Supplemental Digital Content is available in the text
Keywords: cardiogenic shock, dopamine, meta-analysis, norepinephrine
Abstract
Background:
Guidelines recommend that norepinephrine (NA) should be used to reach the target mean arterial pressure (MAP) during cardiogenic shock (CS), rather than epinephrine and dopamine (DA). However, there has actually been few studies on comparing norepinephrine with dopamine and their results conflicts. These studies raise a heat discussion. This study aimed to validate the effectiveness of norepinephrine for treating CS in comparison with dopamine.
Methods:
We performed a meta-analysis of randomized controlled trials (RCTs) to assess pooled estimates of risk ratio (RR) and 95% confidence interval (CI) for 28-day mortality, incidence of arrhythmic events, gastrointestinal reaction, and some indexes after treatment.
Results:
Compared with dopamine, patients receiving norepinephrine had a lower 28-day mortality (RR 1.611 [95% CI 1.219–2.129]; P < .001; P heterogeneity = .01), a lower risk of arrhythmic events (RR 3.426 [95% CI 2.120–5.510]; P < .001; P heterogeneity = .875) and a lower risk of gastrointestinal reaction (RR 5.474 [95% CI 2.917–10.273]; P < .001; P heterogeneity = 0). In subgroup analyses on 28-day mortality by causes of CS, there were more benefits from norepinephrine than dopamine in 2 subgroups.
Conclusions:
Our analysis revealed that norepinephrine was associated with a lower 28-day mortality, a lower risk of arrhythmic events, and gastrointestinal reaction. No matter whether CS is caused by coronary heart disease or not, norepinephrine is superior to dopamine for correcting CS on the 28-day mortality.
1. Introduction
It has been well established that patients with cardiogenic shock (CS) have poor prognoses. Cardiac pump failure, decreased tissue perfusion, increased ventricular end-systolic volume, and increased cardiac end-diastolic pressure are main characteristics of CS, which may cause the ischemia of multiple organs, hepatic and venous congestion, and accelerate multiple system organ failure.[1,2] CS may develop into an irreversibly complex stage without timely medication.[3]
Myocardial infarction (MI) is the main reason of CS.[4] In parallel with the rapid development and broad appliance of percutaneous coronary intervention and administration of circulatory support for MI, it appears that the incidence of CS is on the decrease according to some researches.[5,6]
In spite of this, the mortality from CS remains high.[5] Therefore, optimal administration of vasoactive agents for CS is necessary, which may improve clinical outcomes. Guidelines recommend that norepinephrine (NA) should be used to reach the target mean arterial pressure (MAP) during CS, rather than epinephrine and dopamine (DA).[7–9] But there has not been enough randomized clinical trials (RCTs) to draw a precise conclusion.[10–18] Controversies remain about the optimal administration, such as dopamine and norepinephrine, for the treatment of CS in spite of some randomized controlled trials in this respect.[19,20] A previous trial published in 2014 revealed that the mortality was significantly higher in the dopamine group than in the norepinephrine group[18] while a recent trial published in 2016 revealed that there were no significant differences in the short-term mortality and long-term mortality between the dopamine group and the norepinephrine group.[15] The results conflicts because of the small sample size of individual RCTs and any other reasons, such as low statistical power, which calls for more RCTs and discussion. This meta-analysis is to combine all the relevant trials for more reliable conclusions to provide guidance for clinical treatment by rigorous statistical methods.
2. Methods
We conducted our meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.[21] Since our meta-analysis was based on previously published studies, the ethical approval and patient consent were not required.
2.1. Search strategy and selection criteria
PubMed, Web of Science, Wanfang Database, Weipu Database were searched for literatures concerned up to June 10, 2017 by using “Cardiogenic Shock,” “Dopamine,” and “Norepinephrine” as suitable key words, and then conduct a manual search of reference lists from identified articles in English and Chinese languages. The inclusion criterion of our meta-analysis is a randomized clinical trial, containing the dopamine group and the norepinephrine group, revealing the mortality at 28 days, the incidence of arrhythmic events and gastrointestinal reaction, some indexes after treatment.
Two of the authors (QR and Y-FJ) made the same contribution for this meta-analysis by respectively conducting the searching and selecting. Conflicts were discussed with a third investigator (Y-FZ).
2.2. Data extraction and quality assessment
Extraction of study data we are interested in includes: first author, publication year, sample size, definition of CS, the process of correcting CS, the mortality at 28 days, arrhythmic events, useful basic information in each study like age, causes of CS. The stratified data were extracted from both main text and supplementary data of all the included studies. If it was not indicated, efforts were made to contact the authors for detail information.
Bias were assessed by the Cochrane Collaboration's tool based on methodological items, which contains randomization, blinding, allocation concealment.[22]
2.3. Outcomes
The primary outcome of this meta-analysis is 28-day mortality. The secondary outcome is the incidence of gastrointestinal reaction, arrhythmic events, including atrial fibrillation (AF), ventricular tachycardia, ventricular fibrillation (VF), and indexes after treatment, such as heart rates (HR), mean arterial pressure (MAP), lactic acid (LAC), cardiac index (CAI), and urine volume (UV) after treatment.
2.4. Statistical analysis
For each study included in this meta-analysis, relative risks (RRs) and 95% confidence intervals (CIs) were calculated. We combined odds ratios (ORs) and 95% CIs for summary estimates under a fixed or random-effect model according to the quantification of the heterogeneity calculated with the I2 test. When I2 exceeds 50%, a random-effect model for pooled analysis was chosen, or fixed-effect model (Mantel–Haenszel method) was applied. Sensitivity analysis was performed by combining RRs repeatedly with omission of each study to identify potential alternation of the overall meta result. We have also investigated publication bias via calculating Begg and Egger test values and drawing Begg funnel plot. We also performed Harbord test and a contour-enhanced funnel plot to further clarify the underlying heterogeneity. Besides, we conducted subgroup analyses to further analyze possible significant heterogeneity by grouping studies according to mean ages, sample sizes, races, causes of CS, and different types of arrhythmia. The Meta-analysis was performed by Stata version 14.0 (Stata Corporation, College Station, Texas).
3. Results
3.1. Literature search and selection
The selection and exclusion of the 4 databases identified 256 records in total, which is shown in Fig. 1. After removing 70 duplicated studies, there were 186 studies left for screening and 151 of records were excluded. Thirty five studies were read by full-text, and 26 of full-text articles were excluded, including studies with insufficient datas (n = 1),[8] reviews (n = 21), studies not relevant to our topic (n = 4). There were eventually 9 studies[10–18] of 510 patients eligible for this meta-analysis, randomized to receive dopamine (n = 269) or norepinephrine (n = 241) for correcting CS.8 (wang,qiu,he,gu, tan, xiong,li,jin) of these studies were published in Chinese from 2010 to now and 1 (Bahloul) in English.
Figure 1.
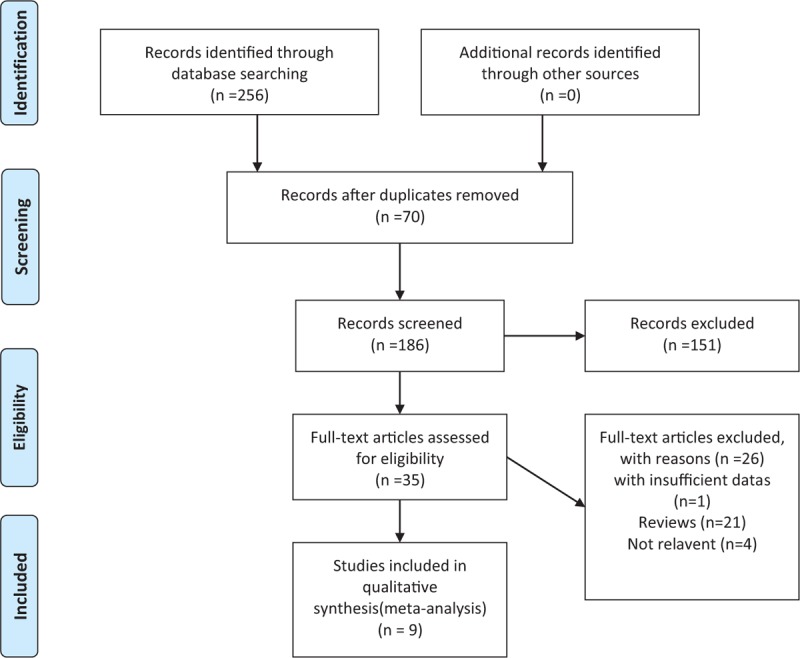
The PRISMA flow diagram of the study selection and exclusion. Nine studies were ultimately included. PRISMA = preferred reporting items for systematic reviews and meta analyses.
3.2. Study characteristics and study quality
Main characteristics of these studies concerned are demonstrated in Table 1 for meta-analysis. The sample sizes of all the included studies ranges from 22 to 80. The races of the studies include Asian (n = 8)[10–17] and African (n = 1).[18] The ages of the including patients range from 35 to 91. Patients of 3 studies[11–13] suffered from CS because of coronary heart disease, patients of 4 studies because of mixed causes, such as coronary heart disease, dilated cardiomyopathy, rheumatic heart disease, severe myocarditis, and so on. All studies used intravenous pump for both groups. The diagnosis indexes of CS in 4 studies[10,14–16] include systolic blood pressure or pulse pressure, tissue hypoperfusion, hemodynamic indexes. The diagnosis indexes in 2 studies[11,18] only includes systolic blood pressure or pulse pressure, tissue hypoperfusion. The diagnosis indexes in 3 studies[12,13,17] didn’t definite CS clearly. The administrations of vasoactive agents in each study were listed in Table 1.
Table 1.
Baseline characteristics of included studies in this meta-analysis.

3.3. DA or NA and events of 28-day mortality
The primary outcome (events of 28-day mortality) was demonstrated in 7 of the 9 studies concerned demonstrated. There were 135 deaths at 28 days in 329 patients included in 7 studies (41.0%). The heterogeneity was not obvious (I2 = 11.7%; P = .01). As I2 <50%, a fixed effect model was used to estimate the risk ratio (RR) and 95% CI (Fig. 2A). The mortality at 28 days was 50.3% (91/181) in the dopamine group while the mortality at 28 days was 29.7% (44/148) in the norepinephrine group (RR: 1.611, 95% CI: 1.219–2.129, P < .001). In all patients concerned, patients who received dopamine for correcting CS had a 61.1% higher risk of 28-day mortality than those who received norepinephrine. Cumulative meta-analysis of 28-day mortality validated that NA was superior to DA on the 28-day mortality.
Figure 2.
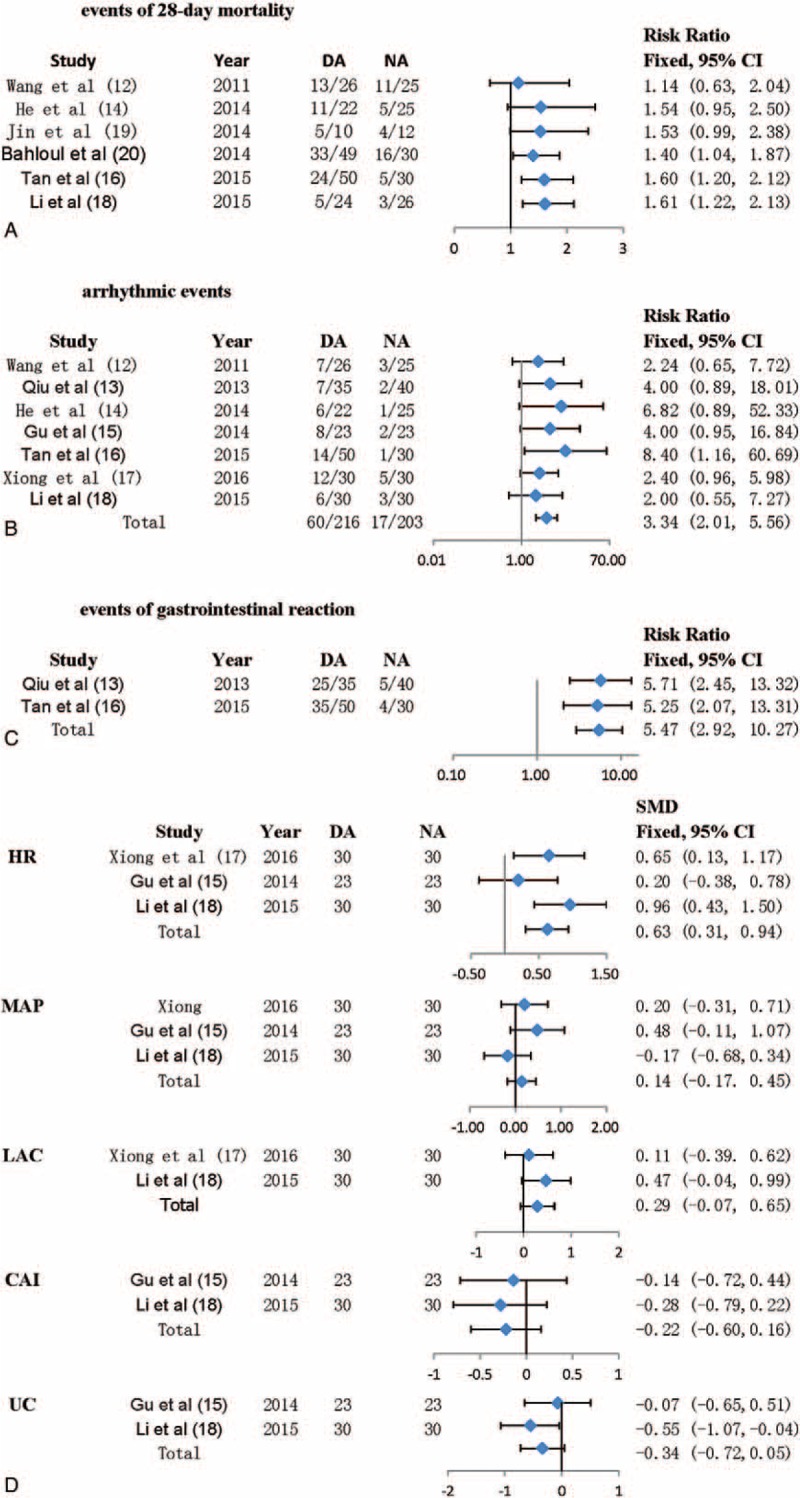
Risk estimates of primary and secondary outcomes for NA versus DA. Forest plots show results for 28-day mortality (A), incidence of arrhythmic events (B), gastrointestinal reaction (C), and some indexes after treatment (D). A fixed-effect model was applied to estimate RR and 95% CI.
3.4. DA or NA and arrhythmic events
Arrhythmic events, which include atrial fibrillation, ventricular tachycardia, and ventricular fibrillation, were reported in 8 of the 9 studies concerned. There were 86 arrhythmic events in 441 patients included in these 8 studies (19.50%). The heterogeneity was not statistically significant (I2 = 0%; P = .875). As I2 <50%, a fixed effect model was used to estimate the risk ratio and 95% CI (Fig. 2B). The arrhythmic events were 29.65% (67/226) in the dopamine group while the arrhythmic events were 8.34% (19/215) in the norepinephrine group (RR: 3.426, 95% CI: 2.130–5.510, P < .001). In all patients concerned, patients who received dopamine for correcting CS had a 2.34 higher risk of arrhythmic events than those who received norepinephrine.
3.5. DA or NA and events of gastrointestinal reaction
Two of the 9 studies concerned reported events of gastrointestinal reaction. There were 69 arrhythmic events in 155 patients included in 2 studies (44.52%). The heterogeneity was not statistically significant (I2 = 0%; P = 0). As I2 <50%, a fixed effect model was used to estimate the risk ratio and 95% CI (Fig. 2C). The events of gastrointestinal reaction were 70.59% (60/85) in the dopamine group while the arrhythmic events were 12.86% (9/70) in the norepinephrine group (RR: 5.474, 95% CI: 2.917–10.273, P < .001). In all patients concerned, patients who received dopamine for correcting CS had a 4.474 higher risk of gastrointestinal reaction than those who received norepinephrine.
3.6. DA or NA and heart rates (HR), mean arterial pressure (MAP), lactic acid (LAC), cardiac index (CAI), urine volume (UV) after treatment
HR, MAP, LAC, CAI, UV after treatment were reported in 3 of the 9 studies concerned. There were 166 patients in the 3 studies. The heterogeneities of all the indexes were not statistically significant (HR: I2 = 43.8%, P = .169; MAP: I2 = 28.4%, P = .247; LAC: I2 = 0, P = .33; CAI: I2 = 0, P = .71; UV: I2 = 32.6%, P = .223). As I2 <50%, fixed effect models were used to estimate the standardized mean difference (SMD) and 95% CI (Fig. 2D). The SMDs and 95% CI of all the indexes after treatment were listed in Fig. 2D (HR: SMD = 0.625, 95% CI: 0.31–0.938, P < .001; MAP: SMD = 0.140, 95% CI: −0.166–0.446, P > .001; LAC: SMD = 0.290, 95% CI: −0.071–0.650, P > .001; CAI: SMD = −0.219, 95% CI: −0.601–0.163, P > .001; UV: SMD = –0.339, 95% CI: −0.724–0.046, P > .001). In all patients concerned, patients who received dopamine for correcting CS had a higher HR than those who received norepinephrine and there were no significant differences in MAP, LAC, CAI, UV between 2 groups.
3.7. Subgroup analyses on dopamine or norepinephrine and events of 28-day mortality
Subgroup analyses were also conducted to discovery and evaluate potential clinically significant heterogeneity by dividing these studies into several groups according to mean age, sample size, races, and causes of CS (Fig. 3A).
Figure 3.
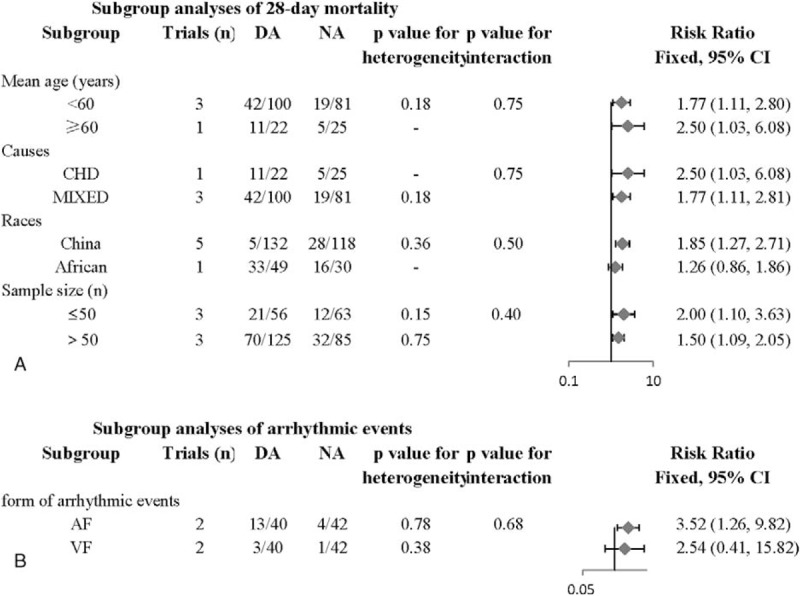
Subgroup analyses for the effect of NA versus DA on CS. (A) Subgroup analyses of 28-day mortality. (B) Subgroup analyses of arrhythmic events. A fixed-effect model was applied to estimate RR and 95% CI. CS = cardiogenic shock.
First, in subgroup analysis based on mean age, 3 studies (wang,tan,li) with 181 patients reported the events of 28-day mortality in the age of <60 years old. The 28-day mortality was 42.0% (42/100) in the dopamine group while the mortality was 23.5% (19/81) in the norepinephrine group (RR: 1.77, 95% CI: 1.11–2.81, P = .016 < .05), and the heterogeneity of this group (<60 years) were not statistically significant (P = .178, I2 = 42.0%). One trial (he) with 47 patients reported the events of 28-day mortality in the age of >60 years old. The 28-day mortality was 50.0% (11/22) in the dopamine group while the mortality was 20.0% (5/25) in the norepinephrine group (RR: 2.50, 95% CI: 1.03–6.08, P = .043 < .05). Monte Carlo permutation test was conducted for meta-regression, and P = .75, which indicated that there was no significant between-group heterogeneity and drawn a conclusion that different ages didn’t affect the heterogeneity.
Next, in subgroup analysis based on sample sizes, 3 studies (he,li,jin) with 119 patients reported the events of 28-day mortality in the sample size of ≤50 patients. The 28-day mortality was 37.5% (21/56) in the dopamine group while the mortality was 19.05% (12/63) in the norepinephrine group (RR: 1.997, 95% CI: 1.098–3.632, P = .024 < .05), and the heterogeneity of this group (≤50 patients) were not statistically significant (P = .75, I2 = 0%). Another there trials (wang,tan,Bahloul) with 210 patients reported the events of 28-day mortality in the sample size of >50 patients. The 28-day mortality was 56.0% (70/125) in the dopamine group while the mortality was 37.65% (32/85) in the norepinephrine group (RR: 1.496, 95% CI: 1.093–2.047, P = .012 < .05). Monte Carlo permutation test was conducted for meta-regression, and P = .75, which indicated that there was no significant between-group heterogeneity and drawn a conclusion that different sample sizes didn’t affect the heterogeneity.
Thirdly, in subgroup analysis based on races, 5 studies (wang, he, tan,li,jin) with 250 patients reported the events of 28-day mortality in the races of Chinese. The 28-day mortality was 43.94% (58/132) in the dopamine group while the mortality was 23.73% (28/118) in the norepinephrine group (RR: 1.853, 95% CI: 1.267–2.710, P = .001 < .05), and the heterogeneity of this group (Chinese) were not statistically significant (P = .365, I2 = 7.2%). One trial (Bahloul) with 79 patients reported the events of 28-day mortality in the races of African. The 28-day mortality was 67.35% (33/49) in the dopamine group while the mortality was 53.33% (16/30) in the norepinephrine group (RR: 1.611, 95% CI: 1.219–2.129, P = .238 > 0.05). Monte Carlo permutation test was conducted for meta-regression, and P = .50, which indicated that there was between-group heterogeneity and drawn a conclusion that different races affect the heterogeneity.
Finally, in subgroup analysis based on causes of CS shown in Fig. 4, 1 study[12] with 47 patients reported the events of 28-day mortality in the CS caused by CHD. The 28-day mortality was 50% (11/22) in the dopamine group while the mortality was 20% (5/25) in the norepinephrine group (RR: 2.500, 95% CI: 1.028–6.078, P = .043 < .05). Another 3 trials[10,14,16] with 181 patients reported the events of 28-day mortality in the mixed causes of CS. The 28-day mortality was 42.0% (42/100) in the dopamine group while the mortality was 23.46% (19/81) in the norepinephrine group (RR: 1.767, 95% CI: 1.112–2.806, P = .016 < .05). Monte Carlo permutation test was conducted for meta-regression, and P = .40, which indicated that there was no significant between-group heterogeneity and drawn a conclusion that different causes of CS didn’t affect the heterogeneity.
Figure 4.

Cumulative analyses of CS. (A) Cumulative analysis in the CS caused by coronary heart disease. (B) Cumulative analysis in CS caused by mixed diseases. CS = cardiogenic shock.
3.8. Subgroup analyses on dopamine or norepinephrine and arrhythmic events
Subgroup analyses were conducted to detect and evaluate potential clinically significant heterogeneity by dividing these studies into several groups according to different arrhythmias (Fig. 3B). In subgroup analysis based on different arrhythmias, 2 studies[15,17] with 82 patients reported the events of atrial fibrillation. The incidence of atrial fibrillation was 32.5% (13/40) in the dopamine group while the incidence was 9.52% (4/42) in the norepinephrine group (RR: 3.524, 95% CI: 1.264–9.824, P = .016 < .05), and the heterogeneity of this group (AF) were not statistically significant (P = .779, I2 = 0%). These 2 studies also reported the events of ventricular fibrillation. The incidence of ventricular fibrillation was 7.5% (3/40) in the dopamine group while the incidence was 2.38% (1/42) in the norepinephrine group (RR: 2.543, 95% CI: 0.409–15.820, P = .317 > .05). Monte Carlo permutation test was conducted for meta-regression, and P = .68, which indicated that there was no significant between-group heterogeneity and drawn a conclusion that different arrhythmias didn’t affect the heterogeneity.
3.9. Sensitivity analysis
The sensitivity analysis was conducted to discover whether the lack of each study will change the pooled OR quantitatively. After the individual study was removed, no results were changed. It proves that the overall meta-analysis results were reliable.
3.10. Assessment of bias
It's no doubt that publication bias is another common problem need to be solved. So the bias was estimated by calculating Begg test and Egger test, drawing the trim and fill funnel plot (Fig. 5A). Obviously, all the studies were distributed symmetrically on both the 2 sides from the funnel plot, which indicated that the publication bias was low in our meta-analysis (Begg, P = .45; Egger, P = .16; all values of P > .05). Harbord test and a contour-enhanced funnel plot were also conducted to further define the potential heterogeneity. It turned out that inter-study heterogeneity might exist (Fig. 5A). Therefore, subgroup analyses were conducted carefully and the result was shown in Fig. 3.
Figure 5.
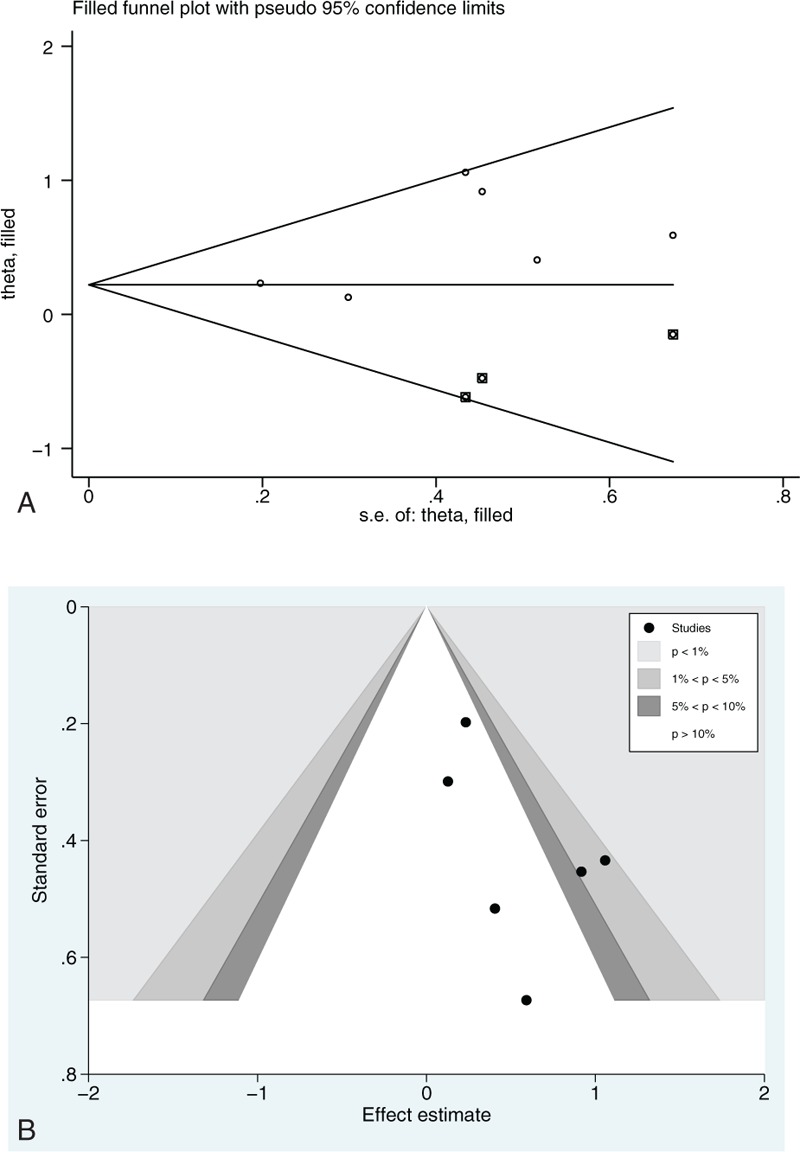
Begg funnel plot with pseudo 95% confidence limits: (A) trim and fill analysis; (B) a contour-enhanced funnel plot. 28-day mortality was extracted for analysis. logOR = logarithm of odds ratio, s.e. = standard error.
4. Discussion
In general, we found that there was a significant decrease in norepinepherine group for correcting CS in 28-day mortality, arrhythmic events, events of gastrointestinal reaction in overall patients compared with the dopamine group. A series of subgroup analyses were conducted according to main basic characteristics of studies concerned. However, in some studies which didn’t report the events of 28-day mortality,[11,13,15] arrhythmic events,[18] events of gastrointestinal reaction[10,12,13,15–18] in 2 groups, we couldn’t calculate the estimated effect and study a statistically significant decrease in the norepinepherine group compared with the dopamine group. We could only study the general tendency. The individual results were different in each study concerned. Six studies[10,12,14,16–18] revealed that NA was superior to DA in the 28-day mortality while another study[15] indicated that there was no significant difference between DA and NA in Intensive-Care-Unit mortality and mortality during hospitalization. Due to the limited number of patients included in each study, these data should be handled carefully. As a result, a contour-enhanced funnel plot was conducted to further detect the potential heterogeneity on the basis that all the 6 studies were symmetrically distributed on both sides of the Begg funnel plot. It was clearer that all the studies were distributed symmetrically after applying trim and fill analysis. It demonstrated that the publication bias was low in our meta-analysis. At last, the inter-study heterogeneity was founded by the contour-enhanced funnel plot. Subgroup analysis and formal interaction tests with meta-regression analyses by predefined variables were conducted to find out the sources of heterogeneity.
In the subgroup analyses according to mean ages, sample sizes and causes of CS, we discovered that NA was better than DA in the 28-day mortality for correcting CS no matter how old the patients is, what the sample size it is, and what the causes of the patients are. Nevertheless, among the Chinese patients, NA was superior to DA in the 28-day mortality while it's not clear which is better for norepinephrine or dopamine among the African patients.
In the subgroup analyses based on different arrhythmic events, we found that DA was inferior to NA when correcting CS in the incidence of atrial fibrillation, while there was no adequate evidence that NA is superior to DA in the incidence of ventricular fibrillation.
These results were confirmed in the formal interaction test with meta-regression and rigorous discussion. Firstly, the results of subgroup analyses were exactly consistent with the assumptions that NA was superior to DA in the 28-day mortality, arrhythmic events, events of gastrointestinal reaction in overall patients for correcting CS as the conclusions of subgroup analyses were not contradictory statements. Secondly, the P values of heterogeneity after subgroup analyses were higher than the overall analysis in the subgroups based on mean ages and causes of CS, which indicated lower bias. It made the conclusions of subgroup analyses more convincing. Thirdly, the P value for interaction based on the different races was statistically significant. It indicated that heterogeneity might come from the races of the patients involved. Finally, NA was superior to DA for correcting CS and could be used to clinical treatment. As NA was associated with the lower 28-day morality than DA in subgroups based on mean ages and causes of CS, the root causes of this result need to be discussed.
Although both DA and NA belong to catecholamine and can increase blood pressure for correcting CS, the main mechanisms of DA and NA are totally different. NA is an endogenous agent of sympathetic nervous system, with a powerful effect on stimulating α-adrenergic receptor and less effect on β-adrenergic receptor. It can shrink the blood vessels, increase cardiac output slightly, and improve stroke volume, thereby raise mean arterial pressure and slow the heart rate by a compensatory vagal reflex. NA is a powerful vasoconstrictor which has a dose-dependent effect on the cardiovascular system. The recommended dose is from 0.01 to 3.3 mg/kg/min. DA is a predrug of NA, whose effect also depends on the dose used. When it is used at low doses (<2 μg/kg/min), it stimulates D1 receptor and then expends the renal vessels, improves the volume of urine. When it's used at middle doses (2–10 μg/kg/min), it agitates β receptors, and then increases cardiac contractility and heart rate. When it's used at high doses (10–20 μg/kg/min), it activates α receptor, shrinks the blood vessels, and then causes increased afterload. These effects may overlap particularly in critically ill patients.[2,23]
As we all know, the vasoconstrictive effects of NA may lead to decreased blood flow of kidney, viscera, and peripheral vessel, and then cause increased afterload, especially in those patients who have not been fully recovered yet. On the other hand, previously, DA at a low dose was thought to protect renal in addition to raising blood pressure by increasing cardiac output and improving renal perfusion. That's why we chose norepinephrine instead of dopamine to treat CS previously.
However, at present, norepinephrine is suggested to be used as the first choice for correcting CS instead of dopamine (weak recommendation, low quality of evidence).[24] The resent studies and reasons are listed as follows. In the SOAP II trial[8] which grouped the patients involved based on etiologies of shock, the mortality was lower in the NA group than DA group in the subgroup of CS probably because of the dysrhythmias caused by DA. Besides, a clinical trial with randomized 328 critically ill patients with early renal dysfunction to low-dose dopamine or placebo, concluded that there was no difference between the 2 groups in peak serum creatinine, other renal outcomes, survival, and hospital stay.[25]
There are some advantages in this meta-analysis. Firstly, as far as we know, this is the first meta-analysis about comparison of NA to DA in CS. Secondly, the patients concerned cover different ages and different causes of CS which may affect the prognosis of CS. We can find something more clinically meaningful from it. The last thing, all the studies concerned was randomized controlled trials of high quality and convincing conclusions.
There are also limitations in our meta-analysis. Firstly, the sample sizes of the studies so far are so small with a number less than 100 that we can’t calculate the effect of DA and NA on patients accurately. In a study with a larger sample size,[8] there is a lack of specific data on cardiogenic shock. We tried to contact the author for data but failed, which may affect the analysis of all the data. This requires more researches with large samples to compare the 2 drugs. Secondly, there is no specific information of the patients involved in the meta-analysis, such as atrial fibrillation, ischemia of peripheral vessels, the changes of creatinine, the events of coronary spasm, which may have an influence on the status of NA for providing blood pressure support in CS and long-term prognosis of patients with CS. Thirdly, we grouped the patients based on the mean ages and whether the CSs were from coronary heart disease or not. Fourthly, the target blood pressure after using DA or NA was not defined clearly. The physician in charge determined it according to their clinical experience, which may lead to bias as a confounding factor. Fifthly, the effect of DA depends on its dose. The maximum dose of DA used may impact the whole statistical results.
Our meta-analysis may, in a way, point the way for future research. Furthermore, there are something meaningful need to be addressed. Can norepinephrine and dopamine be combined effectively for correcting CS?[20] As we all know, CS is a pump failure with a balance between cardiac output and total peripheral resistance. Patients of CS can be at different stages with different cardiac output and total peripheral resistance.[20] We can combine the efficacy for cardiac output of DA with the efficacy for total peripheral resistance of NA to correct CS effectively after dilation of blood volume. If the 2 drugs are combined, what are the appropriate doses and ideal combinations? How can the data of hemodynamic monitoring be used effectively to guide the combination? More randomized controlled trials which compare NA with DA in patients with CS are needed to be conducted to guide clinical treatment.
This meta-analysis makes a conclusion that NA is associated with less 28-day morality, arrhythmic events, the incidence of gastrointestinal reaction than DA, and are not affected by ages and etiologies. More studies with randomized controlled trials which compare NA with DA in patients of CS with different characteristics are needed to be conducted to guide clinical treatment effectively.
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CAI = cardiac index, CHD = coronary heart disease, CI = confidence interval, CS = cardiogenic shock, DA = dopamine, HR = heart rates, LAC = lactic acid, MAP = mean arterial pressure, MI = myocardial infarction, NA = norepinephrine, OR = odds ratio, RCT = randomized clinical trials, RR = risk ratio, SMD = standardized mean difference, UV = urine volume, VF = ventricular fibrillation.
QR and YJ have contributed equally to this work.
Funding: Our work received financial support from National Natural Science Foundation of China (81170174), Jiangsu Province's Key Provincial Talents Program (RC2016056), and Natural Scientific Fund of Jiangsu province (BK20161226). The funders had no roles in study design, data collection and analysis, preparation of the manuscript, or decision to publish. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosures: We declare no relationships with industry.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008;117:686–97. [DOI] [PubMed] [Google Scholar]
- [2].Nativi-Nicolau J, Selzman CH, Fang JC, et al. Pharmacologic therapies for acute cardiogenic shock. Curr Opin Cardiol 2014;29:250–7. [DOI] [PubMed] [Google Scholar]
- [3].Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res 2017;6:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv 2017;10:e004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalmanovich E, Blatt A, Brener S, et al. Trends in the management and outcomes of patients admitted with acute coronary syndrome complicated by cardiogenic shock over the past decade: real world data from the acute coronary syndrome Israeli survey (ACSIS). Oncotarget 2017;8:42876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De LL, Olivari Z, Farina A, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eu J Heart Fail 2015;17:1124–32. [DOI] [PubMed] [Google Scholar]
- [7].Levy B, Perez P, Perny J, et al. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med 2011;39:450–5. [DOI] [PubMed] [Google Scholar]
- [8].Magda S, Margulescu AD. Comparison of dopamine and norepinephrine in the treatment of shock. Maedica (Buchar) 2010;5:69–70. [PMC free article] [PubMed] [Google Scholar]
- [9].Levy B, Bastien O, Karim B, et al. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care 2015;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang GJ, Guo YJ, Hou J, et al. Comparison of the effect of dopamine and norepinephrine on cardiogenic shock. Chin J Pract Med 2011;38:75–6. [Google Scholar]
- [11].Qiu HX, Lin HL, Gu Y, et al. Comparison of dopamine and norepinephrine on treating patients with cardiogenic shock caused by coronary heart disease. Chin J Mod Drug Appl 2013;7:139–40. [Google Scholar]
- [12].He YH, Chen L, Jiao YL, et al. The contrast between dopamine and norepinephrine in the treatment of cardiogenic shock. Northern Pharma 2014;2:51. [Google Scholar]
- [13].Gu XF. Contrastive analysis of the therapeutic effect of IABP with dopamine and norepinephrine for treating acute myocardial infarction with cardiogenic shock. Chin J Mod Drug Appl 2014;13:98–9. [Google Scholar]
- [14].Tan ZH. The comparison of dopamine and norepinephrine for treating cardiogenic shock. Mod Diagn Treat 2016;27:67–8. [Google Scholar]
- [15].Xiong RC, Yu Z, Sun J, et al. Efficacy of dopamine and norepinephrine in patients with cardiogenic shock. Chin J Mult Organ Dis 2016;15:919–22. [Google Scholar]
- [16].Li JD, Liu LX, Guo M. Effects of norepinephrine and dopamine on haemodynamics and tissue perfusion in patients with cardiogenic shock. Med Theory Pract 2015;28:3171–3. [Google Scholar]
- [17].Jin X. The comparison between dopamine and norepinephrine in shock. Chin Tradit Med Technol 2014;1:186. [Google Scholar]
- [18].Bahloul M, Tounsi A, Ben AN, et al. Does change of catecholamine use improve the outcome of patients with shock admitted to intensive care unit? Am J Ther 2014;21:358–65. [DOI] [PubMed] [Google Scholar]
- [19].Kapur NK, Davila CD, Jumean MF. Integrating interventional cardiology and heart failure management for cardiogenic shock. Interv Cardiol Clin 2017;6:481–5. [DOI] [PubMed] [Google Scholar]
- [20].He B. Cardiogenic shock: dopamine or norepinepherine? It's a question. Cardiol Plus 2017;2:1–4. [Google Scholar]
- [21].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med 2011;183:847–55. [DOI] [PubMed] [Google Scholar]
- [24].Møller MH, Claudius C, Junttila E, et al. Scandinavian SSAI clinical practice guideline on choice of first-line vasopressor for patients with acute circulatory failure. Acta Anaesthesiol Scand 2016;60:1347–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial∗. Lancet 2000;356:2139–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


