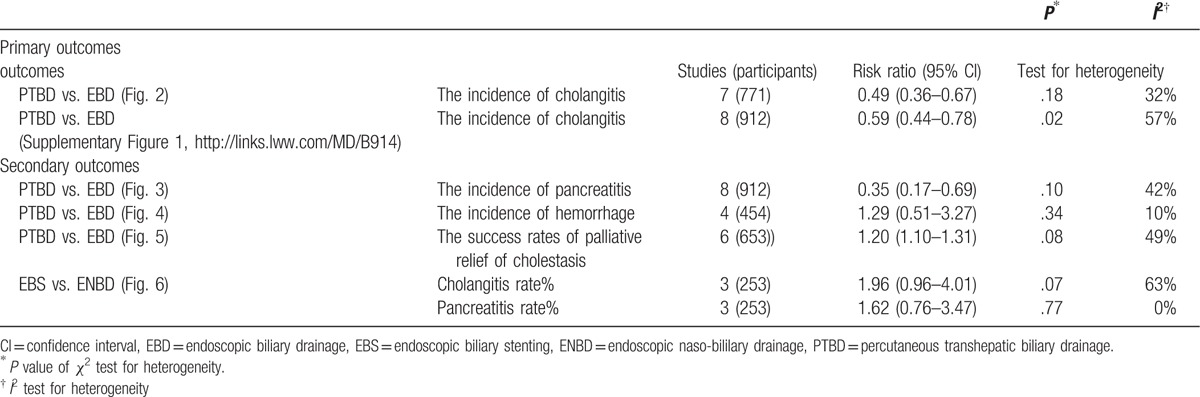
Supplemental Digital Content is available in the text
Keywords: endoscopic biliary drainage, endoscopic biliary stenting, endoscopic nasobiliary drainage, klatskin tumor, percutaneous transhepatic biliary drainage
Abstract
The operative treatment combined with preoperative biliary drainage (PBD) has been established as a safe Klatskin tumor (KT) treatment strategy. However, there has always been a dispute for the preferred technique for PBD technique. This meta-analysis was conducted to compare the biliary drainage-related cholangitis, pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis between percutaneous transhepatic biliary drainage (PTBD) and endoscopic biliary drainage (EBD), to identify the best technique in the management of KT.
PubMed, EMBASE, and Web of Science were searched systematically for prospective or retrospective studies reporting the biliary drainage-related cholangitis, pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis in patients with KT. A meta-analysis was performed, using the fixed or random-effect model, with Review Manager 5.3.
PTBD was associated with lower risk of cholangitis (risk ratio [RR] = 0.49, 95% confidence interval [CI]: 0.36–0.67; P < .00001), particularly in patients with Bismuth-Corlette type II, III, IV KT (RR = 0.50, 95% CI: 0.33–0.77; P = .05). Compared with EBD, PTBD was also associated with a lower risk of pancreatitis (RR = 0.35, 95% CI: 0.17–0.69; P = 0.003) and with higher successful rates of palliative relief of cholestasis (RR = 1.20, 95% CI: 1.10–1.31; P < .0001). The incidence of hemorrhage was similar in these 2 groups (RR 1.29, 95% CI: 0.51–3.27; P = .59). The risk of biliary drainage-related cholangitis (RR = 1.96, 95% CI: 0.96–4.01; P = .06) and pancreatitis (RR = 1.62, 95% CI: 0.76–3.47; P = .21) was similar between endoscopic nasobiliary drainage groups and biliary stenting.
In patients with type II or type III or IV KT who need to have PBD, PTBD should be performed as an initial method of biliary drainage in terms of reducing the incidence of procedure related cholangitis, pancreatitis, and improving the rates of palliative relief of cholestasis. Well-conducted randomized controlled trials with a universial criterion for PBD are required to confirm these findings.
1. Introduction
Klatskin tumor (KT), also named as perihilar cholangiocarcinoma or hilar cholangiocarcinoma, was first recognized as a distinct clinical entity first reported by Klatskin on a series of 13 patients in 1975.[1] It is usually classified according to the extent of ductal involvement by tumor, which has been described by Bismuth et al in 1988.[2] This Bisthmuth classification is a standard for making a decision on resectability of KT and selecting the methods before the surgery. Complete resection of the KT, with negative resection margins, offers the best possibility for long-time survival postoperatively.[3,4] However, liver surgery in cholestatic patients with KT is closely associated with high risks of postoperative morbidity and mortality.[5,6]
In the majority of KT, the first observed symptom was obstructive jaundice, which is related to postoperative morbidity and mortality rates; the mainly lethal factor was considered as hepatic insufficiency.[7] Preoperative biliary drainage (PBD) is employed to create a safer operational environment during the KT surgery compared to traditional operation without drainage,[8] which had been reported reduces jaundice and bacterial translocation, improves liver function and nutritional status, and enhances the ability of the liver to regenerate postoperatively.[9,10] Nevertheless, biliary drainage was considered as for being harmful when biliary drainage-related complications further increase the risk of postoperative morbidity and mortality.[11–15]
There are 2 options for PBD, which include percutaneous transhepatic biliary drainage (PTBD) and endoscopic biliary drainage (EBD). Currently, there have been several disputes over the clinical advantages and disadvantages of PTBD versus EBD, with the latter being achieved by either endoscopic nasobiliary drainage (ENBD) or endoscopic biliary stenting (EBS). Internal drainage by EBD is always be considered as a less invasive technique; however, it carries an increased risk of developing cholangitis because of a bacterial contaminant from the gut and increased risk of procedure-related complications, such as duodenum perforation and pancreatitis.[16–19] However, ENBD has demonstrated that associated with a lower incidence of ascending cholangitis than EBS,[20–23] whereas, ENBD catheter may cause discomfort because of nasopharyngeal irritation.[24] As an alternative, PTBD has been reported associated with a high success rate of palliative relief of cholestasis and with a lower risk of cholangitis.[17–19,25] However, hemorrhage, portal vein thrombosis, catheter tract implantation metastasis, and patient discomfort had been widely reported to be associated with the malpractice of PTBD.[14,19,21,26,27] Moreover the latest studies showed that the preoperative cholangitis was an independent prognostic factor in patients undergoing resection for KT.[13–15,24,28] The preferred technique of PBD remains under debate.
According to the reasons mentioned above, a pooled analysis of studies was undertaken in which data were reported for biliary drainage-related complications. The aim was to assess whether the incidence of cholangitis, pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis in the PTBD groups are less than in EBD, especially in patients with type II or III or IV KT, according to The Bismuths classification. These adverse outcomes were also assessed between ENBD groups and EBS in 1 subgroup analysis.
2. Methods
2.1. Literature research
A comprehensive literature search (up to November 8, 2016) was performed using PubMed (2000-Novemeber 8, 2016), EMBASE (2000-Novemeber 8, 2016), and Web of Science (2000-Novemeber 8, 2016), restricted to articles published in English. The following keywords were used: “proximal bile duct cancer or hilar bile duct carcinoma or klatskin tumor or perihilar cholangiocarcinoma or hilar cholangiocarcinoma” AND “percutaneous transhepatic biliary drainage or endoscopic biliary drainage or endoscopic nasobiliary drainage or endoscopic biliary stenting” AND “cholangitis or pancreatitis or complications.” Within the results for those combined keywords, additional filters, publication types were used to exclude the most common article types that did not report clinical study results (reviews and case studies). Animal studies were also excluded where possible (Appendix I for PubMed search strategy). We also searched by hand the references of studies included in the original search to identify studies missed on the initial search. All procedures were approved by the ethics committee for human experiments of the First Hospital of Lanzhou University.
2.2. Study selection criteria
Published studies were included if they met the following criteria: observational design (retrospective or prospective cohort or case control) or interventional design (random or non-randomized); subjects with KT who accepted the management of PBD (PTBD or EBD); the drainage related complications were reported; and patients not treated previously with portal vein embolization. Studies included PTBD or EBD only, and subjects with another biliary tract carcinoma (e.g., distal cholangiocarcinoma, intrahepatic cholangiocarcinoma, gall bladder carcinoma, carcinoma of pancreas, and region lymph node metastases) were excluded.
2.3. Data extraction
Data extraction was carried out independently by 2 investigators (ZT and YY), with the discrepancies resolved by the consensus of these 2 investigators (Any disagreement on a conflicting study was resolved by full discussion). The information including author, year of publication, country, cancer type (Bismuth-Corlette type) and patients number of each type, type of PBD, mean age and range, sex, the success rates of palliative relief of cholestasis, and the incidence of cholangitis, pancreatitis, and hemorrhage were recorded from each study. The methodological quality of comparative observational studies was assessed by using the Newcastle-Ottawa Scale (NOS). A score of 0 to 9 was assigned to each study.[29]
2.4. Definition of outcomes
The primary endpoint of the meta-analysis was the incidence of biliary drainage-related cholangitis. The second endpoint was the incidence of pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis. We assessed the procedure-related cholangitis and pancreatitis based on consensus criteria.[30] The procedure-related hemorrhage was defined arbitrarily as bleeding that required transfusion or additional intervention, and the success rates of palliative relief of cholestasis were defined arbitrarily as a palliation of cholestasis successfully after operation, or patients underwent the PTBD or EBS or ENBD successfully without converting to another type of PBD.
2.5. Statistical analysis
The incidence of drainage-related cholangitis was the primary outcome measure, and the impact of pancreatitis, hemorrhage, and success rates of palliative relief of cholestasis associated with biliary drainage procedure were the second endpoints. The Mantel–Haenszel method was used to pool data of clinical outcomes. Risk ratio (RR) analysis was used to generate an overall effect estimate of both outcomes. The fixed-effect model was used in case of there was low heterogeneity in the variables among the studies. Moreover the random-effect model was used when there was significant heterogeneity. Intention-to-treat data were extracted from all studies. We used the χ2 test to evaluate heterogeneity between trials and the I2 statistic to assess the extent of the inconsistency, wherein an I2 test >50% suggests significant heterogeneity. Statistical heterogeneity was assessed using an I2 test and was categorized into low (<50%), moderate (51%-75%), or high (>75%) according to predefined criteria.[31] Forest plots were generated by using standard techniques to summarize the included studies, with horizontal lines representing 95% confidence interval (CI) and the area of each square indicating the RR point estimate. The overall summary estimate under fixed-effect or random-effect with its 95% CI was shown, and the vertical line was at the null value (RR = 1.0). Publication bias was evaluated for biliary drainage cholangitis analysis by Egger test and funnel plot. Moreover, a P < 0.05 for Egger test was considered representative of significant publication bias. All statistical analyses were carried out with the software Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014).
3. Results
The initial search identified 546 articles based on the searching key words. Nine retrospective cohort studies[17–21,23–25,32] were eligible for inclusion in this systematic review and meta-analysis (Fig. 1), and of them, 1 study[23] just compared the biliary drainage-related complications occurred in EBS groups and ENBD. The remaining studies compared the complications associated with biliary drainage procedure occurred in PTBD groups and EBD. One study reported by Hirano et al[21] with 141 patients with KT included 14 patients with intrahepatic cholangiocarcinoma located at the perihilar region (6 in PTBD group and 8 in EBD group).
Figure 1.
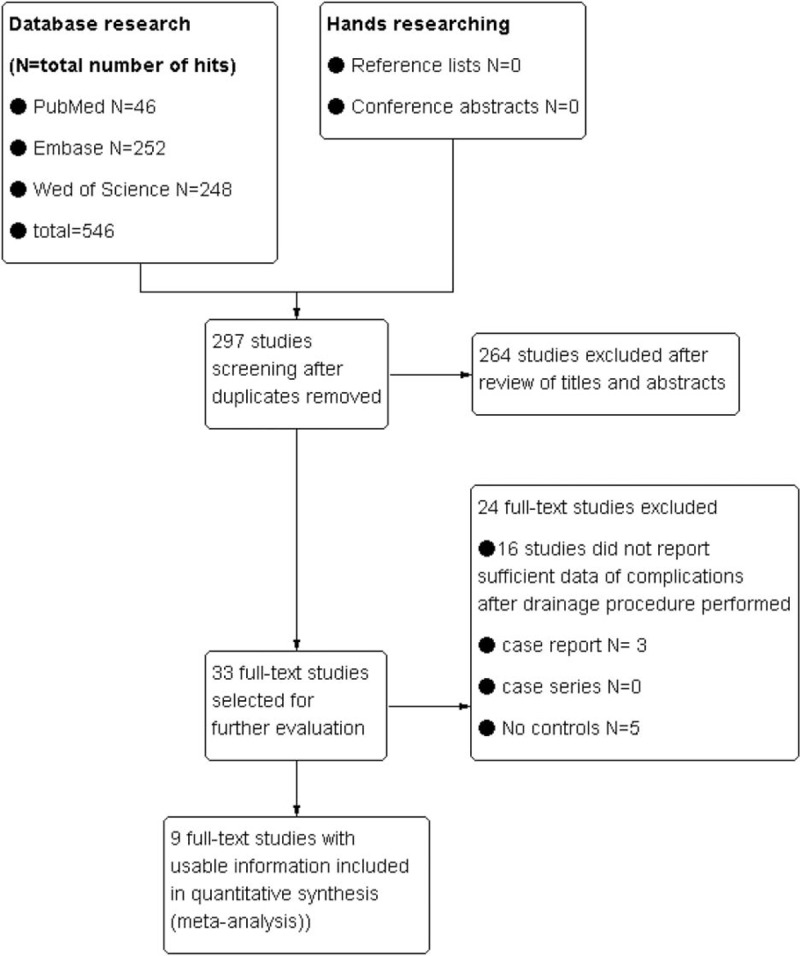
Search flow diagram.
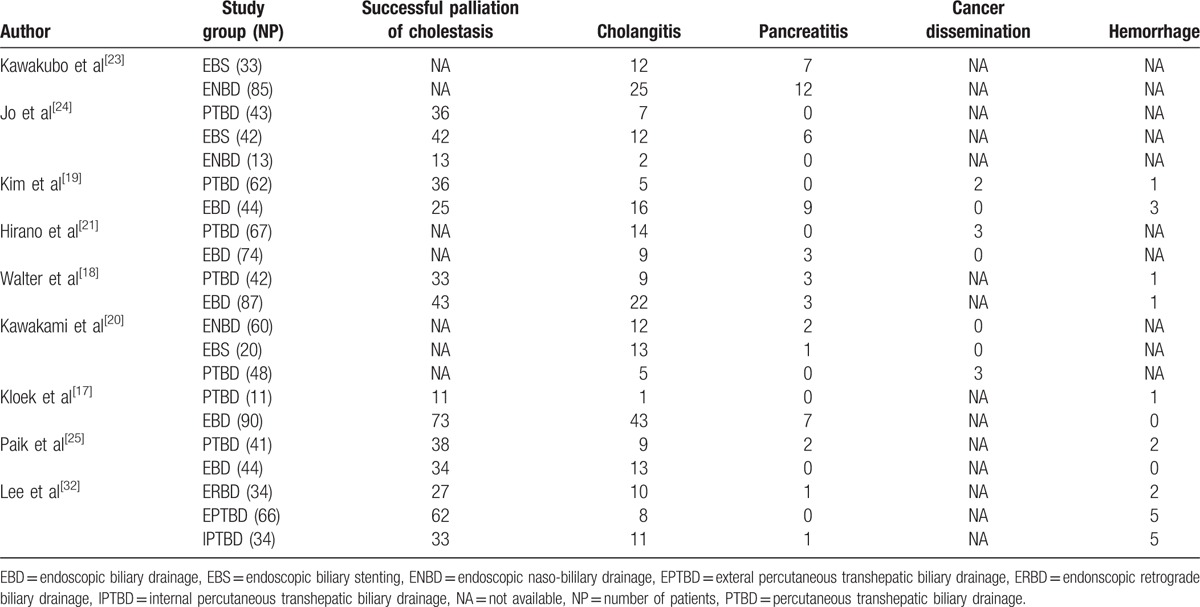
Characteristics of studies included are outlined in Table 1 and Table 2 and “Supplementary Table 1”. The Table 1 provides details about the 9 studies[17–21,23–25,32] that were included in the systematic review and meta-analysis. The incidence of complications associated with PBD was summarized in Table 2, and a total number of 1030 patients with KT were enrolled in the analysis. “Supplementary Table 1” presents the definition of procedure-related cholangitis, pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis reported by studies included in this meta-analysis. No randomized control trial was included, so the quality of the studies included in the meta-analysis was assessed by the NOS scale. Overall, there is an average medium quality of 5 of 9 stars in all studies (range 5–6).
Table 1.
Characteristics of included studies.
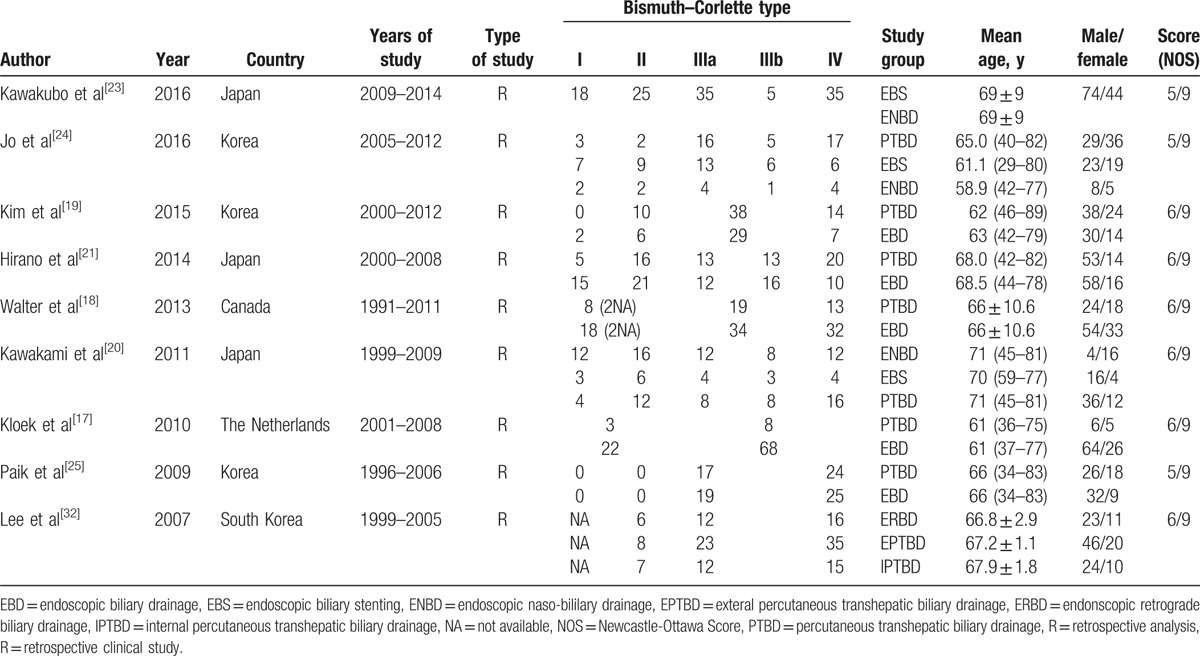
Table 2.
The characteristics of drainage procedure-related complications of included studies.
3.1. Primary endpoint: the incidence of cholangitis in PTBD groups versus in EBD groups
Seven studies[17,18,20,23–25,32] (n = 912 patients) reported the incidence of cholangitis in PTBD groups and EBD. The RR and 95% CI for each study and the pooled RR are shown in Figure 2. The overall summary estimated RR was 0.49 (95% CI: 0.36–0.67; P < .00001). Heterogeneity testing revealed that I2 = 32% and the P for heterogeneity = .18, using a fixed-effect model. In subgroup 2.1.1 (Fig. 2), patients with type II, III, IV KT (RR = 0.50, 95% CI: 0.33–0.77; P = .05). Heterogeneity testing revealed that I2 = 57% and the P for heterogeneity = .10. And in subgroup 2.1.2 (Fig. 2), the summary estimated RR was 0.49 (95% CI: 0.31–0.76; P = .001. Heterogeneity testing revealed I2 = 23% and the P for heterogeneity is .24.
Figure 2.
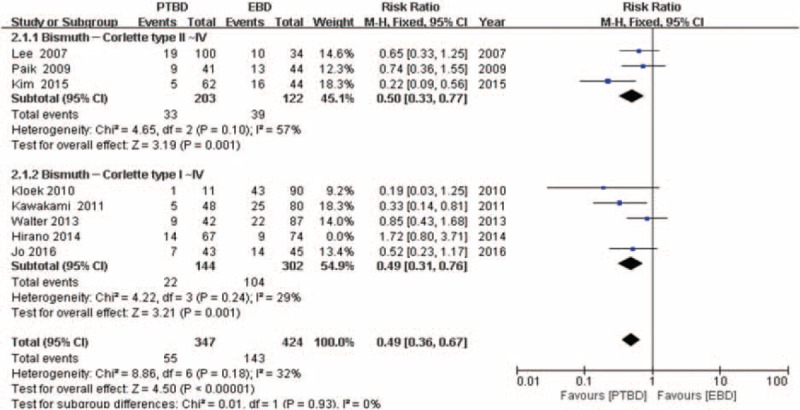
Forest plot for the incidence of cholangitis (percutaneous transhepatic biliary drainage vs. endoscopic biliary drainage).
3.2. Secondary endpoints: the incidence of pancreatitis, hemorrhage, and the success rates of palliative relief of cholestasis
Eight studies[17–21,24,25,32] provided the data (n = 912 patients) on the incidence of pancreatitis, and the RR and 95% CI for each study and the pooled RR are shown in Figure 3. The overall summary estimated RR was 0.35 (95% CI: 0.17–0.69; P = .003). Heterogeneity testing revealed I2 = 42% and the P for heterogeneity = .10, by using a fixed-effect model.
Figure 3.
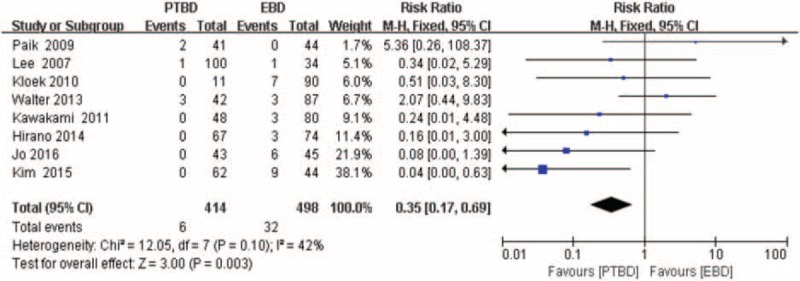
Forest plot for the incidence of pancreatitis (percutaneous transhepatic biliary drainage vs. endoscopic biliary drainage).
The hemorrhage data were provided in 5 studies,[17–19,25,32] but 1 study[17] reported by Kloek et al was excluded from the analysis of the incidence of hemorrhage, as it did not meet the eligibilility criteria for hemorrhage. The RR and 95% CI for remaining 4 studies and the pooled RR are shown in Figure 4. The overall summary estimated RR was 1.29 (95% CI: 0.51–3.27; P = .59). Heterogeneity testing revealed that I2 = 10% and the P for heterogeneity = 0.34, using a fixed-effect model.
Figure 4.
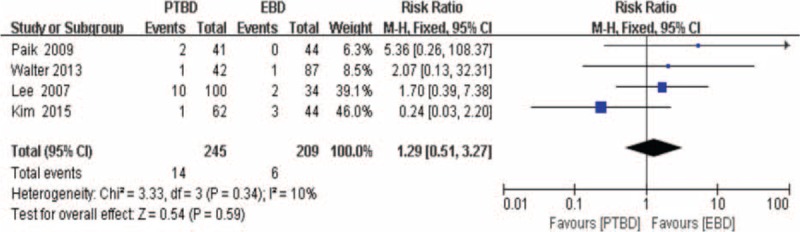
Forest plot for the incidence of hemorrhage (percutaneous transhepatic biliary drainage vs. endoscopic biliary drainage).
Success rates of palliative relief of cholestasis had been extracted from 6 studies[17–19,24,25,32] (n = 653). As described in Figure 5, the overall summary estimated RR was 1.20 (95% CI: 1.10–1.31; P < .0001). Heterogeneity testing revealed that I2 = 49% and the P for heterogeneity <.0001, using fixed-effect model.
Figure 5.
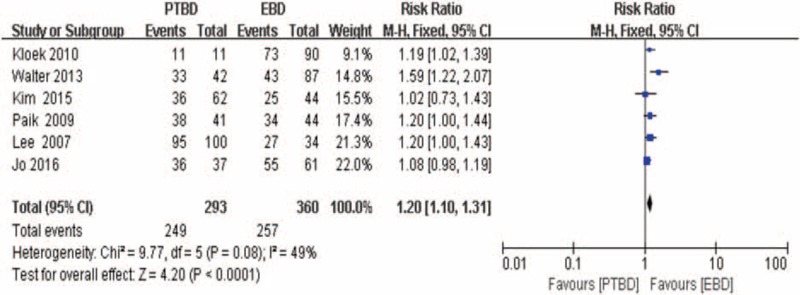
Forest plot for the successful rates of palliative relief of cholestasis (percutaneous transhepatic biliary drainage vs. endoscopic biliary drainage).
In ENBD versus EBS, the incidence of cholangitis and pancreatitis were reported by 3 studies.[20,23,24] As described in Figure 6, the overall summary estimated RR of cholangitis was 1.96 (95% CI:0.96–4.01; P = .06). Heterogeneity testing revealed that I2 = 63% and the P for heterogeneity = .07, using a random-effect model. Moreover, the pooled RR of pancreatitis was 1.62 (95% CI: 0.76–3.47; P = .21). Heterogeneity testing revealed that I2 = 0% and the P for heterogeneity = .77, using a fixed-effect model.
Figure 6.
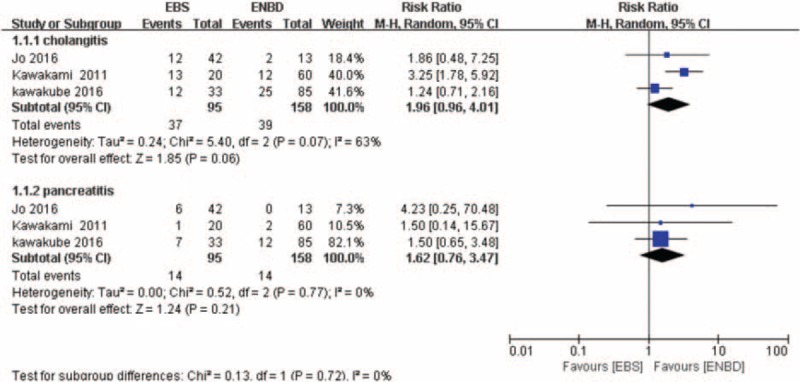
Forest plot for the incidence of cholangitis and pancreatitis (endoscopic biliary stenting vs. endoscopic naso-bililary drainage).
Table 3 provides a summary of results.
Table 3.
Summary of results.
3.3. Publication bias
The funnel plot (Fig. 7) shows no evidence of noticeable asymmetry. Egger test similarly showed no publication bias (Egger t value = 0.000, P = .282).
Figure 7.
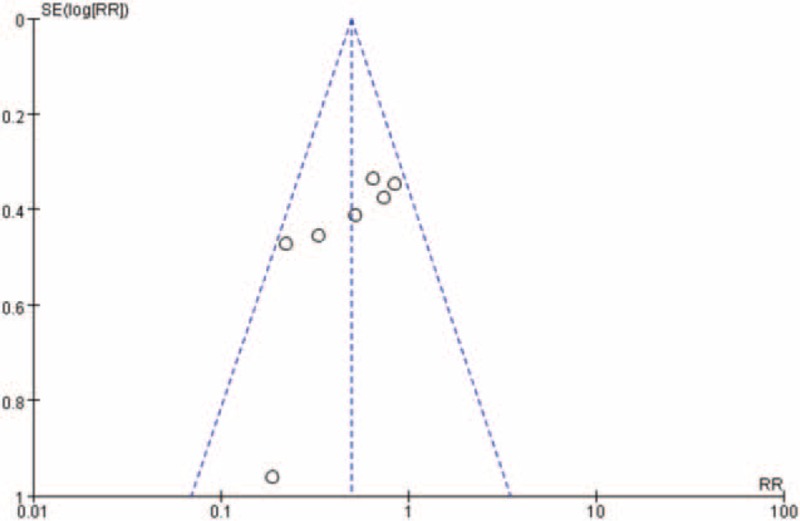
Funnel plot for publication bias (the incidence of cholangitis meta-analysis between percutaneous transhepatic biliary drainage groups and endoscopic biliary drainage).
3.4. Additional ananlyses
Comparing the incidence of cholangitis between PTBD and EBD, there was high heterogeneity (I2 up to 57%), as we included 8 studies[17–21,23–25,32] (Supplementary Figure 1). The RR was 0.59 (95% CI: 0.37–0.92; P = .02). Visual inspection of the forest plot showed that 1 study[21] reported by Hirano et al[21] with 141patients with KT was obviously discrepant from the remaining studies, a similar outcome was represented after this study was removed, and the RR was 0.49 (95% CI: 0.36–0.67; P < .00001). Heterogeneity testing revealed that I2 = 32% and the P for heterogeneity was .18. The exact cause of this heterogeneity may be related to the elements in the selection of populations, compared with other 7 studies; 14 subjects in the study reported by Hirano et al had been diagnosed with KT and intrahepatic cholangiocarcinoma located in the perihilar region.
4. Discussion
Complete resection combined surgery has been representing the only treatment for KT with curative intent[36–39]; herein, the chemotherapy and neoadjuvant therapy were widely reported to remain poorly characterized, and lack postoperative benefit.[33–36] However, varying definition of resectability criteria, surgical approach, and operational strategies have been applied to regions, in particular between Western (USA and Europe) and Eastern centers.[33] It is diffusely accepted that extensive liver resection and combined vascular resection in the early stage of KT may increase the negative histological margins ratios and improve survival in the East Asia.[12,15,37,38] Whereas, more aggressive hepatic resection for patients with KT is also associated with high postoperative mortality and morbidity, with postoperative hepatic insufficiency emerging as the main complication and liver function failure emerging as the most risk factors to increase the mortality rate.[15,39] Preoperative cholangitis and insufficient future liver remnant are major determinants of hepatic insufficiency and postoperative liver failure-related death.[15]
The potential advantages of PBD include its potential ability to reverse cholestasis-hepatic and synthetic toxicity as well as improve nutritional status, immune function and the ability of the liver to regenerate postoperatively. There is no clear guideline exists for KT treatment method not merely because of lack of substantial evidence of which type of biliary drainage is the optimal choice for patients with KT; moreover, PBD is a technically difficult procedure in KT.
In this meta-analysis, we first time extracted all published data comparing the complications associated with biliary drainage in patients with KT and pooled together in our literature. The primary outcome showed that PTBD is closely associated with lower risk of cholangitis in patients with KT compared with that of EBD (Fig. 2), especially in patients with type II or type III or type IV KT. Although only 2 of 44 cases belong to Type I KT in EBD group reported by Kim et al,[19] other types of KT account for the vast majority, reaching 98%. The estimated RR of 4 studies in subgroup 2.1.2 (Fig. 2) shows that there are also significant differences in the incidence of cholangitis between the PTBD and EBD groups, but the type II-IV KT accounts for significant proportion of a total number of patients compared to type I (up to 80%). Eight studies including 912 patients with KT provide cholangitis data. The overall incidence of cholangitis was 16.67% (69/414) of patients in PTBD groups compared to 30.52% (152/498) in the EBD.
The second outcomes showed that PTBD is associated with lower risk of pancreatitis and with a higher success rate of palliative relief of cholestasis compared with that of EBD. The hemorrhage rate was similar in these 2 groups. The incidence of cholangitis and pancreatitis were similar (ENBD vs. EBD) showed by another subgroup analysis (Fig. 6). Although the 3 of 9 studies included in this review reported the few incidence of seeding metastasis in PTBD group (8/414, 1.93%), and no data on the prevalence of seeding metastasis was reported in EBD groups, it is a fact that the seeding metastasis may not be limited to PTBD; seeding metastasis associated with endoscopic drainage methods also have been reported.[40]
Despite several encouraging data were extracted, this meta-analysis still has limitations and strengths. First, this is a study-level meta-analysis of retrospective observational studies; therefore, the differences in measurements among literature still should not be underestimated, including the indication for PBD, the drainage time, the diameter of drainage tube, the biliary stent size, the operation time of drainage procedure and the level of proficiency of operators, and the timing of using antibiotics and the type of antibiotics. Second, most available literatures did not report the complications of each type of KT completely, and 4 studies[19,20,23,34] included in this meta-analysis just provided a total number of complications in EBD group; the complication of ENBD or EBS still remains unclear. Third, there were just 2 patients with type I KT of 1 study incorporated into subgroup 2.1.1 (Fig. 2) based on the percentage values; the limited sample number restricted the accuracy of the study. Fourth, although the definition of procedure-related cholangitis and hemorrhage among studies included in the meta-analysis met the predefined criteria, the definition of procedure-related cholangitis and hemorrhage are not unanimous among these included studies. Finally, only published studies were included in this meta-analysis, and the population data were only obtained from 4 countries (Japan, Korea, Canada, The Netherlands), so these results should be interpreted with caution as in most meta-analysis.
However, despite the limitation listed, this study still the first systematic analysis assessing the biliary drainage-related complications for patients with KT, and the low heterogeneity and highly significant results were obtained with minimal evidence of bias for biliary drainage-related complications analysis.
Genomic, epigenetic, and molecular characterization of KT in an individual patient might provide valuable information on pathogenesis, prognosis, and chemosensitivity, thus indicating the best characterized therapeutic options for each patient as well as new potent. However, as of now, imaging characteristics of KT directly affect the choice of radical resection since the limited understanding of the pathogenesis of KT, especially in molecular pathology. Although KT typically involves the biliary confluence according to imaging characteristics (Bismuth–Corlette type), in most cases, it extends proximally to second- and third-order biliary branches. Therefore, for KT with obstructive jaundice and poor condition, the efficient and sufficient PBD are needed to create a safer environment before surgery.
In conclusion, this systematic review and meta-analysis provide the evidence that PTBD did not increase the risk of procedure related hemorrhage, and for patients with type II or type III or IV KT who need to have PBD, PTBD should be performed as the initial method of biliary drainage in terms of reducing the incidence of procedure related cholangitis, pancreatitis, and improving the rates of palliative relief of cholestasis.
Further randomized control trials with universial criteria for PBD, which are based on the Bismuth-Corlette classification (i.e., the drainage time, diameter of drainage tube, the biliary stent size, the operation time of drainage procedure, the level of proficiency of operators, the definition of procedure-related complications, the timing of using antibiotics and the type of antibiotics), should be performed to give further clear and more credible conclusions.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, EBD = endoscopic biliary drainage, EBS = endoscopic biliary stenting, ENBD = endoscopic nasobiliary drainage, KT = Klatskin tumor, NOS = Newcastle-Ottawa Scale, PBD = preoperative biliary drainage, PTBD = percutaneous transhepatic biliary drainage, RR = risk ratio.
Author Contributions: ZT:study concept and design, collection and extraction of data, statistical analysis, and interpretation of data, and drafting of the manuscript, critical revision of the manuscript for significant intellectual content; YY: collection and extraction of data, analysis, and interpretation of data, critical revision of the manuscript for significant intellectual content; WM and XL: study the concept and design, critical revision of the manuscript for significant intellectual content, and obtained funding and study supervision.
The study was funded by West Light Foundation of The Chinese Academy of Science ([2015] 90). The funding sources had no role in the design conduct of the study, collection, analysis, management and interpretation of the data, or preparation, review, or approval of the manuscript.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170–8. [PubMed] [Google Scholar]
- [2].Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988;12:39–47. [DOI] [PubMed] [Google Scholar]
- [3].Byrnes V, Afdhal N. Cholangiocarcinoma of the Hepatic Hilum (Klatskin Tumor). Curr Treat Options Gastroenterol 2002;5:87–94. [DOI] [PubMed] [Google Scholar]
- [4].Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg 2014;399:693–705. [DOI] [PubMed] [Google Scholar]
- [5].Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26–34. [DOI] [PubMed] [Google Scholar]
- [6].Kaiser GM, Paul A, Sgourakis G, et al. Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg 2013;79:90–5. [PubMed] [Google Scholar]
- [7].Nagino M, Kamiya J, Uesaka K, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg 2001;25:1277–83. [DOI] [PubMed] [Google Scholar]
- [8].Iacono C, Ruzzenente A, Campagnaro T, et al. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191–204. [DOI] [PubMed] [Google Scholar]
- [9].van der Gaag NA, Kloek JJ, de Castro SM, et al. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg 2009;13:814–20. [DOI] [PubMed] [Google Scholar]
- [10].Gouma DJ. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013;100:283–4. [DOI] [PubMed] [Google Scholar]
- [11].Sakata J, Shirai Y, Tsuchiya Y, et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbecks Arch Surg 2009;394:1065–72. [DOI] [PubMed] [Google Scholar]
- [12].Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129–40. [DOI] [PubMed] [Google Scholar]
- [13].Yokoyama Y, Ebata T, Igami T, et al. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery 2014;156:1190–6. [DOI] [PubMed] [Google Scholar]
- [14].Wiggers JK, Coelen RJ, Rauws EA, et al. Preoperative endoscopic versus percutaneous transhepatic biliary drainage in potentially resectable perihilar cholangiocarcinoma (DRAINAGE trial): design and rationale of a randomized controlled trial. BMC Gastroenterol 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ribero D, Zimmitti G, Aloia TA, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg 2016;223:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].dos Santos JS, Junior WS, Modena JL, et al. Effect of preoperative endoscopic decompression on malignant biliary obstruction and postoperative infection. Hepatogastroenterology 2005;52:45–7. [PubMed] [Google Scholar]
- [17].Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010;14:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors? J Vasc Interv Radiol 2013;24:113–21. [DOI] [PubMed] [Google Scholar]
- [19].Kim KM, Park JW, Lee JK, et al. A comparison of preoperative biliary drainage methods for perihilar cholangiocarcinoma: endoscopic versus percutaneous transhepatic biliary drainage. Gut Liver 2015;9:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol 2011;46:242–8. [DOI] [PubMed] [Google Scholar]
- [21].Hirano S, Tanaka E, Tsuchikawa T, et al. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:533–40. [DOI] [PubMed] [Google Scholar]
- [22].Paik WH, Loganathan N, Hwang JH. Preoperative biliary drainage in hilar cholangiocarcinoma: When and how? World J Gastrointest Endosc 2014;6:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kawakubo K, Kawakami H, Kuwatani M, et al. Lower incidence of complications in endoscopic nasobiliary drainage for hilar cholangiocarcinoma. World J Gastrointest Endosc 2016;8:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jo JH, Chung MJ, Han DH, et al. Best options for preoperative biliary drainage in patients with Klatskin tumors. Surg Endosc 2017;31:422–9. [DOI] [PubMed] [Google Scholar]
- [25].Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc 2009;69:55–62. [DOI] [PubMed] [Google Scholar]
- [26].Sakata J, Shirai Y, Wakai T, et al. Catheter tract implantation metastases associated with percutaneous biliary drainage for extrahepatic cholangiocarcinoma. World J Gastroenterol 2005;11:7024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maguchi H, Takahashi K, Katanuma A, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2007;14:441–6. [DOI] [PubMed] [Google Scholar]
- [28].Kitahata Y, Kawai M, Tani M, et al. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg 2014;208:1–0. [DOI] [PubMed] [Google Scholar]
- [29].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [30].Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383–93. [DOI] [PubMed] [Google Scholar]
- [31].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee SH, Park JK, Yoon WJ, et al. Optimal biliary drainage for inoperable Klatskin's tumor based on Bismuth type. World J Gastroenterol 2007;13:3948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. [DOI] [PubMed] [Google Scholar]
- [34].Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther 2006;23:1287–96. [DOI] [PubMed] [Google Scholar]
- [35].Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391–8. [DOI] [PubMed] [Google Scholar]
- [36].Zaydfudim VM, Wang AY, de Lange EE, et al. IgG4-associated cholangitis can mimic hilar cholangiocarcinoma. Gut Liver 2015;9:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013;83:268–74. [DOI] [PubMed] [Google Scholar]
- [38].Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sano T, Shimada K, Sakamoto Y, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg 2006;244:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].ten Hoopen-Neumann H, Gerhards MF, van Gulik TM, et al. Occurrence of implantation metastases after resection of Klatskin tumors. Dig Surg 1999;16:209–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


