Abstract
Although elevated resting heart rate is related to poor outcomes in heart failure (HF) with reduced ejection fraction, the association in HF with preserved ejection fraction (HFpEF) remains inconclusive. Therefore, we conducted a dose–response meta-analysis to examine the prognostic role of heart rate in patients with HFpEF.
We searched PubMed and Embase databases until April 2017 and manually reviewed the reference lists of relevant literatures. Random effect models were used to pool the study-specific hazard ratio (HR) of outcomes, including all-cause death, cardiovascular death, and HF hospitalization.
Six studies with 7 reports were finally included, totaling 14,054 patients with HFpEF. The summary HR (95% confidence interval [CI]) for every 10 beats/minute increment in heart rate was 1.04 (1.02–1.06) for all-cause death, 1.06 (1.02–1.10) for cardiovascular death, and 1.05 (1.01–1.08) for HF hospitalization. Subgroup analyses indicated that these positive relationships were significant in patients with sinus rhythm but not in those with atrial fibrillation. There was also evidence for nonlinear relationship of heart rate with each of the outcomes (All P for nonlinearity < .05).
Higher heart rate in sinus rhythm is a risk factor for adverse outcomes in patients with HFpEF. Future trials are required to determine whether heart rate reduction may improve the prognosis of HFpEF.
Keywords: ejection fraction, heart failure, heart rate, meta-analysis
1. Introduction
Elevated resting heart rate is considered as a risk factor for morbidity and cardiovascular mortality in a broad spectrum of cardiovascular diseases.[1] In patients with heart failure (HF) and reduced ejection fraction (HFrEF), with or without HF symptoms or signs, increased heart rate has been correlated with adverse outcomes, independently of traditional risk factors.[2–4] Accordingly, heart rate reduction with ivabradine has been identified as an effective therapy for patients with HFrEF.[5] In view of these findings, the European Society of Cardiology recommends ivabradine for symptomatic HFrEF patients in sinus rhythm with heart rates remaining ≥75 beats/minute (bpm) despite optimal evidence-based treatment.[6]
HF with preserved ejection fraction (HFpEF) represents up to half of HF cases and exhibits similar prognostic profile to HFrEF.[7] However, it is unclear whether higher heart rate is also associated with poor outcomes in patients suffering from HFpEF. Currently, there are few investigations that have been performed to address this issue, and the results remain inconsistent. Some studies showed that elevated resting heart rates have unfavorable prognostic impacts on HFpEF,[8–11] while others not.[12,13] Thus, we carried out a dose–response meta-analysis to comprehensively evaluate the prognostic role of heart rate in patients with HFpEF. We hypothesized that higher heart rate is associated with poor outcomes in HFpEF patients.
2. Methods
2.1. Search strategy
This meta-analysis was conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology guidelines.[14] We carried out a systematical literature search in PubMed and Embase databases from inception to April 2017 to identify eligible studies, using the search strings as follows: (“heart failure with preserved ejection fraction” OR “heart failure with normal ejection faction” OR “diastolic heart failure”) AND (“heart rate”). Moreover, the reference lists of relevant articles and reviews were manually scrutinized to find additional studies that were eligible for inclusion.
2.2. Included criteria
We included clinical studies if they met the following requirements: the study was designed as cohort study, case–control study, or post hoc analysis of randomized trials; with follow-up durations of ≥1 year; the exposure of interest was heart rate; the outcomes included all-cause death, cardiovascular death, and HF hospitalization; the adjusted risk estimates of outcomes, such as hazard ratios (HRs), were reported for ≥3 categories of heart rate. Reviews, comments, duplicated publications, non-English articles, and pooling analyses of original studies were excluded.
2.3. Data collection and quality assessment
Two reviewers independently extracted the study characteristics, including study author, publication year, study design, sample size, location, inclusion criteria for ejection fraction, heart rhythm, follow-up duration, and variables adjusted in multivariable analysis. We also recorded the maximally adjusted risk estimates of outcomes for each category of heart rate. If necessary, the corresponding author of the study was contacted for missing information. The methodological quality of included studies was appraised using the Newcastle-Ottawa Scale (NOS).[15] With this scale, every study can score up to 9 points: 4 for study group selection, 2 for comparability between group, and 3 for ascertainment of outcomes. A study with a NOS score of ≥7 was considered of high quality. Any disagreements between the 2 reviewers were handled by consulting with a third reviewer.
2.4. Statistical method
We used HRs with 95% confidence intervals (CIs) to report the summary risk estimates. Due to the different cut-off points for categories of heart rate across studies, we calculated a HR with 95% CI for every 10 bpm increase in heart rate for each study. The strategy proposed by Greenland and Longnecker[16] and Orsini et al[17] was used to compute the trend from the correlated estimates for log HR across categories of heart rate. In addition, a potential curvilinear relationship between heart rate and risk of adverse outcomes was investigated by using restricted cubic splines with 3 knots at 25th, 50th, and 75th percentiles of the exposure distribution. The P-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero.
For each study, the mean or median heart rate for each category was assigned to each corresponding risk estimate. When the mean or median heart rate was unavailable, the midpoint of the upper and lower boundaries in each category was used as the average heart rate. If the lowest or highest category was open-ended, we assumed the width of the category to be the same as of the adjacent category.[18] If the lowest category was not adopted as a reference, we recalculated the HRs with 95% CIs relative to the lowest category according to the method described by Hamling et al.[19]
The statistical heterogeneity among studies was detected by the Cochrane Q test with a significant level of P < .1. We also reported the heterogeneity as low, moderate, and high with I2 values of <25%, 25% to 75%, and >75%, respectively. The study-specific HR was pooled using random effect model. Subgroup analyses were conducted according to study design, location, sample size, and heart rhythm, with differences between subsets confirmed by the Altman and Bland test.[20] We also carried out a sensitivity analysis by omitting study one at a time. Potential publication bias was evaluated by funnel plot and Egger test. All data analyses were realized with STATA 13.0 (StataCorp, College Station, TX) and R 3.2.5 (The R Foundation for Statistical Computing, Vienna, Austria) softwares, and 2-sided P values < .05 were considered of significance.
2.5. Ethics statement
This study was a secondary analysis regarding human subject data published in the public domain, thus no ethical approval was required.
3. Results
3.1. Search process
The process of literature search and selection is exhibited in Fig. 1. Briefly, we identified 5514 records in the preliminary search. After scanning the titles or abstracts, 5469 articles were excluded. The remaining 45 publications underwent full-text screening, of which 39 that failed to meet the inclusion criteria were removed. In total, 6 studies[8–13] with 7 reports that were published between 2010 and 2017 were finally included.
Figure 1.
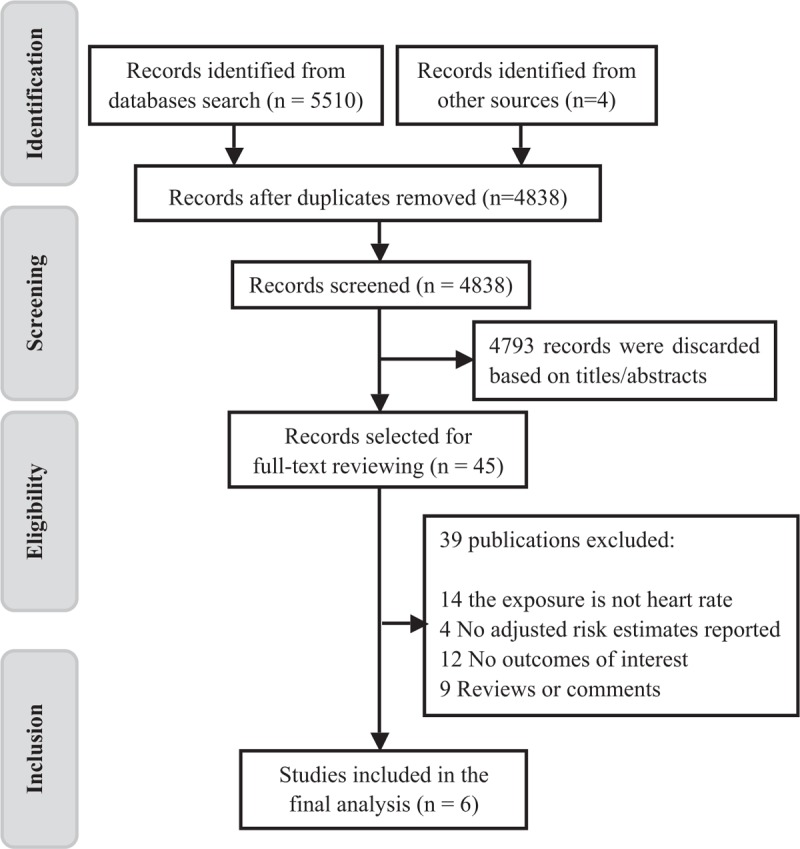
Flow diagram of study search process.
3.2. Study characteristics
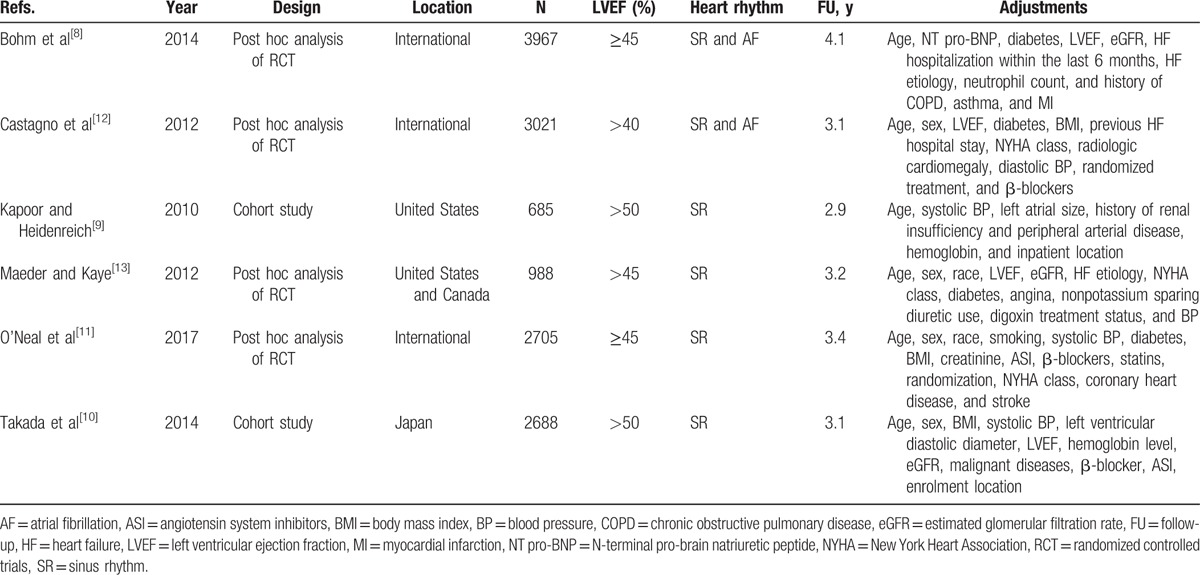
As shown in Table 1, the set of eligible studies consists of 2 cohort studies and 4 post hoc analyses of randomized trials, totaling 14,054 patients with HFpEF. Of the included studies, 2 were conducted in North America, 1 in Japan, and the remaining 3 were international, with follow-up durations ranging from 2.9 to 4.1 years. The most commonly adjusted variables among the studies were age, sex, and ejection fraction. The study quality scores varied from 7 to 9 with a mean NOS score of 7.8, suggesting the presence of high methodological quality.
Table 1.
Baseline characteristics of included studies.
3.3. All-cause death
All studies have reported the risk of all-cause death, with moderate heterogeneity among the studies (I2 = 57%, P = .03). The summarized HR of all-cause death for an increase in heart rate of 10 bpm was 1.04 (95% CI: 1.02–1.06; Fig. 2). By using a restricted cubic spline model, we also found a curvilinear association between heart rate and all-cause mortality (P for nonlinearity = .001; Fig. 3).
Figure 2.
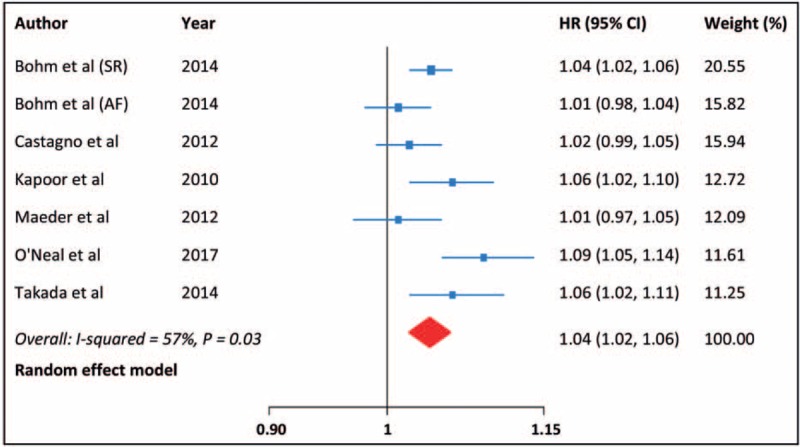
All-cause death for each 10 bpm increase in heart rate.
Figure 3.
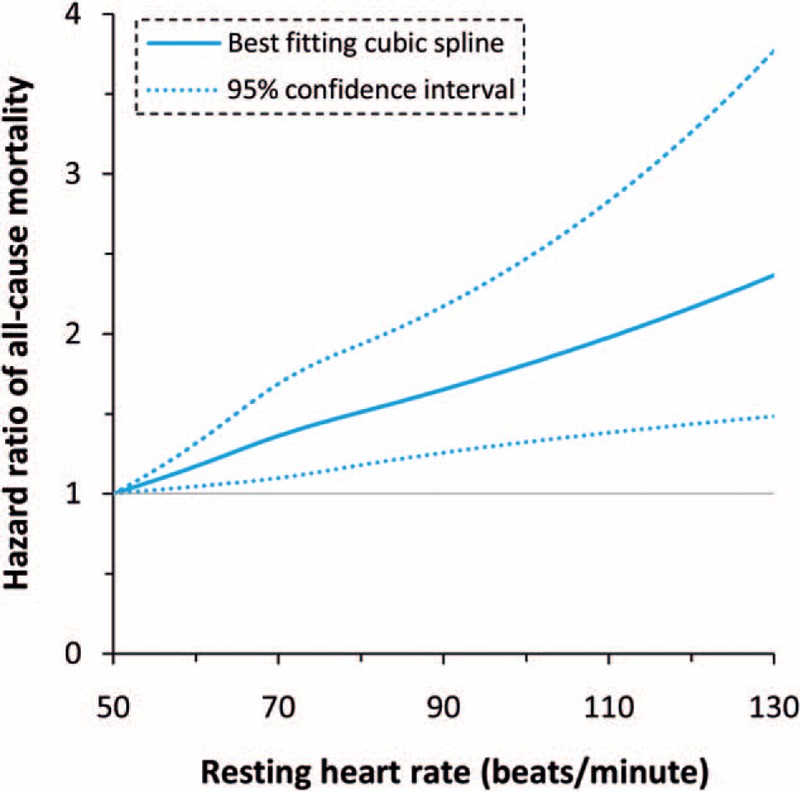
Dose–response analysis of the association of heart rate with all-cause death.
Subgroup analyses demonstrated that the positive relationship of heart rate with all-cause mortality was significant in patients with sinus rhythm (HR: 1.05, 95% CI: 1.03–1.07), but not in those with atrial fibrillation (AF) (HR: 1.01, 95% CI: 0.98–1.04; P for interaction = .03). The increased risk of death for higher heart rate was not modified by study design, location, and sample size. Leave-one-out sensitivity analysis had no influence on the result. There was no indication of publication bias from the funnel plot (Fig. 4) and Egger test (P = .58).
Figure 4.
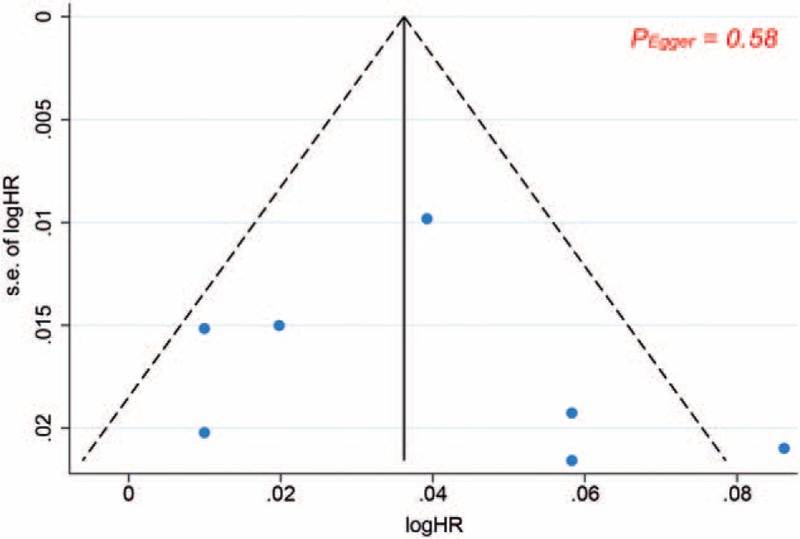
Funnel plot analysis for all-cause death.
3.4. Cardiovascular death and HF hospitalization
The risk estimates of cardiovascular death and HF hospitalization were provided in 4 and 5 reports, respectively; there was moderate heterogeneity across the reports. The pooled HR (95% CI) for each 10 bpm increment in heart rate was 1.06 (1.02–1.10) for cardiovascular death and 1.05 (1.01–1.08) for HF hospitalization (Fig. 5). In addition, there was also an evidence of curvilinear relationship between heart rate and each of the outcomes (cardiovascular death: P for nonlinearity = .04, HF hospitalization: P for nonlinearity = .02; Fig. 6).
Figure 5.
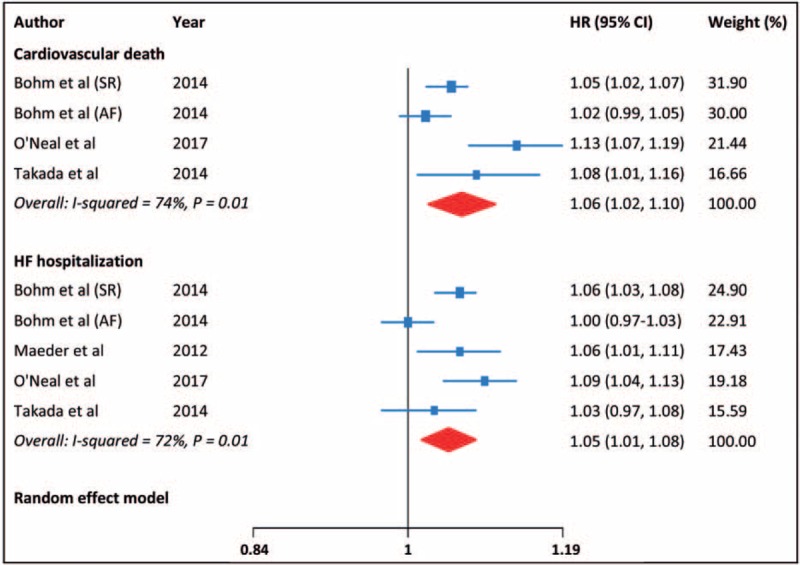
Cardiovascular death and HF hospitalization for each 10 bpm increase in heart rate.
Figure 6.
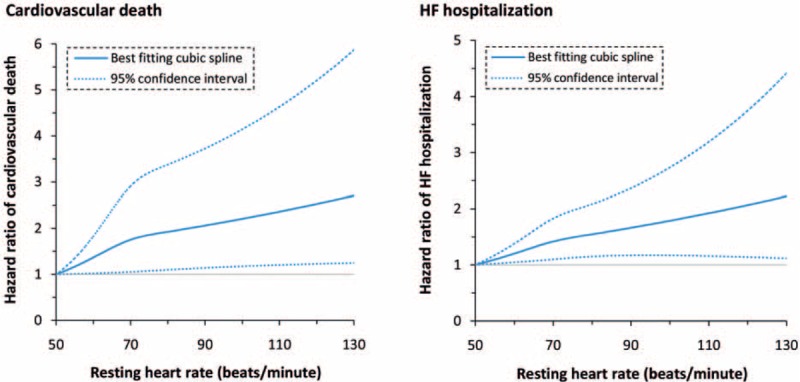
Dose–response analysis of the association of heart rate with cardiovascular death and HF hospitalization.
Similarly, stratified analyses indicated that cardiac rhythm, but not other aforementioned variables, may have an interaction with the risks of cardiovascular death and HF hospitalization (P for interaction = .056 and P for interaction = .001, respectively). Exclusion of single study in sequence had no influence on our findings. There was no evidence of publication bias from funnel plots (Fig. 7) and Egger tests (P = .35 and P = .90, respectively).
Figure 7.
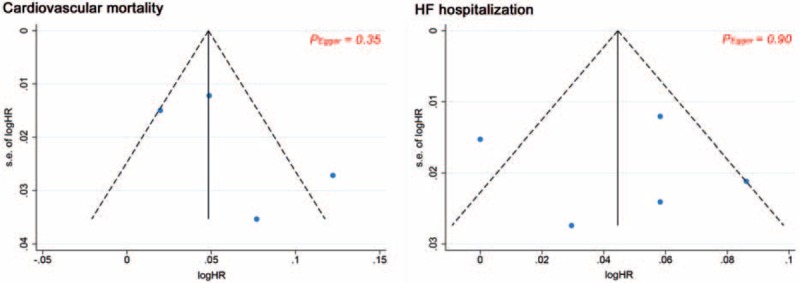
Funnel plot analyses for cardiovascular death and HF hospitalization.
4. Discussion
There are limited studies that have investigated the relationship between heart rate and unfavorable outcomes in HFpEF. The present meta-analysis indicates that in patients with HFpEF, each 10 bpm increase in heart rate is associated with increased risks of all-cause death, cardiovascular death, and HF hospitalization. Moreover, this positive association is significant in patients with sinus rhythm but not in those with AF.
In line with our results, some other studies not included in this meta-analysis also revealed the adverse impact of increased heart rate on HFpEF outcomes. Komajda et al[21] analyzed data of the same cohort included in Bohm study,[8] and found that an increase in heart rate of 5 bpm was related to increased risks of all-cause death (HR: 1.06, 95% CI:1.03–1.09) and HF death or hospitalization (HR: 1.05, 95% CI: 1.01–1.08) in patients with HFpEF. Additionally, an investigation of 145,221 admissions for HF demonstrated that higher admission heart rate was an independent predictor for worse in-hospital outcomes, irrespective of ejection fraction.[22]
Several mechanisms have been proposed to explain the relationship between higher heart rate and poor outcomes in HF. Patients with elevated heart rates have a relatively high prevalence of certain comorbidities,[23] the prognostic impacts of which were not completely controlled for in the multivariate analyses. Some of the comorbidities (e.g., diabetes) may also lead to autonomic neuropathy that could contribute to the increased heart rates. Whatever the cause, elevated heart rate is a reflection of increased energy cost,[24] blunting of the positive force–frequency relationship,[25] and myocardial ischemia due to shortened diastole.[26] These 3 factors alone or in combination may deteriorate the already impaired myocardial relaxation in patients with HFpEF. In addition, increased heart rate is directly associated with higher effective arterial elastance, a lumped parameter of pulsatile and resistive afterload, resulting in disordered ventricular–arterial coupling,[27] which is considered as an important pathophysiological factor in the development of HFpEF.[28] Accordingly, in an experimental model of HFpEF, a reduction in heart rate by If-inhibition has been shown to improve arterioventricular interaction and elastance, as well as diastolic function.[29]
Interestingly, we identified a lack of predictive value of increased heart rate in patients with AF. Although there were limited studies available for the subanalysis of AF, the significant interaction between cardiac rhythm and prognosis associated with heart rate suggests that this result is a possible one. Besides, a similar finding was also obtained in many previous studies of HFrEF patients.[30,31] More directly, a recent meta-analysis of individual patient data showed that in patients with chronic HF, heart rate did not have the same prognostic value in patients with AF as it did in those with sinus rhythm.[32] One plausible explanation is that a lower ventricular rate in patients with AF may indicate disorders in conducting system, which is a poor prognostic sign.[31] Furthermore, HF patients with AF are at intrinsically higher risk for unfavorable outcomes, in particular in those with HFpEF,[33] and this elevated baseline risk may attenuate the effect of heart rate.
If correct, our findings also raise the possibility that heart rate reduction may confer benefits for patients with HFpEF, as it does for those with HFrEF. Clinical data from randomized trials regarding the therapeutic effects of heart rate reduction are, however, relatively scarce. In the J-DHF (Japanese Diastolic Heart Failure) study, carvedilol had neutral effects on the prognosis of HFpEF patients overall; but the investigators pointed out that the standard dose (>7.5 mg/day), not the low dose, prescription of carvedilol might be effective.[34] For heart rate reduction with ivabradine, a previous study showed that it could improve the excise capacity in patients with HFpEF.[35] Nevertheless, in 2 recent randomized trials, ivabradine did not improve the cardiac function and symptoms in HFpEF subjects.[36,37] Of note, all these clinical trials have a small sample size and are limited in short-term outcomes, perhaps leading to an insufficient power to detect the true effect of ivabradine.
There are several limitations that should be acknowledged. First of all, the majority of included studies are post hoc analyses of randomized trials, the cohorts in which may not represent the real world populations. Second, moderate heterogeneity is present in the pooling analyses of outcomes, possibly due to the difference in eligibility criteria for ejection fraction and heart rhythm. Third, the adjusted variables in multivariate models are different among studies. Important factors including comorbidities and medications were not controlled for in some studies.
5. Conclusion
In summary, the present meta-analysis shows that in patients with HFpEF, higher heart rate in sinus rhythm but not in AF is associated with poor outcomes. However, our findings need to be interpreted cautiously because of the limitations mentioned above. Additional large, well-designed trials are needed to determine whether heart rate reduction may improve the prognosis of HFpEF.
Footnotes
Abbreviations: AF = atrial fibrillation, bpm = beats/minute, CI = confidence interval, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, HR = hazard ratio, NOS = Newcastle-Ottawa Scale.
XS and RL contributed equally to this work.
Funding: This work was supported by grants from the National Key Research and Development Program of China (grant no. 2016YFA0101103).
The authors have no conflicts of interest to disclose.
References
- [1].Bohm M, Reil JC, Deedwania P, et al. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med 2015;128:219–28. [DOI] [PubMed] [Google Scholar]
- [2].Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010;376:886–94. [DOI] [PubMed] [Google Scholar]
- [3].Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817–21. [DOI] [PubMed] [Google Scholar]
- [4].DeVore AD, Schulte PJ, Mentz RJ, et al. Relation of elevated heart rate in patients with heart failure with reduced ejection fraction to one-year outcomes and costs. Am J Cardiol 2016;117:946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- [6].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- [7].Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- [8].Bohm M, Perez AC, Jhund PS, et al. Relationship between heart rate and mortality and morbidity in the irbesartan patients with heart failure and preserved systolic function trial (I-Preserve). Eur J Heart Fail 2014;16:778–87. [DOI] [PubMed] [Google Scholar]
- [9].Kapoor JR, Heidenreich PA. Heart rate predicts mortality in patients with heart failure and preserved systolic function. J Card Fail 2010;16:806–11. [DOI] [PubMed] [Google Scholar]
- [10].Takada T, Sakata Y, Miyata S, et al. Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the CHART-2 Study. Eur J Heart Fail 2014;16:309–16. [DOI] [PubMed] [Google Scholar]
- [11].O’Neal WT, Sandesara PB, Samman-Tahhan A, et al. Heart rate and the risk of adverse outcomes in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol 2017;24:1212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Castagno D, Skali H, Takeuchi M, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol 2012;59:1785–95. [DOI] [PubMed] [Google Scholar]
- [13].Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol 2012;155:249–56. [DOI] [PubMed] [Google Scholar]
- [14].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [15].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed May 10, 2017. [Google Scholar]
- [16].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [17].Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57. [Google Scholar]
- [18].Hartemink N, Boshuizen HC, Nagelkerke NJ, et al. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol 2006;163:1042–52. [DOI] [PubMed] [Google Scholar]
- [19].Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- [20].Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Komajda M, Carson PE, Hetzel S, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE). Circ Heart Fail 2011;4:27–35. [DOI] [PubMed] [Google Scholar]
- [22].Bui AL, Grau-Sepulveda MV, Hernandez AF, et al. Admission heart rate and in-hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J 2013;165:567.e6–74.e6. [DOI] [PubMed] [Google Scholar]
- [23].van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16:103–11. [DOI] [PubMed] [Google Scholar]
- [24].Conway MA, Allis J, Ouwerkerk R, et al. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 1991;338:973–6. [DOI] [PubMed] [Google Scholar]
- [25].Hasenfuss G, Holubarsch C, Hermann HP, et al. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J 1994;15:164–70. [DOI] [PubMed] [Google Scholar]
- [26].Colin P, Ghaleh B, Monnet X, et al. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 2004;308:236–40. [DOI] [PubMed] [Google Scholar]
- [27].Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86:513–21. [DOI] [PubMed] [Google Scholar]
- [28].Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003;107:714–20. [DOI] [PubMed] [Google Scholar]
- [29].Reil JC, Hohl M, Reil GH, et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J 2013;34:2839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mulder BA, Damman K, Van Veldhuisen DJ, et al. Heart rate and outcome in heart failure with reduced ejection fraction: differences between atrial fibrillation and sinus rhythm-A CIBIS II analysis. Clin Cardiol 2017;40:740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cullington D, Goode KM, Zhang J, et al. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail 2014;2:213–20. [DOI] [PubMed] [Google Scholar]
- [32].Simpson J, Castagno D, Doughty RN, et al. Is heart rate a risk marker in patients with chronic heart failure and concomitant atrial fibrillation? Results from the MAGGIC meta-analysis. Eur J Heart Fail 2015;17:1182–91. [DOI] [PubMed] [Google Scholar]
- [33].Atwood JE, Myers J, Sullivan M, et al. Maximal exercise testing and gas exchange in patients with chronic atrial fibrillation. J Am Coll Cardiol 1988;11:508–13. [DOI] [PubMed] [Google Scholar]
- [34].Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013;15:110–8. [DOI] [PubMed] [Google Scholar]
- [35].Kosmala W, Holland DJ, Rojek A, et al. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol 2013;62:1330–8. [DOI] [PubMed] [Google Scholar]
- [36].Pal N, Sivaswamy N, Mahmod M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 2015;132:1719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Komajda M, Isnard R, Cohen-Solal A, et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur J Heart Fail 2017;doi: 10.1002/ejhf.876. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


