Abstract
Background
Intrinsic cardiac nerve activities (ICNA) and the skin nerve activities (SKNA) are both associated with cardiac arrhythmias in dogs.
Objective
To test the hypothesis that ICNA and SKNA correlate with postoperative cardiac arrhythmias in humans.
Methods
Eleven patients (60±13 years old; 4 female) were enrolled in this study. Electrical signals were simultaneously recorded from electrocardiogram (ECG) patch electrodes on the chest wall and from two temporary pacing wires placed during open heart surgery on the left atrial epicardial fat pad. The signals were filtered to display SKNA and ICNA. Premature atrial contractions (PAC) and premature ventricular contractions (PVC) were manually determined. The SKNA and ICNA of the first 300 min of each patient were calculated min-by-min to determine baseline average amplitudes of nerve activities and to determine their correlation with arrhythmia burden.
Results
We processed 1365±973 min of recording per patient. Low-amplitude SKNA and ICNA were present at all time, while the burst discharges were observed much less frequently. Both SKNA and burst ICNA were significantly associated with the onset of PACs and PVCs. Baseline average ICNA (aICNA), but not SKNA (aSKNA), had a significant association with PAC burden. The correlation coefficient (r) between aICNA and PAC burden was 0.78 (P<0.01). A patient with the greatest aICNA developed postoperative atrial fibrillation.
Conclusions
ICNA and SKNA can be recorded from human patients in the postoperative period. Baseline magnitude of ICNA correlates with PAC burden and development of postoperative atrial fibrillation.
Keywords: Arrhythmias, Atrial fibrillation, Autonomic nervous system, Cardiac electrophysiology, premature atrial contractions, premature ventricular contractions
Introduction
Autonomic nerve activity is known to be important in cardiac arrhythmogenesis both in animal models and in humans. We have documented that it is feasible to directly record both intrinsic cardiac nerve activities (ICNA) and skin nerve activities (SKNA) in ambulatory dogs and relate the nerve activities to spontaneous heart rate variations and the occurrence of arrhythmias.1, 2 ICNA from the superior left ganglionated plexi and the ligament of Marshall invariably precedes the onset of paroxysmal atrial fibrillation (AF).1 These findings suggest a causal relationship between nerve activities and atrial arrhythmias in canine models. In human patients, it was possible to record sympathetic nerve activities with microelectrodes.3, 4 However, microneurography techniques require invasive procedures and cannot be done in ambulatory subjects. We recently developed a new method (neuECG) to simultaneously record the electrocardiogram (ECG) and SKNA in humans.5 This new method enables the investigators to record the SKNA and ECG (neuECG) in patients for a prolonged period of time to study the relationship between sympathetic tone and cardiac arrhythmia. During open heart surgery, the fat pads that contain the superior left ganglionated plexi and the ligament of Marshall are readily accessible. Rossi et al6 have previously implanted temporary pacing wires to an epicardial fat pad for up to five days after cardiac surgery. The authors reported no complications related to the temporary pacing lead implantation. Based on these previous studies, we hypothesize that it is both safe and feasible to record human ICNA from the epicardial fat pad through a temporary pacing wire in the immediate postoperative period. We also hypothesize that both ICNA and SKNA are associated with the postoperative arrhythmias, including premature atrial contractions (PAC), premature ventricular contractions (PVC) and AF. The purpose of the present study was to test the hypotheses that both SKNA and ICNA are associated with postoperative cardiac arrhythmias.
Methods
Surgical Preparation and Recording
This research protocol was approved by the Institutional Review Board of the Indiana University School of Medicine. Written and informed consent was obtained from each patient. There were no change of routine medical care. At the end of the open heart surgery, two temporary pacing wires (TPW32, Ethicon Inc., Somerville, NJ) were threaded through the epicardial fat pad at the junction of the left superior pulmonary vein and the left atrium (Figure 1). The wires were exteriorized to the left thorax. The remainder of the surgery and post-operative care were performed according to the clinical needs. The exteriorized temporary pacing wires were connected to ADInstruments (ADI, Sydney, Australia) ML 135 Dual Bio Amp amplifier for continuous recordings after surgery. In all patients, an additional surface electrocardiogram (ECG) with the same amplifier was simultaneously recorded. The SKNA was recorded using the traditional ECG patch with the method described in a previous study.5 The data from both channels were simultaneously digtized at 10,000 samples/s by ADI PowerLab data acquisition system and recorded continuously on a portable computer. The recording was made continuously until when the patient was discharged from the intensive care unit or at 72 hours after surgery, whichever came first. The patients were followed in hospital and again in a month as an outpatient. All complications were documented.
Figure 1.
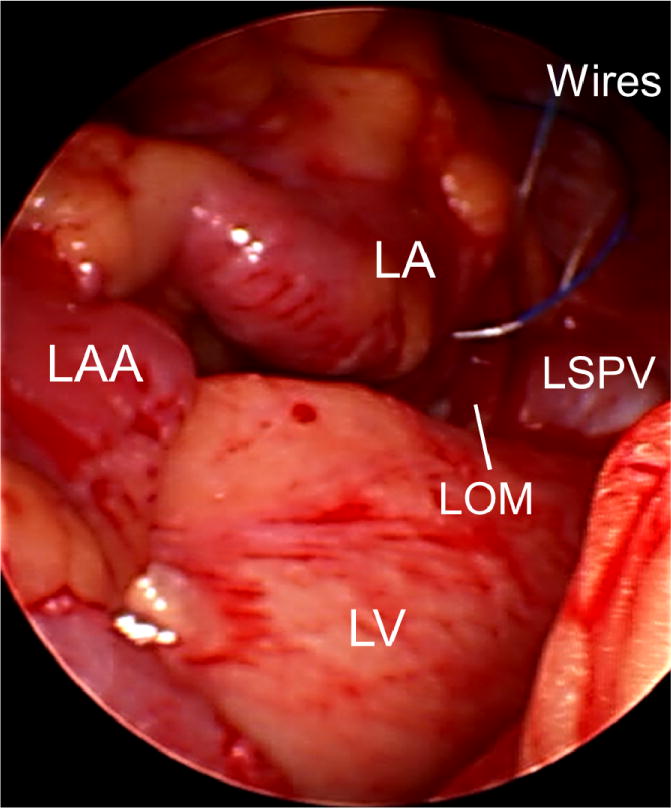
Placement of the temporary pacing wires during surgery. At the end of the open heart surgery, two temporary pacing wires were threaded through the epicardial fat pad at the junction of the left superior pulmonary vein (LSPV) and left atrium (LA). The uninsulated portion of the temporary pacing wires went through the fat pad (yellow) near the LOM and the orifice of the LSPV. LAA, left atrial appendage; LOM, ligament of Marshall; LV, left ventricle.
Data Analyses
The signals were analyzed off-line according to the methods developed from previous studies.5 We manually evaluated all recordings to exclude the periods with artifacts. We then analyzed the first 20-min of each hour to determine the activities of PVC/PAC, if any, in that time period. Data segments of overall poor signal quality or contains significant amount of artifacts were excluded from analyses. We then manually determined the presence or absence of intermittent spontaneous bursts of impulses of nerve activities and arrhythmias at all channels in each 30-s window of data. Based on a previous paper that recorded human sympathetic nerve fibers, two forms of nerve activities are present: one being sustained, low-amplitude nerve discharges, the other being “burst-like” grouped discharges of impulses, occurring intermittently.4 These burst discharges were considered to precede the onset of atrial arrhythmias if they were present within 10 s prior to the onset of these arrhythmias. In addition to manual analyses, a custom-designed software was used to automatically import and to further filter the signals to display nerve activities (500–1000 Hz Band-pass filter and 100 Hz High-pass filter for ICNA and SKNA, respectively5). R-wave-triggered averaging was performed in the observation window to construct an electrocardiogram (ECG) template, which was subtracted beat-by-beat in the recording to remove the ECG interference. The voltages of digitized signals were summed to represent total nerve activity of 1-min time segments. The summed voltage was dependent on the sampling rate. To make the quantitative nerve activity sampling rate independent, the integrated nerve activity was divided by 60 (to make it as per s) and then divided by the sampling rate (10000/s) to obtain the average ICNA (aICNA) and average SKNA (aSKNA) for that 1-min window.5
Statistical Analyses
The data are presented as mean and 95% confidence interval (CI). Fisher’s exact test was used to assess the relationship between ICNA and premature atrial contractions (PACs) or premature ventricular contractions (PVCs). Pearson’s correlation coefficients (r) were used to assess correlations between average integrated nerve activities and PACs or PVCs. Unpaired t-test was used to compare the nerve activities among patients who developed postoperative AF and those who did not. The statistics were computed using the PASW Statistics (version 22; SPSS Inc, Chicago, IL).
Results
Eleven patients (60±13 years old; 4 female) undergoing coronary artery bypass grafting (CABG) surgery consented for this study. Among them, 10 were treated postoperatively with amiodarone. Table 1 summarizes patient characteristics and drugs received in the perioperative period. While ICNA and PACs were seen in all patients studied, the frequencies of occurrences varied greatly among patients. Removal of temporary pacing wires caused pain in 1 patient. The electrode removal in the remaining 10 patients was uneventful. There were otherwise no complications related to the study. The surgical incision sites of all patients were healed and no complications were reported at one month outpatient follow up.
Table 1.
Patient Characteristics
| Pt | Age | Sex | Ht (cm) | Wt (kg) | Diagnoses | LAD (cm) | LVEF (%) | Meds | Surgery | aICNA (μV) | PAC Burden (/min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | 180 | 91 | CAD, HTN | 3 | 60 | None | CABG | 0.9 | 0.03 |
| 2 | 50 | F | 178 | 112 | CAD, HTN | 3.9 | 63 | AM | CABG | 1.9 | 0.01 |
| 3 | 63 | F | 165 | 88 | CAD, HTN, DM, MR | 3.6 | 26 | AM, BB | CABG | 1.8 | 0.05 |
| 4 | 39 | F | 160 | 50 | CAD, DM | 3.2 | 68 | AM, BB | CABG | 1.9 | 0.00 |
| 5 | 69 | M | 168 | 113 | CAD, HTN, DM, CKD | 3.9 | 45 | AM, BB | CABG | 1.3 | 0.08 |
| 6 | 43 | M | 175 | 106 | CAD, HTN, DM | 3.5 | 46 | AM, BB | CABG | 3.9 | 0.01 |
| 7 | 56 | M | 178 | 99 | CAD, HTN | NA | 45 | AM | CABG | 2.5 | 0.22 |
| 8* | 75 | M | 175 | 103 | CAD, HTN, OSA | 3.2 | 67 | AM | CABG | 2.8 | 0.01 |
| 9 | 76 | M | 175 | 90 | CAD, HTN, DM | 3.4 | 60 | AM, BB | CABG | 1.5 | 0.30 |
| 10* | 73 | F | 157 | 49 | CAD, HTN, AF | 3.1 | 62 | AM, BB | CABG | 8.4 | 0.71 |
| 11 | 63 | M | 168 | 109 | CAD, HTN, DM | 4 | 60 | AM, BB | CABG | 1.5 | 0.01 |
AM, amiodarone; BB, beta blockers; CAD, coronary artery diseases; CABG, coronary artery bypass grafting; DM, diabetes mellitus; F, female; Ht, height; aICNA, average integrated intrinsic cardiac nerve activities; LAD, left atrial dimension from echocardiography; LVEF, left ventricular ejection fraction; M, male; Meds, medications; MR, mitral regurgitation; NA, not available (no echocardiogram done); OSA, obstructive sleep apnea; PAC, premature atrial contraction; Wt, weight.
the patient who developed postoperative atrial fibrillation.
Characteristics of Human Nerve Activities
Continuous recordings were performed for 3 days in 10 patients and 1 day in 1 patient. A total of 15020 min of data (15020 1-min data windows) were analyzed, with 1365 [CI: 790 to 1939] min of recording per patient. The sustained low-amplitude nerve discharges from the epicardial fat pad were always present, similar to that observed in the peripheral nerves.3 In contrast, the burst discharges were detected in only 369 of 15020 (2.7%) data windows analyzed. These nerve activities (red arrows, Figure 2) were consistent with that observed in canine studies.1 A majority (61%) of these burst discharges were observed during the last day (Day 3) of monitoring period. In addition, there was a significant inter-individual variability of ICNA frequencies. One patient had burst discharges detected in 5% of time (63 of 1280 windows) while another patient in 0.1% of time (2 of 1500 windows). For all patients studied, the burst discharges was present in 2.7±2% of the windows analyzed. There were a total of 640 episodes of burst activities (averaged 1.7 episodes per window when ICNA was present). Of these, 423 (66%) episodes of burst activities were associated with PACs or PVCs. The remaining burst activities were not associated with premature beats from either chamber. More detailed relationships between ICNA and premature contractions are described in the next paragraph. Compared to these ICNA, SKNA were detected more frequently (679 of 15020 data windows, 5.7% of time, blue arrows, Figure 2). Similarly, a majority (69%) of SKNA was observed on Day 3 and the frequencies of nerve activities varied tremendously among individuals. One patient had SKNA detected in 29% of time (251 of 860 windows) while another patient in 0.3% of time (2 of 720 windows). For all patients studied, the SKNA was present in 5.7±8.1% of the windows analyzed.
Figure 2.
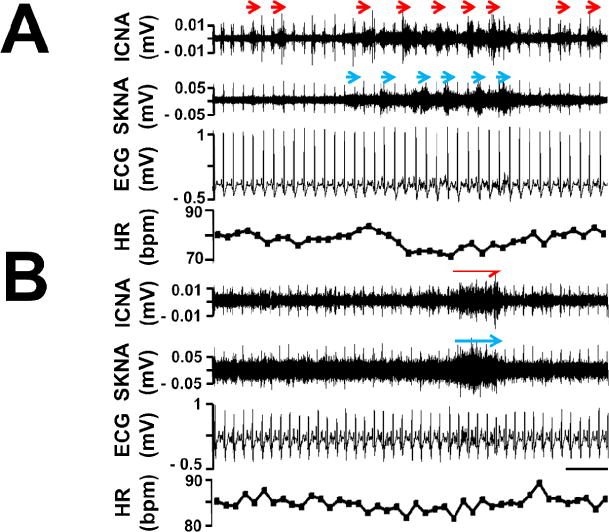
Characteristics of human nerve activities. Panel A shows burst ICNA (red arrows) and SKNA (blue arrows) discharged intermittently in one patient. This patient has burst ICNA and SKNA present in only 0.4% and 0.6% of windows analyzed, respectively. Panel B shows burst ICNA and SKNA discharged simultaneously in another patient. This patient has burst ICNA and SKNA present in only 5.0% and 5.0% of windows analyzed, respectively. Note the sustained low-amplitude nerve activities were present at all time in both channels. Calibration bar = 3 sec.
Nerve Activities and Post-Operative Premature Contractions
From the windows chosen for nerve activities analysis (first 20 min of each hour of available recordings that were free of artifacts), episodes of PACs and PVCs were manually determined. A total of 1425 episodes of PACs were identified (mean 130 per patient, [CI: 3 to 257]). Of them, 216 episodes (15.2%) were preceded by burst ICNA. In comparison, among 1425 control time segments (3 min prior to the PACs), only 68 segments (4.8%) were preceded by burst discharges (P<0.0001). This is also true with the relationships between SKNA and PACs. Of the same 1425 episodes of PACs, 192 (13.5%) were preceded by SKNA; while only 92 control segments (6.5%) were preceded by SKNA (P<0.0001). Figure 3A demonstrates an example of PAC in a patient recovering from CABG. Both the burst ICNA and SKNA clearly preceded the onset of the PAC. Similar, albeit not as robust, association of either ICNA or SKNA with PVCs is observed. A total of 1741 episodes of PVCs were identified (mean 158 per patient, [CI: 7 to 309]). Of them, 207 episodes (11.9%) were preceded by the burst ICNA. In comparison, among 1741 control time segments (3 min prior to the PVCs), 149 episodes (8.6%) were preceded by ICNA (P=0.0014). This is also true with the relationships between SKNA and PVCs. Of the same 1741 episodes of PVCs, 233 (13.4%) were preceded by SKNA; while only 119 control segments (6.8%) were preceded by SKNA (P<0.0001). Figure 3B demonstrates an example of PVC in a different patient recovering from CABG. Both the burst ICNA and SKNA clearly preceded the onset of the PVC.
Figure 3.
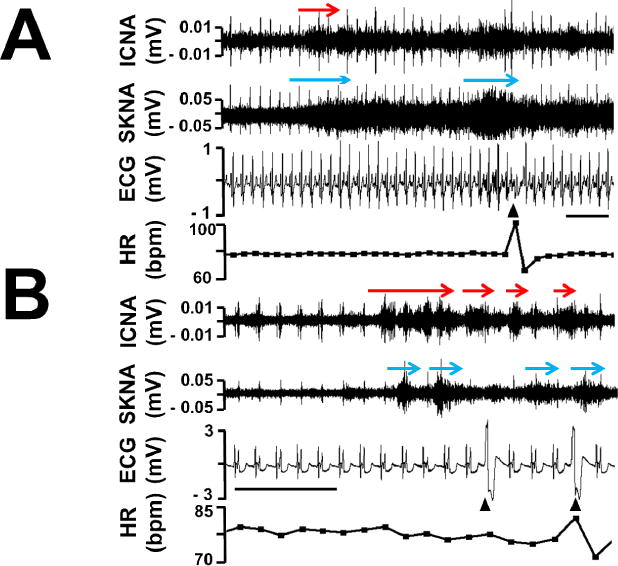
Human nerve activities and PAC and PVC. Panel A shows burst ICNA (red arrows) and SKNA (blue arrows) discharges preceded an episode of PAC (arrowhead). Panel B shows burst ICNA and SKNA discharges preceded an episode of PVC (arrowhead). Calibration bar = 3 sec.
Baseline Nerve Activities and Their Implications of Arrhythmias Burden
Every patient’s first 300 min recordings were processed to represent baseline integrated nerve activities. The aICNA was 2.6 μV (CI: 1.4 to 3.8) and the aSKNA was 2.5 μV (CI: 1.9 to 3.1). Each individual’s integrated nerve activities and their PAC or PVC burden (total episodes of PAC or PVC divided by minutes analyzed) were determined. A significant and strong association was found between the value of aICNA and PAC burden (r=0.78, P<0.01). A significant but weak association was found between the frequency of burst ICNA and PAC burden (r=0.48, P<0.05). By contrast, no significant association was found between aICNA and PVC burden (r=0.06, P=NS). aSKNA had no significant association with either PAC (r=−0.2, P=NS) or PVC burden (r=0.08, P=NS). The patient with highest aICNA at baseline (8.4 μV) also had the highest PAC burden. Moreover, that patient was the only one who developed postoperative AF during the monitoring period in the study. Figure 4 shows the nerve activities during AF in this particular patient. ICNA seemed more active during the AF although SKNA was also observed. This patient had sinus with PAC bigeminy and developed salvos of AF. A grouped burst discharges of the ICNA were observed at the onset of AF, as shown in Figure 4A, but this was not always observed. Among the remaining 10 patients, 1 developed postoperative AF 3 days after surgery, after being discharged from the intensive care unit and disconnected from the recording equipment. The aICNA of the two patients who developed postoperative AF was 5.6 μV (CI: 0.1 to 11.1) which was significantly larger than patients without postoperative AF (1.9 μV [CI: 1.3 to 2.5]), P<0.05.
Figure 4.
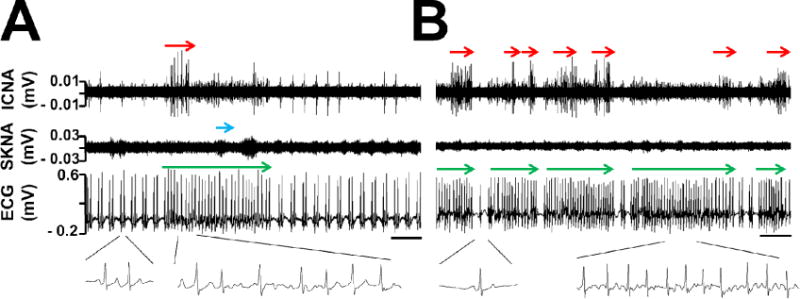
ICNA and postoperative atrial fibrillation (AF). Panel A shows burst ICNA (red arrow) at the onset of an episode of AF (red arrow). Insets show sinus with PAC bigeminy on the left and AF on the right. Sporadic SKNA (blue arrow) was present during AF. Panel B shows salvos of AF with clear sinus beat(s) (inset) in between. Burst ICNA (red arrows) occurred during but not at the onset of AF episodes (green arrows), suggesting passive activation from the real trigger elsewhere. Calibration bar = 5 sec.
Discussion
This is the first-in-man study showing that 1) it was feasible to record both ICNA and SKNA from human patients, 2) two forms of ICNA were recorded: one being sustained, low-amplitude baseline nerve activities that are always present; the other being grouped burst discharges, 3) burst discharges of ICNA and SKNA were significantly associated with the onset of PACs and PVCs and 4) baseline aICNA is positively associated with PAC burden in the postoperative period and was significantly associated with the development of postoperative AF.
Cardiac Autonomic Innervation
The cardiac autonomic innervation had both extrinsic and intrinsic components.7, 8 The extrinsic autonomic innervation came from the paravertebral sympathetic ganglia and the vagus nerve. The intrinsic autonomic innervation came from the ganglionated plexi distributed mostly within the fat pads on the epicardium.7, 8 Immunohistopathological studies showed that both sympathetic and parasympathetic components co-exist in the ganglionated plexi.9 Adrian and Bronk10 were probably the first to record mammalian sympathetic nerve activities, documenting an association between sympathetic discharges and physiological responses, such as vasoconstriction, heart rate and respiration. Subsequent studies showed the feasibility of recording extrinsic cardiac sympathetic nerve activities in the stellate ganglion of various animal models as well as from ganglionated plexi in the fat pad, documenting a direct relationship between nerve activities and pathophysiological responses, including cardiac arrhythmias.1, 11–16 The latter studies also documented the importance of ICNA in cardiac arrhythmogenesis. While autonomic nerve activities are important in the development cardiac arrhythmias, no one has reported patterns of intrinsic cardiac nerve discharges in human patients, or correlated the nerve discharges with spontaneous atrial tachyarrhythmias. In the present study, we have documented the first successful recording of ICNA from human patients. The results show that the ICNA is significantly associated with the development of spontaneous atrial arrhythmias. Because most of our understanding of ICNA physiology came from animal experiments, it was reassuring that the characteristics of the ICNA in humans were similar to that reported in anesthetized and ambulatory dogs.1
Characteristics of Human Nerve Activities in the Postoperative Period
Although baseline ICNA and SKNA were present all the time, we detected burst ICNA in only 2.7% of the windows analyzed. This was much less frequent than that recorded in ambulatory dogs. In the latter experimental models, we were able to detect burst LOM nerve activities in 7.5% and SLGP nerve activities in 17.1% of the windows analyzed.1 Similarly, SKNA was recorded in 5.9% of the windows, whereas it was recorded more than 12.5% of time in a canine study.17 This difference might be explained by the timing of recordings. In ambulatory animals,14 we found that the stellate ganglion nerve activities were infrequently observed in the first few days after surgery but became very frequent one week later.14 The mechanisms by which nerve activities were difficult to detect in the immediate postoperative period could be due to surgical trauma associated with electrode implantation. It was also known that both propofol and isoflurane markedly suppress muscle sympathetic nerve activities in humans.18 There might be residual effects of these anesthetic agents on nerve activities in the immediate postoperative period. In addition, sedation and analgesics given during the postoperative periods might also reduce nerve activities. These possible mechanisms are supported by the fact that the majority of the burst discharges are present during the last day of the monitoring period. Because of the above limitations, the nerve discharges reported in this study may not be representative of the ICNA or SKNA of the general population.
ICNA and Atrial Arrhythmias
We found that 15.2% of the PACs were preceded by the burst ICNA. Given this type of grouped discharges was only present in 2.7% of the windows analyzed, this association was unlikely to be due to chance occurrence. Not only that the patient with largest aICNA developed postoperative AF, but that the aICNA of the two patients who developed post-operative AF was significantly higher than that of other patients who did not. Because the heart has multiple fat pads and ganglionated plexi,7 it is somewhat surprising that ICNA recorded from a single fat pad has such high arrhythmic implications. A possible explanation is that the ganglionated plexi at different locations might communicate with each other, and also communicate directly with the extrinsic cardiac nervous systems. More firing from one site, hence the larger aICNA, may indicate simultaneous firing of multiple nerve structures, which facilitate future arrhythmias. On the other hand, at least from the patient who developed postoperative AF, the ICNA was rarely a direct trigger of the AF, in sharp contrast to canine model, where ICNA was an invariable trigger of paroxysmal AF.1 As shown in Figure 4B, the ICNA was observed mostly after the onset of each episode of AF. This reflects one of the limitations of the current study that only one site of intracardiac nerve structure is being recorded, and that narrowly-spaced “near-field” recording may have failed to record the trigger of AF in that particular patient. In addition, the mechanisms of postoperative AF may be more complex and are not entirely dependent on the presence of abnormal autonomic nerve activities.19 A more recent study20 shows that botulinum toxin injection into epicardial fat pads during coronary artery bypass graft surgery provided substantial suppression of atrial tachyarrhythmias postoperatively. These findings suggest that increased burst ICNA discharges maybe causally related to the development of postoperative atrial arrhythmias, including AF.
Baseline aSKNA did not correlate with PAC burden
We found that baseline aICNA, but not aSKNA, strongly correlated with PAC burden during the 3-day monitoring period. It is possible that the inflammatory response in the immediate postoperative period had significantly increased the magnitude of ICNA while the systemic sympathetic tone was not proportionally increased. Therefore, only aSKNA correlated with the PAC burden. In contrast, elevated aSKNA was strongly associated with paroxysmal atrial tachyarrhythmias that occur without preceding open heart surgery.21
Limitations of the Study
The incidence of postoperative AF had declined significantly in recently years due to the development of effective pharmacological prophylaxis, including the use of amiodarone, beta blockers, colchicine and other pharmacological agents.22–24 These effective prophylactic therapy had reduced the incidence of postoperative AF to as low as 12%.23, 24 Because we did not withdraw standard pharmacological prophylaxis, it was not surprising that only one patient in the current study developed postoperative AF in the monitor period. With only two patients developing postoperative AF, it is difficult to generate any meaningful conclusion to test if ICNA is important in postoperative AF. However, because ICNA had not been previously recorded in humans, we propose that these data provide significant new insights into the neural mechanisms of cardiac arrhythmogenesis and might be helpful in designing further experiments to study the neural mechanisms of heart rhythm disorders in humans. The number of patients enrolled is small. While 11 patients are sufficient to document the feasibility of nerve recording, further studies are needed to document the relationship between ICNA and postoperative AF. There is potential for bias because not all recordings on each patient were analyzed. The disparity of total time processed between patients were mainly affected by the quality of the recordings. Finally, the relationship between blood pressure changes and neural activity would be interesting, but we did not simultaneously record the blood pressure during the study.
Conclusions
Both ICNA and SKNA can be recorded from human patients in the postoperative period. Baseline aICNA has a high correlation with PAC burden and may predict the risks of development of postoperative AF.
Acknowledgments
Study data were collected and managed using REDCap electronic data capture tools hosted at Indiana University.25 We thank Amy Chang for her assistance.
Funding Sources This study was supported in part by a Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology (Dr Shen), NIH Grants R42DA043391 (Dr Everett), P01HL78931, R01HL71140 (Dr Chen), a Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology (Dr Everett), a Medtronic-Zipes Endowment and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative (Dr Chen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Shien-Fong Lin and Peng-Sheng Chen have equity interest in Arrhythmotech, LLC.
References
- 1.Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm. 2015;12:1324–1332. doi: 10.1016/j.hrthm.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallbo AB, Hagbarth KE. Impulses recorded with micro-electrodes in human muscle nerves during stimulation of mechanoreceptors and voluntary contractions. Electroencephalogr Clin Neurophysiol. 1967;23:392. [PubMed] [Google Scholar]
- 4.Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968;74:96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 5.Doytchinova A, H J, Y Y, et al. Simultaneous non-Invasive Recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm. 2017;14:25–33. doi: 10.1016/j.hrthm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi P, Bianchi S, Barretta A, Della Scala A, Kornet L, De Paulis R, Bellisario A, D’Addio V, Pavaci H, Miraldi F. Post-operative atrial fibrillation management by selective epicardial vagal fat pad stimulation. J Interv Card Electrophysiol. 2009;24:37–45. doi: 10.1007/s10840-008-9286-2. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 2010;7:994–996. doi: 10.1016/j.hrthm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa H, Scherlag BJ, Wu R, Po S, Lockwood D, Yokoyama K, Herring L, Lazzara R, Jackman WM. Addition of selective ablation of autonomic ganglia to pulmonary vein antrum isolation for treatment of paroxysmal and persistent atrial fibrillation. Circulation. 2006;110:III–459. [Google Scholar]
- 9.Anderson CR, Bergner A, Murphy SM. How many types of cholinergic sympathetic neuron are there in the rat stellate ganglion? Neuroscience. 2006;140:567–576. doi: 10.1016/j.neuroscience.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Adrian ED, Bronk DW, phillips G. Discharges in mammalian sympathetic nerves. Journal of Physiology. 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavian G, Kopelman D, Shenhav A, Konyukhov E, Gardi U, Zaretzky A, Shofti R, Finberg JP, Hashmonai M. In vivo extracellular recording of sympathetic ganglion activity in a chronic animal model. ClinAutonRes. 2003;13(Suppl 1):I83–I88. doi: 10.1007/s10286-003-1121-3. [DOI] [PubMed] [Google Scholar]
- 12.Watson AM, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol RegulIntegrComp Physiol. 2004;286:R1051–R1056. doi: 10.1152/ajpregu.00708.2003. [DOI] [PubMed] [Google Scholar]
- 13.Jardine DL, Charles CJ, Ashton RK, Bennett SI, Whitehead M, Frampton CM, Nicholls GM. Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. JPhysiol. 2005;565:325–333. doi: 10.1113/jphysiol.2004.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung BC, Dave AS, Tan AY, Gholmieh G, Zhou S, wang DC, Akingba G, Fishbein GA, Montemagno C, Lin SF, Chen LS, Chen PS. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2006;3:78–85. doi: 10.1016/j.hrthm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Armour JA. Activity of in situ middle cervical ganglion neurons in dogs, using extracellular recording techniques. CanJ Physiol Pharmacol. 1985;63:704–716. doi: 10.1139/y85-116. [DOI] [PubMed] [Google Scholar]
- 17.Boyden PA, Dun W, Robinson RB. Cardiac Purkinje fibers and arrhythmias; The GK Moe Award Lecture 2015. Heart Rhythm. 2016;13:1172–1181. doi: 10.1016/j.hrthm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellgren J, Ponten J, Wallin BG. Percutaneous recording of muscle nerve sympathetic activity during propofol, nitrous oxide, and isoflurane anesthesia in humans. Anesthesiology. 1990;73:20–27. doi: 10.1097/00000542-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Yadava M, Hughey AB, Crawford TC. Postoperative Atrial Fibrillation: Incidence, Mechanisms, and Clinical Correlates. Heart Fail Clin. 2016;12:299–308. doi: 10.1016/j.hfc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Pokushalov E, Kozlov B, Romanov A, et al. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. J Am Coll Cardiol. 2014;64:628–629. doi: 10.1016/j.jacc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 21.Uradu A, Wan J, Doytchinova A, Wright KC, Lin AY, Chen LS, Shen C, Lin SF, Everett Tt, Chen PS. Skin Sympathetic Nerve Activity Precedes the Onset and Termination of Paroxysmal Atrial Tachycardia and Fibrillation. Heart Rhythm. 2017 doi: 10.1016/j.hrthm.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koniari I, Apostolakis E, Rogkakou C, Baikoussis NG, Dougenis D. Pharmacologic prophylaxis for atrial fibrillation following cardiac surgery: a systematic review. J Cardiothorac Surg. 2010;5:121. doi: 10.1186/1749-8090-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikroulis D, Didilis V, Konstantinou F, Tsakiridis K, Vretzakis G, Bougioukas G. Diltiazem versus amiodarone to prevent atrial fibrillation in coronary surgery. Asian Cardiovasc Thorac Ann. 2005;13:47–52. doi: 10.1177/021849230501300111. [DOI] [PubMed] [Google Scholar]
- 24.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine Reduces Postoperative Atrial Fibrillation: Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) Atrial Fibrillation Substudy. Circulation. 2011;22:2290–2295. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


