Abstract
Lead is a neurotoxicant of immense public health importance. Epidemiology studies suggest that heavy metal exposure may be associated with an increased risk of cognitive decline, yet few studies to date have assessed the effect of adult lead exposure on cognitive behavior in animal models. Here, we exposed 6-week-old male C57BL/6 mice to 0.2% lead acetate via drinking water for 12 weeks starting at 6 weeks of age and then assessed for deficits in hippocampus-depending spatial memory and impairment of adult hippocampal neurogenesis. Lead did not cause locomotor deficits or anxiety in the open field test. However, we found that adult, subchronic lead exposure was sufficient to cause deficits in spatial short-term memory and these deficits persisted through at least 2 months post-lead exposure. Furthermore, we observed that lead-treated mice had fewer adult-born, mature neurons in the dentate gyrus of the hippocampus compared to control animals, suggesting that lead exposure during adolescence and adulthood may impair the neuronal differentiation of adult-born cells. These data suggest that adult lead exposure is sufficient to cause persistent deficits in spatial short-term memory and impair key processes in adult hippocampal neurogenesis.
Keywords: Lead, behavior, adult hippocampal neurogenesis, learning and memory
1. Introduction
Through a process called adult hippocampal neurogenesis, adult neural precursor cells in the dentate gyrus (DG) of the hippocampus continuously generate neurons throughout adulthood, and these adult-born neurons contribute to hippocampus-dependent learning and memory [1–3]. Importantly, the hippocampus is one of the earliest affected brain regions in patients with Alzheimer’s disease (AD), and the perturbation of adult hippocampal neurogenesis may cause deficits in hippocampus-dependent learning and memory, accelerate cognitive decline, and contribute to AD pathogenesis [4]. While various factors have been shown to modulate adult neurogenesis [1, 2, 5], little is known about the effects of neurotoxicants in this process.
Both animal and epidemiological studies have reported an association between lead exposure and accelerated cognitive decline and/or AD-associated neuropathology in adults [6–8]. While several studies have characterized the effect of developmental or lifetime lead exposure on adult neurogenesis in rats [9–11], only one study to date has characterized the effect of postnatal-only lead exposure on adult neurogenesis [12]. Schneider et al. (2005) observed reduced BrdU+ cells in the dentate gyrus of postnatal rats exposed to 1500 ppm lead acetate for 30–35 days, suggesting that postnatal lead exposure may decrease adult-born cell proliferation [12]. However, they did not assess the effect of lead on adult-born cell survival, maturation, or differentiation, or assess for functional changes in cognitive behavior. Therefore, additional study is required in order to determine the critical window for lead-induced effects on adult hippocampal neurogenesis.
We previously studied the effect of lead exposure on adult neurogenesis in vitro using adult neural precursor cells from adult male C57BL/6 mice, and found that lead significantly increases apoptosis, inhibits proliferation, and impairs spontaneous neuronal differentiation [13]. In addition, we studied whether there is a gene-environment interaction between in vivo adult lead exposure and the E4 allele of Apolipoprotein E (ApoE4) using humanized ApoE3 and ApoE4 knock-in (KI) mice [14]. We found that ApoE4-KI mice exposed to lead develop more severe or earlier hippocampus-dependent learning and memory deficits and significantly impaired adult-born neuron maturation and differentiation in the hippocampus compared to ApoE3-KI mice [14]. However, no study to date has characterized the effect of in vivo, adult lead exposure on both cognitive behavior and adult neurogenesis in non-transgenic, wild-type mice. Thus, the goal of this study was to characterize the effect of subchronic lead exposure during adolescence and adulthood on hippocampus-dependent learning and memory and adult hippocampal neurogenesis in C57Bl/6 male mice.
2. Materials and Methods
2.1. Mice and reagents
Four-week-old male C57Bl/6 mice were obtained from Taconic (Hudson, NY) and allowed to acclimate for two weeks. All animals were housed in standard conditions (12 h light/dark cycle) with food and water provided ad libitum. The University of Washington Institutional Animal Care and Use Committee approved all animal protocols. On a weekly basis, animal drinking water with 0.2% lead (II) acetate (Cat. 316512, Sigma-Aldrich, St. Louis, MO) was prepared from a stock lead acetate solution. Preparation, use, and disposal of hazardous agents was carried out according to the Environmental Health and Safety Office at the University of Washington.
2.2. Lead exposure
Mice were exposed to normal drinking water (control) or 0.2% lead acetate (lead-treated) via drinking water for 12 weeks, starting at 6 weeks of age (n= 9–10 mice per treatment). Water consumption and body weight were recorded weekly during the lead exposure period. After 12 weeks of lead exposure, all of the animals were then switched to normal drinking water for the remaining duration of the study. A separate cohort of C57BL/6 mice (n= 3 mice per treatment) was exposed to 0.2% lead acetate for 12 weeks and then sacrificed to measure peak blood lead levels.
2.3. Open field test
The open field test was conducted to assess locomotor activity and anxiety after the cessation of the lead exposure as previously described [14]. Briefly, each animal was placed into a TruScan Photo Beam Tracking arena (Colbourn Instruments, Whitehall, PA) and the animal was allowed to freely explore the arena for 20 min. The animal’s movement was monitored with two sets of infrared breams and data was automatically collected by TruScan 2.0 software (Colbourn Instruments).
2.4. Novel object location test
Hippocampus-dependent, short-term spatial memory was assessed using the 1 h novel object location (NOL) test, as previously described [14]. Briefly, during the training period, each animal was individually placed into an open, plexiglass arena and allowed to freely explore the two objects for 5 min before being returned to its home cage. One hour later, the animal was returned to the arena with the same two objects; one object remained in the same location as in the training session and one object had been moved to a novel location. Training and testing sessions were video recorded, and an experimenter blinded to the animal’s genotype and treatment scored the amount of time each animal spent actively investigating the different objects.
2.5. Blood lead analysis
Blood was collected at the end of the 12-week lead exposure from the lead analysis cohort (n= 3 mice per treatment). The Environmental Health Laboratory at the University of Washington measured blood lead levels using inductively coupled plasma mass spectrometry (Agilent 7500ce, Agilent Technologies, Santa Clara, CA).
2.6. BrdU administration
After the cessation of the lead exposure, mice were dosed with 5-bromo-2'-deoxyuridine (BrdU, Cat. B9285, Sigma-Aldrich, St. Louis, MO) at various time points prior to sacrifice (n= 3 mice per treatment/time-point). To assess differentiation, mice were dosed 5X in 1 day (every 2 hours) by intraperitoneal (i.p.) injection with 100 mg/kg BrdU and sacrificed either 3 or 7 weeks later. To assess proliferation, mice were dosed i.p. with 100 mg/kg BrdU 1X 2 hours prior to sacrifice.
2.7. Immunohistochemistry
Primary antibodies and dilutions used in immunohistochemistry were: rat monoclonal anti-BrdU (1:500, Bio-Rad Laboratories AbD Serotec, Raleigh, NC) and mouse monoclonal anti-NeuN (1:1000, Millipore, Billerica, MA). Goat anti-rat and goat anti-mouse Alexa Fluor-conjugated secondary antibodies as well as Hoechst 33342 (2.5 μg/ml) were from Invitrogen (Carlsbad, CA). All primary and secondary antibodies were diluted in blocking buffer (10% goat serum and 1% BSA). Mice were anesthetized with ketamine/xylazine and euthanized by decapitation. Immunohistochemistry was performed on 30 μm thick coronal brain sections as previously described [14].
2.8. Tissue preparation and quantification and imaging of immunostained cells
Quantification of immunostained cells was performed as previously described [2, 14]. Briefly, every 8th serial section (30 μm) was immunostained for each marker or marker combination. Marker-positive cells were quantified by a blinded experimenter, and the total number of marker-positive cells per DG was estimated by multiplying the number of marker positive cells by 8 (total number marker-positive cells per DG = total number of marker-positive cells from 9 coronal sections×8). Double-positive cells were defined as overlapping fluorescent signals in a single cell using a Z-series stack. All marker-positive cells from ≥ 9 coronal sections per mouse (n=3–4 mice per treatment) were quantified for each marker or marker combination. Images were captured with an Olympus Fluoview-1000 laser scanning confocal microscope with 0.75 (20X) numerical aperture lenses. Optical Z-sections (1 μm thick) were collected and processed using ImageJ software (NIH, Bethesda, MD). Images were uniformly adjusted for color, brightness, and contrast with Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA).
2.9. Statistical analysis
Statistical analyses were conducted using GraphPad Prism software (version 6.0h for Mac, GraphPad Software Inc., San Diego, CA, USA). Student’s t-test with two-tailed analysis (α = 0.05) was used for pair-wise comparison of the means for the behavior and cellular data. Two-way ANOVA with repeated measures (α = 0.05) was used to analyze the body weight and water consumption data. Data represent mean ± SEM., n.s. not significant, *, p < 0.05; ** p < 0.01; *** p < 0.001.
3. Results
3.1. Blood lead levels
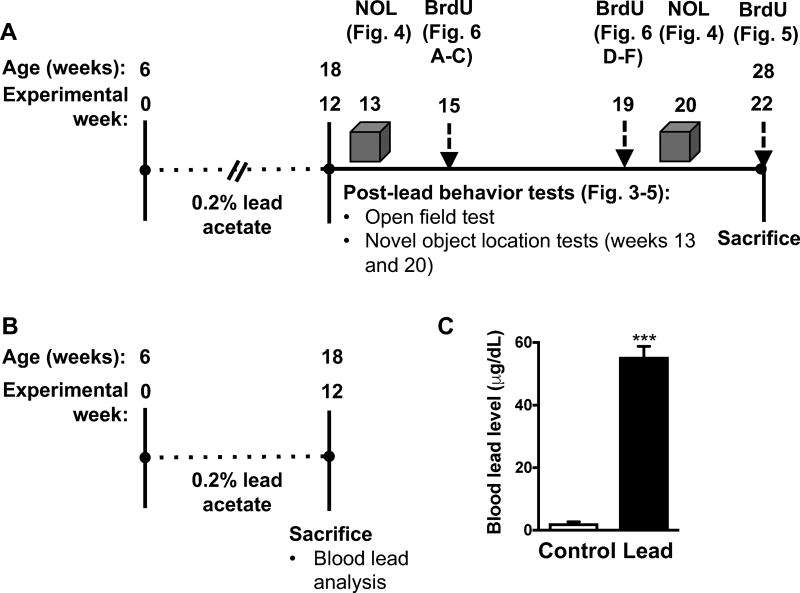
To model subchronic lead exposure, we exposed 6-week-old male C57BL/6 mice to 0.2% lead acetate or normal drinking water for 12 weeks. We chose to start the exposure at six weeks of age because at this age, early postnatal neurogenesis is complete and the DG is considered mature (Bayer et al. 1993). We used 0.2% lead acetate because this concentration has commonly been used to study lead neurotoxicity in rodents [15, 16] and because this lead concentration would achieve blood lead levels comparable to levels reported in occupationally exposed men who experienced learning and memory impairment (40–60 μg/dL) [17]. Furthermore, we previously observed that this lead concentration causes persistent learning and memory deficits in ApoE3-KI and ApoE4-KI mice [14]. In this study, the blood lead levels immediately after the cessation of the lead exposure were 2.2 ± 0.5 μg/dL and 55.47 ± 3.3 μg/dL in control and lead-treated mice, respectively (Fig. 1).
Figure 1. Experimental design and timeline and blood lead analysis.
(A) The same cohort of 6-week-old male C57BL/6 mice were used for the behavior and cellular studies (n= 9–10 per treatment). Following behavior tests, subsets of mice were dosed with BrdU at three different time points prior to sacrifice (n=3 mice per time point). (B) A separate cohort of mice were exposed to 0.2% lead acetate or normal drinking water for 12 weeks, sacrificed, and blood was collected via cardiac puncture and used for blood lead analysis (n=3–4 per treatment). (C) Blood lead levels at the end of the lead exposure. Mean ± SEM; n= 3–4 per treatment. Student’s t test: *** p < 0.001.
3.2. Lead does not cause reduced water consumption, weight loss, locomotor deficits, or anxiety in male C57BL/6 mice
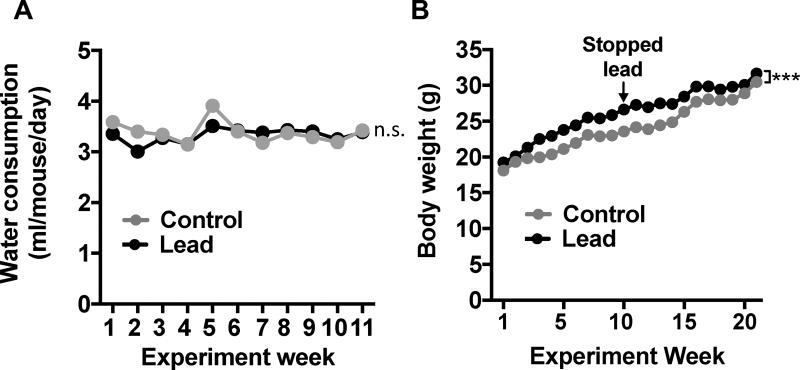
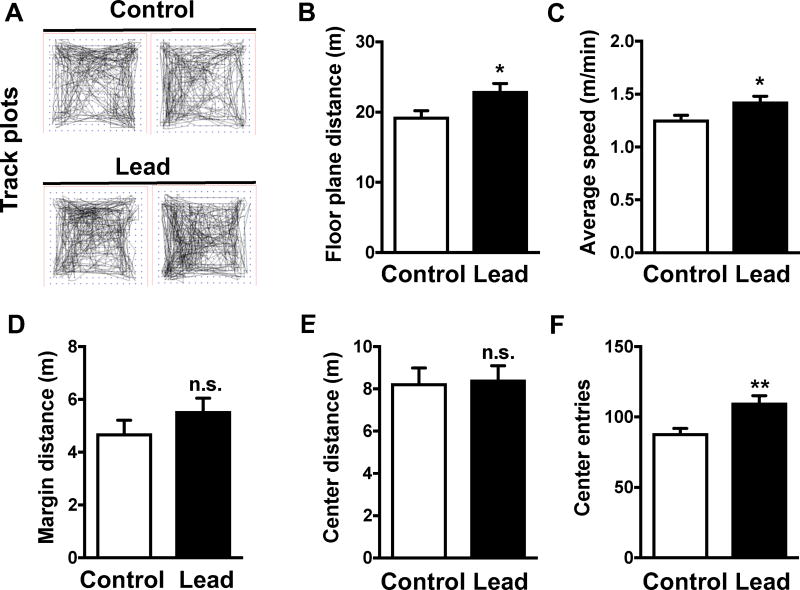
We did not observe any significant difference in water consumption between lead-treated and control mice (Fig. 2A). Lead-treated mice weighed slightly more than control animals at most time points during and post-lead exposure (Fig. 2B). However, this small but statistically significant difference in weight did not cause locomotor deficits in the open field test (Fig. 3). We found that the lead-treated mice traveled a greater floor plane distance and moved faster than control animals in the open field test (Fig. 3A-C). The open field test can also be used to assess anxiety, which is characterized by an animal traveling less distance in the center and traveling a greater distance in the margin of the open field. The lead-treated mice traveled a similar distance as controls in both the margin and center of the open field (Fig. 3D-E), but lead-treated animals did make significantly more center entries than control animals. Thus, subchronic lead exposure did not impair locomotor activity, but similar to our previous findings in ApoE3-KI and ApoE4-KI mice [14], causes a slight, but significant, increase in hyperactivity and produces an anxiolytic phenotype.
Figure 2. Lead does not cause weight loss or a reduction in water consumption.
(A) Water consumption and (B) body weight data. Mean ± SEM; n= 9–10 per treatment. Two-way repeated measures ANOVA: n.s., not significant; *** p < 0.001.
Figure 3. Lead-treated mice do not show overt locomotor deficits or anxiety.
The open field test was conducted after the 12-week lead exposure. (A) Representative track plots from control and lead-treated mice. Open field endpoints included (B) floor plane distance, (C) average speed, (D) margin distance, (E) center distance, and (F) center entries. Mean ± SEM; n= 9–10 per treatment. Two-tailed t test: n.s., not significant; * p < 0.05; ** p < 0.01.
3.3. Young adult and adult lead exposure causes persistent deficits in spatial working memory
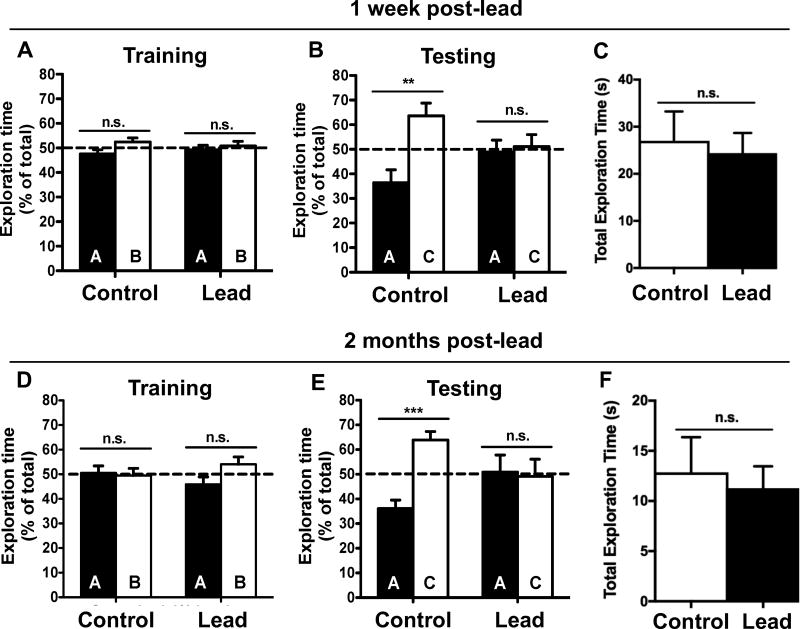
We conducted a 1 h novel object location (NOL) test one week after the lead exposure to assess hippocampus-dependent spatial working memory. None of the mice exhibited a preference for either object or location (Fig. 4A) during training. During testing, the control mice spent significantly more time exploring the novel object location (C) compared to the familiar object location (A), indicating that the control mice remembered the original object locations (Fig. 4B). However, the lead treated mice spent a similar amount of time exploring the novel and familiar object locations (Fig. 4B), suggesting the mice exposed to lead had impaired spatial working memory. Importantly, there was no significant difference in the total exploration time between control and lead-treated mice (Fig. 4C).
Figure 4. Lead exposure starting in late adolescence through adulthood causes persistent deficits in short-term spatial memory.
A one hour novel object location (NOL) test was performed at two time points (1 week and 2 months) post-lead. (A,D) Training and (B,E) test sessions at 1 week and 2 months post-lead, respectively. Total exploration time at (C) 1 week and (F) 2 months post-lead. Mean ± SEM; n= 9–10 per treatment. Two-tailed t–test: n.s. not significant, ** p < 0.01; *** p < 0.001.
To determine if this deficit was transient or persistent, we conducted the 1 h NOL test again, 2 months post-lead exposure. None of the animals exhibited object or location preferences during training (Fig. 4D). In the test session, the control mice exhibited intact spatial working memory and spent significantly more time investigating the object in the novel location (Fig. 4E). However, the lead-treated mice did not discriminate between the novel and familiar object locations (Fig. 4E). Again, there was no significant difference in total exploration time between control and lead-treated mice (Fig. 4F). These data suggest that lead exposure during late-adolescence and adulthood is sufficient to cause spatial working memory deficits in C57BL/6 male mice and that these deficits persist after the cessation of lead exposure.
3.4. Assessment of adult hippocampal neurogenesis after the cessation of lead exposure
We assessed whether lead exposure impairs critical processes in adult hippocampal neurogenesis in C57Bl/6 male mice. After the cessation of lead exposure, we administered BrdU to mice either 7 weeks, 3 weeks, or 2 hours prior to sacrifice (Fig. 1). We used BrdU and NeuN immunostaining to identify adult-born cells and mature neurons, respectively, and assessed the effect of lead on adult-born cell differentiation, survival, and proliferation.
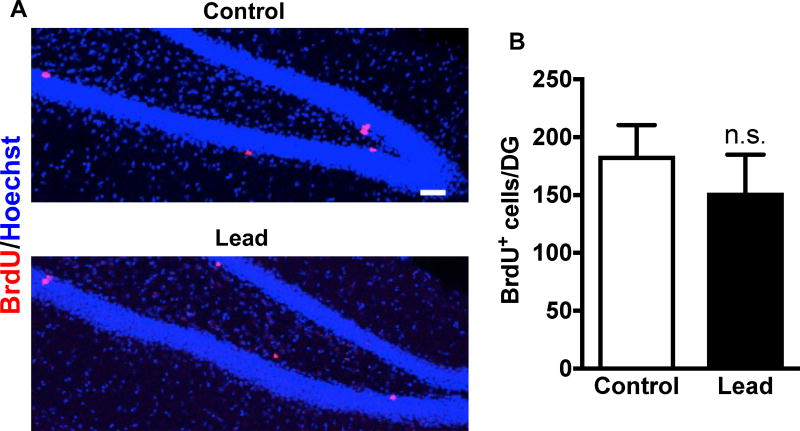
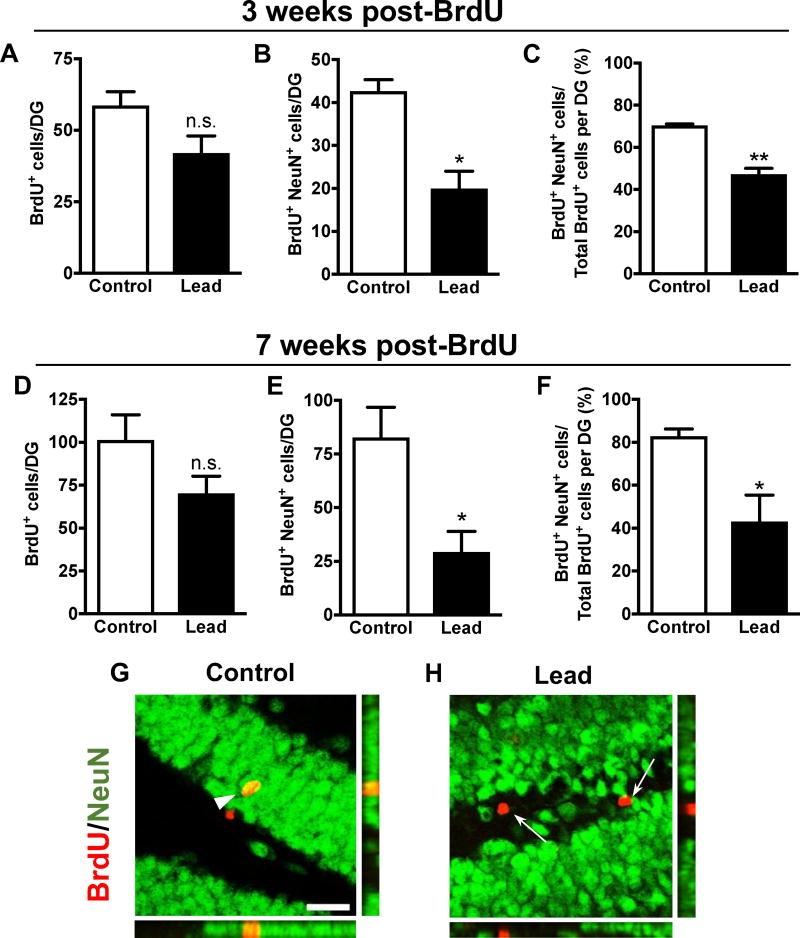
At 10 weeks post-lead exposure (2 hours post-BrdU), there was no statistically significant difference in the total number of BrdU+ cells in the DG of the hippocampus between control and lead-treated mice (Fig. 5), suggesting no impairment in adult-born cell proliferation in C57BL/6 mice at this time point. Lead did not impair adult-born cell survival because there was no statistically significant decrease in the total number of BrdU+ cells in the DG at 3 and 7 weeks post-BrdU (Fig.6A, D). However, there was a statistically significant decrease in the total number of adult-born mature neurons (BrdU+NeuN+ cells) (Fig. 6B, E) and the proportion of adult-born cells that differentiated into mature neurons (BrdU+NeuN+/total BrdU+ cells) in lead-treated mice at both 3 and 7 weeks post-BrdU (Fig. 6C, F), suggesting that lead inhibits neuronal differentiation of adult-born cells in the hippocampus of C57BL/6 mice.
Figure 5. Adult-born cell proliferation in vivo was not impaired 10 weeks post-lead exposure.
Ten weeks after the lead exposure, mice were dosed with 100 mg/kg BrdU and sacrificed 2 hours later. (A) Representative confocal images of BrdU (red) immunostaining in the DG of a control and lead-treated mouse. (B) Quantification of the total BrdU+ cells per DG. Mean ± SEM; n= 3 mice per treatment. Two-tailed t–test: n.s. not significant. Scale bar, 50 μm.
Figure 6. Neuronal differentiation of adult-born cells in vivo is impaired post-lead exposure.
Mice were dosed with 100 mg/kg BrdU (5X in one day) either 3 weeks or 7 weeks prior to sacrifice. Quantification of the total number of BrdU+ cells, total number of BrdU+NeuN+ cells, and the percent of total BrdU+ cells that are BrdU+NeuN+ per DG at (A-C) 3 weeks post-BrdU and (D-F) 7 weeks post-BrdU. Representative confocal images and orthographic panels of BrdU (red) immunostaining in the DG of a (G) control and (H) lead-treated mouse 7 weeks post-BrdU. Mean ± SEM; n= 3 mice per treatment at each BrdU time point. Two-tailed t–test: n.s. not significant, * p < 0.05; ** p < 0.01. Scale bar, 25 μm. Arrowheads: BrdU+/NeuN+ cells. Arrows: BrdU+/NeuN- cells.
4. Discussion
Several studies have characterized the effect of lead on adult-born cell proliferation in the hippocampus of rodents using early-life [9–11], lifetime [9], postnatal-only [12], or adult-only lead exposure paradigms [14]. These studies consistently found that lead decreased adult-born cell proliferation in the dentate gyrus, with the exception of Gilbert et al. (2005), which did not observe an effect of developmental-only or lifetime lead exposure on adult-born cell proliferation [9]. However, the rats in this study were dosed with BrdU twice a day for 12 days, which would have labeled multiple cell cycles and made it difficult to distinguish the effects of lead on cell proliferation and survival.
In the current study, we did not observe a decrease in adult-born cell proliferation using a single dose of 100 mg/kg BrdU at 10 weeks after the cessation of lead exposure, but we did observe a significant decrease in the neuronal differentiation of 3- and 7-week-old adult-born cells at 10 weeks post lead exposure. Thus, while lead inhibition of adult cell proliferation may be reversible after exposure stops, lead impairment of neuronal differentiation may persist after the cessation of lead exposure. The latter may underlie the long-term deleterious effects of lead on cognition.
The functional consequences of impaired adult neurogenesis may vary, depending on the species, strain, sex, and method used to reduce adult neurogenesis [18]. We previously reported that female and male ApoE4-KI mice exposed to lead (starting at 8 weeks old) also develop persistent deficits in the NOL test, starting at 7 and 11 weeks into a 12-week lead exposure, respectively [14]. In our current study, we did not test C57BL/6 male mice in the NOL test until after the conclusion of the lead exposure, so we cannot determine if the “wild-type” mice are more or less sensitive than mice expressing the ApoE4 allele to the effects of lead on short-term spatial memory. However, in our previous study, we did not observe NOL deficits in the lead-treated ApoE3-KI female and male mice until 3 or 6 months post-lead exposure, respectively. Thus, it appears that the “wild-type” male mice expressing the mouse ApoE gene are more sensitive than ApoE3-KI mice to the effects of adult lead exposure on short-term spatial memory. Additional study to further characterize the effect of lead, sex differences, and mouse vs. human isoforms of ApoE on hippocampus-dependent learning and memory is needed.
Together, data presented in this study suggest that in vivo exposure to lead starting in late-adolescence through adulthood is sufficient to impair key processes in adult hippocampal neurogenesis and cause persistent deficits in hippocampus-dependent learning and memory.
Highlights.
Adult mice were exposed to lead for 12 weeks to model subchronic lead exposure during adolescence and adulthood
Subchronic lead exposure during adolescence and adulthood was sufficient to cause persistent deficits in spatial short-term memory
Lead-exposed mice had fewer adult-born, mature neurons in the dentate gyrus of the hippocampus compared to control animals, suggesting lead exposure may impair adult-born neuron differentiation
Acknowledgments
We thank the members of the Xia laboratory for critically reading the manuscript and scientific discussions. This work was supported by NIH grants (R01 MH95840 and ES026591 to Z.X., T32ES015459 to A.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors wish to declare no conflict of interest.
References
- 1.Pan YW, et al. Inhibition of Adult Neurogenesis by Inducible and Targeted Deletion of ERK5 Mitogen-Activated Protein Kinase Specifically in Adult Neurogenic Regions Impairs Contextual Fear Extinction and Remote Fear Memory. J Neurosci. 2012;32:6444–6455. doi: 10.1523/JNEUROSCI.6076-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, et al. Genetic Activation of ERK5 MAP Kinase Enhances Adult Neurogenesis and Extends Hippocampus-Dependent Long-Term Memory. J Neurosci. 2014;34(6):2130–47. doi: 10.1523/JNEUROSCI.3324-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng W, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart WF, et al. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66(10):1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- 7.Weisskopf MG, et al. Cumulative lead exposure and prospective change in cognition among elderly men: the VA Normative Aging Study. Am J Epidemiol. 2004;160(12):1184–93. doi: 10.1093/aje/kwh333. [DOI] [PubMed] [Google Scholar]
- 8.Bihaqi SW, Zawia NH. Enhanced taupathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb) Neurotoxicology. 2013;39:95–101. doi: 10.1016/j.neuro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert ME, et al. Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci. 2005;86(2):365–74. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- 10.Verina T, Rohde CA, Guilarte TR. Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience. 2007;145(3):1037–1047. doi: 10.1016/j.neuroscience.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaako-Movits K, et al. Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. International Journal of Developmental Neuroscience. 2005;23(7):627–635. doi: 10.1016/j.ijdevneu.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Schneider JS, et al. Inhibition of progenitor cell proliferation in the dentate gyrus of rats following post-weaning lead exposure. Neurotoxicology. 2005;26(1):141–5. doi: 10.1016/j.neuro.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom A, Wang H, Xia Z. Lead decreases cell survival, proliferation, and neuronal differentiation of primary cultured adult neural precursor cells through activation of the JNK and p38 MAP kinases. Toxicol In Vitro. 2015;29(5):1146–55. doi: 10.1016/j.tiv.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engstrom AK, et al. Gene-environment interaction between lead and Apolipoprotein E4 causes cognitive behavior deficits in mice. Mol Neurodegener. 2017;12(1):14. doi: 10.1186/s13024-017-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bihaqi SW, et al. Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology. 2014;44:114–20. doi: 10.1016/j.neuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoud AM, et al. Early-Life Exposure to Lead (Pb) Alters the Expression of microRNA that Target Proteins Associated with Alzheimer's Disease. J Alzheimers Dis. 2016 doi: 10.3233/JAD-151018. [DOI] [PubMed] [Google Scholar]
- 17.Baker EL, et al. Occupational lead neurotoxicity: a behavioural and electrophysiological evaluation. Study design and year one results. Br J Ind Med. 1984;41(3):352–61. doi: 10.1136/oem.41.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227(2):391–9. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]