Abstract
Chlorpyrifos (CPF) is an organophosphourus insecticide applied to cotton fields by adolescents employed by the Egyptian Ministry of Agriculture. Urinary 3,5,6-trichloro-2-pyridinol (TCPy) is a biomarker of CPF exposure that has substantial variability among these applicators. In order to identify predictors of CPF exposure, we conducted a longitudinal study of 43 adolescent pesticide applicators in Egypt from April 2010 to January 2011 in Egypt. Urinary TCPy was quantified at 25 time-points, prior to, during, and following application. We used log-linear regression and a best subset selection approach to identify the exposure determinants that were most predictive of cumulative TCPy and participants’ highest TCPy values (peak exposure). Applicators had cumulative urinary TCPy levels ranging from 167 to 49,8208 µg/g creatinine. Total hours applying CPF (semi-partial r2 = 0.32), and total hours in the field applying other pesticides (semi-partial r2=0.08) were the strongest predictors of cumulative TCPy. Applicators had peak urinary TCPy levels ranging from 4 to 5,715 µg/g creatinine. The amount of time applying pesticides prior to blood draw was the strongest predictor of peak TCPy (semipartial r2=0.30). We also observed evidence that wearing clean clothes to work was associated with lower longitudinal TCPy. Our results suggest there is an opportunity for targeted interventions, particularly related to hygiene or implementation of personal protective equipment usage to reduce CPF exposure among adolescent pesticide workers.
Keywords: organophosphates, chlorpyrifos, biomonitoring, occupational exposure, pesticide applicators
Introduction
Chlorpyrifos (CPF) is one of the most commonly applied organophosphorus pesticides (OPs) in the world (Foxenberg et al., 2011). Exposure to OPs, including CPF, is a public health concern because of the acute detrimental neurological impacts associated with acetylcholinesterase inhibition (Keifer and Firestone, 2007) and the potential of chronic exposures to produce deficits in neurobehavioral performance (Eskenazi et al., 2014; Khan et al., 2014; Rauh et al., 2011; Rohlman et al., 2016). OP exposure has also been associated with increased risk of developing certain cancers (Lerro et al., 2015) and poor respiratory function (Chakraborty et al., 2009; Fieten et al., 2009; Hoppin et al., 2002; Hoppin et al., 2006; Ohayo-Mitoko et al., 2000). These concerns are amplified when considering children and adolescents because they have a higher body surface area to volume ratio, which increases their dermal absorption of toxic compounds (Phillips et al., 1993). Furthermore, human and animal studies have demonstrated that younger individuals express lower levels of the OP detoxifying enzyme, paraoxonase (PON1) (Costa et al., 1999).
Previous studies in agricultural pesticide applicators in Egypt and Indonesia have reported that the primary route of exposure to CPF is through dermal absorption (Fenske et al., 2012; Kishi et al., 1995). An individual’s internal dose of CPF is determined by the amount of CPF an individual is exposed to, which can be altered by workplace behaviors and hygiene, and their capability for metabolizing xenobiotics. In addition to the use of personal protective equipment (PPE) (Coble et al., 2011; Krenz et al., 2015), the method of pesticide mixing (Krenz et al., 2015), frequency and duration of spraying, pesticide formulation (granular vs. liquid) (Thomas et al., 2010a; Thomas et al., 2010b), contact with contaminated surfaces (Thomas et al., 2010b), and equipment malfunction (Alexander et al., 2006; Thomas et al., 2010b) can contribute to exposure. Personal hygiene (Curwin et al., 2002), laundering of clothing (Kishi et al., 1995), and eating while applying (Thomas et al., 2010b) have also been demonstrated to impact biomarkers of OP exposure.
Residential use of OPs (Fenske et al., 2002), diet (Beamer et al., 2012; Munoz-Quezada et al., 2012), parents who are employed in agriculture (Fenske et al., 2002; Lu et al., 2000; Rohitrattana et al., 2014), and living in close proximity to a farm (Fenske et al., 2002; Lu et al., 2000; Munoz-Quezada et al., 2012; Rohitrattana et al., 2014) are predictors of increased concentrations of OP metabolites in urine among children. We have previously reported that adolescents who apply pesticides, including CPF, seasonally in Egypt had higher urinary concentrations of TCPy and lower blood butyrylcholinesterase (BChE) activity when compared with non-applicators of similar age (Crane et al., 2013; Khan et al., 2014). Although occupation is the primary source of OP exposure among adults, (Munoz-Quezada et al., 2012) occupational exposure to OPs among adolescents has not been thoroughly evaluated. Determinants of exposure to OPs may differ between adolescents and adults. For instance, adolescent farmworkers in the US have been observed to engage in riskier work practices than their adult counterparts. These practices include engaging in higher pesticide exposure habits, such as less hand washing, wearing wet clothing, or wearing shorts instead of long pants (Kearney et al., 2015). In order to develop potential interventions to reduce CPF exposure among adolescents, determinants of exposure must be identified.
Our objective was to identify the determinants of CPF exposure among adolescent pesticide applicators by examining the relationship between urinary TCPy concentrations throughout a 10-month study period and workplace activities, hygiene habits, PPE use, and duration of applications. We expect that our results will aid in identifying methods to reduce exposure to CPF among adolescent applicators, a population that is particularly vulnerable to the deleterious effects of OPs.
Materials and Methods
Study population and setting
We conducted a longitudinal study of Egyptian adolescents from April 2010 to January 2011 that included 57 applicators and 38 non-applicators. The analyses reported herein are restricted to 43 applicators that had information on body surface area. Of the applicators included in these analyses nine were 18 years old and one was 19 years old, the rest were under age 18. Details regarding the study setting and pesticide application process of this cohort have been described previously (Khan et al., 2014). Briefly, applicators employed seasonally by the Ministry of Agriculture to spray pesticides were recruited from two field stations in the region. Duties of the applicators included mixing pesticides and filling backpack sprayers, which were then used to apply pesticides to cotton fields in the Nile delta. Although CPF was the primary pesticide applied, participants also reported applying other agents, including bacillus thuringiensis, chlorfluazuron, penconazole, propamocarb hydrochloride, profenofos, atrazine, alpha cypermethrin, diflubenzuron, lambda cyhalothrin, and spinosad.
At enrollment participants completed a self-administered questionnaire with research staff available to provide clarification if needed to obtain information regarding participants’ age; education; medical history; usual PPE use, including waterproof gloves; and pesticide use in their homes and gardens. Thirty-four subsequent assessments were conducted throughout the study at one of two field stations where participants donated spot urine samples for TCPy analyses; completed a brief follow-up questionnaire that queried recent symptoms, personal hygiene, and work behaviors, including hours worked and pesticides applied. Quantifications of urinary TCPy were available for baseline and 24 subsequent study sessions. Not all participants attended each session. Written consent was obtained from all participants and their legal guardian. The study was approved by the Oregon Health & Science University Institutional Review Board, and by the Medical Ethics committee of the Faculty of Medicine, Menoufia University.
The typical work schedule was from 8am–12pm and 3pm–7pm six days per week. Spot urine specimens were collected at field stations during the lunch break where they were placed on wet ice and transported to Menoufia University where they were stored at −20° C until they were shipped on dry ice to the University at Buffalo for analysis.
Laboratory measurements
Negative-ion gas chromatography-mass spectrometry was used to quantify urinary TCPy. Samples were hydrolyzed with hydrochloric acid, extracted with toluene, and derivatized using N-(tert-butyldimethylsilyl)-N-methyltrifluoro-acetamide (Sigma Aldrich, USA). A 1 mL aliquot of each urine specimen was thawed and mixed prior to the addition of 100 ng of internal standard (13C-15N-3,5,6-TCPy). TCPy values were corrected for creatinine and are expressed as µg TCPy/g creatinine. Urinary creatinine was quantified using the Jaffe reaction. The within-run precision for TCPy analyses was excellent, as demonstrated by a <2% coefficient of variation and an intraclass correlation coefficient between analytical replicates of 0.997 (Farahat et al., 2011). Participants provided an average of 19 samples over the course of the study. The limit of detection for the method was 0.5 ng/ml urine. The isotope-labeled analogue of 3,5,6-TCPy (13C-15N-3,5,6-TCPy) was used as the internal standard to account for matrix effects. The same degree of ion suppression or enhancement (if there is any resulted from the matrix) will be observed for the target native TCPy and its isotopically labeled analogue. The ratio of the two signals should not be affected, allowing for correct quantification in different matrices.
Statistical Analyses
Cumulative urinary TCPy excretion was estimated by calculating the area under the curve. Each participant’s excretion curve was graphed and integrated using the trapezoid rule to calculate the total TCPy excreted over the course of the study via Stata’s pharmacokinetic function (StataCorp. 2009. Stata Statistical Software: Release 11, StataCorp LP: College Station, TX, USA). Log-linear regression models were used to identify predictors of cumulative TCPy excretion (cumulative exposure model) as well as predictors of the highest TCPy concentration an individual experienced (peak exposure model). Concentrations of TCPy were natural log-transformed in these models. Beta coefficients from these models were transformed using the formula {[(exp(beta)) − 1]*100} to be presented as percent change in geometric mean urinary TCPy per unit increase of the characteristic.
Predictors considered for inclusion in the cumulative exposure model were age; body surface area (BSA), which was calculated using the DuBois formula (BSA= 0.00718 × height cm0.725 × weight kg0.425)(Verbraecken et al., 2006); total hours applying CPF; total hours applying pesticides other than CPF; and applying pesticides at home. We also considered what clothing participants reported usually wearing while working in the field during the baseline questionnaire. These clothing options were pants, shorts to the knee, length of shirtsleeves, hat or head covering, closed shoes or open sandals, socks, bare feet, and bandana or neck scarf over the face. The response options were always, often, sometimes, seldom, or never, which were parameterized as ordinal values.
We assessed differences in the peak TCPy a participant excreted by workplace and hygiene behaviors using the Kruskal Wallis test. We restricted peak TCPy analyses to applicators who worked the day of their peak TCPy excretion. Potential predictors considered for inclusion in the peak exposure log-linear regression model, were, age, BSA, days applying pesticides in the past week, hours applying pesticides on the day of assessment, use of PPE when applying pesticides, time of shower after work the day before assessment, wearing clean work clothes the day of assessment, pesticide the applicator applied on the day of assessment (CPF or another pesticide), mixing pesticides in the past week, device used to mix pesticides in the past week (hand, stick, or other object) and time of hand washing after work the day before assessment.
For both the cumulative and peak models we used the adjrsq model selection option in SAS software’s PROC REG (version 9.3, Copyright © 2006–2010 SAS Institute Inc., Cary, NC, USA) to identify the subset of the covariates described above that generated the highest adjusted R2.
For a longitudinal analysis, we used generalized estimating equations (GEE) to assess the association between TCPy measured at each study visit and reported exposures. TCPy was log-transformed and the resulting beta coefficients were transformed using the same formula described above. The GEE model was constructed assuming Gaussian distribution of the (log-transformed) response with an identity link function and exchangeable correlation structure for repeated measures drawn from the same subject and clustered for field station. We included covariates that were identified as the best subset of predictors of peak TCPy. All statistical analyses were conducted using SAS version 9.3 or Stata release 11.
Results
Demographic information and baseline information regarding workplace attire are displayed in Table 1. The mean age of applicators was 16 years and most reported always wearing long pants, long sleeved shirts, and closed footwear (shoes or boots, not open sandals) while applying.
Table 1.
Demographic information for Egyptian adolescent pesticide applicators (n = 43)n = 43)
| Mean (SD) | Min | Max | |||
|---|---|---|---|---|---|
| Age (years) | 16 (1.7) | 12 | 19 | ||
| Body surface area (m2) | 1.6 (0.2) | 1.1 | 1.8 | ||
| Years of education | 9.7 (1.9) | 4 | 12 | ||
| Total hours applying CPF | 13.5 (11.9) | 0 | 41.0 | ||
| Total hours applying other pesticides | 15.5 (14.5) | 0 | 59.0 | ||
| GM | Min | Max | |||
| Peak TCPy (µg/g creatinine) | 862 | 4 | 5,715 | ||
| Cumulative TCPy (µg/g creatinine) | 13,641 | 167 | 49,8208 | ||
| N (%) | |||||
| Applies pesticides at home | 34 (79.1) | ||||
| Never smoked cigarettes | 42 (97.7) | ||||
| Attire reported as worn while applying: | Always | Often | Sometimes | Seldom | Never |
| Long pants (to the feet) | 28 (65.1) | 10 (23.3) | 4 (9.3) | 1 (2.3) | 0 |
| Short pants (to the knee) | 1 (2.3) | 2 (4.7) | 1 (2.3) | 19 (44.2) | 20 (46.5) |
| Short-sleeved shirt | 2 (4.7) | 1 (2.3) | 6 (14) | 14 (32.6) | 20 (46.5) |
| Long-sleeved shirt | 23 (53.5) | 10 (23.3) | 10 (23.3) | ||
| Jacket over shirt | 1 (2.3) | 1 (2.3) | 9 (20.9) | 2 (4.7) | 30 (69.8) |
| Hat or head covering | 22 (51.2) | 3 (7.0) | 4 (9.3) | 9 (20.9) | 5 (11.6) |
| Shoes or boots (not open sandals) | 20 (46.5) | 6 (14.0) | 7 (16.3) | 9 (20.9) | 1 (2.3) |
| Sandals (part of feet exposed) | 12 (27.9) | 7 (16.3) | 24 (55.8) | ||
| Socks | 10 (23.3) | 4 (9.3) | 7 (16.3) | 10 (23.3) | 12 (27.9) |
| Bare Feet | 3 (7.0) | 11 (25.6) | 29 (67.4) | ||
| Bandana (neck scarf) over face | 2 (4.7) | 1 (2.3) | 40 (93.0) |
Abbreviations: CPF, chlorpyrifos; Min, minimum; Max, maximum; GM, geometric mean; TCPy, urinary 3,5,6-trichloro-2-pyridinol
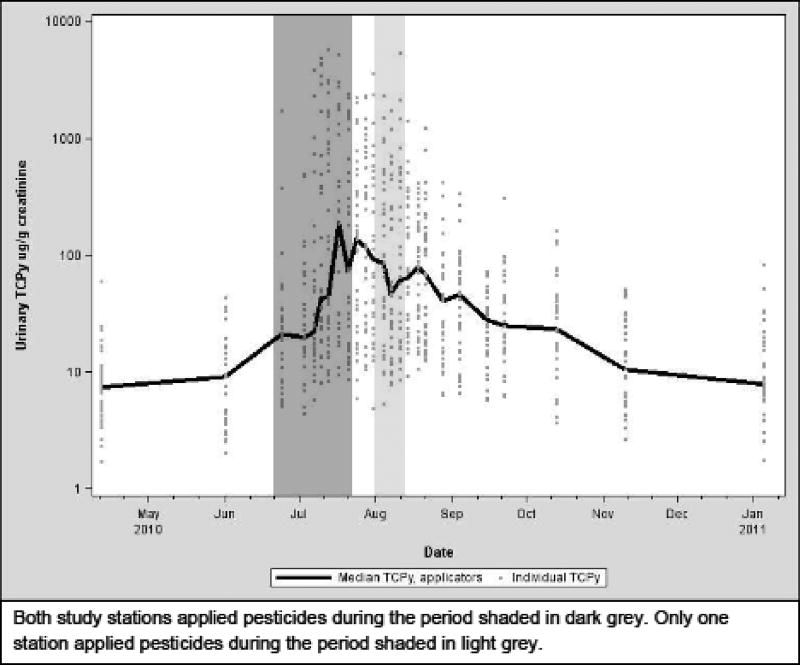
TCPy was detected in 100% of urine samples. The distribution of urinary TCPy by study day is displayed in Figure 1. Concentrations of urinary TCPy tended to increase during CPF application; however, there was substantial variability in individual concentrations of TCPy.
Figure 1.
Urinary TCPy concentrations in individual adolescent applicators, prior to, during and following application of chlorpyrifos.
Predictors of cumulative TCPy that were identified using the best subset approach are displayed in Table 2. Total hours applying CPF and pesticides other than CPF were the strongest predictors of cumulative TCPy (semi-partial r2 = 0.32 and 0.08, respectively). Wearing a long-sleeved shirt to work was non-significantly associated with higher cumulative TCPy (semi-partial r2 = 0.04), while wearing shoes or boots and socks and years of education were associated with lower TCPy (semi-partial r2 = 0.06 for all three covariates).
Table 2.
Predictors of cumulative urinary TCPya among adolescent applicators (n = 43)
| Characteristic | β | 95% CI | % differenceb | 95% CI | Semi-partial R2 |
|---|---|---|---|---|---|
| Intercept | 4.28 | (−1.30; 9.86) | |||
| Total hours applying chlorpyrifos | 0.07 | (0.03; 0.12) | 7.59 | (3.14; 12.23) | 0.32 |
| Total hours applying pesticides other than chlorpyrifos | 0.05 | (0.01; 0.08) | 4.80 | (1.26; 8.47) | 0.08 |
| Long sleeved shirt | 0.66 | (−0.25; 1.56) | 92.60 | (−22.37; 377.84) | 0.04 |
| Shoes or boots (not open sandals) | −0.53 | (−1.14; 0.08) | −41.06 | (−68.00; 8.57) | 0.06 |
| Socks | −0.50 | (−0.91; −0.08) | −39.28 | (−59.91; −8.05) | 0.06 |
| Years of education | −0.36 | (−0.66; −0.06) | −30.28 | (−48.50; −5.61) | 0.06 |
| Pants short to your knee | 0.56 | (0.02; 1.11) | 75.28 | (1.57; 202.49) | 0.02 |
| Body surface area (0.5 m2) | 1.76 | (−0.03; 3.54) | 479.28 | (−2.88; 3,355.18) | 0.002 |
| Mixing pesticides at home | 1.62 | (0.16; 3.08) | 405.40 | (17.39; 2,075.84) | 0.002 |
| Model R2 | 0.65 |
Parameters and corresponding 95% confidence intervals were estimated using log-linear regression.
Refers to predicted percent change in geometric mean TCPy per one unit increase in characteristic
Twenty-six applicators worked on the day of their peak exposure and were included in peak exposure analyses. The results of Kruskal Wallis test for differences in the median TCPy at peak exposure are displayed in Table 3. Participants who had worked in pesticide application for a greater number of days over the past week had higher concentrations of TCPy when compared with those who worked fewer days. The median peak TCPy among those working 1–2 days in the week prior to assessment was 325 µg/g creatinine while the median peak TCPy of those working greater than five days in the week prior was 3564 µg/g creatinine (p=0.008). Applying CPF less hours on the day of assessment or less days in the week prior and limiting exposure to CPF by wearing clean clothing to work was associated with lower concentrations of urinary TCPy, although these estimates were not statistically significant (p-value = 0.10 and 0.11, respectively).
Table 3.
Median concentrations of peak urinary TCPy among adolescent applicators by self-reported work and hygiene responses
| N | Peak urinary TCPy (µg/g creatinine) Median (IQR) |
Pvalue | ||
|---|---|---|---|---|
| Hours worked in pesticide application today | ||||
| 2–3 | 4 | 65 | (2 682) | 0.10 |
| 4–5 | 22 | 1 650 | (2 938) | |
| Days worked in pesticide application over the past week | ||||
| 1–2 | 10 | 325 | (762) | 0.008 |
| 3 | 5 | 1,760 | (2,746) | |
| 4 | 4 | 2,013 | (2,429) | |
| 5–6 | 7 | 3,564 | (4,488) | |
| Using personal protective equipment when applying pesticides | ||||
| No | 25 | 905 | (2,526) | 0.32 |
| Yes | 1 | 3,564 | ||
| Time of bathing | ||||
| Immediately after work | 24 | 1,227 | (2,761) | 0.70 |
| Before going to bed | 2 | 2,844 | (4,914) | |
| Wearing clean clothing to work | ||||
| No | 7 | 2,914 | (4,490) | 0.11 |
| Yes | 19 | 720 | (2,171) | |
| Pesticide mixing | ||||
| Do not mix pesticides | 2 | 318 | (425) | 0.39 |
| A stick | 19 | 905 | (3,219) | |
| Hand | 5 | 1,749 | (1,917) | |
| Pesticide applied | ||||
| Chlorpyrifos | 19 | 1,550 | (3,035) | 0.28 |
| Other | 7 | 512 | (2,102) | |
Abbreviations: IQR, inter-quartile range.
Pvalues for differences in the distribution of peak urinary TCPy between groups were estimated using Kruskal-Wallis test.
The results of linear regression models to predict urinary TCPy at peak exposure are presented in Table 4. The strongest predictor of TCPy was hours applying pesticides the day of assessment (semi-partial r2 = 0.30) and number of days worked in pesticide application in the past week (semi-partial r2 = 0.23).
Table 4.
Predictors of peak urinary TCPy among adolescent applicators (n =26)a
| Characteristic | β | 95% CI | % differenceb | 95% CI | Semi-partial R2 |
|---|---|---|---|---|---|
| Intercept | −1.83 | (−6.52; 2.85) | |||
| Hours applying pesticides today | 0.91 | (0.18; 1.64) | 148.41 | (19.52; 416.31) | 0.30 |
| Number of days worked in pesticide application in the past week | 0.50 | (0.17; 0.84) | 65.07 | (17.99; 130.94) | 0.23 |
| Body surface area (0.5m2) | 1.24 | (0.06; 2.41) | 244.00 | (6.67; 1009.41) | 0.07 |
| Mixing pesticides with hand instead of stick | 0.53 | (−0.36; 1.42) | 70.02 | (−30.39; 315.30) | 0.06 |
| Wearing clean clothes to work today | −0.65 | (−1.77; 0.47) | −47.92 | (−83.04; 59.98) | 0.01 |
| Applying CPF vs. another pesticide | −0.71 | (−1.86; 0.43) | −51.02 | (−84.37; 53.52) | 0.03 |
| Model R2 | 0.70 |
Parameters and corresponding 95% confidence intervals were estimated using log-linear regression
Refers to predicted percent change in geometric mean TCPy per one unit increase in characteristic
The results of the longitudinal analysis of TCPy using the same predictors as identified as contributing to peak TCPy are presented in Table 5. Higher BSA was associated with higher urinary TCPy; a 0.5 m2 higher BSA was associated with a 251.52% higher urinary TCPy (95% CI: 12.51; 998.19). Wearing clean clothes to work was associated with lower urinary TCPy over the course of the study (−32.86%, 95%CI: −52.97; −4.17). Applying CPF instead of another pesticide was associated with 39.40% higher urinary TCPy (95% CI: 7.22; 81.23). A one-day increase in the number of days worked in pesticide application in the past week was associated with an 10.19% increase in urinary TCPy (95% CI: 1.29; 19.87).
Table 5.
Predictors of longitudinal measures of urinary TCPy among adolescent applicators (n =43)
| Characteristic | β | 95%CI | % differencea | 95% CI |
|---|---|---|---|---|
| Intercept | 0.38 | (−3.34; 4.10) | ||
| Body surface area (0.5m2) | 1.26 | (0.12; 2.40) | 251.52 | (12.51; 998.19) |
| Wearing clean clothes to work today | −0.40 | (−0.75; −0.04) | −32.86 | (−52.97; −4.17) |
| Applying CPF vs. another pesticide | 0.33 | (0.07; 0.59) | 39.40 | (7.22; 81.23) |
| Mixing pesticides with hand instead of stick | −0.19 | (−0.64, 0.26) | −17.40 | (−47.43, 29.80) |
| Hours applying pesticides today | 0.17 | (−0.02; 0.35) | 18.08 | (−2.09; 42.40) |
| Number of days worked in pesticide application in the past week | 0.10 | (0.01; 0.18) | 10.19 | (1.29; 19.87) |
Parameters and corresponding 95% confidence intervals were estimated using generalized estimating equations.
Percent difference refers to predicted percent change in geometric mean TCPy per one-unit increase in characteristic
Discussion
We found that the strongest predictor of cumulative TCPy concentrations among adolescent applicators was total hours applying CPF. Additionally, total hours applying a pesticide other than CPF were also strongly predictive of cumulative TCPy. These findings are consistent with drift or bystander contact to CPF even when applicators are not applying CPF. This is important when considering epidemiologic studies on occupational exposure to pesticides, because self-reported workplace behaviors may not capture exposure to CPF when other pesticides are being applied at the same time
We have previously reported that during the CPF spraying season, applicators in our study have urinary TCPy concentrations eight times as high as Egyptian adolescents from the same region who do not apply pesticides (Crane et al., 2013). Previous studies of adult applicators in the US have demonstrated that several factors may impact the amount of CPF exposure an individual experiences. For instance, mixing pesticides has been associated with increased exposure to OPs, as demonstrated by decreased BChE activity (β=−3.97 p= 0.002), and not wearing chemical resistant boots was associated with decreased BChE activity (β= − 16.63, p<0.001) (Krenz et al., 2015). Wearing gloves and other protective clothing during farm work has previously been demonstrated to reduce exposure to OPs (Bradman et al., 2009; Salvatore et al., 2008).
The systematic lack of PPE use by Egyptian pesticide applicators makes comparison with other studies and inferences regarding effectiveness of potential PPE-based interventions in this population problematic. No association between BChE and work regimen, hours of work, years of pesticide use, backpack application, reporting of adverse symptoms, or the use of PPE was reported in a study of 112 adult farmworkers in Brazil (Oliveira Pasiani et al., 2012). The authors of this study suggest that PPE may be used improperly or not at all in hot weather, which is plausible in our study as well. Adolescent applicators in Egypt also did not wear clothing that resists permeation, degradation, and penetration by chlorpyrifos and their clothes would become saturated with pesticides (Figure 2). On the other hand, we did observe that wearing clean clothing to work was associated with decreased peak TCPy excretion and that wearing closed-toe shoes or boots was associated with lower cumulative TCPy, although this estimate, although results were statistically not significant, which could be due to our small sample size. Our findings suggest that hygiene-based interventions may be useful in reducing exposure in regions where applicators are working in hot weather and PPE-adherence may be difficult.
Figure 2.
Image of study participant spraying CPF, note absence of PPE and saturation of clothing
We used three models of exposure, cumulative, peak, and longitudinal. We opted to use these approaches because they are summary measures of exposure that pertain to different exposure-disease models. Occupational epidemiology studies often use cumulative exposure because it is assumed to be proportional to target tissue dose, making it a good estimate for irreversible biologic damage (Kriebel et al., 2007). For instance, occupational exposure to OPs has generally been adversely associated with neurobehavioral performance. However, biomarkers of OP exposure or effect are inconsistently associated with neurobehavioral deficits. The short half-life of metabolites used to estimate CPF exposure could result in non-differential misclassification of exposure, which could partially explain inconsistent findings (reviewed by:(Rohlman et al., 2011)). A cumulative model of exposure that incorporates variability of exposure may be more appropriate when considering neurobehavioral outcomes. However, a peak exposure model may be more informative when assessing adverse events potentially associated with acute exposures as well as when developing interventions to reduce workplace exposure. The advantage of the longitudinal analysis is that it could identify if individual variability in workplace behavior explains short-term changes in TCPy. In our study, most participants practiced consistent workplace behaviors, thus the results of the longitudinal analysis were largely the same as the peak exposure analysis.
As with all studies ours has limitations that should be considered when interpreting our results, particularly the small sample size, which reduced our statistical power. Not all study participants completed every study session, which led to a further reduction in sample size in the peak exposure analysis. We had limited information regarding sources of exposure to CPF outside of the workplace. However, pesticide use in the home or garden was accounted for less than 0.2% of the variability of cumulative TCPy suggesting that the primary source of CPF exposure was from occupational application activities. All of the information regarding workplace activities were self-reported by the pesticide applicators. Self-reported information may result in exposure misclassification. Farmworkers in the US were found to over-report hygiene when compared with observed behaviors (Walton et al., 2016). In our study, it seems unlikely that the participants were over-reporting their PPE use or hygiene practices since most did not use PPE and our observed inverse associations between personal hygiene and urinary TCPy are in the direction we hypothesized. However, if participants were not certain of which pesticides they were applying or confused by the question there may be non-differential misclassification, which usually but not always leads to attenuation in measures of association. We also did not have information regarding the quantity of CPF used by the applicators, which could be useful in elucidating the remaining between-participant variability in urinary TCPy.
Strengths of our study include the longitudinal design and repeated measuring of urinary TCPy, a CPF specific metabolite and objective marker of CPF exposure. The lack of use of PPE by study participants provides a unique setting to assess the impact of hygiene and duration of work on exposure to CPF. Additionally, we had a geographically stable population and a well-defined period of CPF exposure that was fairly homogenous with regards to workplace duties, age, and education level.
In conclusion, we found that cumulative TCPy was associated with total hours worked while CPF was being applied. We also found that Egyptian adolescent pesticide applicators did not generally use PPE and reported fairly consistent hygiene practices. Given the high exposures of this population, efforts directed towards reducing exposure to CPF via personal hygiene and PPE-based interventions that would be effective under the extremely warm weather conditions our applicators work in should be considered.
Acknowledgments
We thank the Egyptian Ministry of Agriculture and the adolescent farmworkers and their parents for their participation, and Mahmoud Abdel-Gawad Esmaeil, Mohammed Fouad El-Sayed Abdel Haleem, and Tameem Aboeleinin and the other members of the research team for their dedication and efforts in the conduct of the project. This work was supported by funding from the Fogarty International Center and the National Institute of Environmental Health Sciences (NIEHS) grants: R211ES017223 and R01ES022163. Catherine L. Callahan was supported by the National Cancer Institute (NCI) grant R25CA113951.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This article was prepared while Catherine Callahan was employed at the University at Buffalo. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.’
Conflict of interest.
The authors declare no conflict of interest.
References
- Alexander BH, et al. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J Expo Sci Environ Epidemiol. 2006;16:447–56. doi: 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- Beamer PI, et al. Relative pesticide and exposure route contribution to aggregate and cumulative dose in young farmworker children. Int J Environ Res Public Health. 2012;9:73–96. doi: 10.3390/ijerph9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, et al. Community-based intervention to reduce pesticide exposure to farmworkers and potential take-home exposure to their families. J Expo Sci Environ Epidemiol. 2009;19:79–89. doi: 10.1038/jes.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, et al. Chronic Exposures to Cholinesterase-inhibiting Pesticides Adversely Affect Respiratory Health of Agricultural Workers in India. Journal of Occupational Health. 2009;51:488–497. doi: 10.1539/joh.l9070. [DOI] [PubMed] [Google Scholar]
- Coble J, et al. An updated algorithm for estimation of pesticide exposure intensity in the agricultural health study. International journal of environmental research and public health. 2011;8:4608–4622. doi: 10.3390/ijerph8124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, et al. The role of paraoxonase (PON1) in the detoxication of organophosphates and its human polymorphism. Chem Biol Interact. 1999;120:429–38. doi: 10.1016/s0009-2797(99)00055-1. [DOI] [PubMed] [Google Scholar]
- Crane AL, et al. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J Expo Sci Environ Epidemiol. 2013;23:356–62. doi: 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin B, et al. Pesticide use and practices in an Iowa farm family pesticide exposure study. J Agric Saf Health. 2002;8:423–33. doi: 10.13031/2013.10222. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res. 2014;134:149–57. doi: 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, et al. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119:801–6. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, et al. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int J Occup Environ Health. 2012;18:198–209. doi: 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- Fenske RA, et al. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environmental Health Perspectives. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieten KB, et al. Pesticide exposure and respiratory health of indigenous women in Costa Rica. Am J Epidemiol. 2009;169:1500–6. doi: 10.1093/aje/kwp060. [DOI] [PubMed] [Google Scholar]
- Foxenberg RJ, et al. Cytochrome P450-specific human PBPK/PD models for the organophosphorus pesticides: Chlorpyrifos and parathion. Toxicology. 2011;285:57–66. doi: 10.1016/j.tox.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, et al. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med. 2002;165:683–9. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, et al. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol. 2006;163:1129–37. doi: 10.1093/aje/kwj138. [DOI] [PubMed] [Google Scholar]
- Kearney GD, et al. Work safety climate, safety behaviors, and occupational injuries of youth farmworkers in North Carolina. American journal of public health. 2015;105:1336–1343. doi: 10.2105/AJPH.2014.302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer MC, Firestone J. Neurotoxicity of Pesticides. Journal of Agromedicine. 2007;12:17–25. doi: 10.1300/J096v12n01_03. [DOI] [PubMed] [Google Scholar]
- Khan K, et al. Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open. 2014;4:e004177. doi: 10.1136/bmjopen-2013-004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, et al. Relationship of pesticide spraying to signs and symptoms in Indonesian farmers. Scand J Work Environ Health. 1995;21:124–33. doi: 10.5271/sjweh.19. [DOI] [PubMed] [Google Scholar]
- Krenz JE, et al. Determinants of butyrylcholinesterase inhibition among agricultural pesticide handlers in Washington State: an update. Ann Occup Hyg. 2015;59:25–40. doi: 10.1093/annhyg/meu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel D, et al. Exposure and dose modelling in occupational epidemiology. Occup Environ Med. 2007;64:492–8. doi: 10.1136/oem.2006.030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerro CC, et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med. 2015;72:736–44. doi: 10.1136/oemed-2014-102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, et al. Pesticide Exposure of Children in an Agricultural Community: Evidence of Household Proximity to Farmland and Take Home Exposure Pathways. Environmental Research. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Munoz-Quezada MT, et al. Predictors of exposure to organophosphate pesticides in schoolchildren in the Province of Talca, Chile. Environ Int. 2012;47:28–36. doi: 10.1016/j.envint.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayo-Mitoko GJ, et al. Self reported symptoms and inhibition of acetylcholinesterase activity among Kenyan agricultural workers. Occup Environ Med. 2000;57:195–200. doi: 10.1136/oem.57.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Pasiani J, et al. Knowledge, attitudes, practices and biomonitoring of farmers and residents exposed to pesticides in Brazil. Int J Environ Res Public Health. 2012;9:3051–68. doi: 10.3390/ijerph9093051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, et al. Distributions of total skin surface area to body weight ratios for use in dermal exposure assessments. J Expo Anal Environ Epidemiol. 1993;3:331–8. [PubMed] [Google Scholar]
- Rauh V, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohitrattana J, et al. Organophosphate pesticide exposure in school-aged children living in rice and aquacultural farming regions of Thailand. J Agromedicine. 2014;19:406–16. doi: 10.1080/1059924X.2014.947457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, et al. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268–76. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, et al. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex. 2016;74:383–95. doi: 10.1016/j.cortex.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore AL, et al. Occupational behaviors and farmworkers’ pesticide exposure: findings from a study in Monterey County, California. American journal of industrial medicine. 2008;51:782. doi: 10.1002/ajim.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KW, et al. Assessment of a pesticide exposure intensity algorithm in the agricultural health study. J Expo Sci Environ Epidemiol. 2010a;20:559–69. doi: 10.1038/jes.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KW, et al. Urinary biomarker, dermal, and air measurement results for 2,4-D and chlorpyrifos farm applicators in the Agricultural Health Study. J Expo Sci Environ Epidemiol. 2010b;20:119–34. doi: 10.1038/jes.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbraecken J, et al. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55:515–524. doi: 10.1016/j.metabol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Walton AL, et al. Observed and self-reported pesticide protective behaviors of Latino migrant and seasonal farmworkers. Environmental Research. 2016;147:275–283. doi: 10.1016/j.envres.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]