidence of the parathyroid gland being mistakenly dissected in CLN specimens was 2.9-fold higher than that in the thyroid specimens (261/90). Despite this, CLNM increased the recurrence rate that might lead to a second operation. Thus, it would not only increase the risk of recurrent laryngeal nerve damage and parathyroid glands injury but also aggravate the psychological and economic burden of the patients. Literature[23,24] reported CLNM may not have a significant impact on the survival rate of the patients, but according to the latest data released by the official China Cancer Center, China 5-year survival rate of thyroid cancer was 67.5%,[25] lower than the United States (98.2%)[26] and Europe (86.5%).[27] At present, there was still a great deal of deficiency of thyroid cancer in the diagnosis and treatment in China, and more active surgery and treatment were needed. In order to minimize the risk and maximize the benefits to the patients, surgeons should select individualized more active prophylactic CLND for high-risk groups of CLNM rather than a single treatment therapy for all patients.
Due to anatomical locations, the sensitivities of preoperative US, CT, and other imaging examinations in CLNM assessment are relatively low.[15] Hence, the optimal examining methods for CLNM are yet controversial. In the present study, the retrospective analysis of the large sample size of clinicopathological data was conducted, and the univariate model was used to identify the risk factors of CLNM. Furthermore, to eliminate the influence of confounding factors and improve the reliability of the conclusion based on univariate analysis, multivariate logistic regression analysis was used for screening the independent risk factors of CLNM.
4.1. Gender
In thyroid cancer, women encounter a potentially higher incidence of the disease. However, several studies suggested that LNM rate in men was higher than that in women, and could be used as an independent predictor of LNM.[5,28,29]
In the present study, women had a lower rate of CLNM (38.0% < 51.4%), which could be used as an independent predictor of CLNM (odds ratio [OR] = 0.610, 95% confidence interval [CI]: 0.504–0.740) in multivariate analysis, in agreement with the previous reports.
4.2. Age
Age is constantly a major risk factor in various staging systems of differentiated thyroid carcinoma (DTC), such as TNM, age, metastasis, extratrothyroidism, and size, and Metastasis, age, complete, invasion, and size. Multiple studies have shown that age ≤45 years could be used as an independent predictor of LNM.[5,28,30] Ito et al[31] speculated that the LNM rate was higher with age <40 years. In this study, the 4 groups were divided according to age, and the results showed high incidences in both groups 41 to 60 and 21 to 40 years. In the univariate and multivariate analysis, age can be used as an independent predictor of CLNM (OR = 0.510, 95% CI: 0.449–0.579). The CLNM rates among different age groups were compared: <20 years group (90.9%), >21–40 years group (51.3%), >41–60 years group (32.1%), and >60 years group (30.0%). Among them, the CLNM rate in <20 years group was as high as 90.9%. Thus, we proposed that the treatment of such patients with routine prophylactic CLND be rather beneficial.
4.3. Tumor size and extrathyroidal invasion
The tumor size and extrathyroidal invasion were considered as vital influencing factors for the progression of PTC.[11] As a criterion for evaluating the treatment plan and surgical procedures, large tumor diameters were correlated with the LNM rate, thereby increasing the T staging. In the 2015 ATA guidelines, thyroid nodules <1 cm were not recommended for assessment, the T1a DTC patients were recommended only unilateral lobectomy, both T1 and T2 patients were not recommended prophylactic CLND, and only PTC patients with thyroid nodules >4 cm complicated with extrathyroidal invasion were considered for prophylactic CLND.[9] In the present study, the CLNM rates of T1b patients, T2 patients, patients with thyroid nodules >4 cm, and those complicated with extrathyroidal invasion reached 58.4%, 71.2%, 93.8%, and 73.6%, respectively. In all patients with thyroid nodules >1 cm and patients complicated with extrathyroidal invasion, the CLNM rates were high. Therefore, this study did not agree with the recommended prophylactic CLND surgery in the 2015 edition of the ATA guidelines. The univariate and multivariate regression analysis showed that the maximum size of PTC and extrathyroidal invasion was closely related to CLNM. The tumor size (OR = 2.514, 95% CI: 2.220–2.846) and extrathyroidal invasion (OR = 3.541, 95% CI: 2.611–4.801) could be used as independent predictors of CLNM.
Studies of the US cancer registry data from surveillance, epidemiology, and end results program (SEER) showed that total 33,886 cases of PTC were registered from 1983 to 2006 while papillary thyroid microcarcinoma (PTMC) consisted of only 10,629 cases, accounting for 31.3%, and other papillary carcinomas with diameter >1 cm comprised up to 23,257 cases, accounting for nearly 70%.[32] Due to the limitations of the US equipment, a large number of PTMCs were not diagnosed until the tumor diameter progressed to >1 cm. Nevertheless, all the PTCs with tumor diameters >1 cm were progressed from PTMC; PTMC and PTC exhibited identical biological features. Therefore, PTMC could not be considered safe. PTMC with diameter <1 cm accounted for 30.0% to 60.0% of PTC and the lymph node metastasis rate could be as high as 12.0% to 64.0%.[33–36] In the present study, PTMC patients accounted for 64.6% (2113 cases), and CLNM rate was 28.6%, similar to the previous studies. Although the CLNM of tumors with smaller diameter was low, 2/30 cases with CLNM persisted in unexpected cancer with a diameter of 0.1 cm. Therefore, we speculated that the PTMC patients with smaller size should not be treated with routine surgical procedures but individualized therapies according to their age, sex, location of the tumor, and other predictors.
4.4. Multifocality
That intrathyroidal metastasis often occurs in PTC, and multifocality is one of its remarkable features. According to the published reports, multifocal carcinoma accounted for 20.3% to 33.5% of PTC,[28,30,37] and could be used as an independent predictor of LNM.[33] In the univariate analysis, multifocal carcinoma accounted for 33.5%, and the CLNM rate was 48.9%. The CLNM rates of unilateral multifocal carcinoma and bilateral multifocal carcinoma were 39.3% and 51.3%, respectively, and the difference was statistically significant (P = .001). However, in multivariate analysis, the results showed that multifocality could not be used as an independent predictor of CLNM (P = .186), which differed from the previous results. This phenomenon may be attributed to the differences in the operation scope. The greater the resection scope of the thyroid gland, the higher the possibility of the unexpected multifocal carcinoma, which may lead to selection bias. In the current study, total or near-total thyroidectomy in all cases accounted for 87%, which rendered further stringency to the inclusion criteria.
4.5. Tumor location
Presently, the lymphatic drainage mechanism of the thyroid gland is unclear, and the relationship between the tumor location and CLNM is yet controversial. In gastric cancer, colon cancer, and other gastrointestinal tumors, LNM is closely associated with the regional tumor lymph return path. Thus, we speculated that LNM of PTC was related to the lymph return path in thyroid region. The results confirmed our hypothesis. According to the different thyroid regions occupied by multifocal carcinomas, the CLNM were multiple bilateral regions 51.3% > multiple unilateral regions 47.2% > single region 36.7%; the difference was statistically significant (P = .003), and the rate of metastasis was increased when tumors occupied a large number of thyroid regions.
Zhang et al[38] suggested that the risk of CLNM reduced when the tumor was located in the upper thyroid, whereas Xiang et al[39] showed that the risk increased when the tumor was located in the middle thyroid. In this study, the interferences of multifocality and other factors were excluded. According to the preoperative US examination and the intraoperative observation by naked eyes, tumors confined to a single region were divided into 4 categories by their locations. The results showed that the CLNM rate was higher (41.6%) when the tumor was located in the lower thyroid, and lowest (28%) (P < .001) when in the upper thyroid. We speculated that the tumors in the lower thyroid rapidly metastasized to CLN, which was inconsistent with the result from the study by Xiang et al. Due to the morphological thyroid features, restricted according to the upper, middle, and lower parts of the 1/3 division, the middle volume was larger than the upper and lower parts. These features led to a greater number of middle thyroid tumors than the other 2 parts; however, the lack of an adequate sample size may interfere with the results of the study.
Whether the tumors occupying only 1 region were distributed by multifocality and analyzed further, the results showed no statistical significance (P = .68). We postulated that PTC CLNM was closely related to the occupied regions of the thyroid, rather than the number of tumors. Thus, the tumor locations necessitate intensive focus during the US examination. In the case of multifocal carcinomas, additional attention is essential on the thyroid locations occupied by cancer foci, rather than the tumor numbers.
4.6. Initial symptoms
In the present study, 2647 patients (80.9%) without clinical symptoms presented tumors in the US medical examination, and their CLNM rate was 37.0%, which is lower than that of the patients (CLNM rate of 56.4%) with clinical symptoms (palpability, compression, pain, and distant metastasis); the difference was statistically significant (P < .001). However, in multivariate logistic regression analysis, the symptoms could not be used as independent predictors of CLNM (P = .055). Compared with the group without clinical symptoms, the duration of the disease was longer, and the tumor diameter was larger in the group with clinical symptoms. As the US examination was advantageous due to cost-effectiveness and no damage to the human body, we speculated that regular thyroid examination by US could detect the tumors early, leading to early treatment, thereby effectively reducing the LNM rate, and thus narrowing the scope of surgery.
4.7. Observation
Through prospective studies of 5-year and 10-year follow-ups, Ito et al[31] found that the rates of new LNM in low-risk patients were only 1.7% and 3.8%, respectively. In the present study, 1478 patients who were not highly suspected of malignancy by US examination were closely observed. The results of univariate and multivariate regression analysis assessed whether observation could or not be used as an independent predictor of CLNM (OR = 0.760, 95% CI: 0.649–0.888). The CLNM rate was lower in the observation group than the direct operation group. The median time from the detection of the tumor to the pathological confirmation of malignancy in the observation group was 12.0 (7.0, 24.0) months. However, whether the patient being observed or not was strongly related to the clinical symptoms and tumor diameter, and it may be affected by the limitations of the surgeon, which may easily cause bias in the hospitalization rate. We assumed that the appearance time of tumor malignancy was unrelated to CLNM or extrathyroidal invasion. Patients encountering difficulty in being identified as benign or malignant should be closely observed. There was no evidence of disease progression (CLNM and extrathyroidal invasion) by observation. Thus, it was recommended to review the US examination with a cycle shorter than 12 months.
This study was a retrospective study of a large sample of single center, had strict inclusion criteria, unified and standard process, and all the surgeries were performed by experienced specialists, could provide some reference for the selection of clinical treatment in patients with PTC. Chinese hospitals had a major problem in patient management and follow-up after surgery compared with the United States standard cancer registration system, and we also developed a follow-up software based on Internet and physician + patient participation that based on this study. However, due to the shorter follow-up time, there has been no long-term recurrence, mortality, quality of life of patients and other information, could not provide more guidance for the operation. We expect further validation using multicenter and large-sample prospective studies to provide valuable CLNM diagnostic methods and optimal surgical procedures for patients with PTC.
In summary, the results of this study showed that males, younger age, greater tumor diameter, and extrathyroidal invasion were independent risk factors of CLNM. PTC CLNM was related to multiple thyroid regions occupied by tumors and not to multifocality. When the tumor occupied only a single region, the location in the lower part of the thyroid gland could be used as a predictive factor for CLNM. For patients who were difficult to diagnose as benign or malignant, no evidence of disease progression was found by observation. For the high-risk PTC patients with CLNM, prophylactic CLND may be beneficial. Nevertheless, the present data was a single center retrospective analysis, and the long-term prognostic factors including recurrence rate and mortality rate were lacking. Therefore, the results of this research may only serve as a tool in predicting CLNM.
Footnotes
Abbreviations: ATA = American Thyroid Association, CLND = central lymph node dissection, CLNM = central lymph node metastasis, DTC = differentiated thyroid carcinoma, epidemiology, and end results program, FNAB = fine-needle aspiration biopsy, LNM = lymph node metastasis, PTC = papillary thyroid cancer, SEER = surveillance, US = ultrasound.
The authors state that they have no conflicts of interest.
References
- [1].Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].WHO/IARC. World Cancer Report. Lyon: IARC Press; 2014. [Google Scholar]
- [3].Dal Maso L, Lise M, Zambon P, et al. Incidence of thyroid cancer in Italy, 1991–2005: time trends and age–period–cohort effects. Ann Oncol 2011;22:957–63. [DOI] [PubMed] [Google Scholar]
- [4].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [5].Mao LN, Wang P, Li ZY, et al. Risk factor analysis for central nodal metastasis in papillary thyroid carcinoma. Oncol Lett 2015;9:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cramer JD, Fu P, Harth KC, et al. Analysis of the rising incidence of thyroid cancer using the surveillance, epidemiology and end results national cancer data registry. Surgery 2010;148:1147–52. discussion 1152-1153. [DOI] [PubMed] [Google Scholar]
- [7].Howlader N, Noone AM, Krapcho M. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute; 2012. Available at: http://seer.cancer.gov/statfacts/html/thyro.html Accessed March 15, 2013. [Google Scholar]
- [8].Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf) 2012;76:131–6. [DOI] [PubMed] [Google Scholar]
- [11].Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th ed.New York: Springer Verlag; 2010. [Google Scholar]
- [12].Chéreau N, Buffet C, Trésallet C, et al. Recurrence of papillary thyroid carcinoma with lateral cervical node metastases: predictive factors and operative management. Surgery 2016;159:755–62. [DOI] [PubMed] [Google Scholar]
- [13].Lan X, Sun W, Zhang H, et al. A meta-analysis of central lymph node metastasis for predicting lateral involvement in papillary thyroid carcinoma. Otolaryngol Head Neck Surg 2015;153:731–8. [DOI] [PubMed] [Google Scholar]
- [14].Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 2011;121:487–91. [DOI] [PubMed] [Google Scholar]
- [15].Lee DW, Ji YB, Sung ES, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol 2013;39:191–6. [DOI] [PubMed] [Google Scholar]
- [16].Khokhar MT, Day KM, Sangal RB, et al. Preoperative high-resolution ultrasound for the assessment of malignant central compartment lymph nodes in papillary thyroid cancer. Thyroid 2015;25:1351–4. [DOI] [PubMed] [Google Scholar]
- [17].Kim E, Park JS, Son KR, et al. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008;18:411–8. [DOI] [PubMed] [Google Scholar]
- [18].Kandil E, Noureldine SI, Abbas A, et al. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery 2013;154:1346–52. discussion 1352–1353. [DOI] [PubMed] [Google Scholar]
- [19].Carty SE, Cooper DS, Doherty GM, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 2009;19:1153–8. [DOI] [PubMed] [Google Scholar]
- [20].Moo TA, McGill J, Allendorf J, et al. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg 2010;34:1187–91. [DOI] [PubMed] [Google Scholar]
- [21].Yan H, Zhou X, Jin H, et al. A study on central lymph node metastasis in 543 cN0 papillary thyroid carcinoma patients. Int J Endocrinol 2016;2016:1878194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer 2006;106:524–31. [DOI] [PubMed] [Google Scholar]
- [23].Lang BH, Wong CK, Tsang JS, et al. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol 2014;21:850–61. [DOI] [PubMed] [Google Scholar]
- [24].Wang TS, Cheung K, Farrokhyar F, et al. A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol 2013;20:3477–83. [DOI] [PubMed] [Google Scholar]
- [25].Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921–30. [DOI] [PubMed] [Google Scholar]
- [26].Surveillance E aERP. SEER stat fact sheets: thyroid cancer; 2015. Available at: http://seer.cancer.gov/statfacts/html/thyro.html Accessed June 17, 2015. [Google Scholar]
- [27].De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol 2014;15:23–34. [DOI] [PubMed] [Google Scholar]
- [28].Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012;97:1250–7. [DOI] [PubMed] [Google Scholar]
- [29].Nam IC, Park JO, Joo YH, et al. Pattern and predictive factors of regional lymph node metastasis in papillary thyroid carcinoma: a prospective study. Head Neck 2013;35:40–5. [DOI] [PubMed] [Google Scholar]
- [30].Malandrino P, Pellegriti G, Attard M, et al. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab 2013;98:1427–34. [DOI] [PubMed] [Google Scholar]
- [31].Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aschebrook-Kilfoy B, Ward MH, Sabra MM, et al. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid 2011;21:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee J, Song Y, Soh EY. Central lymph node metastasis is an important prognostic factor in patients with papillary thyroid microcarcinoma. J Korean Med Sci 2014;29:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chung YS, Kim JY, Bae JS, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: results of therapeutic lymph node dissection. Thyroid 2009;19:241–6. [DOI] [PubMed] [Google Scholar]
- [35].Cho SY, Lee TH, Ku YH, et al. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery 2015;157:111–8. [DOI] [PubMed] [Google Scholar]
- [36].Zhao Q, Ming J, Liu C, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol 2013;20:746–52. [DOI] [PubMed] [Google Scholar]
- [37].Kim HJ, Park HK, Byun DW, et al. Number of tumor foci as predictor of lateral lymph node metastasis in papillary thyroid carcinoma. Head Neck 2015;37:650–4. [DOI] [PubMed] [Google Scholar]
- [38].Zhang LY, Liu ZW, Liu YW, et al. Risk factors for nodal metastasis in cN0 papillary thyroid microcarcinoma. Asian Pac J Cancer Prev 2015;16:3361–3. [DOI] [PubMed] [Google Scholar]
- [39].Xiang D, Xie L, Xu Y, et al. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery 2015;157:526–33. [DOI] [PubMed] [Google Scholar]


