Abstract
Background
Systemic absorption of phthalates and parabens has been demonstrated after dermal application of body lotion, and medical devices such as intravenous bags and tubing have been identified as a source of exposure to di(2-ethylhexyl) phthalate (DEHP). However, use of products during medical procedures such as aqueous gel applied during obstetrical ultrasound in pregnancy has not been investigated as a potential source of endocrine disrupting chemical (EDC) exposure. Human studies have associated EDCs with various adverse pregnancy outcomes. There is a need to identify sources of inadvertent exposure to EDCs especially during vulnerable developmental periods such as pregnancy.
Objectives
We conducted a pilot study to determine whether use of gel during routine obstetrical ultrasound increased urinary concentrations of phthalate and phenol biomarkers.
Methods
We recruited 13 women from the Massachusetts General Hospital who provided spot urine samples at the time of their second trimester anatomic survey. The first sample was collected prior to the procedure (pre-exposure, time 1), and two additional samples were obtained at approximately 1–2 hours (time 2) and 7–12 hours (time 3) post-exposure following the scan.
Results
Urinary concentrations of several DEHP metabolites and metabolite of diisononyl cyclohexane-1,2-dicarboxylate (DINCH) increased across time. For example, the geometric mean concentrations of mono(2-ethyl-5-hydroxyhexyl) phthalate increased from 3.1 ng/ml to 7.1 ng/ml (p-value=0.03) between time 1 and time 3. We also observed significant differences in concentrations of metabolites of butylbenzyl phthalate (BBzP), di-n-butyl phthalate (DnBP), and di-isobutyl phthalate (DiBP). For example, mono-n-butyl phthalate (metabolite of DnBP) decreased from 3.5 ng/ml to 1.8 ng/ml (p-value=0.04) between time 1 and time 2, but then increased to 6.6 ng/ml (p-value=0.002) at time 3. Propylparaben concentrations increased from 8.9 ng/ml to 33.6 ng/ml between time 1 and time 2 (p-value=0.005), followed by a decrease to 12.9 ng/ml at time 3 (p-value=0.01). However, we cannot rule out the possibility that some of the observed differences are due to other sources of exposure to these compounds.
Conclusions
While additional research is needed, this pilot study potentially identifies a previously unknown source of phthalate and paraben exposure among pregnant women undergoing routine ultrasound examination.
Keywords: phthalates, phenols, parabens, obstetric ultrasound, trans-dermal absorption
BACKGROUND
Phthalates, parabens, triclosan, and benzophenone-3 are endocrine disrupting chemicals (EDCs) with widespread use in many personal care products including cosmetics, lotions, creams, perfumes/cologne, and gels (Ferguson et al. 2017). Use of personal care products (PCPs) is an important route of exposure to some EDCs (Ferguson et al. 2017; Duty et al. 2005; Philippat et al. 2015). Systemic absorption of parabens and phthalates has been demonstrated after dermal application of body lotion (Janjua et al. 2007) and medical devices such as intravenous tubing and bags have been identified as a source of di(2-ethylhexl) phthalate (DEHP) exposure (Green et al. 2005; Latini et al. 2009). However, use of other products during medical examinations or procedures, such as aqueous gel used to facilitate trans-abdominal imaging during obstetrical ultrasound in pregnancy has not been investigated as a possible source of EDC exposure.
Both phthalates and parabens have properties appealing to manufacturers of personal care products. Low molecular weight phthalates are often used as solubilizing agents and are added to products containing fragrance or perfume as they help bind the scent and color. Parabens are widely used as preservatives to increase shelf life in moisturizers, skin lotions, and other personal care products. Ingredients listed by the manufacturers of aqueous ultrasound gel products used during ultrasound include propylparaben, fragrance, and dyes. Therefore, ultrasound gel used in gynecological and obstetric exams may be a potentially important yet unrecognized source of exposure given its use during vulnerable developmental periods such as pregnancy.
Accumulating epidemiologic evidence has associated EDCs with adverse reproductive health outcomes in humans, including infertility, implantation failure, pregnancy loss, reduced clinical pregnancy rates, preterm birth, preeclampsia, and poorer child development (Gore et al. 2015; Woodruff et al. 2008). Studies have shown that the developing embryo and fetus are particularly sensitive to potential adverse effects of EDCs (Gore et al. 2015; Wittassek et al. 2009). Preterm neonates in intensive care units have substantially higher urinary concentrations of DEHP, di-isononyl phthalate (DiNP), butylbenzyl phthalate (BBzP) and bisphenol A compared to full term infants, suggesting higher exposure and potentially differential metabolism by gestational age at birth (Green et al. 2005; Calafat et al. 2009; Weuve et al. 2006; Huygh et al. 2015). Phthalates and phenols can cross the placenta and have been detected in amniotic fluid, cord blood, and newborn meconium (Wittassek et al. 2009; Philippat et al. 2013). Furthermore, several phthalate metabolites have been detected in the urine of both preterm and full-term infants at post-natal day 7(Frederiksen et al. 2014).
Despite widespread use of phthalates, parabens, and other phenols in cosmetics and PCPs, there are limited data on systemic human absorption of these compounds through the skin (Janjua et al. 2007). To our knowledge no study has investigated whether ultrasound gel products used during routine obstetric ultrasound scans are a source of exposure to these chemicals. A better understanding of potentially unknown sources of exposure during critical developmental windows such as periconception and pregnancy is important and provides data that may be used to reduce exposure. The objective of this study was to determine whether exposure to gel during routine obstetrical ultrasound examinations increased urinary concentrations of 17 individual phthalate metabolites, 2 metabolites of diisononyl cyclohexane-1,2-dicarboxylate (DINCH), and 11 phenols.
METHODS
Study Cohort
The Environment and Reproductive Health (EARTH) Study is a prospective preconception cohort of couples from the Massachusetts General Hospital (MGH) Fertility Center. The study was designed to evaluate the effects of environmental exposures and diet on fertility and pregnancy outcomes. The EARTH Study has been ongoing since 2004 and has recruited approximately 800 women and 500 men to date. Women 18 – 46 years are eligible to participate and may enroll independently or as a couple. Participants are followed from study entry throughout their fertility care, pregnancy, and delivery. At enrollment, participants completed a study staff-administered sociodemographic, lifestyle, and medical history questionnaire. They also completed a more comprehensive questionnaire on family, medical, reproductive and occupational history, stress, product use, smoking history, and physical activity.
Pilot Study Design
EARTH Study participants who were pregnant and scheduled for their routine 20-week anatomic survey ultrasound between December 2014 and December 2015 were approached to take part in this pilot study at the MGH obstetrical outpatient unit. The pilot was designed to investigate potential exposure at the time of participant’s routine ultrasound at approximately 17 to 20 weeks gestation. Participants provided a total of three spot urine samples: the first two were obtained following trained study staff instructions on the obstetrical unit at the MGH. Sample 1 (S1) was collected immediately prior to the start of the ultrasound scan (pre-exposure, time 1) and sample 2 (S2) 1–2 hours after the commencement of the scan (post-exposure, time 2). Participants used a home urine collection kit that we provided to collect the third sample (S3) at 7–12 hours post ultrasound (post-exposure, time 3). The kit included instructions, a collection cup, ice packs, questionnaire, and pre-paid overnight mail back labels. All participants also completed self-reported questionnaires at the time of each urine collection to identify product use and food/beverage consumption. The ultrasound technician conducting the scan also completed a brief form identifying the length of the ultrasound, the products used (manufacturer name and product), whether the ultrasound was only trans-abdominal or if trans-vaginal imaging was also necessary, the number of fetuses, and approximate gestational age. Technicians additionally collected a sample of the gel applied during the procedure, which was stored for future analyses. Trained study staff described the study protocol to all participants in detail and answered questions, and participants provided written informed consent. The study was approved by the Institutional Review Boards of MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Phthalate and Phenol Measurement
All urine samples were collected in a polypropylene specimen cup and analyzed for specific gravity with a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), divided into aliquots, and frozen for long-term storage at −80 °C. Samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA) for quantification of urinary phthalate and phenol biomarker concentrations, as well as metabolites of diisononyl cyclohexane-1,2-dicarboxylate (DINCH, a replacement chemical for DEHP), using online solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Silva et al. 2007; Ye et al. 2005). The urinary concentrations of the following 19 biomarkers were measured: monomethyl phthalate (MMP), primary metabolite of dimethyl phthalate (DMP); monoethyl phthalate (MEP), primary metabolite of diethyl phthalate (DEP); mono-n-butyl phthalate (MBP) and mono-3-hydroxy-n-butyl phthalate (MHBP), metabolites of di-n-butyl phthalate (DBP); mono-isobutyl phthalate (MiBP) and mono-hydroxyisobutyl phthalate (MHiBP), metabolites of DiBP, monoisononyl phthalate (MNP) and monocarboxyisooctyl phthalate (MCOP), metabolites of DiNP; mono(3-carboxypropyl) phthalate (MCPP), metabolite of di-n-octyl phthalate (DOP) and of other high molecular weight phthalates; monobenzyl phthalate (MBzP), metabolite of BBzP; mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), metabolites of DEHP; monocarboxyisononyl phthalate (MCNP), metabolite of di-isodecyl phthalate, and cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHiNCH) and cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH), two oxidative metabolites of DINCH.
The urinary concentrations of the following eleven phenols were also quantified: 2,4-dichlorophenol; 2,5-dichlorophenol; butylparaben; propylparaben; ethylparaben; methylparaben; triclosan; benzophenone-3; bisphenol A (BPA); bisphenol S (BPS); bisphenol F (BPF).
The limits of detection (LOD) for phthalates and DINCH metabolites ranged from 0.1 ng/ml (MiBP, MEOHP, MCNP, MCOP, MCPP, MECPP, MEHHP) to 0.6 ng/ml (MEP). For the other biomarkers the LOD range was 0.1 ng/ml (propylparaben, butylparaben, BPS, 2,5-dichlorophenol) to 1.7 ng/ml (triclosan). Instrumental reading values were used for concentrations below the LOD. The molar sum of four DEHP metabolites was calculated by dividing each metabolite concentration by its molecular weight and then summing: ΣDEHP= [(MEHP*(1/278.34)) + (MEHHP*(1/294.34)) + (MEOHP*(1/292.33)) + (MECPP*(1/308.33))]. We then multiplied the molar sum by the molecular weight of MECPP (308.33) to convert ΣDEHP to ng/ml.
Baseline Characteristics and Other Covariates
Socio-demographic characteristics of study participants including age, race, education, smoking status, and parity were obtained from the EARTH Study baseline enrollment questionnaire. A study staff member measured participants’ height and weight at study entry. Body Mass Index (BMI) was calculated as pre-pregnancy weight (kilograms) divided by height (meters) squared. Smoking status was self-reported at baseline and categorized as never smoked vs. ever smoked, defined as a current or former smoker. The underlying cause of infertility was diagnosed by a physician using the Society for Assisted Reproductive Technology definitions.
Relevant information on the pregnancy including gestational age and duration of ultrasound was obtained from the ultrasound technician form. Length of ultrasound was estimated in minutes using start time and end time as recorded by the ultrasound technicians at the MGH obstetrical unit. The number of food and beverages items consumed, and personal care products used between sample intervals (S1 to S2, and S2 to S3) were counted and the median was estimated from the self-reported questionnaire data.
Statistical Analysis
We present the demographic and clinical characteristics among study participants as mean (± SD) or number (%). Urinary biomarker concentrations were adjusted for urinary dilution by multiplying the biomarker concentration by [(SGp-1)/(SGi-1)], where SGi is the specific gravity of the participant’s sample and SGp is the mean specific gravity for all female EARTH participants (N=739) included in the study (Pearson et al. 2009). The specific gravity-adjusted biomarker concentrations were natural log-transformed to standardize the distribution and reduce the influence of outliers. The geometric mean of the biomarker concentrations was calculated for each of the three collection times. We estimated differences in log biomarker concentrations at pre-exposure time 1 (S1) vs. post-exposure time 2 (S2), pre-exposure time 1 (S1) vs. post-exposure time 3 (S3), as well as post-exposure time 2 (S2) vs. post-exposure time 3 (S3) using a signed rank test.
RESULTS
We enrolled 13 pregnant women into the pilot study, but excluded one woman who provided only a single urine sample. The 12 women included in this analysis were on average 34.6 years of age and had a pre-pregnancy BMI of 23.8 kg/m2. Women were predominantly Caucasian (83%), had college (42%) or graduate degrees (58%) and were never smokers (75%). A quarter had a female cause of infertility (25%) and 67% were nulliparous (Table 1). Women provided urine samples immediately before (S1) and twice after routine anatomical ultrasound scan at a median of 1.2 hours (S2) and 8.6 hours (S3) (Table 2). Measurements at time 1 took place between 7:00 AM and 4:30 PM. We obtained a total of 34 urine samples: 10 women provided all three samples and two women provided only the first two samples. All participants underwent trans-abdominal ultrasound (mean 18.7 weeks gestation) and one of 12 women additionally underwent trans-vaginal ultrasound. The average duration of the ultrasound was 37 minutes (range 13 to 60 minutes). A single woman reported eating a food item (an apple), six women reported drinking only water, and four women reported using a personal care product (hand soap) between S1 and S2. All women completing the self-reported questionnaire reported eating, drinking and using personal care products between S2 and S3 (Appendix Table 3).
Table 1.
Study characteristics among 12 pregnant women in the Ultrasound Gel Exposure Study.
| Characteristic | Women N=12 |
|---|---|
| Age (years) | |
| Mean (SD) | 34.6 (4.0) |
| Age>35, n (%) | 6 (50) |
| Race, n (%) | |
| White | 10 (83) |
| Black | 1 (8) |
| Asian | 1 (8) |
| Body Mass Index (BMI) kg/m2 | |
| Mean (SD) | 23.8 (3.6) |
| BMI >25, n (%) | 5 (42) |
| Education, n (%) | |
| < College | 0 (0) |
| College Graduate | 5 (42) |
| Graduate Degree | 7 (58) |
| Smoking Status, n (%) | |
| Never | 9 (75) |
| Ever (former or current) | 3 (25) |
| Infertility Diagnosis, n (%) | |
| Male Factor | 4 (33) |
| Female Factor | 3 (25) |
| Unexplained | 5 (42) |
| Nulliparous, n (%) | |
| Yes | 8 (67) |
| Gestational age at time of ultrasound | |
| Mean (min-max) | 18.7 weeks (17.6 – 19.6) |
| Number of twin gestations, n (%) | |
| 2 (17%) |
Table 2.
Number of hours (hrs) between pre-exposure urine sample (S1 at Time 1) and post-exposure urine sample (S2 at Time 2); and between pre-exposure urine sample (S1 at Time 1) and post-exposure urine sample (S3 at Time 3).
| Time | N | Mean time (SD) | Median time | Min-Max |
|---|---|---|---|---|
| Post-exposure S2 at Time 2 | 12 | 1.2 hrs (0.42) | 1.2 hrs | 40 min – 1.9 hrs |
| Post-exposure S3 at Time 3 | 10 | 10.6 hrs (5.1) | 8.6 hrs | 6.0 hrs – 23 hrs |
Phthalates and DINCH
The percentage of urine samples with detectable concentrations of phthalate and DINCH biomarkers ranged from 26% (MCOCH) to 100% (MCNP, MCOP, MECPP, MEP, MiBP) (Appendix Table 1). The geometric mean of the SG-adjusted urinary biomarker concentrations ranged from a low of 0.43 ng/ml (S1, MCOCH) to a high of 32.2 ng/ml (S3, MEP) (Appendix Table 1). We observed significant increases in concentrations between time 1 and time 3 for DEHP metabolites MECPP, MEHHP, MEOHP and the DINCH metabolite MHiNCH (Table 3 and Appendix Figure 1). For example, following ultrasound gel application, the geometric mean concentration of MEHHP increased from 3.1 ng/ml at time 1 to 7.1 ng/ml (p-value=0.03) at time 3 (Table 3 and Appendix Figure 1, Panel B). We also observed significant increases in geometric mean concentrations between time 2 and time 3 for ΣDEHP (and individual metabolites MECPP, MEHHP, MEOHP) as well as MHiBP, MiBP, MBzP, and MBP. For example, MBP concentration decreased significantly from 3.5 to 1.8 ng/ml (p-value=0.04) between time 1 and time 2, and then increased to 6.6 ng/ml (p-value=0.002) at time 3 (Table 3 and Appendix Figure 1, Panel D). Urinary concentrations of metabolites MEP, MNP, MHINCH increased sequentially across the three time periods, while MCOCH and MHBP increased and then decreased only marginally at time 2 and time 3, respectively (Table 3).
Table 3.
Distribution of specific gravity-adjusted phthalate and DINCH metabolite concentrations (ng/ml) from 12 Ultrasound Gel Exposure Study pilot participants providing 34 urine samples.
| Biomarker | Sample 1 Geomean (SD) |
Sample 2 Geomean (SD) |
Sample 3 Geomean (SD) |
Time Comparison p-value1,* | ||
|---|---|---|---|---|---|---|
| T1-T2 | T2-T3 | T1-T3 | ||||
| Low Molecular Weight Phthalate | ||||||
| MMP | 2.2 (0.69) | 1.8 (0.60) | 3.3 (0.99) | 0.42 | 0.25 | 0.43 |
| MEP | 25.5 (10.3) | 24.1 (9.4) | 32.2 (10.8) | 0.79 | 0.92 | 0.92 |
| MBP | 3.5 (1.1) | 1.8 (0.40) | 6.6 (0.80) | 0.04* | 0.002* | 0.13 |
| MiBP | 3.5 (1.1) | 1.8 (0.40) | 6.5 (0.79) | 0.13 | 0.002* | 0.08 |
| MHBP | 0.57 (0.13) | 0.63 (0.10) | 0.59 (0.14) | 0.97 | 1.0 | 1.0 |
| MHiBP | 2.5(0.55) | 1.8 (0.27) | 3.0 (0.54) | 0.15 | 0.02* | 0.32 |
| High Molecular Weight Phthalate | ||||||
| MCPP | 0.84 (0.21) | 0.73 (0.21) | 1.5 (0.46) | 0.20 | 0.04* | 0.06 |
| MBzP | 1.3 (0.68) | 0.8 (0.20) | 2.4 (0.53) | 0.20 | 0.004* | 0.13 |
| MEHP | 0.90 (0.20) | 0.98 (0.14) | 1.8 (0.39) | 0.68 | 0.05* | 0.08 |
| MEOHP | 2.6 (0.50) | 1.8 (0.36) | 5.5 (1.0) | 0.18 | 0.002* | 0.04* |
| MNP | 0.55 (0.13) | 0.78 (0.20) | 1.1 (0.43) | 0.11 | 0.98 | 0.08 |
| MEHHP | 3.1 (0.68) | 2.2 (0.46) | 7.1 (1.4) | 0.38 | 0.002* | 0.03* |
| MECPP | 4.7 (0.75) | 3.8 (0.67) | 9.0 (1.9) | 0.20 | 0.002* | 0.02* |
| MCOP | 7.6 (1.5) | 6.4 (1.9) | 10.7 (3.1) | 0.27 | 0.08 | 0.19 |
| MCNP | 1.6 (0.25) | 1.4 (0.20) | 2.2 (0.50) | 0.20 | 0.10 | 0.16 |
| sumDEHP | 1.0 (0.22) | 1.1 (0.15) | 2.0 (0.45) | 0.85 | 0.04* | 0.08 |
| DINCH (DEHP Substitute) | ||||||
| MHiNCH | 0.72 (0.22) | 0.90 (0.31) | 0.74 (0.43) | 0.38 | 0.06 | 0.01* |
| MCOCH | 0.43 (0.08) | 0.76 (0.23) | 0.55 (0.24) | 0.01* | 0.38 | 0.70 |
ABBREVIATIONS: mono-methyl phthalate (MMP); monoethyl phthalate (MEP); mono-n-butyl phthalate (MBP); mono-isobutyl phthalate (MiBP); mono-3-hydroxy-n-butyl phthalate (MHBP); mono-hydroxyisobutyl phthalate (MHiBP); mono(3-carboxypropyl) phthalate (MCPP); monobenzyl phthalate (MBzP); mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); monoisononyl phthalate (MNP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); monocarboxyisooctyl phthalate (MCOP); monocarboxyisononyl phthalate (MCNP); di(2-ethylhexyl) phthalate (DEHP); cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHiNCH); cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH).
Time 1 (T1); Time 2 (T2); Time 3 (T3) and
connotes significant p-value (<0.05) using signed-rank test of significance.
Note: Mean (SD) specific gravity by sample: Sample 1: 1.016 (0.005); Sample 2: 1.009 (0.006); Sample 3: 1.017 (0.008)
Phenols
The percentage of urine samples with detectable concentrations ranged from 26% (BPF) to 100% (2,4-dichlorophenol, methylparaben, propylparaben, benzophenone-3) (Appendix Table 2). The geometric mean of the SG-adjusted urinary phenol concentrations ranged from a low of 0.18 ng/ml (S1, BPS) to a high of 710 ng/ml (S2, benzophenone-3) (Appendix Table 2). We observed a significant almost four-fold increase in propylparaben concentrations between time 1 and time 2: the geometric mean urinary concentration increased from 8.9 to 33.6 ng/ml (p-value=0.005) and then decreased to 12.9 ng/ml at time 3 (p-value=0.16) (Table 4 and Appendix Figure 2, Panel C). Among the 12 participants, 8 showed increased urinary propylparaben concentrations at 1.2-hour post-ultrasound. Furthermore, the highest change in propylparaben was observed among the one woman who additionally underwent a trans-vaginal ultrasound with a nearly seven-fold increase between time 1 and time 2 (Appendix Figure 2, Panel C, subject 2). Excluding the subject with a trans-vaginal ultrasound from the study sample did not materially change the results and the increase in propylparaben concentration remained significant (data not shown). Similar patterns were observed for butylparaben: the geometric mean urinary concentration increased from 0.55 to 1.00 ng/ml (p-value=0.005) between time 1 to time 2 and then decreased back down to 0.50 ng/ml at time 3 (Table 4 and Appendix Figure 2, Panel D). However, the detection frequency for butylparaben was only 50%. The geometric mean of the other phenol concentrations did not differ significantly over the three time periods (Table 4).
Table 4.
Distribution of specific gravity-adjusted phenol concentrations (ng/ml) from 12 Ultrasound Gel Exposure Study pilot participants providing 34 urine samples.
| Biomarker | Sample 1 Geomean (SD) | Sample 2 Geomean (SD) | Sample 3 Geomean (SD) | Time Comparison p-value1* | ||
|---|---|---|---|---|---|---|
| T1-T2 | T2-T3 | T1-T3 | ||||
| Parabens | ||||||
| Methylparaben | 83.5 (37.0) | 116.4 (49.5) | 77.3 (39.4) | 0.15 | 0.25 | 0.63 |
| Ethylparaben | 6.5 (3.1) | 7.5 (3.8) | 4.8 (3.1) | 0.91 | 0.36 | 0.32 |
| Propylparaben | 8.9 (5.0) | 33.6 (14.4) | 12.9 (7.2) | 0.005* | 0.16 | 0.19 |
| Butylparaben | 0.55 (0.35) | 0.99 (0.60) | 0.50 (0.40) | 0.02* | 0.16 | 0.43 |
| Bisphenols | ||||||
| BPA | 0.47 (0.09) | 0.50 (0.08) | 0.58 (0.12) | 0.62 | 0.36 | 0.32 |
| BPF | 0.32 (0.18) | 0.42 (0.17) | 0.39 (0.21) | 0.33 | 0.19 | 0.85 |
| BPS | 0.18 (0.04) | 0.22 (0.05) | 0.21 (0.06) | 0.51 | 0.84 | 0.27 |
| Other Phenols | ||||||
| 2,4-Dichlorophenol | 0.57 (0.26) | 0.64 (0.22) | 0.61 (0.28) | 0.47 | 0.50 | 0.77 |
| 2,5-Dichlorophenol | 1.8 (1.32) | 1.8 (1.10) | 2.4 (1.74) | 0.73 | 0.38 | 0.92 |
| Triclosan | 8.4 (6.1) | 9.6 (6.3) | 8.6 (5.9) | 0.96 | 0.13 | 0.28 |
| Benzophenone-3 | 688 (317) | 710 (320) | 487 (213) | 0.79 | 0.11 | 0.32 |
ABBREVIATIONS: methylparaben (MPB); ethylparaben (EPB); propylparaben (PPB); butylparaben (BPB); bisphenol A (BPA); bisphenol F (BPF); bisphenol S (BPS); 2,4-dichlorophenol (2,4-DCP); 2,5-dichlorophenol (2,5-DCP); triclosan (TCS); benzophenone-3 (BP3).
Time 1 (T1); Time 2 (T2); Time 3 (T3) and
connotes significant p-value (<0.05) using signed-rank test of significance.
Note: Mean (SD) specific gravity by sample: Sample 1: 1.016 (0.005); Sample 2: 1.009 (0.006); Sample 3: 1.017 (0.008)
DISCUSSION
In this pilot study of pregnant women, we found suggestive evidence of systemic absorption of phthalates and propylparaben after dermal application of ultrasound gel used during routine obstetrical scan at 20 weeks gestation. Increases in urinary concentrations of DEHP metabolites and of MBP, MiBP, MHiBP, MBzP, and MCPP of samples taken at a median time of 8.6 hours post-ultrasound are consistent with reported peak urinary concentrations of phthalate metabolite concentrations at 7–12 hours post-ingestion or dermal application (Janjua et al. 2007; Sandanger et al. 2011). Overall, we observed a pattern of an initial small magnitude decrease in the geometric mean concentrations between time 1 and time 2 followed by a larger increase in concentrations from time 2 to time 3 for several phthalate metabolites (MiBP, MBP, MMP, MHiBP, MBzP, MCNP, MCOP, MCPP, MECPP, MEHHP, MEOHP). For MBP and MiBP the geometric mean concentrations nearly doubled from time 1 to time 3 and tripled from time 2 to time 3 (Table 3). One potential explanation for the increase at time 3 but not time 2 may be that dermal application of the gel leads to a delay in the systemic uptake of these phthalates following passage through the skin.
In contrast to phthalates, we observed a nearly quadrupling in urinary propylparaben concentrations between time 1 measurements (8.9 ng/ml) and samples at a median of 1.2-hour post-ultrasound (33.6 ng/ml), followed by a decrease at almost 9 hours after ultrasound (12.9 ng/ml). This increase suggests rapid uptake following dermal application for propylparaben. This is consistent with Janjua et al. 2007 who found that around 70% of the maximal butylparaben blood peak after whole-body topical application was observed at 1-hour post-intervention, demonstrating rapid dermal uptake (Janjua et al. 2007). In addition, metabolism studies using in vitro human liver microsomes have reported short half-lives for parabens, ranging from 0.5–1.5 hours (Jewell et al. 2007; Abbas et al. 2010). Among factors that may influence dermal permeation is the anatomical site of application, determined by the thickness of the stratum corneum and the vascularization of the skin area (Rougier et al. 1986). Thus, the abdominal area presents a medium to high absorption potential (Rougier et al. 1986), and it is well known that during pregnancy there is a remodeling of connective tissue and high vascularization, which might favor abdominal transdermal permeation (Geraghty and Pomeranz 2011). Interestingly, 8 of 12 participants showed increased urinary propylparaben concentrations at 1.2-hour post-ultrasound, and one of these women additionally underwent a trans-vaginal ultrasound, showing the highest change: a nearly seven-fold increase between time 1 and time 2 (Appendix Figure 2, Panel C). This is consistent with the fact that the vaginal wall and vulvar skin present a very thin stratum corneum and a high basal blood flow (Elsner et al. 1989). On the other hand, emulsion formulations can strongly influence the percutaneous absorption of parabens. It has been reported that lipophilic parabens, including propylparaben, increase their skin permeation when dissolved in an aqueous vehicle: with increasing lipophilic character, they become less soluble in the vehicle but more soluble in the stratum corneum (Pozzo and Pastori 1996). Overall, if we assume that the observed increase in propylparaben concentration was due to the ultrasound intervention, and not to other exposure sources, we may have missed the true peak in urine. Moreover, this peak might have occurred at 3 to 5-hours post-ultrasound, potentially explaining why we observed a decline in urinary propylparaben concentrations at 8.6-hours post-ultrasound. While the use of propylparaben is acknowledged in the ultrasound gel formulation, partial confounding with other factors cannot be ruled out. Consequently, our results should be interpreted cautiously as we were limited by a small pilot study sample size and could not adjust for potential confounding factors.
Phthalates and parabens can be found in a myriad of products, and total human exposure occurs through several routes including dermal absorption, ingestion, and inhalation (Giulivo et al. 2016). Biomonitoring data suggest that exposure to phthalates and parabens is ubiquitous across the United States population (Calafat et al. 2010; Zota et al. 2014). Phthalate and parabens are considered non-persistent EDCs with short biological half-lives (Janjua et al. 2007; Albro 1986; Hoppin et al. 2002). Low molecular weight phthalates such as DEP, DiBP, and DnBP are used in PCPs (e.g., lotions) and in pharmaceuticals and lubricants. High molecular weight phthalates such as DEHP are used to impart flexibility to plastics and can be found in vinyl flooring, medical devices, food packaging materials, toys, and electronics. Parabens are widely used as preservatives in food and consumer products like moisturizers, skin lotions, hair/shaving products, food packaging-commodities and industrial products, due to their antimicrobial and anti-fungal properties (Giulivo et al. 2016; Soni et al. 2005). Thus, the main source of human exposure to parabens occurs via dermal absorption of PCPs (Giulivo et al. 2016).
While we found suggestive patterns of increases in urinary concentrations of biomarkers of DEHP, DBP and BBzP at nearly 9-hours after ultrasound and of propylparaben at ~ 1 hour post-ultrasound, there could be other sources of these compounds that may partially confound our results. However, it should be noted that baseline measurements at time 1 were obtained at different times of the day (ranging from 7:00 AM to 4:30 PM) and therefore background exposure sources through food and cosmetics or other PCPs would not all follow the same overall pattern. Of significance, however, is the fact that the manufacturer lists the composition of the aqueous gel product used at MGH obstetrical unit to include: propylparaben, fragrance, and dyes among the ingredients. It is possible that DBP and BBzP compounds are used in the gel product as solvents/fixatives to adhere fragrance and/or color/dye to the gel (Al-Saleh and Elkhatib 2016) and that urinary increases at 8.6 hours post-ultrasound reflect dermal absorption of the product at time of ultrasound imaging. However, it is also possible that observed concentration increases at time 3 also reflect additional exposure through food, cosmetics, and other sources including potential hospital procedures following ultrasound.
The highest increase in propylparaben concentration at time 2 was observed in the sole participant who also underwent a trans-vaginal ultrasound. As women undergoing medically assisted reproduction receive frequent trans-vaginal ultrasound during the monitoring phase of follicles, this previously unrecognized source of exposure during fertility treatment needs further investigation, especially in light of the potential for increased trans-mucosal absorption through the vaginal wall (Machado et al. 2015). Compared to dietary sources, dermal exposure avoids first-pass metabolism, directly reaching systemic circulation. Therefore, the relative contribution of dermal sources to the total internal exposure of the general population might be of equal or even higher toxicological relevance than dietary sources for some EDCs (von Goetz et al. 2017). Although the health relevance of acute EDC exposures during medical procedures such as those seen in the present pilot is unknown, there is a need for further research that considers potential inadvertent exposure, especially during vulnerable periods such as prenatal development.
CONCLUSIONS
The results of this pilot study suggest that aqueous gels used to facilitate trans-abdominal imaging during obstetrical ultrasound in pregnancy might be an unrecognized source of EDC exposure, particularly parabens and some phthalates. More research is needed in order to confirm these findings and determine whether this inadvertent source of exposure is associated with fertility or pregnancy outcomes, especially among subfertile women trying to conceive.
HIGHLIGHTS.
Phthalates and phenols are widely used in personal care products such as cosmetics, lotions, creams, perfumes, and gels.
Use of aqueous gel applied during obstetrical ultrasound in pregnancy has not been investigated as a potential source of phthalate or phenol exposure.
Urinary concentrations of some phthalates and parabens increased following application of ultrasound gel in this pilot study.
While additional research is needed, this study potentially identifies a previously unknown source of phthalate and paraben exposure among pregnant women undergoing routine ultrasound examination.
Acknowledgments
The authors gratefully acknowledge Manori Silva, Ella Samandar, Jim Preau, Prabha Dwivedi, Xiaoliu Zhou, Tolar Powell, and Tao Jia (CDC, Atlanta, GA) for measuring the urinary concentrations of the phthalate and phenols biomarkers. We also acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd, Patricia Morey and Gheed Murtadi, physicians and staff at Massachusetts General Hospital Fertility Center and Obstetrics Unit. A special thanks to all the study participants.
Funding: Work supported by grants ES R01 009718 from the National Institute of Environmental Health Sciences (NIEHS). BJW was supported by the National Institutes of Health (NIH K23 ES021471). CM was supported by a post-doctoral fellowship award from the Canadian Institutes of Health Research.
Appendix Figure 1.
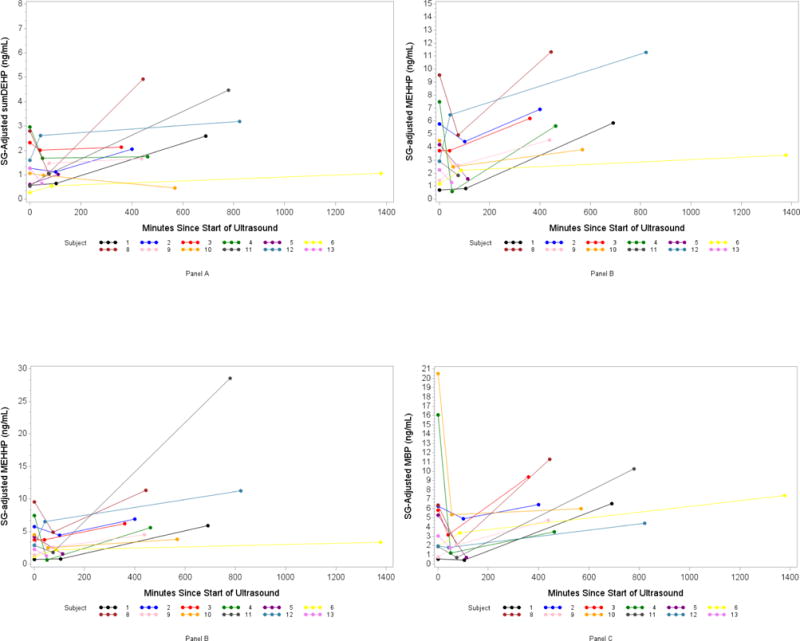
Spaghetti Plots of SG-Adjusted Phthalate Metabolite Concentration (ng/ml) by number of minutes since ultrasound: Panel A (DEHP, di(2-ethylhexyl) phthalate), B (MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate), C (MEOHP, mono(2-ethyl-5-oxohexyl) phthalate), D (MBP, mono-n-butyl phthalate).
Appendix Figure 2.
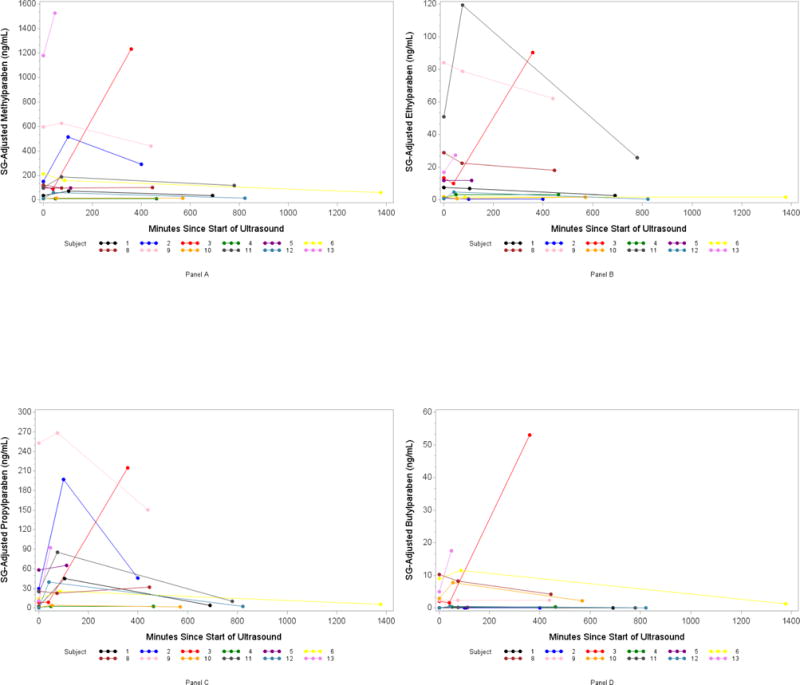
Spaghetti Plots of SG-Adjusted Phenol Concentration (ng/ml) by number of minutes since ultrasound: Panel A (Methylparaben), B (Ethylparaben), C (Propylparaben), D (Butylparaben).
Appendix Table 1.
Distribution of specific gravity-adjusted phthalate and diisononyl cyclohexane-1,2-dicarboxylate (DINCH) metabolite concentrations (ng/ml) for 12 Ultrasound Gel Exposure Study pilot participants providing 34 urine samples.
| Phthalate Metabolite | Detection Frequency | Sample | Geometric Mean (GSD) | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|
| Low Molecular Weight | ||||||||
| MMP | 82% | S1 | 2.2 (0.69) | 0.38 | 1.1 | 2.1 | 6.1 | 11.7 |
| S2 | 1.8 (0.60) | 0.46 | 0.69 | 1.3 | 4.9 | 13.8 | ||
| S3 | 3.3 (0.99) | 0.83 | 1.5 | 4.4 | 7.5 | 11.7 | ||
| MEP | 100% | S1 | 25.5 (10.3) | 6.4 | 6.9 | 17.9 | 85.5 | 273 |
| S2 | 24.1 (9.4) | 5.5 | 7.2 | 21.7 | 49.4 | 468 | ||
| S3 | 32.2 (10.8) | 6.7 | 9.3 | 48.1 | 89.1 | 92.4 | ||
| MBP | 85% | S1 | 3.5 (1.1) | 0.58 | 1.9 | 4.2 | 6.3 | 20.5 |
| S2 | 1.8 (0.40) | 0.49 | 1.8 | 2.1 | 3.4 | 5.3 | ||
| S3 | 6.6 (0.80) | 3.5 | 4.7 | 6.5 | 9.4 | 11.3 | ||
| MiBP | 100% | S1 | 3.5 (1.1) | 1.3 | 2.7 | 3.8 | 9.0 | 14.7 |
| S2 | 1.8 (0.40) | 1.0 | 2.2 | 2.7 | 4.7 | 7.0 | ||
| S3 | 6.5 (0.79) | 3.3 | 4.8 | 7.0 | 8.5 | 15.1 | ||
| MHBP | 50% | S1 | 0.57 (0.13) | 0.19 | 0.33 | 0.48 | 1.1 | 1.8 |
| S2 | 0.63 (0.10) | 0.23 | 0.42 | 0.73 | 0.74 | 1.8 | ||
| S3 | 0.59 (0.14) | 0.17 | 0.45 | 0.65 | 1.2 | 1.2 | ||
| MHiBP | 91% | S1 | 2.5(0.55) | 0.43 | 1.9 | 2.1 | 4.6 | 7.3 |
| S2 | 1.8 (0.27) | 0.73 | 1.3 | 1.8 | 2.4 | 5.8 | ||
| S3 | 3.0 (0.54) | 0.95 | 2.4 | 3.1 | 3.6 | 8.5 | ||
| High Molecular Weight | ||||||||
| MCPP | 91% | S1 | 0.84 (0.21) | 0.34 | 0.41 | 0.76 | 1.3 | 4.3 |
| S2 | 0.73 (0.21) | 0.24 | 0.33 | 0.49 | 1.6 | 6.0 | ||
| S3 | 1.5 (0.46) | 0.83 | 0.87 | 1.0 | 1.4 | 17.8 | ||
| MBzP | 79% | S1 | 1.3 (0.68) | 0.21 | 0.45 | 0.81 | 2.9 | 139 |
| S2 | 0.8 (0.20) | 0.26 | 0.45 | 0.52 | 1.8 | 3.9 | ||
| S3 | 2.4 (0.53) | 0.91 | 1.1 | 2.8 | 4.5 | 6.0 | ||
| MEHP | 65% | S1 | 0.90 (0.20) | 0.27 | 0.5 | 1.0 | 1.7 | 2.6 |
| S2 | 0.98 (0.14) | 0.26 | 0.6 | 0.87 | 1.3 | 1.9 | ||
| S3 | 1.8 (0.39) | 0.43 | 1.5 | 1.8 | 2.8 | 4.4 | ||
| MEOHP | 97% | S1 | 2.6 (0.50) | 0.72 | 2.0 | 2.6 | 4.4 | 6.0 |
| S2 | 1.8 (0.36) | 0.43 | 1.1 | 1.8 | 2.6 | 5.9 | ||
| S3 | 5.5 (1.0) | 2.7 | 3.9 | 4.9 | 6.6 | 21.0 | ||
| MNP | 41% | S1 | 0.55 (0.13) | 0.08 | 0.19 | 0.38 | 0.75 | 5.0 |
| S2 | 0.78 (0.20) | 0.08 | 0.27 | 0.52 | 0.87 | 4.7 | ||
| S3 | 1.1 (0.43) | 0.35 | 0.43 | 0.80 | 0.94 | 25.9 | ||
| MEHHP | 97% | S1 | 3.1 (0.68) | 0.72 | 1.8 | 3.3 | 5.1 | 9.6 |
| S2 | 2.2 (0.46) | 0.81 | 1.4 | 2.4 | 4.1 | 6.5 | ||
| S3 | 7.1 (1.41) | 3.4 | 4.6 | 6.1 | 11.3 | 28.5 | ||
| MECPP | 100% | S1 | 4.7 (0.75) | 1.6 | 3.24 | 4.4 | 7.9 | 9.6 |
| S2 | 3.8 (0.67) | 1.8 | 2.5 | 3.2 | 5.9 | 15.6 | ||
| S3 | 9.0 (1.9) | 3.4 | 6.7 | 8.0 | 12.2 | 34.5 | ||
| MCOP | 100% | S1 | 7.6 (1.5) | 3.1 | 4.1 | 7.4 | 13.9 | 25.3 |
| S2 | 6.4 (1.9) | 0.87 | 3.4 | 5.9 | 14.0 | 36.7 | ||
| S3 | 10.7(3.1) | 3.9 | 5.8 | 8.8 | 12.0 | 114 | ||
| MCNP | 100% | S1 | 1.6 (0.25) | 0.87 | 1.1 | 1.5 | 2.0 | 4.6 |
| S2 | 1.4 (0.20) | 0.73 | 0.93 | 1.3 | 2.0 | 3.9 | ||
| S3 | 2.2 (0.50) | 0.76 | 1.2 | 2.1 | 4.6 | 6.5 | ||
| sumDEHP | – | S1 | 1.0 (0.22) | 0.28 | 0.56 | 1.2 | 2.0 | 2.9 |
| S2 | 1.1 (0.15) | 0.53 | 0.81 | 1.0 | 1.6 | 2.6 | ||
| S3 | 2.0 (0.45) | 0.45 | 1.7 | 2.1 | 3.2 | 4.9 | ||
| DINCH | ||||||||
| MHiNCH | 71% | S1 | 0.72 (0.22) | 0.19 | 0.31 | 0.67 | 1.3 | 9.2 |
| S2 | 0.90 (0.31) | 0.15 | 0.43 | 0.85 | 1.1 | 21.3 | ||
| S3 | 0.74 (0.43) | 0.18 | 0.52 | 1.1 | 3.0 | 72.5 | ||
| MCOCH | 26% | S1 | 0.43 (0.08) | 0.10 | 0.26 | 0.34 | 0.52 | 2.4 |
| S2 | 0.76 (0.23) | 0.11 | 0.33 | 0.46 | 1.0 | 7.0 | ||
| S3 | 0.55 (0.24) | 0.09 | 0.24 | 0.36 | 0.65 | 18.0 |
Number of samples: S1 (n=12); S2 (n=12); S3 (n=10).
ABBREVIATIONS: mono-methyl phthalate (MMP); monoethyl phthalate (MEP); mono-n-butyl phthalate (MBP); mono-isobutyl phthalate (MiBP); mono-3-hydroxy-n-butyl phthalate (MHBP); mono-hydroxyisobutyl phthalate (MHiBP); mono(3-carboxypropyl) phthalate (MCPP); monobenzyl phthalate (MBzP); mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); monoisononyl phthalate (MNP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); monocarboxyisooctyl phthalate (MCOP); monocarboxyisononyl phthalate (MCNP); di(2-ethylhexyl) phthalate (DEHP); Diisononyl cyclohexane-1,2-dicarboxylate (DINCH), cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHiNCH); cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH).
Appendix Table 2.
Distribution of specific gravity-adjusted phenol biomarker concentrations (ng/ml) for 12 Ultrasound Gel Exposure Study pilot participants providing 34 urine samples.
| Phenol Metabolite | Detection Frequency | Sample | Geometric Mean (GSD) | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|
| 2,4-DCP | 100% | S1 | 0.57 (0.26) | 0.15 | 0.24 | 0.38 | 0.78 | 50.1 |
| S2 | 0.64 (0.22) | 0.22 | 0.29 | 0.52 | 0.80 | 17.8 | ||
| S3 | 0.61 (0.28) | 0.14 | 0.18 | 0.60 | 1.14 | 16.5 | ||
| 2,5-DCP | 97% | S1 | 1.8 (1.32) | 0.11 | 0.29 | 1.29 | 3.62 | 1618 |
| S2 | 1.8 (1.10) | 0.26 | 0.39 | 1.25 | 2.47 | 634 | ||
| S3 | 2.4 (1.74) | 0.18 | 0.91 | 1.24 | 4.55 | 538 | ||
| M-PB | 100% | S1 | 83.5 (37.0) | 9.97 | 23.4 | 104 | 181 | 1182 |
| S2 | 116.4 (49.5) | 9.53 | 66.4 | 96.2 | 350 | 1526 | ||
| S3 | 77.3 (39.4) | 9.53 | 14.6 | 78.9 | 291 | 1234 | ||
| E-PB | 74% | S1 | 6.5 (3.1) | 0.77 | 1.36 | 9.70 | 22.9 | 84.1 |
| S2 | 7.5 (3.8) | 0.38 | 2.32 | 8.49 | 24.8 | 119 | ||
| S3 | 4.8 (3.1) | 0.32 | 1.52 | 2.81 | 25.8 | 90.2 | ||
| P-PB | 100% | S1 | 8.9 (5.0) | 0.54 | 1.49 | 12.9 | 27.8 | 253 |
| S2 | 33.6 (14.4) | 2.17 | 15.8 | 42.4 | 89.0 | 268 | ||
| S3 | 12.9 (7.2) | 2.06 | 2.6 | 8.13 | 46.0 | 215 | ||
| B-PB | 50% | S1 | 0.55 (0.35) | 0.04 | 0.07 | 1.09 | 3.99 | 10.3 |
| S2 | 0.99 (0.60) | 0.04 | 0.18 | 1.04 | 8.1 | 17.6 | ||
| S3 | 0.50 (0.40) | 0.03 | 0.04 | 0.80 | 2.44 | 53.0 | ||
| BP-3 | 100% | S1 | 687.9 (317) | 55.4 | 139 | 1084 | 1949 | 8375 |
| S2 | 709.6 (320) | 43.4 | 422 | 979 | 2502 | 4264 | ||
| S3 | 487.3 (213) | 42.4 | 211 | 839 | 1508 | 2475 | ||
| BPA | 79% | S1 | 0.47 (0.09) | 0.16 | 0.27 | 0.48 | 0.84 | 1.45 |
| S2 | 0.50 (0.08) | 0.22 | 0.36 | 0.48 | 0.82 | 1.05 | ||
| S3 | 0.58 (0.12) | 0.15 | 0.52 | 0.60 | 0.81 | 1.82 | ||
| BPF | 26% | S1 | 0.32 (0.18) | 0.08 | 0.11 | 0.15 | 0.37 | 63.3 |
| S2 | 0.42 (0.17) | 0.11 | 0.17 | 0.36 | 0.64 | 17.3 | ||
| S3 | 0.39 (0.21) | 0.09 | 0.15 | 0.20 | 0.61 | 8.25 | ||
| BPS | 68% | S1 | 0.18 (0.04) | 0.05 | 0.09 | 0.21 | 0.34 | 0.87 |
| S2 | 0.22 (0.05) | 0.08 | 0.10 | 0.22 | 0.35 | 1.30 | ||
| S3 | 0.21 (0.06) | 0.06 | 0.12 | 0.18 | 0.49 | 0.85 | ||
| TCS | 62% | S1 | 8.38 (6.1) | 0.92 | 1.12 | 4.32 | 126 | 797 |
| S2 | 9.63 (6.3) | 0.98 | 2.54 | 3.73 | 134 | 597 | ||
| S3 | 8.57 (5.9) | 0.71 | 2.14 | 4.02 | 83.2 | 295 |
Number of samples: S1 (n=12); S2 (n=12); S3 (n=10).
ABBREVIATIONS: methylparaben (M-PB); ethylparaben (E-PB); propylparaben (P-PB); butylparaben (B-PB); bisphenol A (BPA); bisphenol F (BPF); bisphenol S (BPS); 2,4-dichlorophenol (2,4-DCP); 2,5-dichlorophenol (2,5-DCP); triclosan (TCS); benzophenone-3 (BP3).
Appendix Table 3.
Number of food, beverages, and personal care products used by participants between pre-exposure urine sample (S1 at Time 1) and post-exposure urine sample (S2 at Time 2); and between post-exposure urine sample (S2 at Time 2) and post-exposure urine sample (S3 at Time 3).
| Time | N | Food Items1 Median (min-max) |
N | Beverage Items2 Median (min-max) |
N | Personal Care Products3 Median (min-max) |
|---|---|---|---|---|---|---|
| S1 to S2 | 12 | 0 (0 – 1) | 12 | 0.5 (0 – 1) | 12 | 0 (0 – 1) |
| S2 to S3 | 9 | 7 (2 – 7) | 10 | 1 (1 – 1) | 9 | 1 (0 – 3) |
Only one woman (n=1/12) reported eating between S1 and S2. All women (n=9/9) reported eating between S2 and S3.
Six women (n=6/12) reported drinking between S1 and S2 (all 6 reported the beverage to be water). All 10 women (n=10/10) reported drinking between S2 and S3.
Four women (n=4/12) reported using a personal care product between S1 and S2. Eight women (n=8/9) reported using personal care products between S2 and S3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors declare no actual or potential competing interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services or CDC.
References
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. Journal of exposure science & environmental epidemiology. 2017;27(3):326–332. doi: 10.1038/jes.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental health perspectives. 2005;113(11):1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environmental research. 2015;140:369–376. doi: 10.1016/j.envres.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environmental science & technology. 2007;41(15):5564–5570. doi: 10.1021/es0628755. [DOI] [PubMed] [Google Scholar]
- Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environmental health perspectives. 2005;113(9):1222–1225. doi: 10.1289/ehp.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Del Vecchio A, Barducci A, Ferri M, Chiellini F. Di-(2-ethylhexyl)phthalate leakage and color changes in endotracheal tubes after application in high-risk newborns. Neonatology. 2009;95(4):317–323. doi: 10.1159/000181161. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine reviews. 2015;36(6):E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary. Fertility and sterility. 2008;89(2 Suppl):e1–e20. doi: 10.1016/j.fertnstert.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J, Kolossa-Gehring M, Schafer SD, Klockenbusch W, Dobler L, et al. Fetal exposure to phthalates–a pilot study. International journal of hygiene and environmental health. 2009;212(5):492–498. doi: 10.1016/j.ijheh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environmental health perspectives. 2009;117(4):639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Sanchez BN, Calafat AM, Schettler T, Green RA, Hu H, et al. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environmental health perspectives. 2006;114(9):1424–1431. doi: 10.1289/ehp.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygh J, Clotman K, Malarvannan G, Covaci A, Schepens T, Verbrugghe W, et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environment international. 2015;81:64–72. doi: 10.1016/j.envint.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. Prenatal exposure to environmental phenols: concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environmental health perspectives. 2013;121(10):1225–1231. doi: 10.1289/ehp.1206335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Kuiri-Hanninen T, Main KM, Dunkel L, Sankilampi U. A longitudinal study of urinary phthalate excretion in 58 full-term and 67 preterm infants from birth through 14 months. Environmental health perspectives. 2014;122(9):998–1005. doi: 10.1289/ehp.1307569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. Journal of exposure science & environmental epidemiology. 2009;19(3):336–342. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- Sandanger TM, Huber S, Moe MK, Braathen T, Leknes H, Lund E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: the NOWAC postgenome study. Journal of exposure science & environmental epidemiology. 2011;21(6):595–600. doi: 10.1038/jes.2011.22. [DOI] [PubMed] [Google Scholar]
- Jewell C, Bennett P, Mutch E, Ackermann C, Williams FM. Inter-individual variability in esterases in human liver. Biochemical pharmacology. 2007;74(6):932–939. doi: 10.1016/j.bcp.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Abbas S, Greige-Gerges H, Karam N, Piet MH, Netter P, Magdalou J. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in man. Drug metabolism and pharmacokinetics. 2010;25(6):568–577. doi: 10.2133/dmpk.dmpk-10-rg-013. [DOI] [PubMed] [Google Scholar]
- Rougier A, Dupuis D, Lotte C, Roguet R, Wester RC, Maibach HI. Regional variation in percutaneous absorption in man: measurement by the stripping method. Archives of dermatological research. 1986;278(6):465–469. doi: 10.1007/BF00455165. [DOI] [PubMed] [Google Scholar]
- Geraghty LN, Pomeranz MK. Physiologic changes and dermatoses of pregnancy. International journal of dermatology. 2011;50(7):771–782. doi: 10.1111/j.1365-4632.2010.04869.x. [DOI] [PubMed] [Google Scholar]
- Elsner P, Oriba HA, Maibach HI. Physiology of the skin of the vulva: new aspects. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 1989;40(7):411–417. [PubMed] [Google Scholar]
- Pozzo AD, Pastori N. Percutaneous absorption of parabens from cosmetic formulations. International journal of cosmetic science. 1996;18(2):57–66. doi: 10.1111/j.1467-2494.1996.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Giulivo M, Lopez de Alda M, Capri E, Barcelo D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environmental research. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environmental health perspectives. 2010;118(5):679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental health perspectives. 2014;122(3):235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro PW. Absorption, metabolism, and excretion of di(2-ethylhexyl) phthalate by rats and mice. Environmental health perspectives. 1986;65:293–298. doi: 10.1289/ehp.8665293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environmental health perspectives. 2002;110(5):515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Elkhatib R. Screening of phthalate esters in 47 branded perfumes. Environmental science and pollution research international. 2016;23(1):455–468. doi: 10.1007/s11356-015-5267-z. [DOI] [PubMed] [Google Scholar]
- Machado RM, Palmeira-de-Oliveira A, Gaspar C, Martinez-de-Oliveira J, Palmeira-de-Oliveira R. Studies and methodologies on vaginal drug permeation. Advanced drug delivery reviews. 2015;92:14–26. doi: 10.1016/j.addr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- von Goetz N, Pirow R, Hart A, Bradley E, Pocas F, Arcella D, et al. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regulatory toxicology and pharmacology: RTP. 2017;85:70–78. doi: 10.1016/j.yrtph.2017.02.004. [DOI] [PubMed] [Google Scholar]


