Abstract
Ischemic stroke is a leading cause of death worldwide. A key secondary cell death mechanism mediating neurological damage following the initial episode of ischemic stroke is the upregulation of endogenous neuroinflammatory processes to levels that destroy hypoxic tissue local to the area of insult, induce apoptosis, and initiate a feedback loop of inflammatory cascades that can expand the region of damage. Stem cell therapy has emerged as an experimental treatment for stroke, and accumulating evidence supports the therapeutic efficacy of stem cells to abrogate stroke-induced inflammation. In this review, we investigate clinically relevant stem cell types, such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), very small embryonic-like stem cells (VSELs), neural stem cells (NSCs), extraembryonic stem cells, adipose tissue-derived stem cells, breast milk-derived stem cells, menstrual blood-derived stem cells, dental tissue-derived stem cells, induced pluripotent stem cells (iPSCs), teratocarcinoma-derived Ntera2/D1 neuron-like cells (NT2N), c-mycER(TAM) modified NSCs (CTX0E03), and notch-transfected mesenchymal stromal cells (SB623), comparing their potential efficacy to sequester stroke-induced neuroinflammation and their feasibility as translational clinical cell sources. To this end, we highlight that MSCs, with a proven track record of safety and efficacy as a transplantable cell for hematologic diseases, stand as an attractive cell type that confers superior anti-inflammatory effects in stroke both in vitro and in vivo. That stem cells can mount a robust anti-inflammatory action against stroke complements the regenerative processes of cell replacement and neurotrophic factor secretion conventionally ascribed to cell-based therapy in neurological disorders.
Keywords: cell death, secondary injury, cell transplantation, apoptosis, chronic inflammation
1. Introduction
In 1907, Ross Granville Harrison transplanted a sample of neural progenitor cells into a droplet of frog lymph (Nicholas, 1961, Maienschein, 2011). He hoped to demonstrate that neural fibers were the products of innate outgrowth from the neuroblast instead of the neuroblast’s response to an environmental matrix. As experimental controls, Harrison placed progenitor cells from other embryonic tissues in similar media. He was astounded by what he observed under the microscope. Though these progenitor cells were grown in complete isolation, they had developed into nerve cells bristling with dendritic spines, contractile muscle fibers, and epidermal cells with working cilia. Harrison believed he had quieted a debate among his contemporaries with these results, but he had actually accomplished something much more remarkable. Harrison had produced the first in vitro cultures of stem cells (Maienschein, 2011).
The study of self-proliferating, pluripotent cell lines would continue over subsequent decades under the mantle of cancer research. In the 1960s, while attempting to delineate the cellular origin of teratoma, a tumor that appeared to derive from undifferentiated, totipotent cells of the germ line, Leroy Stevens would embark on a series of experiments that led to the isolation and culture of the first embryonic stem cells (Lewis, 2000). Contemporaneously, Ernest McCullough and James Till characterized the first adult-derived stem cells, hematopoietic bone marrow cells, in their studies of the impact of radiation on hematopoiesis in mice (Till and McCullough, 1961). Together, these discoveries laid the foundation for a new field in clinical science: regenerative medicine.
Initial studies using embryonic and adult-derived stem cells to repair damaged tissues assumed a therapeutic paradigm of cell replacement, wherein stem cells implanted at the site of injury would simply replace damaged cells and proliferate in their place. However, laboratory evidence soon revealed that the cell replacement paradigm was largely unfounded (Dailey et al., 2013). In response, researchers in the field shifted their investigations to the secretory activities of stem cells (Nishino and Borlongan, 2000; Dailey et al., 2013; Drago et al., 2013). These studies revealed that both autologous and allogenic stem cells secreted trophic factors with significant anti-inflammatory, neuroprotective, angiogenic, and restorative properties (Nishino and Borlongan, 2000, Yasuhara et al., 2006; Dailey et al., 2013; Drago et al., 2013). With the discovery of stem cells’ diverse trophic and paracrine functions, from controlling neuroinflammation to mobilizing endogenous stem cell populations via biobridges, researchers are now focused on determining the most therapeutically relevant cell lines for treating specific diseases (Yasuhara et al., 2006; Dailey et al., 2013; Drago et al., 2013; Tajiri et al., 2014). Though stem cell therapy has been investigated as a regenerative treatment for various types of tissue damage, cell therapy has long been regarded as an especially relevant solution for the maintenance and regeneration of the brain. Until the 1990s, it was widely believed that the cells of CNS were incapable of self-renewal. With the discovery of endogenous reservoirs of neural stem cells in the forebrain subventricular zone and dentate gyrus, this perception was reworked (Ma et al., 2009). The cells of the adult mammalian CNS were found to display the capacity for proliferation after disruption, including enhanced axonal growth, self-renewal, and the recovery of lost functionality (Arvidsson et al., 2002; Song et al., 2002, Ma et al., 2009). Recent advances in regenerative medicine have demonstrated that exogenous and endogenous cell therapies can promote neurogenesis, angiogenesis, and synaptogenesis in neuronal tissues adversely impacted by a suite of neurological disorders (Mazzini et al., 2003; Lindvall et al., 2004, Yasuhara et al., 2006; Kim et al., 2009; Shinozuka et al., 2013). Because many CNS disorders, such as Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, and amyotrophic later sclerosis, include neurodegenerative pathologies, regenerative medicine stands as an appealing treatment model for these conditions.
1.1. Stroke as a therapeutic target for stem cell therapy
More pertinently, cell therapy has gained national attention as a treatment for a notoriously recalcitrant disorder: ischemic stroke. The pathologic category of stroke can be either hemorrhagic or ischemic (Borlongan et al., 2012; Truelsen et al., 2015). Hemorrhagic stroke is characterized by rupture of the cerebral vasculature followed by intracranial bleeding. The origin of the hemorrhagic event can be used to further classify hemorrhagic stroke as either an intracerebral hemorrhage or a subarachnoid hemorrhage (Broderick et al., 1993). An intracerebral hemorrhage occurs when an artery in the brain parenchyma bursts, leaking blood into the surrounding tissue, and encouraging the development of a hematoma when left untreated (Broderick et al., 1993). Often preceded by a cerebral aneurysm, a subarachnoid hemorrhage occurs when a damaged vessel on the surface layer of the brain diverts blood into the subarachnoid space. This can increase the fluid pressure in the brain, resulting in swelling, hydrocephalus, and vasospasm (Broderick et al., 1993). Ischemic stroke, on the other hand, is the most ubiquitous sub-class of stroke, accounting for 87 percent of stroke cases in the United States alone (Broderick et al., 1993; Go et al., 2013). Ischemic stroke occurs when a region of brain tissue is deprived of oxygen due to a decrease in local blood flow, often as the result of an occluding event, such as embolism or thrombus formation. Ischemic stroke can also be brought about by systemic hypoperfusion (Broderick et al., 1993).
Currently, ischemic stroke patients suffer from relatively limited treatment options. Tissue plasminogen activator (tPA), a thrombolytic agent, can be administered only during the acute onset of stroke pathology, and its inconsistent efficacy combined with this narrow therapeutic window means stroke patients cannot depend on tPA treatment for assured functional recovery (Dailey et al., 2013; Zhang et al., 2015). Moreover, available surgical interventions aim to address the direct triggers of ischemic events, often by lowering the general risk of clot formation. Therefore, these treatment options are primarily preventive in scope (Dailey et al., 2013). Stem cell therapy, on the other hand, can target the subacute and chronic phases of ischemic stroke, thereby providing stroke patients a potential solution to the management of chronic symptoms associated with neural ischemia, such as long-term neuroinflammation and localized necrosis (Jin et al., 2013).
The ischemic cascade following stroke can be divided into three key phases. The acute phase occurs directly after and in the first few hours proceeding the occluding event (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011). In this phase, lack of blood flow to the area of infarct creates a region of oxidative stress and excitotoxicity. Reactive oxygen species (ROS) are formed which damage tissue and vasogenic edema forms in the area of infarct due to the movement of water into the intracellular space, primarily driven by disruption of ionic homeostasis (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011). Na+ and Ca+2 accumulation in cells at the ischemic core leads to cell death, while cells in the ischemic penumbra may survive the insult but begin expressing signals associated with neuronal injury. In the subacute phase, which occurs directly after the acute phase and lasts for the first few days after stroke onset, neuroinflammation is upregulated, with the release of cytokines, chemokines, cellular adhesion molecules (CAMs), and matrix metalloproteases (MMPs) from injured neurons and auxiliary cells, such as microglia and astrocytes (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011; Acosta et al 2015). Expression of MMPs, specifically, increases the permeability of the BBB, allowing peripheral leukocytes to invade the area of injury, where they upregulate present inflammatory processes (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011). CAMs permit leukocytes to adhere to cerebral vessels, allowing those cells to attract more cells to the site of injury. Chronic inflammation continues after the subacute phase and is primarily driven by activated microglia and astrocytes (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011). These endemic brain cells secrete cytokines, chemokines, and CAMs, which recruit more peripheral neutrophils and macrophages through the BBB. Chronic inflammation can lead to cerebral edema and neuronal death, thereby threatening infrastructure throughout the brain (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011).
Importantly, stem cell therapy represents a treatment paradigm uniquely poised to combat both subacute and chronic inflammatory processes. Investigators have long maintained the importance of neuroprotection for the subacute phase of stroke, as inflammation usually accompanies the subacute phase and, if left untreated, may significantly worsen the extent of injury (Borlongan et al 2012). There is also a need for neuroregeneration and the maintenance of anti-inflammatory processes in treating both the subacute and chronic stages of stroke (Lakhan et al., 2009; Ceulemans et al., 2010; Iadecola et al., 2011, Acosta et al., 2015). While subacute administration of stem cells is intended to prevent early secondary cell death by suppressing oxidative stress, mitochondrial impairment, apoptosis, and inflammation, chronic delivery is designed to activate brain rejuvenation and reperfusion by stimulating regenerative mechanisms such as vasculogenesis, neurogenesis, angiogenesis, and synaptogenesis, which can restore cerebral infrastructure, such as the BBB, and sequester inflammatory insults (Park et al., 2009; Dailey et al., 2013; Acosta et al., 2015). By assisting the damaged brain in recovering from an ischemic event by moderating endogenous neuroinflammation and encouraging reinnervation, stem cell therapy stands to fill an alarmingly bleak gap in known subacute and chronic treatments for stroke patients.
Over the years, a variety of transplantable cells have been examined in laboratory studies, including fetal cells, NT2N cells, CTX0E3, embryonic stem cells, neural stem/progenitor cells, umbilical cord blood, amnion, adipose, and induced pluripotent stem cells (Borlongan et al., 1999; Borlongan et al., 2005; Borlongan, 2009; Borlongan et al., 2010; Antonucci et al., 2011; Tajiri et al., 2012; Dailey et al., 2013; Maya-Espinosa et al., 2015; Stevanato et al., 2016). While some of these cell types have been investigated in clinical trials for ischemic stroke, current preclinical studies and clinical trials have concentrated predominantly on the cellular derivatives of bone marrow. Bone marrow-derived stem cells, including mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), SB623, multipotent adult progenitor cells MAPCs, and multilineage-differentiating stress enduring (Muse) cells, sport a robust safety profile in other disease indications (Yamei et al., 2007). Moreover, bone-marrow derived stem cells, especially MSCs, have been studied extensively in animal models. In this review, we will discuss contemporary advances in cell therapy with an eye to the history of the field, the development of known stem cell lines, and the societal views that influence and continue to influence the discipline. Our goal is to provide an overview of modern research into stem cell therapy for ischemic stroke with the hopes that it may inform the improvement of applied cell therapies for this debilitating disorder with regards to the treatment of neuroinflammatory insults. We will present a detailed discussion of the current cell types available for translational therapy, as well as the advantages and disadvantages of each in treating neuroinflammation. We will examine the literature examining the efficacy of each cell line both in vitro and in vivo. We will complement our reviews by describing modern multidisciplinary approaches to cell therapy, including the supplementation of cell treatments with pharmaceuticals and biomaterials. Laboratory evidence assessing whether the same stem cell population will be capable of accomplishing both preventative/protective and regenerative effects in the stroke brain has been shown in animal models of stroke (Borlongan et al., 1998; Tajiri et al., 2014; Chen and Chopp, 2006; Sanchez-Ramos et al., 2000). However, in the clinic, it may be limited to use stem cells as a preventive or protective treatment because most stroke episodes are unpredictable, suggesting that stem cell therapy may be more appropriate as a regenerative biologic. Nonetheless, with the advent of diagnostic tools designed to identify at-risk stroke patients based on family history, genetics, co-morbidy factors (e.g., diabetes, hypertension), it may be possible in the future to contemplate stem cells as preventive/protective therapeutics. Future laboratory research defining the preventive and regenerative potentials of stem cells will be key in guiding the optimal clinical applications of cell therapy.
2. Identifying the Optimal Cell Type for Stem Cell Transplantation
The success of cell transplantation and the ability of the transplant to abrogate stroke-induced neuroinflammation depend on a variety of factors, including route, dosage, and the timing of administration, but the specific cell type employed is principal. NT2N, CTX0E3, embryonic stem (ES) cells, hematopoietic stem cells (HSCs), neural stem cells (NSCs), adult tissue-derived stem cells, and induced pluripotent stem cells (iPSCs) encourage variable levels of histologic and behavioral recovery in animal models of stroke (Dailey et al., 2013). When determining the optimal stem cell for treatment, these disparities in efficacy between available cell types must be considered alongside the ethical and logistical issues that may also constrain their usage. For example, ethical objections and government sanctions make studies on fetal and embryonic cell lines impractical, while difficulties growing an adequate number of cells that display “stemness” limit the expediency of research involving other cell types. Here we summarize the characteristics of the most common stem cell types, discussing their strengths and weaknesses as models for translational research and as mediators of harmful immune responses.
2.1. Embryonic Stem Cells (ES)
ES cells have long been considered the consummate model of “stemness” as they proliferate indefinitely and exhibit the capacity to differentiate into tissues of all three germ layers. However, ES cells are a worrisome cell type for transplantation therapy due to significant ethical concerns regarding their clinical use and reported risks of tumorigenicity. These concerns notwithstanding, ES cells have shown noteworthy therapeutic potential in animal studies of stroke. Direct transplantation of ES cell-derived neuronal progenitor cells in stroke mice coincided with observed repair of neuronal damage, while transplantation of endothelial and mural cells also derived from ES cells was shown to promote cerebral angiogenesis and reduce the infarct area of mice after stroke (Liu et al., 2014; Maya-Espinosa et al., 2015). In addition, cells derived from ES cells are amenable to genetic modification. Genetically modified ES cell-derived cells have been reported to encourage functional recovery upon transplant in ischemic stroke mice models while also providing neuroprotection by overexpressing neuroprotective factors such as BcL-2, adenosine, and myocyte enhancer factor 2C (Shinozuka et al., 2013).
2.1.1. Embryonic Stem Cells: Critical Assessment
Preclinical studies in stroke models (in vitro and in vivo)
In vitro models of ES cell transplantation suggest the hypoxic neural conditions which emerge following an ischemic event may encourage ES-cell derived neural progenitor cells to release neurotrophic factors, including erythropoietin (EPO), as well as upregulate the expression of bcl-2, hypoxia-inducible factor (HIF-1α), erythropoietin receptor (EPOR), neurofilament (NF), and synaptophysin (Theus et al., 2008). Hypoxia pretreatment of ES-NPCs also increased observed neural differentiation and reduced apoptosis and caspase-3 activation in ES-NPCs transplanted in the ischemic brain of rat models as compared to non-pretreated ES-NPC transplants (Maya-Espinosa et al., 2015). The hypoxia-induced neuroregenerative and neuroprotective effects of ES cells may be especially important in the treatment of neuroinflammation, as hypoxic conditions are known to correlate with the development of an inflammatory microenvironment. Toll-like receptors (TLRs), specifically TLR2 and heterodimers TLR2/1 and TLR2/6, are a clade of 10 pattern recognition receptors that recognize ligands expressed by a wide consortium of microbes and upregulate immune reactions via the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interferon regulatory factor (IRF)-dependent pathways in response (Akira, 2006; Okun et al., 2009; Okun et al., 2010). Hypoxia is known to induce TLR2 and TLR6 activation, and (HIF)-1 may encourage this expression through transcriptional modification (Tang et al. 2007). Notably, TLR2 activation in endogenous neural progenitor cells (NPCs) leads to cell death and the release of pro-inflammatory cytokines, such as TNF-α (Covacu et al., 2009; Okun et al., 2010). The fact that ES-cells produce anti-inflammatory signals in response to hypoxic conditions means they might serve to ameliorate these ischemia-induced inflammatory effects, sequestering neuroinflammation driven by TLR2 activation in endogenous neural cell types upon delivery to the area of infarct.
Human embryonic stem cells (hESCs) and embryonic stem cell-derived embryoid body (EB) cells additionally display significant potential to enhance tissue integrity, promote neural repair, and improve functional recovery in animal models of ischemic stroke. In a recent study by Liu and colleagues, the transplantation of human neural stem cells (hNSCs) into the ipsilateral lateral ventricle 7 days after a one-hour transient middle cerebral artery occlusion (MCAO), a well-regarded stroke model in which the rodent middle cerebral artery is blocked by embolism using an intraluminal filament, resulted in improvements in behavioral (rotarod, footfault, and corner-turn) tests and reduced the area of ischemic infarct as compared to controls (Borlongan et al., 1998c; Liu et al., 2014). Moreover, it was observed that the hNSCs differentiated successfully into oligodendrocytes and astrocytes in the corpus collosum, and neural bodies in the peri-infarct parenchyma (Liu et. al, 2014). Neuronal differentiation of hESCs into neural phenotypes may depend, in part, upon the relative neurogenic quality of the cerebral environment to which the hESCs are transplanted (Seminatore et al., 2010). Areas of increased neurogenic effect primarily include areas near to the rostral migratory stream (Arvidsson et al., 2002). Pertinently, ischemic areas may themselves release higher than average concentrations of neurogenic signals, which can then be detected by transplanted hESCs and used to encourage their necessary differentiation into neural phenotypes at the site of injury. Though in vitro models of ES cells demonstrate that hypoxic conditions induce their production of anti-inflammatory signals, in vivo models have shown that pro-inflammatory cytokines, including TNF-alpha and interferon-γ (IFN-γ), present in the inflamed brain can suppress the proliferation and differentiation of ES-derived NPCs (Ideguchi et. al 2008). Interestingly, however, neuroinflammation also accelerates the mobilization of ES-derived NPCs to the site of injury (Ben-Hur et al., 2003). Therefore, complete immunosuppression may not be ideal for the transplantation of ES-cell derived cells, despite negative effects on their growth. They may continue to exhibit anti-inflammatory properties in the neuroischemic environment, as demonstrated in in vitro models, and may more accurately home to the therapeutic target in response to active gradients of endogenous inflammation.
Limitations
The high proliferative capacity of hESCs and their derivatives as well as the diversity of the neural phenotypes to which they can differentiate make them an outwardly appealing candidate for the replacement and repair of neuroblasts and neurons in ischemic neural tissue. However, their proliferative capacity also means that hESCs and their derivatives boast a significant tumorigenic risk (Kawai et al., 2010; Dailey et al., 2013). This prospective tumorigenicity poses a significant challenge to the clinical translation of this cell type, and research suggests the postichemic environment may accelerate aberrant proliferation (Kawai et al., 2010; Seminatore et al., 2010). Moreover, the use of hESCs in clinical research must contend with a daunting cultural stigma. Unfavorable public opinions toward the use of fetal-derived human embryonic stem cells drove the enactment of a moratorium on federal funding for ES-cell research from 2001 to 2009 (Shinozuka et al., 2013). Though this moratorium has since been lifted, it is safe to assume that a societal bias against hESCs still remains. In light of the compounding ethical and societal concerns that surround embryonic stem cell usage and ultimately constrain their clinical availability, this cell type is unappealing as a clinical model for stroke therapy. However, due to recent advancements in genetic engineering, researchers now have feasible alternatives.
Future Directions
While human embryonic stem cells may not represent the future of stem cell research due to the ethical, social, and tumorigenic concerns that limit their clinical usage, the powerful capacity for proliferation and differentiation that make these cells so attractive may be reproduced through the use of induced pluripotent stem cells (iPSCs). iPSCs are somatic cells that have been reprogrammed to revert to their previous pluripotent states. Accordingly, iPSCs retain the ability to differentiate into all cell types in vitro and can be wholly autologous, derived directly from the adult tissues of the stroke patient to which they will be administered. These cells can be transgene and vector free and are generated primarily from human fibroblasts via the induction of a suite of transcription factors, including Oct4, Sox2, Nanog, Klf4, c-Myc and Lin-28 (Yu et al., 2007; Zhao et al., 2008; Mohamad et al., 2013; Wang et al., 2016). Moreover, iPScs can be differentiated into neural stem cells, using known culturing techniques, including the administration of retinoic acid (Mohamad et al., 2013). The transplantation of iPSC-derived neural stem cells in animal models of stroke has produced encouraging results, with neurogenic, angiogenic, and functional benefits comparable to those observed after transplantation with hESC-derived NSCs.
2.2. Adult Tissue-Derived Stem Cells
While adult-derived stem cells subvert the ethical concerns raised by embryonic cell lines, they are not without their own challenges, most prominent among them: the difficulty in obtaining a homogenous cell population. Adult tissues predominantly contain mature cells that have previously differentiated, making the harvesting and purification of rarer pluripotent cell types an important consideration and, at times, rate-limiting step for associated research. This concern in mind, we will continue by summarizing the available categories of adult-tissue derived stem cells.
2.2.1. Bone Marrow-Derived Stem Cells
The bone marrow is a fertile substrate from which multiple cell types have been isolated, including subsets of naturally occurring stem cells and genetically engineered stem/progenitor cells, such as bone marrow-derived mesenchymal stem or stromal cells (BM-MSCs), endothelial progenitor cells (EPCs), notch-transfected mesenchymal stromal cells (SB623), multipotent adult progenitor cells (MAPCs), and multilineage-differentiating stress enduring (Muse) cells. Moreover, bone marrow-derived stem cells show particular aptitude as the cell-type of choice for the treatment of ischemic stroke. Upon injury, stem cells from the bone marrow can perfuse into the peripheral blood and migrate to the site of injury (Borlongan, 2011). They retain the ability to cross the blood-brain barrier and therefore may constitute an important part of the body’s regular neurorestorative response to cerebral damage (Borlongan, 2011). Stem cell types observed in the bone marrow can be subdivided into four categories: hematopoietic stem cells (HSCs), MSCs, EPCs, and very small embryonic-like stem cells (VSELs). We continue our discussion by examining each of these classes in depth.
2.2.1.1. Hematopoietic Stem Cells (HSCs)
HSCs are predominantly identified by the surface marker CD34+ and ancillary markers CD150+, CD244−, and CD48− (Oguro et al., 2013). HSCs retain the capacity to differentiate into blood cell phenotypes and can be mobilized in response to cerebrovascular insult through the release of chemokines by the CNS (Shyu et al., 2004, Ratajczak et al., 2012). Recent evidence from murine models indicates that a variety of chemotactic homing factors, including SDF-1, sphingosine-1-phosphate (S1P), ceramide-1-phosphate (C1P), and adenosine triphosphate (ATP), are responsible for directing mobilized HSCs to areas of ischemic injury (Mocco et al., 2014). Present stroke treatment protocols take advantage of this cytokine-mediated recruitment by administering granulocyte-colony stimulating factor (G-CSF) to induce the mobilization of HSCs (Shyu et al., 2004). The mobilization of HSCs can also be induced by neurotransmitters, namely catecholamine, which can either directly signal the bone marrow through a nerve ending paracrine signal or by sympathetic release into open circulation (Saba et al., 2013). Human histological data for patients with acute stroke agrees with this pattern of mobilization, revealing an increase in the levels of peripheral blood immature hematopoietic CD34+ cells, colony-forming cells, and long-term culture-initiating cells after cerebral insult (Sullivan et al., 2015). Notably, the extent of mobilization appears to correlate with the extent of functional recovery (Dunac et al., 2007). A recent study by Courties and colleagues demonstrated that ischemic stroke activates HSCs via sympathetic stimulation which decreases concentrations of hematopoietic niche factors that encourage quiescence and increases concentrations of noradrenaline in the BM. This response appears to be skewed toward the development of myeloid, instead of lymphoid, progeny (Courties et al., 2015). The potential of ischemic stroke to activate internal HSCs and the elevated mobilization and circulation of HSCs following an ischemic event suggest that HSCs play a restorative function in hypoxic or vascular insult.
2.2.1.1.1. Critical Assessment: HSCs
Preclinical studies in stroke models (in vitro and in vivo)
Though multiple studies have confirmed that HSCs are activated and mobilized in peripheral circulation following ischemic insult, preclinical studies of isolated HSC stem cells in both in vitro cultures and in vivo animal models are limited, especially with regard to ischemic stroke. Some in vitro studies have examined methods with which to enhance the efficiency of isolating HSCs from progenitor cells and mixed mesenchymal tissue as well as to improve their proliferative capacity (Oguro et al., 2013). Notably, engineered niches using biomaterials, such as nanofibers and ECM-based macroporous sponges, have been employed to significant effect to both encourage the differentiation of iPSCs into HSCs and to promote the growth of HSC cell lines (Soffer-Tsur et al., 2016). HSCs are isolated in relatively low numbers from human bone marrow cell populations. Therefore, these studies have aimed to address issues concerning the time necessary to grow clinically relevant cell populations by examining whether HSCs can emerge from pluripotent cell lines and how to create a more conducive environment for the growth of naturally isolated HSCs. In vitro studies of HSCs have also attempted to further characterize the behavior of this cell type in response to injury. A recent paper by Kumar and colleagues analyzed the reaction of HSCs in vitro to hemorrhagic shock (Kumar et al., 2016). Bone-marrow aspirates from patients with trauma hemmoraghic shock and those from healthy patients were grown in vitro, with HSCs populations isolated and enumerated at varying time points following the administration of individual growth factors, including recombinant human erythropoietin (rhEPO), recombinant human granulocyte macrophage-colony-stimulating factor (rhGM-CSF), recombinant human interleukin-3 (rhIL-3), and the combination of all three. Researchers observed that trauma hemmoraghic shock reduced natural HSC population levels below baseline, but that this suppression could be partly rescued with the addition of the aforementioned growth factors (rhEPO, rhGM-CSF, rhIL-3) both alone and in conjunction. Though this work suggests that HSC BM dysfunction may be reversible, the study’s limited sample size also indicates further research must be undertaken before the behavior of HSCs in response to traumatic injury can be confidently ascertained. Notably, due to the limited scope of literature available describing the in vitro behaviors of HSCs, the specific effects of HSCs with regard to neuroinflammation in culture remain unresolved. Nevertheless, significant evidence exists regarding the in vivo effects of this cell type on inflammatory processes, as explained below.
In vivo models of HSC transplantation suggest untoward complications may occur when this cell type is used as a treatment for ischemic stroke, including increased neuroinflammation and decreased functional recovery (Bhatt et al., 2015; Hilgendorf et al., 2015; Kashara et al., 2016). A recent study by Kasahara and colleagues compared the intravenous and intra-arterial injection of bone marrow-derived mononuclear cells (BM-MNCs) and CD133+ cells in mouse models of ischemic stroke (Kasahara et al., 2016). Their results revealed a pattern familiar to HSC transplantation in vivo: a troublesome mixture of functional benefits and adverse effects. Cells injected intra-arterially were shown to encourage neuroinflammation and coincided with the loss of microvascular structures, providing no benefit to cognitive function. Though cells injected intra-venously did not produce similar inflammatory effects and even improved cortical function, the uncertainty of the therapeutic outcome of this cell type may serve as a barrier to enthusiastic research as to their clinical applicability, especially with other, more reliable cell lines available. The dangers of HSC transplantation are corroborated by the results of clinical studies. A long-term review of patients who received allogenic HSC grafts by Hilgendorf and colleagues revealed the therapy can encourage a host of chronic complications, including a higher risk of infection, gender non-specific gonadal dysfunction, lipid metabolic disturbances, and a decreased lifespan (Hilgendorf et al., 2015). Another recent review of allogenic HSC transplant patients suggested that HSCs may lead to central nervous system complications as well, significantly elevating the risk of mortality for patients in which these symptoms manifest (Bhatt et al., 2015). While the body of evidence seems to suggest HSCs can promote inflammation and harmful complications when used as a transplant source, it is important to note that a recent clinical trial employed HSCs as a graft source with results showing the procedure was tolerated well in all patients and improved functional recovery (Banerjee et al., 2014). Immunosuppressive agents were not used in this study (Banerjee et al., 2014). Nevertheless, until significant laboratory evidence is collected which demonstrates that HSC grafts produces no adverse effects in vivo, it seems wise to exercise caution when deciding whether to use this cell type in clinical treatments.
Limitations
While it is evident from observations that HSCs are mobilized during the body’s systemic response to injury that HSCs may exhibit therapeutic potential, the heterogeneous quality of this cell source is a considerable complication in the successful transition of HSC therapy to the clinic (Oguro et al., 2013). Significantly, this admixed and ill-defined cell population may produce adverse effects following transplantation (Bhatt et al., 2015, Hilgendorf et al., 2015, Kashara et al., 2016). As mentioned above, a murine stroke model of HSC transplantation demonstrated that intra-arterial HSC transplants actually encouraged inflammation in the area of infarct and negatively impacted neurologic recovery (Kasahara et al., 2016). Moreover, a recent meta-analysis of clinical trials involving allogenic HSC transplantation suggested that HSCs may encourage central nervous system complications in human patients (Bhatt et al., 2015). In addition, HSC transplantation may actually promote the incidence of cerebral infarct (Hsiao et al., 2014). Following a hematopoietic stem cell transplant after chemotherapy for acute lymphoblastic leukemia, a 35-year old patient developed a thromboembolism after displaying an elevated Factor VIII count. The hematopoietic stem cell transplant was likely responsible for the abnormal Factor VIII levels, which in turn can encourage thrombin formation or induce acquired protein C resistance, thereby putting a patient at risk for thromboembolic stroke (Hsiao et al., 2014).
Future Directions
Future studies must examine the intracellular and environmental signals that induce HSC differentiation into specific cell types so as to control for cases in which these factors could encourage HSCs to express pro-inflammatory phenotypes. Purification techniques that select for therapeutic HSCs while selecting against potentially dangerous phenotypes are a crucial necessity and may help to isolate usable HSC lines. In addition, prospective studies should explore the optimal route, timing of HSC administration, and dosage in experimental animal models. Until these details are more clearly defined, clinical trials of HSC transplantation should be approached with caution.
2.2.1.2. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
Mesenchymal stem cells (MSCs), otherwise known as mesenchymal stromal cells, can be harvested from nearly any tissue type and are identified by the phenotypic markers CD29+, CD44+, CD105 +, CD73+, CD90+, CD106+, CD166+, CD14−, CD34−, and CD45− (Mafi et al., 2011; Li et al., 2016). Different from BM-MSCs, there is a subpopulation within the bone marrow of CD34+ mononuclear cells (MNCs) which stands as an equally potent donor cell source with therapeutic applications for ischemic pathologies. Bone marrow-derived mesenchymal stem cells (BM-MSCs) are a multipotent sub-class of MSCs, harvested from bone marrow, which exhibit the capacity to differentiate into mesenchymal tissues, including osteogenic, chondrogenic, and adipogenic cells (Wang et al., 2016). Transplantation of BM-MSCs has been shown to encourage improvements in neurologic function following cerebral ischemia in stroke models (Lee et al., 2016). BM-MSCs may promote functional improvements in part due to the secretion of neurotrophic factors, which in turn stimulate endogenous cerebral repair processes. The active neurotrophic factors secreted by BM-MSCs include: hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF, FGF-2), and insulin growth factor-1 (IGF-1) (Eckert et al., 2013, Chen et al., 2015, Shichinohe et al., 2015). BM-MSCs also promote endogenous neurogenesis by encouraging the recruitment of primary stem cells from the subventricular zone (SVZ) and subgranular zone (SGZ) to site of injury and diminishing the rate of apoptotic insult in the penumbral zone of the principal lesion (Li et al., 2016). It remains to be determined if BM-MSCs differentiate into functional neurons, especially in light of their low survival time after transplantation, but their influence on neurogenesis is clear. In addition to secretion of the neurotrophic factors listed above, BM-MSCs may also improve rates of angiogenesis, thereby helping to encourage perfusion at the site of injury (Lee et al., 2016; Li et al., 2016).
MSCs are the most studied extraembryonic cell type and a particularly appealing paradigm for autologous transplantation. This distinction owes itself in part to the accessibility of MSCs, as they are naturally available in all mesenchymal tissues, including bone marrow, placenta, teeth, and adipose tissue. While MSCs can be harvested from all of the preceding tissues, evidence suggests that MSCs may exhibit varying functionality depending on their respective locations of origin and the means by which they are extracted, isolated, and proliferated. Certain therapies may be more effective with MSCs derived from a specific location and future studies should consider this nuance. Notably, MSCs may carry a risk of tumorigenicity. In a study in which primary BM-MSCs were transplanted in mice, a sarcoma developed in the lungs after delivery (Tolar et al., 2007). Moreover, BM-MSCs secrete a factor which may influence tumor development, transforming growth factor β (TGF-β), which has been shown to enhance the ability of breast cancer cell lines to migrate when secreted by BM-MSCs (McAndrews et al., 2015). Breast cancer cells also induce de novo secretion of the chemokine CCL5 from BM-MSCs, a factor which in turn acts in a paracrine fashion to enhance the motile, invasive, and metastatic potential of active cancer cells (Karnoub et al., 2007). The nuanced functionality of mesenchymal cells harvested from differing tissue sources may also come into play in determining the extent of their tumorigenicity, with MSCs from specific tissue lines encouraging different levels of effect (Hass et al., 2011; Subramanian et al., 2012). For example, when grown in the presence of cancer cells, human Wharton’s jelly umbilical cord-derived MSCs do not develop into tumor progenitor cells while some BM-MSCs do (Subramanian et al., 2012).
2.2.1.2.1. Critical Assessment: BM-MSCs
Preclinical studies in stroke models (in vitro and in vivo)
The potential therapeutic effects of BM-MSCs have been well characterized across in vitro and in vivo studies of ischemic stroke. Investigations of BM-MSCs in culture have examined approaches that encourage the expansion of this cell type, the exosomic mechanisms BM-MSCs employ to rescue ischemic tissues, and the effects of biomaterials on the efficiency of BM-MSC proliferation, differentiation, and treatment. A suite of growth factors and differentiation-inducing molecules have been identified that promote BM-MSC proliferation and differentiation toward neural cell types. Notably, hBM-MSCs cultured in fetal calf serum with platelet lysate (PL) and G-CSF demonstrate accelerated expansion rates as compared to controls (Yamauchi et al., 2014). Furthermore, PL has been shown to provide an increase to the growth rates of MSC populations grown outside of fetal bovine serum, while still ensuring the chromosomal stability of the cell line over multiple generation. Moreover, additional trophic factors which improve the proliferation and differentiation of BM-MSCs into neurons and neuron-like cells in vitro have been identified, including melatonin, ATP, neurotrophin-3 (NT-3), rolipram, β-mercaptoethanol (BME), CHIR99021 (CHIR), lithium chloride, and others (Tu et al., 2014; Abdullah et al., 2016; Joe and Cho, 2016; Narcisi et al., 2016; Shuai et al., 2016; Yan et al., 2016). Co-culturing BM-MSCs with active neurons or culturing BM-MSCs on media conditioned by mature neurons also appears to promote their differentiation towards neural cell types (Tu et al., 2014; Kil et al., 2016). For example, hBM-MSCs co-cultured with astrocytes displayed a significant tendency to differentiate into neural lineages, while hBM-MCSs cultured on media conditioned by choroid plexus epithelial cells showed higher rates of induction toward dopaminergic cell types than controls (Tu et al., 2014; Aliaghaei et al., 2016). Biomaterials also have been investigated for their potential role in improving BM-MSC proliferation and increasing the efficacy of their transplantation. Nanofibrous, ECM-based structures and engineered delivery systems, such as biodegradable polycaprolactone microcarriers, may encourage the expansion and engraftment of BM-MSCs, suggesting biomimetic BM-MSC-designed niches represent an appealing adjunctive strategy to encourage both BM-MSC proliferation and BM-MSC-based therapy (Yan et al., 2015; Bhardwaj and Webster, 2016; Shekaran et al 2016).
In vitro models also provide strong evidence that BM-MSCs exhibit neuroprotective, anti-inflammatory, neurogenic, and angiogenic effects in models of ischemic injury. When BM-MSCs are co-cultured in oxygen-glucose deprivation (OGD)-injured neuron models, they reduce rates of apoptosis and necroptosis, downregulating necroptosis-related receptor interacting protein kinase1 and 3 and deactivating caspase-3, an enzyme involved in apoptosis (Kong et al., 2016). It is widely hypothesized that BM-MSCs function via a paracrine model to rescue neural tissue, secreting exosomes which provide trophic support to the surrounding tissues. These exosomes, when isolated from BM-MSCs, appear to be 50–100 nm in size, display CD81, CD9, and Alix exosome-associated proteins, and have a lipid membrane identity consisting of cholesterol, sphingomyelin, and phosphatidylcholine (Lai et al., 2010). BM-MSCs express multiple trophic factors which may function to protect and support neurons in ischemic conditions, including BDNF, NGF, thrombospondin1, pantraxin3, VEGF, bFGF, and placental growth factor, as well as therapeutic microRNAs, such as microRNA 133b (Eckert et al., 2013; Park et al., 2015; Shichinohe et al., 2016; Xin et al., 2016). Importantly, BM-MSCs play a potent role in the sequestration of neuroinflammation. In organotypic hippocampal cultures exposed to ischemic insult, implanted BM-MSCs reduced markers of microglial activation and levels of astrogliosis, evidence of anti-inflammatory properties (Zhong et al., 2003). Moreover, when BM-MSCs are exposed to inflammatory cytokines, such as interferon-γ, they develop a specific immunoregulatory phenotype, resulting in the enhanced suppression of T cells or natural killer cells in the surrounding tissue (Zimmermann et al., 2016). In addition, BM-MSCs reduce leukocytes proliferation rates and affect their differentiation profiles when the cell types are co-cultured (Bartholomew et al., 2002; Duffy et al., 2011).
The safety, biocompatibility, and therapeutic potential of BM-MSCs have also been confirmed in multiple in vivo studies of ischemic stroke. The results of these animal studies suggest that BM-MSC transplantation promotes angiogenesis, synaptogenesis, neurogenesis, and the mobilization of endogenous stem cells, while also providing anti-apoptotic and neuroprotective effects to cells in the area of infarct (Eckert et al., 2013; Guihong et al., 2016). With evidence suggesting that exogenous BM-MSCs do not operate via a cell replacement paradigm, the BM-MSC exosome, comprising a broad array of neurotrophic factors, anti-inflammatory cytokines, and regenerative microRNAs, seems to represent the primary therapeutic mechanism by which BM-MSCs rescue tissues in vivo (Lai et al., 2010; Kong et al., 2016; Guihong et al., 2016). Promisingly, a systematic review of every BM-MSC transplantation study available on PubMed and Web of Science revealed that 38 out of 39 studies that reported behavioral outcomes recorded results that showed BM-MSC transplantation produced significant improvements (Wang et al., 2016). Functionally, BM-MSC engraftment corresponds to increased proliferation of endogenous stem and progenitor cells in the subventricular and subgranular zones, while also promoting the activation and proliferation of astroglia, which may amplify neurorestorative effects by secreting brain-derived neurotrophic factor and glial cell-derived neurotrophic factors (Eckert et al., 2013, Guihong et al., 2016). BM-MSCs also produce ECM components, including fibronectin, which may work to encourage synaptogenesis (Eckert et al., 2013). BM-MSCs secrete an array of proangiogenic trophic factors, including VEGF, basic fibroblast growth factor, and placental growth factor, which also encourage angiogenesis within both the penumbra and area of infarct in experimental models of stroke (Eckert et al., 2013). The ability of BM-MSCs to sequester neuroinflammation in animal models is also well categorized. BM-MSCs exhibit significant immunomodulatory effects in vivo, modulating T-cell proliferation rates, activating a T-regulatory cell phenotype (CD8+CD28− Treg), and producing a general suppressive effect on CD4+ and CD8+ T cells (Castro-Manrreza and Montesinos, 2015, Liu et al., 2015). BM-MSC transplantation reduces levels of pro-inflammatory cytokines, including interleukin(IL)-1β and IL-6, upregulates the expression of anti-inflammatory cytokines, such as IL-4, IL-10, and transforming growth factor-β1, and lessens the number of activated microglia in ischemic tissue (Chen et al., 2013; McGuckin et al., 2013; Castro-Manrreza and Montesinos, 2015; Laranjeira et al., 2015).
Limitations
Concerns remain as to the safety of BM-MSCs in the clinical setting. First, BM-MSCs are primarily administered via either intracranial transplantation through stereotactic delivery or via intravascular injection preceding through intravenous or intra-arterial routes (Eckert et al., 2013, Guihong et al., 2016; Wang et al., 2016). Intracranial transplantation is an invasive process that can engender additional areas of infarct in the stroke brain, especially since most treatment programs generally require multiple injections. In addition, though intravascular delivery is a less invasive treatment program, the majority of the BM-MSCs transplanted this way never reach the ischemic area of interest. It stands to be determined the precise mechanism and efficiency with which BM-MSCs traverse the blood-brain barrier, and the organ systems and regions in systemic circulation to which they migrate. Moreover, the potential tumorigenic risks of BM-MSC transplantation deserve further examination (Karnoub et al., 2007; Subramanian et al., 2012). Finally, an insufficient number of studies have investigated the potential synergistic effect of rehabilitation therapy with BM-MSC transplantation as well as the various modifications that can be made to BM-MSCs, both environmental and genetic. Rehabilitation is the most common therapy employed post-stroke in human patients and has been demonstrated clinically to improve functional recovery (Nishino and Borlongan, 2000; Trialists, 2004).
Future Directions
The efficacy of BM-MSC transplantation can be greatly improved if future studies address the current gaps in knowledge regarding the shortcomings of this cell type. First, in order to improve target migration, future efforts might characterize the pathway by which BM-MSCs reach the area of infarct, with consideration paid to the cellular signaling pathways that lead them there as well as the processes that determine how they are distributed in other areas of the body. Accordingly, future research should also fully address both the effects of current intracranial delivery regimes and devise new methods that reduce invasive damage. Additionally, BM-MSC transplantation strategies currently suffer from a lack of knowledge as to the optimal time window for administration, as well as the most optimal dosage for both acute and chronic treatments. Though a recent study by Toyoshima and colleagues suggests the optimal therapeutic window for BM-MSC transplantation is 24 hours after recanalization (Toyoshima et al., 2015), investigators examining the translational relevance of BM-MSC therapy should expand on these findings. This data is crucial to the optimization of transplant regimes in both preclinical and, importantly, clinical trials. Future research must also acknowledge that their mechanism of action most likely does not involve direct cell replacement. Future studies should focus on determining the specific molecular mechanisms of action by which therapeutic action occurs. BM-MSC efficacy may also be improved through the investigation and eventual application of environmental and genetic modifications. First, hypoxia preconditioning of BM-MSCs has been shown to promote their proliferation, angiogenic effects, and migration to areas of infarct, with a subsequent increase in reperfusion to ischemic regions (Chacko et al., 2010; Wei et al., 2013; Yu et al., 2013). Moreover, hyperbaric oxygen therapy has been suggested as a potential environmental modification that enhances the mobilization of BM into circulation as well as anti-inflammatory effects in damaged tissue (Thom et al., 2006; Pan et al., 2009). Research into hypoxia preconditioning should be explored further in animal models of stroke. Researchers should also determine the optimal treatment regime for hyperbaric oxygen therapy and BM-MSC transplantation models. Future research should better define the treatment time window (acute/chronic) necessary to encourage therapeutic effect, with consideration paid to the accessibility and practicality of chronic treatment protocols when translating the therapy to a clinical setting.
BM-MSCs modified by gene transfections that encourage their differentiation into valuable neural identities and/or enhance their expression of signals that promote endogenous stem cell proliferation and differentiation represent another powerful model for improving cell therapy for stroke (Yasuhara et al., 2009). Genetically modified BM-MSCs have been shown improve angiogenesis, endogenous neurogenesis, reduce infarct size, and decrease inflammation, while encouraging MSC survival in animal models of stroke (Kurozumi et al., 2005; Horita et al., 2006; Onda et al., 2009; Yasuhara et al., 2009; Li et al., 2016). However, not all bioactive gene transfections employ factors that produce therapeutic results. Future research must examine the optimal genetic modification for BM-MSCs used to treat ischemic stroke as well as examining these cell lines in clinical trials. Moreover, as mentioned above, preclinical studies can enhance their applicability of a cell line in the clinical setting by demonstrating how BM-MSC transplant programs respond in conjunction with adjunctive therapies, such as physical rehabilitation (Nishino and Borlongan, 2000; Trialists, 2004).
2.2.1.3. Endothelial Progenitor Cells (EPCs)
EPCs are a multipotent class of stem cells that can differentiate into mature endothelial cells. EPCs express HSC markers CD34 or CD133 and endothelial cell markers, including protein vascular endothelial growth factor receptor 2 (VEGRF2), CD31, Von Willebrand factor, vascular endothelial cadherin (VE-cadherin or CD 144), Tie2, c-kit/CD117 and CD62E (E-selectin) (Zhao et al., 2013). EPCs can be further subdivided into two main classes according to culture characteristics: early HSCs and late HSCs (Hur et al., 2004; Fadini et al., 2012) Early HSCs manifest after 4–10 days of culturing mononuclear cells from peripheral blood, while late EPCs, or endothelial colony forming cells (ECFCs), appear after long-term culturing (>14 days) of mononuclear cells. Studies suggest early EPCs secrete angiogenic growth factors, while late EPCs express higher levels of VE-cadherin and kinase insert domain receptor and can themselves integrate into regenerating vasculature (Hur et al., 2004). EPCs are a particularly attractive paradigm for the treatment of ischemic stroke due to the vascular weakness that often precedes ischemic events in the brain. An early study found that endogenous EPCs which were mobilized into the peripheral blood by GM-CSF migrated to the newly vascularized endothelium of surgically induced ischemic hind limb injury in rabbits, suggesting EPCs may play a part in promoting angiogenesis in areas of ischemic insult (Takahasi et al., 1999). These results were reinforced by further studies in which free-circulating bone marrow-derived EPCs selectively mobilized to sites of neovascularization where they also differentiated into mature endothelial cells (Tilling, Chowienczyk, and Clapp, 2009, Zhao et al., 2013). In fact, a correlational study in human ischemic stroke patients found that the concentration of circulating EPCs predicts the extent of improvement on the National Institute of Health Stroke Scale for large-artery atherosclerosis and small-vessel disease etiologic subtypes (Martí-Fàbregas et al., 2013). Moreover, EPC transplantation may have general neurorestorative effects, as intravenous infusion of autologous EPCS after MCAO in rabbits preceded functional improvement, a decrease in the number of apoptotic cells, elevated microvessel density in the ischemic peri-infarct, and a smaller infarct area (Chen et al., 2008). It is important to note that research into the therapeutic potential of EPCs is still nascent, and therefore further laboratory investigations must be conducted before their putative effects can be confidently affirmed.
2.2.1.3.1. Critical Assessment: EPCs
Preclinical studies in stroke models (in vitro and in vivo)
In vitro studies of EPCs have primarily focused on determining the trophic factors and molecular signals that enhance EPC proliferation rates, mobilization, and vascularization capacities in culture. Treatment of cultured EPCs with norepinephrine has a positive dose-dependent influence on proliferation rate in the S-phase, as well as acting to improve migratory activity (Jiang et al., 2014). Moreover, the administration of norepinephrine encourages the phosphorylation of Akt and eNOS in EPCs, suggesting the Akt/eNOS pathway may play a role in the mediation of proliferative and migratory behaviors observed in culture (Jiang et al., 2014). A variety of statins have also improved the functional behaviors of EPCs when administered in vitro. Kallistatin treatment resulted in a decrease in tumor necrosis factor-α–induced apoptosis in cultured EPC populations as well as a reduction in caspase-3 activity (Gao et al., 2014). Kallistatin also encouraged EPC proliferation, migration, adhesion, and vascular tube formation, concomitant with an observed increase in Akt, glycogen synthase kinase-3β, endothelial NO synthase phosphorylation, endothelial NO synthase expression, matrix metalloproteinase-2 synthesis, and expression of VEGF and NO (Gao et al., 2014). Atorvastatin and rosuvastatin improve the neovascularization ability of EPCs in vitro, with vascularization corresponding to the up-regulation of C-X-C chemokine receptor type 4 (CXCR4) (Chiang et al., 2015). The means by which statins influence the vasculogenic properties of EPCs may involve an interaction with the stromal cell-derived factor-1α/CXCR4 pathway and NO (Chiang et al, 2015). Moreover, VEGF and SDF significantly enhance the migration of outgrowth endothelial cells (OECs) and circulating angiogenic cells (CACs) in culture, as well as encouraging the adhesion capacity of these cell types, though CACs are more sensitive to the effects of these treatments (Anderson et al., 2015). The terminologies of endothelial progenitor cells (EPCs) and circulating angiogenic cells (CACs) may connote phenotypic feature overlaps, as well as differences between these cells. In an attempt to clarify the distinction between these cell populations, it is important to consider their distinct cellular and molecular characteristics, and to their developmental stage. Based on different reports, early circulating endothelial progenitor cells are considered to be a heterogeneous population originating from myeloid hematopoietic cells, which share many phenotypic characteristics with early immature immune cells conferring a paracrine effect on angiogenic processes (Pearson., 2010; Kachamakova-Trojanowska et al., 2015). Populations of circulating endothelial progenitor cells with these features are termed CACs (Kachamakova-Trojanowska et al., 2015). On the other hand, the real “EPCs” closely resemble mature endothelial cells exhibiting a greater differentiation potential which contributes to the development of neovessels (Kachamakova-Trojanowska et al., 2015). It has been hypothesized that S1P, a bioactive lysophospholipid, may also work to facilitate EPC-driven neovascularization (Williams et al., 2015). When OECs are exposed to S1P and VEGF treatment, they display elevated proliferation rates, an increase in 3D sprouting, a reduction in sprouting time, and an increase in directed migration under normoxic conditions. Importantly, OECs significantly increase S1P receptor expression when exposed to hypoxic conditions in vitro (Williams et al., 2015). This dynamic response means that, under hypoxic conditions, OECs respond significantly more robustly to S1P administration, with 6.5 times and 25 times the rates of sprouting and directed migration observed in hypoxic EPCs treated with S1P as compared to normoxic EPCs treated with the same concentrations of S1P (Williams et al., 2015). Studies have determined that the primary neurotrophic and angiogenic factors secreted by EPCs in vitro are BDNF and VEGF (Liu et al., 2010, Zhao et al., 2013). These trophic molecules provide mechanistic evidence for the observation that EPCs do not appear to directly combat inflammation in culture, as their primary mechanisms of therapeutic action instead are focused on neurogenesis, synaptogenesis, and vasculogenesis. Nevertheless, their transplantation may aid in reducing neuroinflammation through indirect pathways or through yet uncharacterized molecular processes in in vivo models, as discussed below.
In animal models, EPCs appear to contribute significantly to neovascularization via differentiation-mediated vasculogenesis. They also promote angiogenesis via in situ migration and proliferation of endogenous endothelial cells according to the release of intracellular signals including VEGF, HGF, angiopoietin-1 (ANG-1), SDF-1α, IGF-1, and eNOS (Aicher et al., 2003; Zhao et al., 2013; Peplow, 2014; Balaji et al., 2015; Bai et al., 2015). The body’s natural mobilization of EPCs from a quiescent to proliferative state following ischemic events and their subsequent homing to the site of cerebral injury along a gradient of SDF-1 strongly suggest these cells play a vital role in the maintenance of damaged endothelial tissue (Shen et al., 2012). Moreover, administering granulocyte-stimulating factor has been shown to be to mobilize endogenous EPCs (Zhao et al., 2013). Importantly, EPC transplantation can improve in cerebral microvascular density, regional cortical blood flow, and functional recovery in stroke models (Chen et al., 2008; Li et al., 2015). EPCs appear to have therapeutic potential in both the acute and chronic stages of stroke pathology. In the acute phase, the neuroprotective growth factors secreted by EPCS, including VEGF, SDF-1, and IGF-1, may protect endothelial cells and neurons threatened by ischemia-induced damage (Zhao et al., 2013). In the chronic stage of stroke, EPCs may encourage neovascularization, neurogenesis, and therefore the restoration of cerebral infrastructure (Zhao et al., 2013). It is precisely this process of restoring cerebral infrastructure which may allow EPCs to exhibit an anti-inflammatory effect in vivo. Specifically, EPCs may function to repair the blood brain barrier (BBB), which is often damaged following cerebral ischemia, by differentiating into the brain endothelial cells which compose it (Neuwelt et al., 2011; Wong et al., 2013; Garbuzova-Davis et al., 2014). When the BBB is damaged and its permeability increases, elevated numbers of circulating inflammatory cells can reach the area of injury, thereby encouraging local neuroinflammation (Neuwelt et al., 2011; Borlongan et al., 2012; Wong et al., 2013; Garbuzova-Davis et al., 2014). EPC administration may serve to protect the ischemic zone from this added effect, as transplanted EPCs have been shown to preserve mitochondrial morphology in endothelial cells of the ischemic brain, promote therapeutic pinocytotic activity, reduce perivascular edema, and support the integrity of microvessels, including those of the BBB (Garbuzova-Davis et al., 2017). Co-administration of pharmaceuticals may also improve the ability of EPCs to sequester neuroinflammation. When EPCs were administered with RWJ 67657, a p38 mitogen-activated protein kinase inhibitor, in an in vivo model of diabetic ischemic stroke they exerted a significant anti-inflammatory effect (Bai et al., 2015). This data, combined with what is known regarding EPC-based facilitation of BBB repair, suggests this cell type has promise as a modulator of inflammation, though further research is necessary to characterize the specific mechanism and extent of this action. Specifically, research needs to address studies in the literature that suggest EPCs encourage neuroinflammation by releasing pro-inflammatory compounds, such as IL-8 and MCP-1(Hur et al., 2004; van der Strate et al., 2007; Moubarik et al., 2011; Zhao et al., 2013).
Limitations
Sporting a robust exosome and confirmed therapeutic effects in vivo, EPCs are an attractive therapy for ischemic stroke. However, while generally safe and biocompatible, certain aspects of EPC transplantation should be investigated further before they can achieve clinical success. First, EPC transplantation may carry a risk of increasing atherosclerotic plaque levels in patients with hyperlipidemia, as EPC transplantation increased aortic plaque size in apolipoprotein E knockout mice (George et al., 2005). Conversely, locally administered EPCs have been shown to inhibit atherosclerotic plaque formation in healthy animals and circulating EPCs have been shown to exert a related inhibitory effect, suggesting this subject deserves further study (Kunz et al., 2006; Ma et al., 2009). Moreover, EPC transplantation therapies suffer from non-refined methods of harvesting and purification. Currently, no method exists to fractionate EPC cell populations so as to isolate EPCS with specific vasculogenic, angiogenic, and differentiation capabilities. Instead, quality and quantity-controlled culture systems are used to select for cells that generally exhibit these faculties: unfractionated mononuclear cells are sub-sampled to generate a population of mononuclear cells enriched in EPCs (Hur et al., 2004; Fadini et al., 2012). However, this method does not produce pure cell cultures, and the resulting admixture may contain cells with uncharacterized effects (Hur et al., 2004; Fadini et al., 2012). Additionally, the vast majority of EPC-based preclinical studies employ acute administration (0–48 hours after stroke onset), suggesting the effects of chronic EPC transplantation warrant elucidation (Chen et al., 2008; Fan et al., 2010; Bai et al., 2015a; Bai et al., 2015b). In addition, no clinical trials have been initiated that use EPCs to treat stroke. Finally, it has been noted in the literature that transplanted EPCs may promote neuroinflammation after ischemic events, an issue which must be addressed if EPC-based therapies are to be considered clinically relevant (Hur et al., 2004; van der Strate et al., 2007; Moubarik et al., 2011; Zhao et al., 2013).
Future Directions
Future research should focus on solving aforementioned issues in EPC administration and harvesting, while more robustly characterizing the effects of EPCs, before this cell type can earn its place in the clinic. Preclinical research should determine the relative risk of atherosclerotic plaque formation for EPC transplants as well as the pre-existing conditions that may promote it. Additionally, future studies should devise better fractionation and isolation techniques, so as to prepare homogenous EPC populations whose effects can be fully characterized and normalized. Moreover, to understand the full value of EPC cell therapy, preclinical studies must be performed that investigate the chronic effects of EPC delivery, instead of solely the benefits of acute administration. In addition, clinical trials affirming the safety and efficacy of EPC transplantation in ischemic stroke should be initiated.
2.2.1.4. Very Small Embryonic-Like Stem Cells (VSELs)
VSELs exhibit phenotypic markers Sca-1+, CD45−, and pluripotent stem cell markers SSEA-1, Oct-4, Nanog, and Rex-1 (Kucia et al., 2007; Kassmer and Krause, 2014). VSELs are characterized by a high nucleus-to-cytoplasm ration, a characteristic they share with embryonic stem cells, and their nuclei contain one-type chromatin (euchromatin) (Ratajczak et al., 2012; Kassmer and Krause, 2014). VSELs are mobilized from adult tissues in a manner similar to HSCs upon ischemic insult and are released into peripheral blood, suggesting they may play a role in endogenous repair processes (Ratajczak et al., 2012). On account of their categorically low concentrations when released in peripheral blood, VSELs are widely considered to be epiblast-derived pluripotent stem cells which are deposited during early embryonic development for the purpose of acting as a cache of restorative tissue that is drawn from through adulthood (Ratajczak et al., 2012). Pertinently, the brain possesses a comparatively large relative proportion of cells exhibiting the VSEL phenotype (Ratajczak et al., 2012). VSELs can differentiate into neurons, oligodendrocytes, and microglia (Havens et al., 2014). Their potential for neurogenesis thus makes them valuable candidates for stroke therapy (Grymula et al., 2014). However, transplantation studies are limited by the extremely low yield of VSELs that can be viably obtained by current harvesting protocols (Shin et al 2013). This paucity demands extensive proliferation prior to transplantation. Moreover, VSEL concentrations decrease with age, a trend which can further decrease the inherently low harvest yield (Kucia et al., 2006; Shin et al., 2013). Despite these challenges to the process of obtaining an adequate number of VSELs, the potential therapeutic scope of VSEL treatments is arguably vast.
2.2.1.4.1. Critical Assessment: VSELs
Preclinical studies in stroke models (in vitro and in vivo)
Because VSELs are challenging to isolate and expand to therapeutically relevant dosages, in vivo and in vitro studies utilizing this cell type are relatively limited, especially with regard to the delivery of VSELs as a therapy for ischemic stroke. Nevertheless, there is evidence that VSELs can differentiate into neurons, oligodendrocytes, and microglia in vitro, indicating these cells may be valuable as donor grafts for the regeneration of CNS tissue after stroke (Havens et al 2014). In a paper by Kucia and colleagues, GFP-positive VSELs co-cultured with non-GFP bone marrow cells in cardiac, neural, and pancreatic differentiation media were shown to differentiate into the cell types of their respective media, with VSELs grown on neural media developing into multiple neural subtypes, including glia (Kucia et al., 2006). It is important to note, however, that no GFP-negative controls were used in this study, so what may have appeared as a cell staining positive for differentiation-dependent markers could have been an artifact of autoflourescence. A more recent study of VSEL differentiation suggests that VSELs can differentiate along the hematopoietic lineage when co-cultured with OP9 stromal cells (Ratajczak et al., 2011). This study additionally found that VSELs are highly resistant to radiation damage, as opposed to HSCs (Ratajczak et al., 2011). VSELs in culture have also been shown to exhibit a strong chemotactic attraction to SDF-1, HGF, and leukemia inhibitory factor (Kucia et al., 2006). This gradient-driven mobilization may elucidate, in part, the mechanism by which VSELs home to sites of ischemic injury following their release into peripheral circulation (Kassmer and Krause, 2013). Moreover, VSELs actively express CXCR4, c-met, and leukemia inhibitory factor receptor in culture, as well as embryonic transcription factors Oct-4 and Nano (Kucia et al., 2006). In addition, it has been demonstrated that VSELs have bivalent domains in promoters that encode homeobox-containing transcription factors important to development, including Sox21, Nkx2.2, Dlx1, Lbx1h, Hlxb9, Pax5, and HoxA3 (Shin et al., 2012). The faculties of VSELs relevant to the treatment of neuroinflammation remain to be determined, as the paracrine effects of this cell type have yet to be well characterized in vitro. VSEL-derived BM cells grown in angiogenic media exhibited a mesenchymal phenotype (CD90+, Thy-1 gene positive expression) and did produce pro-inflammatory cytokines, IL-6, IL8 and chemokine (C-C motif) ligand 5 (Guerin et al., 2015). Despite this pro-inflammatory evidence, VSELs sport a diverse portfolio of differentiable phenotypes, suggesting they could very well be induced to take on an immunoregulatory role. Nonetheless, future studies are required to elucidate the full potential of this cell type
In vivo studies confirm that VSELs are mobilized into peripheral blood following tissue injury. Evidence from murine models indicates that VSELs concentrations in the blood are elevated following multiple categories of systemic insult, including hypoxic conditions, the injection of carbon tetrachloride or cardiotoxin to model toxic liver or skeletal muscle damage, and myocardial infarction (Kucia et al., 2008, Bhartiya et al., 2013). Moreover, G-CSF can also act as a powerful signal in vivo for the recruitment and mobilization of VSELs (Kucia et al., 2008). Relevantly, VSELs have been shown to differentiate into HSCs, MSCs, endothelial cells, epithelial cells of the lung, oocytes, and cardiomyocytes in vivo (Dawn et al., 2008; Taichman et al., 2010; Parte et al., 2011; Ratajczak et al., 2011; Wu et al., 2012; Kassmer et al., 2013) Though few transplantation studies examining the therapeutic effects of exogenous VSELs have been published, transplantation of GFP-positive VSELS in mice after myocardial infarction improved ventricular function and cardiac remodeling (Zuba-Surma et al., 2011). Notably, in cases of stroke, increased levels of circulating VSELs have been observed following the insult, suggesting their mobilization could be co-opted as a therapeutic strategy (Paczkowska et al., 2009). Responses of VSELs in vivo to the administration of certain extrinsic factors have also been examined. As mentioned above, G-CSF encourages VSEL mobilization, but chronic increases in plasma-circulating IGF-1 may actually accelerate the depletion of VSEL stores in adult tissues (Kucia et al., 2008; Kucia et al., 2013). Additionally, in an animal model of toxic brain damage induced via the administration of kainic acid, it was demonstrated that not only do VSELs mobilize following the neurotoxic insult but also the bone marrow pool of quiescent VSELs undergoes expansion, suggesting the endogenous proliferation of VSEL stores may occur (Grymula et al., 2014). Evidence of VSELs being mobilized into circulation after injury suggest that these cells may play a role in the mediation of injury-induced damage, including the sequestration of neuroinflammation. This implication warrants further investigation. Considering the pluripotency of this cell type, it would not be surprising to discover that VSELs are an applicable tool for controlling neuroinflammation in ischemic stroke. However, at this time, they are not considered one.
Limitations
The average concentration of circulating VSELs in peripheral blood is exceptionally low: on average 1 cell per 105 monocular BM cells under steady-state conditions (Ratajczak et al., 2012; Shin et al 2013). This relative paucity makes the harvesting and purification of VSELs extremely difficult. Currently, to purify and harvest VSELs from human blood necessitates time-intensive flow cytometry (Ratajczak et al., 2012). This harvesting process does not result in high yields, and therefore the capacity to proliferate VSEL samples to practical concentrations for transplantation therapy poses a considerable problem for time-effective therapies. Together, the need for homogenous, autologous stem cell populations combined with the difficulties of harvesting and amplifying VSEL populations means that it is currently impractical for VSELs to be acutely administered to treat ischemic stroke, since cell populations must be proliferated and transplanted within hours after the onset of the event.
Future Directions
Preclinical research into VSEL-based therapies has only recently begun. Future investigations should work to refine harvesting and purification protocols to improve upon the meagre yields offered by current cytometric filtering techniques (Ratajczak et al., 2012; Shin et al., 2013). Moreover, systems should be proposed that can encourage the efficient and timely proliferation of VSEL populations. Without better harvesting and proliferation protocols, VSELs will remain an impractical cell type for clinical therapy. Future studies should also work to characterize the factors that encourage VSEL differentiation. Finally, for VSELs to begin a transition from the laboratory to the clinic as a therapy for stroke, it is imperative that VSELs be investigated in animal models of ischemia. The ideal route of administration, dosage amount, and imaging system by which to track systematic migration also stand to be determined. Animal studies will provide a clearer picture of exactly what regenerative niche VSEL treatments best occupy in the remediation of ischemic stroke pathology.
2.2.2 Neural Stem Cells (NSCs)
NSCs describe a class of multipotent cells that can differentiate into neurons, astrocytes, and oligodendrocytes (Shi et al., 2015). Their ability to produce the primary cellular phenotypes of the CNS makes them an extremely attractive candidate for stroke therapy. Endogenous populations of NSCs are common to the SGZ of the dentate gyrus and SVZ (Santilli et al., 2010). In the aftermath of a stroke-like injury, there is increased cellular activity in these zones and NSCs actively migrate to the site of ischemic insult (Zhang et al., 2014), a targeted mobilization paradigm with therapeutic potential. However, the means by which NSCs provide functional repair post-stroke remains remain to be fully characterized.
2.2.2.1. Critical Assessment: NSCs
Preclinical studies in stroke models (in vitro and in vivo)
In vitro and in vivo studies of NSCs offer promising insights into the neurogenic, angiogenic, and neuroprotective properties of this cell type. NSCs can be harvested from the adult brain and proliferated in vitro with the addition of bFGF and epidermal growth factor (Santilli et al., 2010; Cai et al., 2014). After the withdrawal of cytokines, NSCs will differentiate into all three primary neural cell types, including neurons, astrocytes, and oligodendrocytes (Shi et al., 2015). Endogenous NSCs have been shown to encourage to encourage angiogenesis in the brain following ischemic insult (Zhang et al., 2011; Zhang et al., 2014). Importantly, hypoxic conditions may act as a therapeutic signal, encouraging NSC differentiation toward neural cell types, thereby promoting neurogenesis. A study by Cai and colleagues examined how NSCs react to hypoxic conditioned media (HCM) and found that NSCs grown on 4% HCM matured more consistently into neurons NSCs grown on 1% HCM and controls, though neurons grown on 1% HCM also displayed higher neuronal counts than controls (Cai et al., 2014). The study authors also found that PI3-K, Akt, and JNK displayed increased phosphorylation levels in NSCs cultured on HCM, which indicated that NSC differentiation into neurons may depend on PI3-K/Akt pathways (Cai et al., 2014). A study by Santilli and colleagues corroborated these findings with an important caveat. In their study, NSCs grown at 1% oxygen returned to a state of quiescence (Santilli et al., 2010). This result indicates that extremely low oxygen concentrations may be noxious to NSC differentiation, implying that the conditioned response of NSCs to hypoxia may warrant further resolution, namely as to the minimum and maximum hypoxic concentrations permissible to ensure a therapeutic effect (Santilli et al., 2010). Other in vitro studies have sought to characterize the signals that mediate NSCs proliferation and differentiation. These studies have demonstrated that neural stem cells respond positively to the overexpression of miR-381, nuclear factor kappa B (NFκB), and Nox4-generated superoxide levels (Zhang et al., 2012, Topchiy et al., 2013, Shi et al., 2015). On the other hand, contact with endothelial cells, mediated via the expression of endothelial proteins ephrinB2 and Jagged1, may encourage the quiescence of NSCs and inhibit their differentiation (Chou et al., 2014). Moreover, high glucose levels have been shown to encourage apoptosis and reduce proliferation of NSCs in oxygen glucose deprivation cultures through the activation of JNK/p38 MAPK pathways and the stimulation of a late G1-S transition in the cells (Chen et al., 2013). The NSC exosome may play an active role in regulating inflammation, with NSCs producing known neuroprotective compounds, including VEGF, BDNF, NGF, and neurotrophins (Lua et al., 2003). However, the precise mechanism of action for the potential anti-inflammatory effects of NSCs has yet to be determined in vitro.
As mentioned above, it is well-categorized in in vivo models that NSCs in the SVZ migrate to the site of ischemic injury after stroke (Hao et al., 2014, Zhang et al., 2014). Following an ischemic cerebral event, they are redirected from the rostral stream into blood vessels that perfuse the area of infarct, with mobilization induced by chemokine signals released from the damaged tissue, including SDF-1, (VEGF), and angiopoietin (Hao et al., 2014). Endogenous NSCs promote neurogenesis as well as improvements to the vascular architecture of the infarct area through interactions with endothelial cells (Wang et al., 2016). Considering the endogenous role NSCs play in endemic regeneration, exogenous implantation of NSCs has significant therapeutic potential to restore neuronal circuits and improve the function of residual penumbral neurons in vivo. In an MCAO rodent model, intravenous transplantation of human NSCs resulted in hNSC-treated ischemic animals showing significant improvements to behavioral function and reduced cerebral infarct volumes as compared to controls (Shen et al., 2010). Exogenous NSC transplants have also been reported to encourage the proliferation of endogenous NSCs and their differentiation into mature neural cell types, as well as to promote angiogenesis in the ischemic boundary zone, with higher concentrations of Willebrand factor-positive proliferating endothelial cells observed in human NSC-treated rats than controls (Zhang et al., 2010). A study examining the effects of NSC transplantation in a model of photothrombic ischemic stroke reported similar results, with exogenous NSC-treated rats displaying behavioral recovery superior to controls (Hou et al., 2016). This study also confirmed that NSCs differentiated into neurons and astrocytes in vivo (Hou et al., 2016). Importantly, NSC survival and differentiation is threatened by inflammatory processes and the action of pro-inflammatory T cells. The expression of glucocorticoid-induced TNF receptor on activated CD4+T cells correlates with a reduction in the number of endogenous NSCs in a murine stroke model (Takata et al., 2012). However, NSCs have also been linked to anti-inflammatory effects in vivo. Intravenous administration of NSCs in a rat stroke model resulted in a decrease in OX-42+ microglia and MPO+ infiltration into the area of injury and also reduced cerebral and splenic expression of TNF-a, IL-6, and NF-kB. NSC transplantation has also decreased leptin receptor activation (Lee et al., 2007). In addition, in a MCAO rat model of stroke, NSCs injected into the ipsilesional hippocampus exhibited a notable acute anti-inflammatory effect, decreasing microglial activation, reducing the expression of proinflammatory factors (TNF-α, IL-6, IL-1β, MCP-1, MIP-1α,, decreasing the number of adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1), and repairing blood-brain barrier damage (Huang et al., 2014). Moreover, co-grafts of NSCs and OECs produced a decrease in inflammatory factor IL-6 and Bcl-2-associated death promoter, an apoptotic signal molecule, expression in an rat model of TBI, leading to significantly improved neurological function (Liu et al., 2014). To ascertain the full anti-inflammatory potential of NSCs, however, the extent to which neuroinflammation impairs NSC survival must be weighed against the pro-proliferative effects of ischemic hypoxia on this cell type.
A recent meta-analysis of NSC-based cell therapies for ischemic stroke confirmed the preclinical efficacy of this treatment by systematically comparing the quality score and effect size of multiple animal studies of NSC transplantation and ischemic stroke (Chen et al., 2016). Across all available studies, transplantation was shown to generally result in functional improvements, with the meta-analysis confirming that NSCs significantly enhance functional and structural recovery in vivo (Chen et al., 2016). However, the meta-analysis noted that the therapeutic efficacy of NSC treatment depends on certain conditions. First, NSC treatments are the most efficacious when the NSCs derive from a donor of the same species as the recipient. Moreover, NSC cells administered acutely (0 to 72h) were much more effective than those administered after this window (Chen et al., 2016).
Limitations
The precise mechanism of action by which NSCs mediate a therapeutic effect remains unresolved and may involve any of the following: stimulation of endogenous NSC proliferation, possible replacement of supporting cells/neurons, or the effects of a therapeutic exosome (Lua et al., 2003; Zhang et al., 2010; Huang et al., 2014; Lio et al., 2014; Shi et al., 2015). Nevertheless, the potential of this cell type as a therapy for ischemic stroke is promising. Importantly, however, the development of NSCs as a clinical treatment model faces particular challenges. First, NSCs are problematic to harvest from the CNS, and autologous treatments may require invasive surgeries prior to therapy (Burns et al., 2009; Shinozuka et al., 2013). In addition, to generate allogenic grafts of NSCs would potentially necessitate the use of fetal cell lines or the induction of the phenotype in an alternative cell source (Burns et al., 2009). NSCs, like some other highly potent stem cell lines, also carry the risk of aberrant proliferation. Tumorigenicity concerns must also be considered alongside the mandate that NSCs cell populations be purified and homogenous upon transplant (Shinozuka et al., 2013). In a case of clinical transplantation where a child with ataxia telangiectasia glioneuroal neoplasm was implanted with a non-homogenous mixture of fetally derived NSCs, the child developed glioneuroal neoplasm four years after transplantation (Shinozuka et al., 2013). Additionally, even though NSCs proliferate at a level that may pose a tumorigenic risk, their proliferation rate is still relatively low for biomedical purposes, such that it makes generating adequate cell numbers for transplant difficult (Shinozuka et al., 2013). Recent innovations, such as developing long-term culturing, immortalization, gene therapy through the insertion of oncogenes, and derivation of NSCs from pluripotent stem cell lines and other tissues, have attempted to circumvent these challenges in NSC transplantation (Shinozuka et al., 2013). However, these new techniques themselves are problematic. For example, long-term culturing may induce conversion to a non-neural cell type, such as a tumor precursor cell (Shinozuka et al., 2013). Moreover, in the case of oncogene therapy, though teratocarcinoma-derived hNT neuron cell lines reached a phase II clinical trial in stroke patients, their transplantation produced no observable neurologic recovery (Shinozuka et al., 2013). Nevertheless, oncogene insertion still holds promise. Recently, stem cell therapeutics company ReNeuron LTD developed a therapy which uses a c-Myc regulator gene and a mutated estrogen receptor transgene to create an immortalized neural cell line that has generated a clinical trial for stroke patients in the UK (Shinozuka et al., 2013). The results of this study may help to advance the resolution of these issues.
Future Directions
Future studies of NSC therapy should improve the harvesting and purification processes so as to promote the facile proliferation of homologous cell populations. Along with this improvement, future work should also focus on characterizing and reducing the tumorigenicity of NSCs. Preclinical studies should continue to determine, with greater accuracy, the optimal transplantation dosage, route of administration, and timing of treatment post-stroke. In addition, further defining the mechanism of action (direct cell replacement, exosome, endogenous cell stimulation) will be crucial to the potential translation of this treatment from the laboratory to the clinic. Moreover, future studies should examine whether NSCs transplanted with other cell types or adjunctive pharmaceutical therapies exhibit a more significant therapeutic profile than NSCs transplanted alone. For example, recombinant human tissue kallikrein produced in the ischemic brain following adenovirus injection of the kallikrein gene inhibits inflammatory cell accumulation and promotes neurogenesis, suggesting that complementary therapies, like gene transfer, could encourage a more tolerable microenvironment wherein NSCs can have greater impact (Xia et al., 2006).
2.2.3. Extraembryonic Stem Cells
The placenta, Wharton’s jelly, the umbilical cord, and the amnion are all rich sources of extraembryonic stem cells, with placental-derived stem cells, amniotic epithelial cells, umbilical cord matrix stem cells, and amnion-derived stem cells having been successfully isolated from these sources (Park et al., 2009; Antonucci et al., 2011; Tajiri et al., 2012; Dailey et al., 2013). Similar to MSCs and NSCs, extraembryonic stem cells are consistent with the various developmental germ layers. For example, amniotic epithelium derives from the ectoderm, while the mesoderm gives rise to amnion-derived MSCs (Dailey et al., 2013). As a result, amnion-derived stem cells have a much greater capacity to give rise to mesodermal cell lineages than ectodermal lineages (Dailey et al., 2013). Accordingly, amnion-derived MSCS have demonstrated greater embryonic specificity, while lacking the potential for endothelial growth (Antonucci et al., 2011; Dailey et al 2013).
Current investigations of extraembryonic stem cell types are predominantly focused on the use of placenta-derived MSCs and umbilical cord blood-derived MSCs (UCB-MSCs) to treat experimental models of ischemic stroke. These extraembryonic MSC-derivatives not only replace damaged cells, they also refine the hostile microenvironment that develops after stroke, thereby improving endogenous neurogenesis long-term (Jones et al., 2007; Park et al., 2009; Ou et al., 2010; Chen et al., 2013; Iskander et al., 2013). Recent studies on the effects of UCB-MSCs in rodent stroke models report improved functional recovery, elevated expression of bFGF and VEGF, evidence of neurogenesis, and increased vascular density in treated animals (Park et al., 2009; Ou et al., 2010; Hocum Stone et al., 2016). Umbilical cord lining mesenchymal stromal cells have also exhibited an immunosuppressive influence on the innate immune cascade prompted by transplanted cells, and also display heightened immunological immaturity in comparison to aged bone marrow mesenchymal stromal cells (Jones et al., 2007; Wang et al., 2009). Furthermore, Wharton’s jelly-derived mesenchymal stromal cells have the capacity to differentiate into CXCR4+, glial, doublecortin+, neuronal, and vascular endothelial cells which function to improve the neuroplasticity of the ischemic brain (Alaminos et al., 2010; Dalous et al., 2012; Becker and Riet, 2016). They may also suppress the immune response through release of leukemia inhibitory factor (LIF) (Najar et al., 2010).
Lately, umbilical cord banking is increasing in popularity in response to the notable therapeutic potential of umbilical cord-derived stem cells in both autologous and allogenic transplantation models (Kurtzberg et al., 2005). Umbilical cord blood describes the mononuclear fraction, which consists of monocytes, hematopoietic progenitors, MSCS, and lymphocytes (Achyut et al., 2014). Despite the heterogeneity of this source, UCB-MSCs are still thought to be immunologically immature (Wang et al., 2009). Accordingly, UCB-MSCs have been observed to mediate the response of the immune system and restrict levels of pro-inflammatory cytokines (Chao et al., 2016; Vellasamy et al., 2016). Recent investigations into the transplantation of umbilical cord blood in stroke models have displayed favorable results, including a reduction in infarct size, improved functional recovery, and increased expression of several neuroprotective factors including VEGF and BDNF (Lim et al., 2011; Park et al., 2015; Hocum Stone et al., 2016; Liang et al., 2016; Zhilai et al., 2016).
2.2.3.1. Critical Assessment: Extraembryonic Stem Cells
Preclinical studies in stroke models (in vitro and in vivo)
The two extraembryonic stem cell lines that display significant potential for the treatment of ischemic stroke are UCB-MSCs and placenta-derived MSCs. UCB-MSCs exhibit the capacity to differentiate into cells of all three germ layers in vitro (Dailey et al., 2013). Notably, multiple studies confirm the successful induction of UCB-MSCs toward neural-like cells upon stimulation with growth factors, including b-FGF and retinoic acid (Li et al., 2012; Jin et al., 2015). Cultured with these agents, UCB-MSCs displayed morphological changes and the expression of neural markers, such as nestin, β-tubulin III, and neurofilament 200 (Li et al., 2012; Jin et al., 2015). Moreover, UCB-MSCs have been shown to differentiate into functional EPCs following induction with VEGF and hFGF, and the formation of vessel-like structures has been observed following a period of growth (Sabry et al., 2016). Placenta-derived MSCs also exhibit the ability to differentiate into endothelial-like cells and smooth muscle-like cells when cultured with VEGF (Makhoul et al., 2016). Placenta-derived MSCs can also be induced so to express neural phenotypes, displaying neuronal markers, such as GFAP, Nestin, or β-Tubulin III (Martini et al 2013).
Important to the potential translation of extraembryonic cell types for the treatment of ischemic stroke, both UCB-MSCs and placenta-derived MSCs are genetically stable under hyperglycemic and ischemic conditions in vitro, only exhibiting quiescent behavior after serum starvation accompanied by hypoxia (Sharma and Bhonde, 2015). UC-MSCs also exhibit a significant effect in suppressing pro-inflammatory immune responses in vitro (Cutler et al., 2010). Co-culturing of UC-MSCs with various immune cells has demonstrated that UC-MSCs suppress the proliferation, differentiation, and immunoglobulin expression of B cells, as well as suppressing the proliferation of T cells and the downregulation of monocyte function, possibly through the expression of PGE2 (Cutler et al., 2010; Che et al., 2012). Placenta-derived MSCs have also been shown to suppress allogenic T-cell proliferation in vitro (Jones et al., 2007). On the other hand, interferon (IFN)-γ and tumor necrosis factor (TNF)-α enhance the ability of placenta-derived MSCs to induce the differentiation of immunoactive CD4(+)IL-10(+)and CD8(+)IL-10(+)Treg subsets and express programmed cell death ligand-2, implying certain microenvironments could encourage placenta-derived MSCs to promote neuroinflammation (Li et al., 2015). Together, these results suggest that extraembryonic stem cells have significant potential to reduce ischemia-induced neuroinflammation in the brain.
In vivo studies of UCB-MSC and placenta-derived MSC transplantation demonstrate these cell lines can also ameliorate ischemic conditions and improve therapeutic outcomes following stroke in animal models. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) exhibit angiogenic, neurogenic, and anti-inflammatory properties in stroke rodents (Park et al., 2009; Ou et al., 2010; Lim et al., 2011; Iskander et al., 2013). Recent research suggests their therapeutic effects may be mediated through the upregulation of thrombospondin1, pantraxin3, and vascular endothelial growth factor under hypoxic conditions (Park et al., 2015). Placenta-derived stem cells also display neurogenic and neuroprotective effects in in vivo rat models of stroke, reducing the size of cortical lesions and improving behavioral recovery following ischemic injury (Chen et al., 2013; Zhang et al., 2014; Liang et al., 2016). In addition, recent research suggests placenta-derived stem cells significantly elevate levels of VEGF, HGF, and BDNF in the ischemic border zone (Chen et al., 2013). Pertinently, hUCB-MSCs may inhibit immune cell migration and activation near the area of cerebral infarct, as well as promote microglial survival (Pimentel-Coelho et al., 2012). Placenta-derived MSCs also reduce inflammation in the area of injury and may encourage the activation of protective microglia (Zhang et al., 2014; Liang et al., 2016). In this way, both UCB-MSCs and placenta-derived MSCs should be considered notable candidate cells for therapeutic control of neuroinflammation following ischemic stroke.
Limitations
When Kranz and colleagues examined placenta-derived mesenchymal stromal cells in an experimental rat model of ischemic stroke, though the transplantation engendered significant functional improvements, only maternally derived placental MSCs were seen to induce significant effects (Kranz et al., 2010). Harvesting these cells from the placenta is itself a constraint regarding the availability of placenta-derived stem cells for transplantation, and therefore evidence that suggests maternal placental cells are necessary to see therapeutic benefits further limits the scope of treatments with this cell line (Kranz et al., 2010). For this reason, placenta-derived mesenchymal stromal cells presently seem more appropriate for neonatal and pediatric treatments than the treatment of adult stroke patients.
hUCB-MSCs do exhibit significant potential to exhibit therapeutic and anti-inflammatory effects following transplantation in models of ischemic stroke. However, the efficacy of hUCB-MSC treatment may depend on the route of administration (Lim et al., 2011). Specifically, Lim and colleagues investigated this question, examining whether hUCB-MSCs could enter the brain, survive, and produce a therapeutic effect following intrathecal transplantation into the lumbar spinal cord and intravenous transplantation into the tail vein in rat models of ischemic stroke (Lim et al., 2011). While each experimental group treated with hUCB-MSCs displayed better neurological recovery than controls, rats receiving intrathecal hUCB-MSC treatments recorded higher numbers of cells having migrated to the ischemic area, higher cell survival, and greater expression of mature neural-lineage markers than rats that received hUCB-MSCs administered intravenously (Lim et al., 2011). This study suggests the efficacy of hUCB-MSC treatment may be limited by the route of administration.
Future Directions
Preclinical studies of placenta-derived stem cells have not yet determined the optimal dosage, timing, or route of administration for cell therapy. Specifically, studies have focused predominantly on the acute transplantation of placenta-derived stem cells. Not only should future research refine the optimal time window for acute administration, but these studies should examine the benefits, if any, of chronic programs. Future studies should compare routes of administration and work to standardize the transplantation procedure. Moreover, studies should examine how placenta-derived stem cells can be optimally employed as a therapy. If placenta-derived stem cells must be used from a maternal line, researchers should examine the practicality of treatment with this cell type and in what situations this cell type might be considered for stroke-specific therapy (Kranz et al 2010). Future studies of hUCB-MSCs, on the other hand, should resolve issues surrounding the optimal route, timing, and dosage for administration. Importantly, preclinical research should examine intrathecal routes as a potential optimum mode of delivery (Lim et al 2011). Future studies should also determine whether the results of hUCB-MSC treatment can be improved through adjunctive therapies, as a recent study by Liang and colleagues demonstrated that an estradiol complement significantly improved the ability of human umbilical cord blood CD34+ to remediate ischemic injury after stroke (Liang et al., 2016).
2.2.4. Other Sources of Adult Stem Cells
2.2.4.1 Adipose Tissue
Adipose tissue-derived MSCs (AD-MSCs) are one of the most abundant MSC subtypes and can be readily obtained without invasive procedures (Puissant et al., 2005; Tobita et al., 2011). Investigations conducted to determine the effects of adipose-derived stem cells in models of ischemic stroke have revealed exogenous transplantation reduces cerebral inflammation, infarct size, and chronic neurodegeneration, while improving neurological function and recovery (Gutiérrez-Fernández et al., 2013). As compared to bone marrow-derived stromal cells, adipose-derived stem cells have a higher proliferative capacity and produce greater quantities of HGF and VEGF (Ikegame et al., 2011). They are also capable of differentiating into vascular endothelial cells, glial cells, and neural cells in vivo (Gutiérrez-Fernández et al., 2013).
2.2.4.1.1. Critical Assessment: Adipose Tissue-Derived Stem Cells
Preclinical studies in stroke models (in vitro and in vivo)
AD-MSCs have been proven safe in animal models of ischemic stroke and their therapeutic efficacy is supported by the results of both in vitro and in vivo studies. In vitro, AD-MSCs can differentiate into cell types relevant to treating neural ischemia, including neural, glial, and vascular endothelial cells (Ikegame et al., 2011). In culture, hAD-MSCs also form vascular-like structures and actively express von Willibrand Factor, a blood glycoprotein important for hemostasis (Planat-Benard et al., 2004). Moreover, in a comparison of trophic factor release from AD-MSCs and bone-marrow derived MSCs cultured on similar media, AD-MSCs were shown to produce higher levels of VEGF and HGF than BM-MSCs (Ikegame et al., 2011). AD-MSCs transplanted in an in vitro model of stroke (oxygen and glucose deprivation) encouraged the expression of anti-inflammatory and immunomodulatory genes in ischemic Neuro2A cells, thereby making the case that adipose-derived stem cells may exert an anti-inflammatory effect in stroke via paracrine activation, i.e. “the bystander effect” (Jeon et al., 2013). The abilities of BM-MSCs and AD-MSCs to control sources of neuroinflammation have also been compared in vitro. BM-MSCs and AD-MSCs were proliferated and co-cultured with T-cells, monocytes, or natural killer (NK) cells (Valencia et al 2016). Each cell type showed similar regulatory properties, with BM-MSCs and AD-MSCs reducing NK-cell proliferation, cytokine secretion, and the expression of activating receptors and cytotoxic molecues, despite only BM-MSCs significantly reducing NK cytotoxic activity (Valencia et al., 2016). BM-MSCs also negatively impacted the proliferation of T-cells and their production of inflammatory cytokines after activation (Valencia et al., 2016). AD-MSCs and BM-MSCs also diminished cytokine production by dendritic-cells and their capacity to induce CD4(+) T-cell proliferation, with AD-MSCs impairing dendritic-cell differentiation more acutely than BM-MSCs. AD-MSCs may also reduce levels of IL-18, TLR-4, and plasminogen activator inhibitor-1 in culture (Leu et al., 2010). These results point to AD-MSCs as a particularly intriguing cell type for the mediation of neuroinflammation, with potent effects that might outstrip MSCs derived from more conventional sources, such as the bone marrow.
AD-MSCs have encouraged therapeutic outcomes following exogenous transplantation in vivo. These cells have been proven to be both safe and biocompatible both as allogenic and xenogenic grafts in rodent models, with no significant differences in recovery observed between rodents treated with foreign or self-derived AD-MSCs (Gutiérrez-Fernández et al., 2015). Gutiérrez-Fernández and colleagues also demonstrated that acute allogenic AD-MSC administration thirty minutes after MCAO in rats increased cellular proliferation, neurogenesis, oligodendrogenesis, synaptogenesis and angiogenesis significantly as compared to controls (Gutiérrez-Fernández et al., 2013). Moreover, the abundance, accessibility, and potential for rapid proliferation exhibited by AD-MSCs are appealing qualities for potential clinical treatments. Currently, intravascular administration is the predominant paradigm for AD-MSC delivery, with some studies in animal models suggesting that AD-MSCs administered this way can improve functional deficits, reduce infarct area, and promote functional recovery (Gutiérrez-Fernández et al., 2013). With regards to the homing and migration of administered AD-MSCS, studies suggest that while AD-MSCs do not migrate fully to the region of cerebral ischemia after intravascular injection, but may instead encourage neuroprotection and neurogenesis from peripheral organs through a robust exosome (Gutierrez-Fernandez et al., 2011; Ikegame et al., 2011; Chung et al., 2012), Exogenously transplanted AD-MSCs have been shown to encourage higher levels of HGF and angiopoietin-1 in ischemic brain tissue in vivo than similarly transplanted bone-marrow derived MSCs (Ikegame et al., 2011). This study in combination with a study by Moon and colleagues in which transplanted AD-MSCs were shown to increase neovascularization in a model of hindlimb ischemia suggest AD-MSCs may promote angiogenesis (Moon et al., 2006; Ikegame et al., 2011). AD-MSCs have also been found to reduce cell death, slowing the rate of apoptosis and improving recovery in the cerebral infract of rats with ischemic stroke (Kim et al., 2007; Gutierrez-Fernandez et al., 2013; Leu et al., 2010). The ability for AD-MSCs to improve neuronal survival, decrease the size of the infarct area, and encourage functional recovery may owe itself in part to the anti-inflammatory effects of AD-MSCs. As observed in vitro, AD-MSCs have significant potential for abrogating neuroinflammation, releasing trophic factors which decrease both the expression of pro-inflammatory cytokines and the proliferative faculties of T-cells (Leu et al., 2010, Jeon et. al. 2013, Valencia et al 2016). Moreover, intra-arterial delivery of AD-MSCs in an induced model of ischemic stroke attenuated inflammation and led to improved functional recovery, especially during the early phase of ischemia (Oh et al., 2015). In another study of AD-MSC transplantation, this time with the cells delivered intravenously, AD-MSCs were shown to significantly upregulate expression of the potent neuroprotective factors BDNF and TrkB (Li et al., 2016). AD-MSCs have also been shown to promote white matter repair in subcortical ischemic stroke via paracrine excretions, indicating a probable exosomic effect on inflammation (Otero-Ortega et al., 2015). AD-MSCs delivered via stereotactic injection in a MCAO mouse model improved spatial learning and memory, as well as exerting a significant effect on neuroinflammatory processes and improving neuronal survival (Zhou et al., 2015). Therefore, AD-MSCs should be investigated as an immunoregulator in future experimental models, with special attention paid to their acute and chronic effects on the inflammatory environment post-stroke.
Limitations
Despite the putative benefits of adipose-derived stem cells in treatment for stroke, they also may produce undesirable side effects. Research has demonstrated that adipose-derived stem cells can encourage the proliferation of existing cancer cells, specifically breast cancer (Eterno et al., 2014). In addition, an early study found that AD-MSCs transplanted in immunodeficient mice produced tumors at a frequency of 50% (Rubio et al., 2005; Gimble et al., 2007)
Future Directions
In order for adipose derived stem cells to be established as an effective and safe therapy for ischemic stroke, further studies must be conducted to firmly establish the relative risks of AD-MSCs with regards to their tumorigenicity and whether they produce extracellular signals that may encourage tumor formation (Gimble et al., 2007; Eterno et al., 2014). To this end, future studies must also make clear the mechanism of action by which AD-MSCs encourage therapeutic recovery and how these cells interact with complex systemic processes when administered in vivo (Gimble et al., 2007). It would greatly improve the targeted design of clinical trials using AD-MSCS if, for example, it was determined whether AD-MSCs exhibit therapeutic effects via their exosome or direct cell replacement. Moreover, fine details such as the optimal time of administration, route, and dosage have not been determined (Gutiérrez-Fernández et al., 2013). In addition, future research should examine how the functional benefits of AD-MSCs vary when they are harvested from different adipose-sources, as well as whether the gender of the source influences functional outcome, as some have reported (Minteer et al., 2012). Therefore, future studies should aim to further describe AD-MSCs, their secretory behavior, and their homing pattern upon administration so as to ensure this cell type is properly understood before clinical translation.
2.2.4.2. Breast milk
Mammary tissue represents another source of transplantable stem cells. Breastmilk-derived stem cells are capable of migrating to the lactating epithelium and into the breastmilk via the mechanical forces of breastfeeding (Cregan et al., 2007; Hassiotou et al., 2012). Stem cells in the breastmilk demonstrate characteristics similar to embryonic stem cells in terms of phenotype and morphology, and are capable of differentiating into cell lines from all three germ layers (Hassiotou et al., 2012). Easy access to breastmilk makes harvesting these stem cells straightforward and limits the need for invasive techniques. Generally, breast milk has been recognized for the immunologic and nutritive advantages it imparts to newborns, and new studies stand to illuminate the potential benefits that stem cells in the breastmilk may also provide. For patients, breastmilk-derived stem cells are promising due, in part, to the ease with which they are harvested (Dailey et al., 2013). Breastmilk-derived stem cells also have the potential for autologous transplantation (Dailey et al., 2013).
2.2.4.2.1. Critical Assessment: Breast milk-derived stem cells
Preclinical studies in stroke models (in vitro and in vivo)
Current data on breast milk-derived mesenchymal stem cells is scarce. The field has identified breast milk as a potential source of therapeutic stem cells, but the application of these cells in ischemic stroke models has not yet been initiated (Thomas et al., 2010; Hassiotou et al., 2012). We were able to locate only two preclinical studies involving breast milk-derived MSCs. In the first study, breast milk-derived MSCs were cultured in vitro to determine the identity of the growth factors they secrete (Kaingade et al., 2016). The study found that breast milk-derived MSCs display increased levels of VEGF and HGF secretion when cultured in human cord serum as compared to synthetic media (Kaingade et al., 2016). The results provide the first evidence as to the exosome of breast milk-derived MSCs, while providing recommendations for culture media that may enhance cell potency, suggesting that serum improves production of VEGF and HFG (Kaingade et al., 2016). The second study examined whether breast milk-derived MSCs could be made to differentiate into neural phenotypes in vitro. The researchers used multiple rounds of culturing and a diverse array of signaling factors, including fibroblast growth factor, endothelial growth factor, B27, and N2, to encourage differentiation (Hassiotou et al., 2012). The results suggest that breastmilk-derived stem cells can be induced to differentiate into all three neural lineages (oligodendrocytes, astrocytes, and neurons) and therefore can mimic the role of neural stem cells in the brain (Hassiotou et al., 2012). Because studies on breast milk-derived stem cells are limited, it remains to be resolved whether this cell type has notable effects on neuroiflammation. From the data available, we can postulate that if these cells, which can differentiate into endothelial-like cells, do regulate the immune environment post-stroke, such effects might arise via restoration of the BBB and its subsequent decreased permeability to circulating inflammatory cell types or general paracrine effects on pro-inflammatory immune sources (Borlongan et al., 2012; Garbuzova-Davis et al., 2014). In addition, because breast milk-derived stem cells can differentiate into astrocytes, which can produce pentraxin 3, a compound that promotes BBB integrity after ischemic stroke, breast milk-derived stem cells may have the capacity to encourage suppression of peripheral immune invasion (Shindo et al., 2016). To this end, an active component in breast milk, milk fat globule-EGF factor 8 protein, has anti-inflammatory effects in models of inflammatory bone loss, suggesting, perhaps, an a role for breast milk-derived components in abrogating neuroinflammation following stroke (Abe et al., 2014). However, evidence as to the anti-inflammatory effects of breast-milk derived stems cells in the brain remains to be elucidated.
Limitations
The clinical applicability of breast-milk derived stem cells is most limited by a lack of knowledge as to their therapeutic function in animal models. While these cells may have potential clinical applications for the treatment of ischemia, specifically in neonatal models, little is known regarding the restorative effects, efficacy, or safety of these cells in vivo (Dailey et al., 2013). As it stands, the optimal route of administration, dosage, timing, and mechanism of action of breast-milk derived stem cells in models of ischemic stroke remains unclear. Moreover, no research has established whether breast-milk derived stem cells can survive in a cerebral environment, cross the blood-brain barrier, or improve neurological recovery after stroke.
Future Studies
Future studies of breast-milk derived stem cells must address the current lack of knowledge as to the function, safety, and efficacy of this cell type in animal models of stroke. Before these cells can come under consideration for clinical trials, researchers must better understand the mechanism of action with which these cells may remediate ischemic insult, while also fully characterizing the potential adverse effects of this treatment. All told, breast-milk derived stem cells require much more time under laboratory scrutiny, namely in animal models of stroke, before they can be approved for clinical application.
2.2.4.3. Menstrual Blood
Stem cell researchers have also recently become interested in the cell population present in the endometrial lining discarded during the female reproductive cycle (Patel et al 2008, Borlongan et al., 2010). Menstrual blood-derived stem cells (MenSCs) have demonstrated multipotency and the capacity to secrete trophic factors, including neurotrophin-3, VEGF, and BDNF in response to oxygen glucose deprivation (OGD) in in vitro stroke models (Patel et al 2008, Borlongan et al 2010). It has also been found that co-culturing of MenSCs with rat primary neurons enhances the survival rates of both cells following OGD (Borlongan et al., 2010). Moreover, intravenous and intracerebral implantation of MenSCs into stroke rats not only improved host cell endurance, but also behavioral functions. (Borlongan et al., 2010). One major benefit of these cells is that they have been successfully tested in in vivo surgical MCAO rat models and did not require immunosuppressants, further reinforcing their potential for clinical translation (Borlongan et al., 2010).
2.2.4.3.1. Critical Assessment: Menstrual blood-derived stem cells
Preclinical studies in stroke models (in vitro and in vivo)
Stem cells derived from the endometrial lining present an appealing autologous model for the treatment of ischemic stroke, especially for female patients. However, few studies exist that characterize these cells in vitro or in vivo. Immunocytochemical assays of cultured menstrual blood reveal that they express embryonic-like stem cell phenotypic markers (Oct4, SSEA, Nanog), and when expanded in appropriate conditioned media, exhibit neuronal phenotypic markers Nestin and MAP2 (Patel et al., 2008, Borlongan et al., 2010). MenSCs cultured in vitro also display high clonogenic and proliferative potential, which are important advantages in the clinical application of this cell type (Xu et al., 2015). It has also been demonstrated that the adherent fraction of menstrual stem cells did not lose its karyotypic normality or develop tumorigenic potential even after 68 in vitro doublings (Meng et al., 2007). Furthermore, menstrual blood-derived stem cells exhibit multipotency and, as mentioned, secrete trophic factors such as VEGF, BDNF, and neurotrophin-3 in vitro after oxygen glucose deprivation (OGD) (Borlongan et al., 2010). In these studies, rat primary neurons were co-cultured with menstrual blood or its conditioned media; co-culturing improved the survival rate of primary neurons (Borlongan et al., 2010). Importantly, the transplantation of MenSCs via intracranial and intravenous routes without immunosuppression have both resulted in a significant reduction of behavioral and histological impairments in rat models of ischemic stroke as compared to controls (Borlongan et al., 2010). In a model of endometrial injury, transplanted MenSCs improved angiogenesis via via Akt and extracellular signal–regulated kinases (ERK) pathways (Zhang et al., 2016). However, the precise mechanism of action by which MenSCs influence the neuroenvironment remains unclear. Pertinently, whether MenSCs have anti-inflammatory effects in ischemic stroke has yet to be confirmed either in vitro or in vivo, but there is circumstantial evidence to suggest they may. As mentioned above, in a study by Borlongan and colleagues, transplantation of MenSCs intracerebrally or intravenously without immunosuppression produced an injury environment that showed no gross signs of neuroinflammation (visualized by Nissl and H&E stains), suggesting the possible influence of an immunosuppressive effect (Borlongan et al., 2010). In a model of inflammatory bowel disease, MenSCs exhibited a significant immunomodulatory and anti-inflammatory effect, regulating the movement of macrophages and NK cells, decreasing the number of immune cells, decreasing the concentration of pro-inflammatory cytokines IL-2 and TNF-α, and increasing the concentration of anti-inflammatory factors IL-4 and IL-10 (Lv et al., 2014). Moreover, since MenSCs share markers with known immunosuppressive MSCs, including CD29, CD44, CD73, CD90, CD105 and MHC-1, suggest there is evidence that future investigations into the anti-inflammatory profile of this cell type will reveal therapeutic benefits (Xu et al., 2015). Nonetheless, until future studies examine these properties, MenSCs cannot be considered a translationally relevant regulator of neuroinflammation in stroke.
Limitations
Currently, research into endometrium-derived stem cells is most limited by a lack of available literature as to the genetic and mechanistic profiles of populations of endometrial stem/progenitor cells and their differentiated progeny. There is evidence to suggest that endometrial cells do not differentiate in vivo and instead retain their stem cell marker, Oct4 (Borlongan et al., 2010). Therefore, their neuroprotective effects may depend on secretory factors of the endometrial stem exosome. However, without more definitive evidence characterizing the mechanism of action and cell identities of endometrial stem cell populations, the optimal role of this cell type in clinical therapy for ischemic stroke remains indistinct.
Future Directions
Future studies should be initiated to further describe the effects of endometrium-derived stem cell treatments in vivo. Preclinical studies of the optimal route, dosage, and timing of administration must be performed, making sure to test both acute and chronic regimes. Moreover, future literature should work to characterize the molecular mechanism and signaling pathways that support the therapeutic potential of these cell. As stated above, the intracerebral injection of stromal-like menstrual blood stem cells in an experimental model of ischemic stroke suggested these cells exert a neuroprotective effect, but tissue analysis revealed that cells migrated to non-injured areas as well as the area of infract, without evidence of differentiation, suggesting endometrial cells may not contribute cerebral rejuvenation through a model other than cell differentiation (Borlongan et al., 2010). Resolving these issues may expedite the translation of endometrium-derived stem cells from the laboratory to the clinic.
2.2.4.4. Dental Tissue
Several dental tissue-derived stem cells have been identified, including periodontal ligament stem cells, post-natal dental pulp stem cells (DPSCs), stem cells from apical papilla, and stem cells from exfoliated deciduous teeth (SHED) (Dailey et al., 2013). These cells exhibit capabilities similar to mesenchymal stromal cells: most notably, the facility to differentiate into multiple cell lines, including neural tissue, odontoblasts, and adipocytes (Leong et al., 2012, Dailey et al., 2013).
2.2.4.4.1. Critical Assessment: Dental tissue-derived stem cells
Preclinical studies in stroke models (in vitro and in vivo)
Stem cells in dental tissue, specifically human exfoliated deciduous tooth-derived cells and dental pulp-derived CD31−/CD146− stem/progenitor cells, show significant promise as an alternative cell source for stem cell treatments of ischemic stroke, as corroborated by the results of in vitro and in vivo models (Yamagata et al., 2012; Takanori et al., 2013; Song et al., 2015). Human dental pulp stem cells (hDPSCs) have been shown in in vitro studies to be amendable to induction toward neuronal cell types and can even differentiate successfully into dopaminergic neurons (Nosrat et al., 2004; Chun et al., 2016). DPSCs have been found to successfully form neurospheres, establish immature neural networks, and differentiate into neuronal lineages in vitro (Ellis et al., 2014). Moreover, hDPSCs secrete a robust suite of neurotrophic factors in vitro, including glial cell line-derived neurotrophic factor (GDNF), NGF, BDNF (Nosrat et al., 2004). In addition to these neurotrophic effects, hDPScs improve the robustness of co-cultured neurons and offer neuroprotection against insult, such as neurotoxin 6-hydroxy-dopamine (Nosrat et al., 2004). In another study, hDPSCs were observed to confer more significant cytoprotective effects on human astrocytes exposed to oxygen-glucose deprivation in vitro than bone-marrow derived mesenchymal stem cells in a dose-dependent manner (Song et al., 2015). These results indicated that hDPSCs reduce OGD-induced GFAP, nestin, and musashi-1 expression in ischemic astrocytes (Song et al., 2015). Furthermore, CM-hDPSCs, like hMSCs, opposed OGD-induced ROS production and interleukin-1ß upregulation (Song et al., 2015). In a study of the potential immunomodulatory effects of hDPSCs co-cultured with phytohemagglutinin activated CD3+, researchers concluded that hDPSCs can exert a potent effect on sources of neuroinflammation, with elevated expression levels of human leukocyte antigen G, HGF-β1, intracellular adhesion molecule −1, IL-6, IL-10, TGF-β1, vascular adhesion molecule-1 and VEGF detected in the co-culture systems (Demircan et al., 2011). Moreover, the in vitro study revealed that hDPSCs reduced the levels of pro-inflammatory cytokines, including IFN)-γ, IL-2, IL-6 receptor, IL-12, interleukin-17A, and TNF-α, expressed by phytohemagglutinin–CD3(+) T cells, while upregulating the expression levels of the anti-inflammatory cytokine, inducible protein −10 (Demircan et al., 2011). Finally, the study concluded that paracrine factors secreted by hDPSCs encouraged apoptosis in phytohemagglutinin–CD3(+) T cells over a 24hr period as compared to controls (Demircan et al., 2011). Together, these results highlight the potential for hDPSCs to suppress neuroinflammation and modulate local immune responses, properties which could significantly improve infarct repair post-stroke.
In animal models, dental tissue-derived stem cells have demonstrated the capacity to improve recovery after ischemic stroke. Dental pulp-derived cells secrete NGF, BDNF, and GNDF in vivo (Leong et al 2012). Accordingly, these cells have been used as paracrine promotors, increasing the survival of neurons in an animal model of spinal cord injury as well as helping to regenerate nerves in murine models (Nosrat et al., 2001). DPSCs grown on (SHED-derived conditioned media were administered intranasally in rats after they underwent MCAO and resultant cerebral ischemia (Takanori et al., 2013). DPSC transplants appeared to encourage the migration of NPCs from the SVZ to the peri-infarct area on days 6 and 16 (Takanori et al., 2013). Elevated levels of doublecortin, neurofilament H, neuronal nuclei, and rat endothelial cell antigen were also observed in the peri-infarct area, suggesting neurogenesis and vasculogenesis had occurred (Takanori et al 2013). DPCSs also significantly improved rat motor function and lessened infarct volume as compared to BM-MSC treatments (Takanori et al., 2013). A recent study by Leong and colleagues examined the effect of hDPSC transplantation on a rodent model of ischemic stroke, demonstrating that hDPSC-treated rats exhibited improved forelimb sensorimotor function 4 weeks after treatment as compared to controls (Leong et al., 2012). Importantly, DPSC cells survived in vivo and had begun to migrate toward the specific location of the cerebral lesion upon observation (Leong et al., 2012). Pertinently, intracerebral administration of SHED in a rat model of perinatal hypoxia-ischemia demonstrated that engrafted SHED could significantly reduce ischemia-induced neuroinflammation (Yamagata et al., 2012). SHED upregulated the expression of anti-inflammatory cytokines, IL-6 and IL-10, while downregulating the expression of pro-inflammatory cytokines, interleukin-1β and TNF-α (Yamagata et al., 2012). These data evincing a positive role in abrogating neuroinflammation, combined with the results of experiments whereby dental tissue-derived stem cells improve functional recovery following ischemic insult, suggests this cell type should be investigated as a tool for sequestering neuroinflammation in the hypoxic stroke brain.
Limitations
While dental tissue-derived stem cells have displayed promising results in current studies, the available literature on their effects is scarce. Current research suggests that dental tissue-derived stem cells exert a therapeutic effect through paracrine signaling rather than direct cell replacement (Nosrat et al., 2004; Demircan et al., 2011; Leong et al., 2012). However, because many other cell types, as discussed above, provide similar benefits by secreting neurotrophic and anti-inflammatory signals, it is difficult to see the advantage of dental tissue-derived stem cells over more accessible stem cells, like EPCs or iPSCs. This inaccessibility owes itself to the limitations inherent in harvesting cells from dental tissue, as compared to other, more ubiquitous tissue sources.
Future Directions
Future studies should work to characterize the mechanism of action, optimal administration route, and optimal time of administration for dental tissue-derived stem cells. Moreover, as investigations into mesenchymal stem cells have revealed, the source from which a specific stem cell is derived can influence the resultant effects of that stem cell sub-type. Therefore, future studies should examine if periodontal ligament stem cells, DPSCs), stem cells from apical papilla, and SHED differ in their functional effects (Dailey et al., 2013). Additionally, for this cell type to achieve clinical success, researchers should propose a protocol whereby these cells can be harvested from human donors and proliferated into an autological cell line in a practical fashion. Future studies may well bring dental tissue to the fore of stem cell therapy. However, the problem of the accessibility of the source tissue suggests this cell type might best be used as an alternative cell therapy.
2.2.4.5. Induced Pluripotent Stem Cells (iPSCs)
While in the past it was believed that stem cells were committed to a phenotype upon differentiation, recent investigations have revealed that stem cells can be manipulated to return to their pluri- or multipotent states (Dailey et al., 2013). For example, embryonic-like stem cells, through the process of retrograde manipulation by transfection of relevant transcription factors, can be regenerated from fibroblasts (Takahashi et al., 2006). This transfection process has also been utilized in models of neural stem cells, umbilical cord blood cells, adipose-derived stem cells, and placental mesenchymal stromal cells with results showing that the process enhances cellular potency in each cell type (Cai et al., 2010; Tat et al., 2010). The resulting cells are known as induced pluripotent stem cells (iPSCs).
One important advantage of retrograde conversion is the improved proliferative capabilities of precursor cells compared to mature cells. Several benefits have been observed in the transplantation of iPSCs to treat stroke, such as an increase in anti-inflammatory cytokines, a decrease in pro-inflammatory cytokines, enhanced sensorimotor functions, and a reduction in infarct size (Chen et al., 2010; Jiang et al., 2011). Despite these observed benefits, administration of iPSCs is not without potential complications. Similar to most stem cells, transplantation brings with it concerns of both tumorigenesis and immunogenicity. In the transfection protocol utilized to produce precursor cells, known oncogenic transcription factors are used (Dailey et al., 2013). As a matter of fact, undifferentiated iPSCS have demonstrated a higher incidence of tumorigenesis than other transplanted cells in ischemic brain tissue (Yamashita et a., 2011). In addition, iPSCs have also been known to provoke an immune response and eventual rejection, even when they are autologous (Zhao et al., 2011). Further research will need to be conducted to determine the risk-to-benefit ratio of iPSCs.
Despite the fact that iPSCS have demonstrated great potential for autologous cell-based treatments, extensive improvements in technology must be reached before iPSCS can become an effective treatment for acute stroke. Specifically, studies must demonstrate the viability of iPSCs following long periods of storage prior to injury, considering the amount of time it takes to develop the right amount of stem cells for a therapeutic injection. Furthermore, it is essential that any additional genetic alterations of iPSCs be closely regulated in the time following transplantation to prevent possible formation of ectopic tissue or tumors.
2.2.4.5.1. Critical Assessment: Induced Pluripotent Stem Cells
Preclinical studies in stroke models (in vitro and in vivo)
Induced pluripotent stem cells are an attractive candidates for the treatment of neurodegenerative disorders due to their capacity to differentiate into a wide array of relevant phenotypes, including neural precursor cells, neurons, and vascular endothelial cells, upon addition of induction factors such as retinoic acid (Yuan et al., 2013; Muffat et al., 2016). iPSC neural progenitor cells have been found to spontaneously differentiate into neurons and astrocytes in vitro, expressing high levels of β-tubulin and glial fibrillary acidic protein (GFAP) (Paşca et al., 2015). Since iPSCs can differentiate into neural stem cells, they also may have the potential to produce the therapeutic benefits of NSCs through the release of the same neuroprotective trophic factors. Moroever, iPSCs may work to reconstitute disturbed neural networks: mature, differentiated iPSCs produce functional sodium and potassium channels, fire action potentials, and express adult neuronal markers (Pasca et al., 2015). In terms of neuroinflammation, iPSCs may play a significant role. A recent in vitro study showed that iPSCs could differentiate into microglia, the primary immunoregulatory cell type in the brain (Muffat et al 2016). Moreover, a study by Hague and colleagues found that iPSCs could develop into antigen-specific regulatory T cells (Tregs), a T cell type involved in the suppression of autoimmunity (Haque et al., 2016). Therefore, iPSCs may mediate cerebral damage caused by over-expressed neuroinflammatory cell types. Notably, since NSCs sport such a robust differentiation profile of potential mature cell types, they have the capacity to express a similarly diverse suite of anti-inflammatory factors and may therefore be programmed to produce the most strategically relevant outcome. iPSCs have also been shown to improve motor function, reduce stroke volume, exhibit anti-inflammatory properties, promote neurogenesis, and encourage angiogenesis when administered in in vivo models of ischemic stroke (Yuan et al., 2013; Chua et al., 2014; Eckert et al., 2015). In a study by Chau and colleagues, the effects of iPSC administration via intracranial injection 7 days after MCAO were examined (Chua et al., 2014). The study found that differentiated iPSCs displayed mature neuronal markers, functional sodium and potassium channels, and were able to initiate action potentials in vivo (Chua et al., 2014). Moreover, the iPSC-NPCs observed in the cerebral environment expressed a variety of neurogenic and angiogenic trophic factors (Chua et al., 2014). iPSC-NPC transplantation resulted in a greater concentration of SDF-1α and VEGF in the peri-infarct region of treated animals (Chua et al., 2014). Moreover, iPSC-NPCs displayed positive staining for neuronal nuclei and glial fibrillary acidic protein (GFAP) up to 14 days after transplantation (Chua et al., 2014). Importantly, iPSC-NSCs have also exhibited significant anti-inflammatory properties in vivo. When iPSC-NSCs were engrafted into the ipsilesional hippocampus acutely (24 hrs after stroke), 48 hrs after stroke mice brains showed a reduction in levels of pro-inflammatory factors (TNF-αIL-6, IL-1β, MCP-1, MIP-1α), a decrease in the numbers of activated microglia, and restoration of the BBB (Eckert et al., 2015). These results, combined with the knowledge that iPSCs can be made to differentiate into most known cell types, suggest iPSCs can be tailored to treat neuroinflammation in the ischemic stroke brain.
Limitations
Tumorigenesis is the greatest risk to iPSC transplantation. Due to genomic instability, iPSCs bear a significant risk of developing into cancerous malignancies (Liang et al 2013). In two studies, iPSCs were shown to produce tridermal teratomas in the postischemic neural tissue of mice subjected to transient MCAO (Kawai et al., 2010; Yamashita et al., 2011). iPSCs were administered acutely in each of these cases and injected directly into the ipsilateral striatum and cortex. These results confirm that iPSCs display tumorigenicity in ischemic stroke models and suggest that the hypoxic environment post-stroke may actually enhance the risk of tumorigenesis for these cells. Another concern regarding iPSC-based therapies is the immunogenicity of iPSC cells. When iPSCs generated from B6 mouse embryonic fibroblasts formed teratomas, these teratomas were immune-rejected by B6 recipients (Zhao et al., 2011; Hao et al., 2014). This data suggests that there is a chance that autologous iPSCs may be met with host rejection via a T-cell mediated immune response.
Future Directions
Though iPSCs have demonstrated regenerative promise, if this cell type is to advance to the clinic, its potential for tumorigenesis must be addressed. Only when this property is controlled can the cell type ever hope to achieve translational success. For this reason, future research should focus predominantly on mediating iPSCs’ tumorigenic risks. In addition, iPSC studies should investigate the immunogenicity of cell types, and whether this property is influenced at all by the induction regime from which iPSC cell lines are generated (Zhao et al., 2011). However, if preclinical research can resolve this two issues, iPSCs exhibit incredible potential as mechanisms of cell therapy for stroke. Accordingly, when the risks of iPSC treatment can be abated or better characterized, clinical trials intended to advance the translation of iPSC therapy from the laboratory to the clinic should be enthusiastically encouraged.
3. Gene-Edited Stem Cells
Genetically modified stem cell lines provide an appealing answer to FDA requirements necessitating homogenous cell populations for human transplantation trials. Genetically modified cell lines are generated so as to proliferate clonally in vitro at a stable rate, allowing for the control of cell identities and the harvest of clinically applicable populations. However, the process of genetic manipulation required to create these homogenous stem cell populations presents an obstacle to receiving FDA clearance on account of potential issues surrounding the genetic stability of the cell line, including ectopic tissue formation and tumorigenesis (Borlongan, 2009).
3.1. NT2N
NT2N (hNT) cells are neurons derived from a clonal human teratocarcinoma cell line (NTera-2 or NT2) (Andrews et al 1984, Andrews et al 1998, Borlongan et al 2006). NT2N cells obtain a permanent postmitotic neuronal phenotype in vitro following retinoic acid treatment, while cultured and grafted hNT cells display a neuronal phenotype with the formation of operative synapsis in vivo and secretion of neurotransmitters (GABA, dopamine) (Pleasure et al., 1992, Dunlop et al 1998, Hartley et al 1999a, b, Lee et al 2000). These hNT neurons are model systems for human neurobiology research.
Studies have used grafted hNT neurons for stroke therapy in rodents (Borlongan et al., 1998a; Borlongan et al., 1998b; Saporta et al., 1999; Borlongan et al., 2006). A phase I clinical trial of grafted hNT neurons has also been reported, which indicated the feasibility and safety of neuron transplantation for patients with motor stroke (Hurlbert et al., 1999). In addition, a study by Nelson and colleagues “Clonal Human (hNT) Neuron Grafts for Stroke Therapy” explored the effects of grafted hNT neurons in one of these trial patients, a 71-year-old man with fixed motor deficits as a result of stroke. 2 × 106 hNT neurons 34 months after infarction were administered via stereotoxic implantation. Unlike 6 of the 12 patients in the trial who exhibited motor recovery, this patient displayed no motor improvements and died of a myocardial infarction 27 months following implantation (Nelson et al., 2002). The brain was removed and processed for neuropathological examination. The data reported confirm that grafted hNT neurons do not form a neoplasm and support that a number of hNT neurons did survive in the brain until his death. While limited previous studies exist to explain the neuropathology of human brain implants, these transplants were used in embryonic human neural cells and for neurodegenerative diseases. On the other hand, hNT cells are extensively researched clonal human neurons possessing many benefits. For example, they do not pose ethical or legal concerns as a human embryo is not involved. Also, unlike xenografts, hNT cells do not have known human pathogens or infectious vehicles. Furthermore, these cells are highly uniform and available in unlimited quantities manufacturing in accordance with procedures for human use, unlike cells cultured from living animals. Lastly, hNT neurons are responsive to genetic engineering, and have been well characterized in vitro as well as in animal models of stroke, Parkinson’s disease, Huntington’s disease, and trauma (Pleasure et al., 1992; Dunlop et al., 1998; Hartley et al., 1999a, b; Philips et al., 1999; Baker et al., 2000; Kondziolka et al., 2000; Lee et al., 2000). These distinct advantages denote hNT cells as a promising approach to treating neurological disorders.
3.1.1. Critical Assessment: NT2N
Preclinical studies in stroke models (in vitro and in vivo)
NT2N cells have been reported to proliferate and fully differentiate in vitro into neuron-like cells displaying large outgrowth processes and the expression neuronal phenotypic markers following treatment with retinoic acid and mitotic inhibitor (Pleasure et al., 1992; Dunlop et al., 1998; Hartley et al., 1999; Lee et al., 2000). Moreover, NT2N cells can be induced to form GABAergic and dopaminergic neuron-like cells in culture (Matsuoka et al., 1997 and Zigova et al., 2000). Furthermore, when NT2N-derived neurons proliferate in culture they form immature neural networks. However, these networks display relatively random firing patterns, as opposed to the synchronized bursts of networks formed by embryonic cortical neurons in culture (Gortz et al., 2004). The survival of NT2N cells increases when co-cultured with fetal astrocytes, suggesting NT2N cells are amenable to neurotrophic factors secreted by astrocytes (Tornatore et al., 1996). NT2N cells secrete neurotrophic factors as well, and, though the exact exosome of this cell type has yet to be characterized, studies have shown, for example, that NT2N cells express neuroprotective GDNF (Lin et al., 1999). Current preclinical studies have focused on optimizing the NT2N model via genetic modification to further improve its survival and therapeutic efficacy upon transplantation. Special attention has been paid to a specific genetic surrogate of the NT2N cell line, NT2N.Nurr1 (Hara et al., 2008). Nurr1 is a transcription factor that induces tyrosine hydroxylase expression, which encourages differentiation towards dopaminergic neural phenotypes (Hara et al., 2008). When NT2N cells are transfected with Nurr1 and treated with retinoic acid and mitotic inhibitors, they display an increased post-mitotic commitment to neural phenotypes and secrete elevated levels of GDNF in vitro (Hara et al., 2008). Pertinently, there is little evidence to suggest NT2N cells exert a significant effect on stroke-induced neuroinflammation. An early in vitro study suggested NT2N cells might secrete immunosuppressive factors, but subsequent research has yet to support or expand on these findings (Hara et al., 2008).
NT2N remain an appealing candidate for the in vivo treatment of neural disorders. Multiple studies of NT2N transplantation in animal models have demonstrated that NT2N cells can improve locomotor and cognitive function following experimental stroke (Borlongan et al., 1998a; Borlongan et al., 1998b; Phillips et al., 1999; Borlongan et al., 2006). Transplantation studies using NT2N.Nurr1 grafts to treat ischemic stroke ischemic stroke also suggest NT2N.Nurr1 may encourage more robust functional improvements than NT2N alone (Hara et al., 2007; Yang et al., 2009). While there is preliminary in vitro evidence to suggest NT2N cells produce immunosuppressive factors, in vivo results seem to suggest the functional outcome of NT2N engraftment is actually significantly improved following induced immunosuppression (Hara et al., 2008). That is to say, NT2N cells may not on their own be a valuable tool for the treatment of neuroinflammation and may depend on a reduction in neuroinflammation to achieve a viable survival rate. Neverthless, the expedited neural commitment observed in NT2N grafts suggests these cells may still have a role in stem cell treatment plans when co-administered with supportive factors.
Limitations
Concerns remain as to the efficacy and safety of NT2N cells. First, the tumorigenic risk of NT2N cells and the conditions that might encourage tumor formation haves yet to be fully characterized. Notably, the anatomical site to which NT2N cells are transplanted may mediate the neoplasticity of these cells (Miyazono et al., 1995). NT2 cells implanted in the sub-arachnoid space and the superficial neocortex proliferated and experienced apoptotic-like cell death, while exhibiting little to no ability to differentiate into neurons (Miyazono et al., 1995). These cells later formed bulky tumors that were lethal 70 days after transplantation. Similarly, NT2N cells transplanted into the lateral ventricles, liver, and muscle, formed large, lethal tumor 10 weeks after implantation (Miyazono et al., 1995). NT2N cells grafted onto the caudoputaminal region displayed reduced proliferation rates, no signs of necrosis, apoptosis, or tumor formation, and differentiated into post-mitotic immature neuron-like cells (Miyazono et al, 1995). The variability in how NT2N cells respond to the host’s tissue environments is a worrisome property of this cell type. Moreover, though multiple studies of NT2N cells have demonstrated functional improvements in animal models, clinical studies have yet to show comparable benefits (Borlongan et al., 1998a; Borlongan et al., 1998b; Phillips et al., 1999; Nelson et al., 2002).
Future Studies
Future studies of NT2N cells and their genetic derivatives should address the limitations listed above. Specifically, preclinical research should examine the neoplastic risk of NT2N implantation, with an eye to the way regional microenvironments might influence abnormal proliferation (Miyazono et al., 1995). Future studies should also investigate the clinical efficacy of NT2N transplantation, the optimal route and timing of administration, and more rigorously determine the optimal dosage. Preclinical studies should also determine whether the NT2N.Nurr1 augmented cell type is a superior model for transplantation, or if its effects are limited to the treatment of specific disorders. To this end, researchers might examine whether NT2N.Nurr1 transplantation produces more significant improvements when targeted in the striatum as compared to other areas of the brain, as suggested by the tendency of NT2N. Nurr 1 to differentiate towards dopaminergic phenotypes and the high proportion of dopaminergic neurons endemic to the striatal region (Hara et al., 2008). Finally, future clinical studies should be initiated to more accurately determine whether NT2N cells have worth as a treatment for human stroke patients, as the evidence currently available suggests they exert a negligible therapeutic influence.
3.2. CTX0E03
CTX0E03 is a genetically engineered cell from a clinical-grade conditional immortalizing human neural stem-cell line produced following transfection with a conditional immortalizing gene, c-mycER(TAM) (Stroemer et al., 2009). Dheeraj Kalladka and associates provide promising initial results of this gene therapy in their clinical trial, “Pilot Investigation of Human Neural Stem Cells in Chronic Ischemic Stroke Patients” (PISCES) in The Lancet, in which they evaluate the safety and efficacy of intracerebral administration of CTX0E03 (Kalladka et al., 2016). The PISCES trial was founded on rigorous preclinical evidence which demonstrated therapeutic potential in rats through improved behavioral results as well as neurogenesis and angiogenesis (Stroemer et al., 2009). The clinical trial designed by Kalladka and associates enlisted men age 60 years or older with chronic stroke who were administered single doses of various quantities of CTX0E03 cells (2 million, 5 million, 10 million, or 20 million) by stereotactic ipsilateral putamen injection (Kalladka et al., 2016). Immunosuppressive agents were not used in this study. 24 months following transplantation, the evaluated subjects displayed some neurological and functional improvements. No cell-related adverse effects were observed. While CTX0E03 cells might prove to have potential as a promising gene therapy approach, this clinical trial did have significant limitations, such as a long period after stroke onset before enrollment began and a considerably small patient pool, rendering their positive outcomes potentially inconclusive (Kalladka et al., 2016). Perhaps the use of placebo controls, such as sham transplantation with burr holes succeeded by rehabilitation therapy, would more effectively provide conclusive results on the safety and efficacy of this therapy (Kalladka et al., 2016). Additionally, close monitoring of the fate of the transplanted cells in stroke patients must also be strictly adhered to for reasons of safety in order to detect formation of cancerous cells or inflammatory responses and reveal adverse effects. Future preclinical studies of CTX0E03 will further demonstrate the potential of this specific gene therapy in treating stroke.
3.2.1. Critical Assessment: CTX0E03
Preclinical studies in stroke models (in vitro and in vivo)
CTX0E03 cells have demonstrated appealing therapeutic properties in vitro and in vivo animal models of stroke and have been proven biocompatible in human clinical trials (Kalladka et al., 2016). Using RT-PCR, Western blot, and ELISA, the angiogenic activity of CTX0E03 cells in culture was investigated (Hicks et al., 2013). The study confirmed that CTX0E03 express high levels of trophic and proangiogenic factors, including ANG-1, ANG-2, EGF, bFGF, HIF-1a, TGF-β1, and VEGF-A (Hicks et al., 2013). Moreover, an investigation into the quantification of the CTX0E03 exosome, demonstrated that it contains, among other molecules, miRNAs that may exert therapeutic effects (Stevanato et al., 2016). In addition, CTX0E03 cells improved human umbilical vein endothelial cell total tubule formation and average tube length in Matrigel tube formation assays as compared to controls, suggesting CTX0E03 encourage angiogenesis (Hicks et al., 2013). In vitro tests of CTX0E03 cells also demonstrate that they are notably resistant to the toxic effects of certain molecules that may be present in models of neurodegeneration, including Aβ1–4, okadaic acid, and a phosphatase 2A inhibitor (Puangmalai et al., 2015). By upregulating angiogenesis in the ischemic brain, CTX0E03 cells could thereotically help to reduce neuroinflammation by restoring the BBB and therefore slowing the delivery of pro-inflammatory immune cells (Borlongan et al., 2012; Garbuzova-Davis et al., 2014). However, angiogenic factors such as angiopoietin-2 can also exacerbate inflammation (Fiedler et al., 2006). Therefore, it remains to be determined in in vitro studies the precise extent to which and mechanism by which CTX0E03 mediate inflammatory conditions. CTX0E03 have been employed in in vivo studies to beneficial effect. In an effort to characterize the mechanism by which CTX0E03 encourages neural regeneration, Hassani and colleagues transplanted CTX0E03 in the brains of rat models of ischemic stroke one week and four weeks after reperfusion, and found that CTX0E03 engraftment encouraged the proliferation of endogenous doublecortin neuroblasts and CD11b+ microglial cells as compared to vehicle-based controls (Hassani et al., 2012). Another study by Pollock and colleagues recorded that the transplantation of CTX0E03 in an MCAO rat model produced significant functional locomotor improvements (Pollock et al., 2006). This study also demonstrated that CTX0E03 survival in vivo was not related to a high rate of proliferation, suggesting these cells are resilient to microenvironments in the stroke brain (Pollock et al., 2006). A dose-dependent experiment by Stroemer and colleagues examined how varying concentrations of CTX0E03 cells injected into the rat putamen would affect recovery (Stroemer et al., 2009). This study found that mid to high doses (45,000 to 450,000 cells) were enough to encourage the recovery of sensorimotor deficits and protect endogenous neurogenesis processes in the SVZ (Stroemer et al., 2009). Because cell survival did not correlate with more robust recovery, the authors surmised that primary regenerative benefits of CTX0E03 are expressed via a paracrine tropic mechanism (Stroemer et al., 2009). The proposed ability of CTX0E03 cells to sequester neuroinflammation in vivo appears to also be mediated via a paracrine mechanism, whereby CTX0E03 cells activate other cell types with more direct immunosuppressive effects. For example, CTX0E03 transplantation has been noted to encourage endogenous NSC proliferation in the SVZ and the proliferation of immunomodulatory CD11b+ microglial cells (Hassani et al., 2012). However, while CTX0E03 cells may produce an immunosuppressive effect in vivo, this property is not sufficiently characterized in the literature, especially to the extent it is for other cell types, implying that CTX0E03 cells should not be used to treat neuroinflammation at this time.
Limitations
The body research on CTX0303 is, at present, too limited to define how this cell type should best be employed as a potential clinical therapy. Notably, the mechanism by which CTX0E03 cells promote neural regeneration remains unclear. A study by Hicks and colleagues points to proangiogenic properties, including an increase in microvessels in the area of infarct following transplantation, while a study by Hassani and colleagues suggests conditionally proliferative microglia might mediate the induction of CTX0E03 cells towards neural phenotypes (Hassani et al., 2012; Hicks et al., 2013). The idea that CTX0E03 also upregulates endogenous neurogenesis via the release of NSCs from the SVZ has been recorded (Hasani et al., 2012). These results have each been presented individually, and there have been no contemporary studies that corroborate these conclusions in such a way as to permit comparability. Moreover, it has been documented that the efficacy of CTX0E03 engraftment may vary according to the implantation site and may also depend on the topology of the stroke legion, with, for example, CTX0E03 cells producing a far more significant therapeutic outcome for strokes that occur in the striatum (Smith et al., 2012).
Future Directions
Future research must focus on defining the regenerative mechanisms of CTX0E03 cells in experimental animal models before researchers can identify how best to employ CTX0E03 cells in the clinical setting. Moreover, future studies should examine the relationship between CTX0E03 cells and the site of their implantation, making sure to characterize areas where CTX0E03 cells may be less or more effective (Smith et al., 2012). Future studies should also work to characterize the optimum timing of CTX0E03 administration (acute or chronic), the ideal route of administration, and the most effective dosages.
3.3. SB623
SB623 cells are modified bone marrow-derived mesenchymal stem cells and were generated as an allogeneic cell therapy for chronic motor deficits caused by stroke. These cells are developed under appropriate manufacturing processes by transient transfection of an expression vector containing the human Notch-1 intracellular domain, a factor thought to promote differentiation toward astroglial phenotypes (Dezawa et al., 2004). The transfection is deemed transient due to the expansion and passing of cells which results in the loss of the transfected plasmid. A study by Steinberg and colleagues evaluated the safety and efficacy of surgical transplantation of SB623 cells (Steinberg et al., 2016). The clinical trial enlisted eighteen patients with stable chronic stroke in a 2-year, open-label, single-arm study. At least one treatment-emergent adverse effect occurred in all patients. Six of them experienced six severe adverse events, none of which related to the cell treatment (Steinberg et al., 2016). Immunosuppression was not utilized in this study. 12-month follow-up evaluations reported a significant improvement from baseline in the European Stroke Scale, National Institutes of Health Stroke Scale, Fugl-Meyer total score, and Fugl-Meyer motor function total score. Additionally, this study gave insight into the temporary survival of SB623 cells, suggesting that the secretion of supportive molecules rather than integration of transplanted stem cells may be more effective in achieving persistent neurological recovery (Steinberg et al., 2016).
However, this study had important limitations, including a small number of patients and a nonrandomized design. Conclusions drawn from this trial with regard to the general chronic stroke population should therefore be interpreted with caution while recognizing the variation in definitions as to what constitutes chronic stable stroke. Overall, the stereotactic implantation of SB623 cells performed by Steinberg and colleagues proved to be safe and well tolerated by patients with moderate adverse events. These findings indicate the safety of SB623 cells as a feasible approach using gene therapy to treat chronic complications from stroke.
3.3.1. Critical Assessment: SB623
Preclinical studies in stroke models (in vitro and in vivo)
SB623 cells display significant therapeutic properties in vitro models of ischemic stroke, which suggest these cells may have neurotrophic, angiogenic, and neuroprotective effects (Aizman et al., 2009; Tate et al., 2010; Dao et al., 2013). In a study by Aizman and colleagues, SB623 cells and human MSC cells were used to create an extracellular matrix on which embryonic rat brain cortical cells were cultured for three weeks (Aizman et al., 2009). Cortical cells cultured this way displayed 1.5 to 3 times higher metabolic activity compared to controls (cells cultured on a poly-D-lysine (PDL) matrix), and the MSC and SB623 cell-derived ECM exhibited a neuroprotective effect, leading to increased survival of neural cells following nutrient and growth factor insult (Aizman et al., 2009). Moreover, MSC and SB623-derived ECM encouraged more diverse differentiation, with embryonic cortical cells growing into neurons, astrocytes, and oligodendrocytes within it, with cells cultured on the control PDL matrix only developing into neurons (Aizman et al., 2009). Another study by Tate and colleagues reinforces the idea that SB623 cells exert a neuroprotective effect, as the presence of SB623 provided significant trophic support to and increased the survival rate of cortical neurons and cells in hippocampal slices cultured under OGD conditions representative of ischemic stroke (Tate et al., 2010). Finally, Dao and colleagues suggested SB623 cells could provide angiogenic benefits to a damaged brain through their identification of angiogenic paracrine factors secreted by SB623 cells in vitro and the observation that co-culturing endothelial cells with SB623 cells improved endothelial cell survival, proliferation, and vascular tube formation under serum-deprived conditions (Dao et al., 2013). SB623 cells have also been shown to exert a direct immunosuppressive effect in vitro, which provides promising evidence for their use as a therapy to sequester neuroinflammation following ischemic stroke (Dao et al., 2011). Though a small number of SB623 display senescent-like behaviors, for the most part, these cells all produce active immunomodulatory factors in vivo, suppressing human T cell proliferation in both the allogenic and xenogenic mixed lymphocyte reaction to an extent comparable with MSCs (Dao et al., 2011). Moreover, co-cultured SB623 cells encouraged the proliferation of anti-inflammatory IL-10 producing T cells and reduced monocyte-dendritic cell differentiation (Dao et al., 2011). In fact, SB623 cells inhibited the maturation of monocyte-dendritic cells to a greater extent than MSCs, suggesting SB623 cells may be an attractive candidate for the treatment of ischemia-induced neuroinflammation (Dao et al., 2011).
SB623 cells have received limited preclinical attention in in vivo animal models as a cell therapy for ischemic stroke. Though SB623 cells have been investigated in animal models to treat Parkinson’s disease and traumatic brain injury (TBI), we could not find any studies that examined the results of SB623 transplantation or injection in animal models of cerebral ischemia (Tajiri et al., 2014, Tate et al., 2015). However, it is useful to note that SB623 cells injected intracerebrally in a model of TBI were responsible for the formation of a biobridge between the neurogenic subventricular zone and the injured cortex, a pathway which recruits new host cells to the site of injury and a hitherto unknown mechanism for endogenous stem cell recruitment in neural repair models (Tajiri et al., 2014). The biobridge concept represents a novel paradigm in stem cell therapy, and the possible role SB623 cells may have in the formation of similar mechanisms in the ischemic brain therefore could represent an important therapeutic tool for stroke (Tajiri et al., 2014). Pertinently, the precise effects of SB623 cells on neuroinflammation have not been delineated in vivo. However, acknowledging the significant anti-inflammatory and immunosuppressive effects SB623 cells exert in vitro, future studies as to the ability of this cell type to sequester neuroinflammation in animal models may discover significant effects.
Limitations
The primary limitation to SB623’s translational potential is the lack of research on this cell type in animal models for stroke. It stands to be determined whether these cells are compatible in rodent models, the best method of administration, the optimal dosage, and the primary regions to which grafts should be targeted. Moreover, the mechanism of action for SB623 cells in vivo requires further clarification. It stands to be investigated how SB623 may interact with systems in the living organism. More importantly, it remains to be determined whether SB623 cells, as a derivate of MSCs, exhibit a significantly more valuable effect than standard MSC cell types. In addition, how SB623 cells may be influenced by adjunctive therapies has yet to be determined. Immunosuppressants exert therapeutic benefits by themselves as standalone therapy (i.e. cyclosporine A), even without stem cells, as shown by us and other groups (Tajiri et al., 2016; Osman et al., 2011; Lulic et al., 2011; Ishii et al., 2013; Furuichi et al., 2003). However, most of these therapeutic effects have been observed when immunosuppressants are delivered prior to or immediately after the brain insults in pre-clinical models of CNS disorders. Moreover, all the clinical trials of stem cells for stroke discussed in our paper did not use immunosuppressants in their patients. Even with the use of immunosuppression in the clinic, they are likely not to promote therapeutic benefits by themselves alone since the cell therapy regimen (albeit immunosuppression treatment) was initiated at post stroke periods.
Future Directions
Future research should focus on delineating exactly what functional benefits SB623 cells can provide in animal models of ischemic stroke. Future studies should examine the dosage, route, and timing of SB623 administration, as well as whether the cells are capable of surviving in the hostile microenvironments of ischemic host tissue. Moreover, it must be determined whether SB623 have tumorigenic properties and, if so, how to best mitigate these adverse effects. Future research might also look into how SB623 migrate in the body, whether they interact trophically or otherwise with other systems of the body, and how they are influenced by adjunctive therapies. Accordingly, current clinical trials of SB623 cells in human stroke subjects are premature. The laboratory science has yet to provide a robust definition of how SB623 cells operate in animal models, and thus future attempts to apply these cells in human models should be approached with necessary caution [Table 3].
Table 3.
Advantages and disadvantages of available stem cell types
| Cell Type | Advantages | Disadvantages | Prospective Clinical Use |
|---|---|---|---|
| Embryonic Stem Cells | Pluripotent High rate of proliferation Angiogenic and neuroprotective effects (Liu et. al 2014, Maya-Espinosa et al. 2015) Potentially therapeutic secretome under hypoxic conditions (Theus et al., 2008, Covacu et al. 2009, Okun et. al 2010) |
Ethical controversy (Shinozuka et al 2013) Risk of tumorigenesis (Kawai et al 2010, Dailey et al 2013) |
Subacute Chronic Intracerebral Allogeneic |
| Hematopoietic Stem Cells (HSCs) | Endogenous mobilization to site of ischemic injury suggests a natural reparative role (Shyu et al 2004, Ratajczak et. al 2012, Mocco et al 2014) Extent of mobilization correlates with functional recovery (Dunac et. al 2007) |
Limited cellular potency (Oguro et. al 2013) Largely heterogeneous population (Oguro et al 2013) Difficult to isolate and proliferate in clinically relevant amounts (Soffer-Tsur et al 2016) May encourage inflammation/adverse effects (Hsiao et al 2014, Bhatt et al 2015, Hilgendorf et al 2015, Kashara et al 2016) Low number of in vitro and in vivo studies |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Mesenchymal Stem Cells (MSCs) | Harvested from nearly any tissue (Mafi et al 2011, Li et. al 2016) Multipotent (Wang et. al 2016) Can be conveniently induced toward neural phenotypes (Tu et al 2014, Abdullah et al 2016, Joe and Cho 2016, Narcisi et al 2016, Shuai et al 2016, Yan et al 2016) Flexible therapeutic potential through genetic modification (Kurozumi et al. 2005, Horita et al. 2006, Onda et al 2009, Yasuhara et al 2009, Li et al 2016) Positive results in in vitro ischemia models (Bartholomew et al 2002, Zhong et al 2003, Duffy et al 2011, Kong et al 2016, Zimmermann, Hettiaratchi, and McDevitt 2016) Neuroregenerative neuroprotective results in in vivo models (Eckert et al 2013, Guihong et al 2016, Wang et al 2016) Significant suppressive effect on neuroinflammation (Liu et al 2015, Castro-Manrreza and Montesinos 2015) Safety record in both animal and clinical trials (Bang et al 2005, Savitz et al 2011, Eckert et al 2013, Banerjee et al 2014, Prasad et al 2014, Guihong et al 2016) Promote endogenous neurogenesis (Eckert et al 2013, Guihong et al 2016) Robust neurotrophic and restorative secretome (Eckert et al 2013, Chen et. al 2015, Shichinohe et al 2015) Multiple routes of administration (Castro-Manrreza and Montesinos 2015, Liu et al 2015, Eckert et al 2013, Guihong et al 2016) |
Further research must be conducted to determine tumorigenic risk (Karnoub et al 2007, McAndrews et al 2015) Tissue source can influence function (Hass et al 2011, Subramanian et al 2012) |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Endothelial Progenitor Cells (EPCs) | Multipotent (Hur et al 2004, Fadini et al 2012, Zhao et al 2013) Encourage angiogenesis in stroke models (Chen et al 2008, Li et al 2015) Potential to exert acute neuroprotection and provide chronic infrastructural repair (Zhao et al 2013) May help repair blood-brain barrier after ischemic stroke, reducing number of invading lymphocytes (Neuwelt et al 2011, Borlongan et al 2012, Wong et al 2013) |
Cell identities have not been adequately characterized (Hur et al 2004, Fadini et al 2012) Potential to stimulate atherosclerotic plaque formation in certain patients (George et al 2005) No direct mechanism reported by which they abrogate neuroinflammation (Liu et al 2010, Zhao et al 2013) EPCs have potential to promote neuroinflammation (Hur et al 2004, van der Strate et al 2007, Moubarik et al 2011, Zhao et al 2013 |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Very Small Embryonic-Like Stem Cells (VSELs) | Pluripotent, can differentiate into neurons, oligodendrocytes, and microglia (Kucia et al 2007, Havens et al 2014, Kassmer and Krause 2014) Can differentiate into a more robust hematopoietic cell than natural HSCs (Ratajczak et al 2011) VSEL concentrations in the blood are elevated following multiple categories of systemic insult, suggesting an endogenous neuroprotective and/or neurogenic role (Kucia et al 2008, Paczkowska et al. 2009, Bhartiya et al 2013) |
Difficult to harvest (Ratajczak et al 2012, Shin et al 2013) Time-intensive proliferation efforts required (Ratajczak et al 2012, Shin et al 2013) Harvestable VSEL concentrations decrease with age (Kucia et al 2006, Shin et al 2013) Potential to produce pro-inflammatory cytokines (Guerin et al 2015) Very few in vitro or in vivo studies examining therapeutic potential |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Neural Stem Cells (NSCs) | Multipotent (Shi et al 2015, Hou et al 2016) Demonstrated anti-inflammatory effect in animal models (Lee et al 2007, Huang et al 2014, Liu et al. 2014) Promote endogenous NSC proliferation (Zhang et al 2010) Angiogenic and neurogenic potential in ischemic brain (Burns et al 2009, Shen et al 2010, Zhang et al 2010, Shinozuka et al 2013, Wang et al 2016) |
May be intolerant of inflammatory and some hypoxic conditions (Santilli et al 2010, Takata et al 2012) Harvesting may necessitate invasive surgery (Shinozuka et al 2013) May require induction from fetal line (Burns et al 2009, Shinozuka et al 2013) Risk of tumorigenesis (Shinozuka et al 2013) |
Subacute Chronic Intracerebral Allogeneic |
| Extraembryonic Stem Cells* *As MSC-derivatives, extraembryonic stem cells share many advantages and disadvantages with MSCs. |
Can be induced toward endothelial and neuronal phenotypes (Li et al 2012, Martini et al 2013; Jin et al 2015, Makhoul et al 2016, Sabry et al 2016) Promote neurogenesis, neuroprotection in models of ischemic stroke (Chen et al 2013, Zhang et al 2014, Liang et al 2016) Inhibit immune cell migration to are of infarct and exhibit anti-inflammatory effect (Pimentel-Coelho et al 2012; Zhang et al 2014, Liang et al 2016) Robust neuroprotective secretome (Chen et al 2013) |
Heterogeneous cell populations (Cell source may influence cell function (Kranz et al 2010) Route of administration may significantly influence therapeutic potential (Lim et al 2011) |
Subacute Chronic Intracerebral Allogeneic |
| Adipose-Derived Stem Cells* *As MSC-derivatives, adipose-derived stem cells share many advantages and disadvantages with MSCs. |
Abundant and can be harvested with minimally invasive procedures (Puissant et al., 2005; Tobita et al., 2011) Greater proliferative capacity than BM-MSCs (Ikegame et al 2011) Produce more VEGF and hepatocyte growth factor (HGF) than BM-MSCs (Ikegame et al 2011) Multipotent, with neuronal and vascular cell potential (Planat-Benard et al 2004, Ikegame et al 2011) Neurogenic and neuroprotective effects in animal models of ischemic stroke (Kim et al., 2007; Ikegame et al 2011; Gutiérrez-Fernández et. al 2015; Otero-Ortega et. al 2015) May promote angiogenesis (Moon et al 2006, Ikegame et al 2011)) Immunmodulatory and anti-inflammatory effects in vitro and in vivo (Leu et al., 2010, Jeon et. al. 2013, Oh et. al 2015, Zhou et. al. 2015, Valencia et al 2016) |
Risk of tumorigenesis and/or paracrine promotion of tumor proliferation (Rubio et al 2005, Gimble et al 2007, Eterno et al 2014) Mechanism of action remains to be determined (Gimble et al 2007, Gutierrez-Fernandez et al 2013) |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Breastmilk-Derived Stem Cells* *As MSC-derivatives, breastmilk-derived stem cells could potentially share advantages and disadvantages with MSCs, but further study is required to adequately characterize this cell type. |
Potentially pluripotent (Hassiotou et al 2012) Simple to harvest (Dailey et al 2013) Potentially therapeutic exosome (Kaingade et al 2016) |
Too few studies both in vitro and in vivo to render significant conclusions as to their clinical applicability or therapeutic efficacy | Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Menstrual Blood-Derived Stem Cells* *As MSC-derivatives, menstrual blood-derived stem cells could potentially share advantages and disadvantages with MSCs, but further study is required to adequately characterize this cell type. |
Multipotent (Patel et al 2008, Borlongan et al 2010) High clonogenic and proliferative potential (Meng et al 2007, Xu et al 2015) Potential immunomodulatory and/or immunosuppressive profile (Borlongan et al 2010, Lv et al 2014, Xu et al 2015) Potential angiogenic effects (Zhang et al 2016) Improve functional recovery after transplantation in ischemic stroke (Borlongan et al 2010) |
Too few studies to render significant conclusions No neuroinflammatory modulation has been directly characterized in vivo |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Dental Tissue-Derived Stem Cells | Multipotent, differentiate into neuronal lineages in vitro (Ellis et al 2014) Provide neuroprotective effects in vitro (Nosrat et al 2004; Song et al 2015) Immunmodulatory and anti-inflammatory effects in vitro (Demircan et al 2011) Stimulate endogenous NPC mobilization, angiogenesis, and neurogenesis in animal models and promote functional recovery (Leong et al 2012, Takanori et al 2013) Potential to abrogate ischemia-induced neuroinflammation in vivo (Yamagata et al 2012) |
Limited published research on cell type/subtypes Practical concerns related to accessing dental tissue source for clinical setting as compared to ease of access provided by more pervasive tissue sources |
Acute Subacute Chronic Intravenous Intra-arterial Autologous Allogeneic |
| Induced Pluripotent Stem Cells (iPSCs) | Pluripotent (Yuan et al 2013, Muffat et al 2016) Differentiate into mature neurons, as well as microglia and astrocytes in vitro (Paşca et al 2015, Muffat et al 2016) Potential to differentiate into immunoregulatory cell types (Haque at el 2016) Improve functional recovery and reduce infarct volume in stroke models (Yuan et al 2013, Chua et al 2014, Eckert et al 2015) Encourage neurogenesis and angiogenesis in ischemic brain (Chua et al 2014) Anti-inflammatory properties in vivo (Eckert et al 2015) |
Significant risk of tumorigenesis (Kawai et al 2010, Yamashita et al 2011, Liang et al 2013) Risk of immunogenicity/host rejection (Zhao et al 2011) |
Acute Subacute Chronic Intravenous Intra-arterial Autologous |
| NT2N (hNT) | Multipotent, with ability to differentiate into several neuronal phenotypes, including dopaminergic neurons (Pleasure et al 1992, Matsuoka et al., 1997, Dunlop et al 1998, Hartley et al 1999a, b, Lee et al 2000, Zigova et al., 2000, Hara et al 2008) Transplantation improves locomotor and cognitive function following ischemic stroke (Borlongan et al 1998a, Borlongan et al 1998b, Phillips et al 1999) Nurr.1 transfection may improve efficacy of therapeutic benefits (Hara et al 2007, Yang et al 2009) |
Tumorigenic risk (Miyazono et al 1995) May be incompatible with transplantation in certain brain regions (Miyazono et al 1995) No evidence of substantial neuroinflammatory effects May be susceptible to inflammatory environments (Hara et al 2008) Inconclusive clinical results (Nelson et al 2002) |
Chronic Intracerebral Allogeneic |
| CTX0E03 | Produce significant levels of angiogenic trophic factors in vitro (Hicks et al 2013) Potential to promote angiogenesis, endogenous neurogenesis, and functional recovery in models of ischemic stroke (Stroemer et al 2009, Hassani et al 2012) Resistant to toxic molecules associated with neurodegeneration (Puangmalai et al 2015) Safety and positive results recorded in clinical trial (Kalladka et al 2016) |
Few studies in vitro or in vivo to render significant conclusions No evidence as to anti-inflammatory or immunomodulatory effects Efficacy may be mediated by region of transplantation in brain (Smith et al 2012) |
Chronic Intracerebral Allogeneic |
| SB623 | Neuroprotective effect in vitro (Aizman et al 2009, Tate et al 2010) Potential to encourage angiogenesis (Dao et al 2013) Anti-inflammatory and immunosuppressive effects in vitro (Dao et al 2011) Potential as a therapeutic tool to encourage biobridge formation in models of ischemic stroke, though no studies have been initiated (Tajiri et al 2014) Clinical trial showed some functional improvements (Steinberg et al 2016) |
Very few studies examining effects in vivo, with no studies found that examined effects in models of ischemic stroke Emergent adverse effect reported in clinical trial (Steinberg et al 2016) | Chronic Intracerebral Allogeneic |
In summary, different types of stem cells were presented in this paper from embryonic to engineered stem cells. Briefly, we noted that embryonic/fetal stem cells have long been considered the yardstick of “stemness” as they are pluripotent and multipotent. However, these cells raise significant ethical concerns regarding their controversial source, as well as logistical issues related to their high risks of tumorigenicity. Next, the adult differentiated stem cells are defined as stem cells from adult tissues, including hematopoietic stem cells, (HSCs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), very small embryonic-like stem cells (VSELs), brain derived-neural stem cells (NSCs), extraembryonic stem cells, adipose tissue-derived stem cells, menstrual blood-derived stem cells, breast milk-derived stem cells and dental tissue-derived stem cells. Furthermore, we discussed induced pluripotent stem cells (iPSCs) harvested from adult differentiated cells that have been manipulated to revert back to their pluri- or multipotent states (Dailey et al., 2013). Next, among the engineered stem cells are NT2N, CTX0E03, and SC623 cells. NT2N cells are neurons derived from a clonal human teratocarcinoma cell line (NTera-2 or NT2) and they are considered neural progenitor cells (Andrews et al 1984, Andrews et al 1998, Borlongan et al 2006). CTX0E03 cells are neuron-like cells derived from fetal brain tissues and they are genetically engineered to achieve a clinical-grade conditional immortalizing human neural stem-cell line via transfection with a conditional immortalizing gene, c-mycER(TAM) (Stroemer et al., 2009). SB623 cells are neuron-like cells from modified bone marrow-derived mesenchymal stem cells, which are developed by transient transfection of an expression vector containing the human Notch-1 intracellular domain, a factor considered to promote differentiation toward astroglial phenotypes (Dezawa et al., 2004) [Figure 1].
Figure 1.
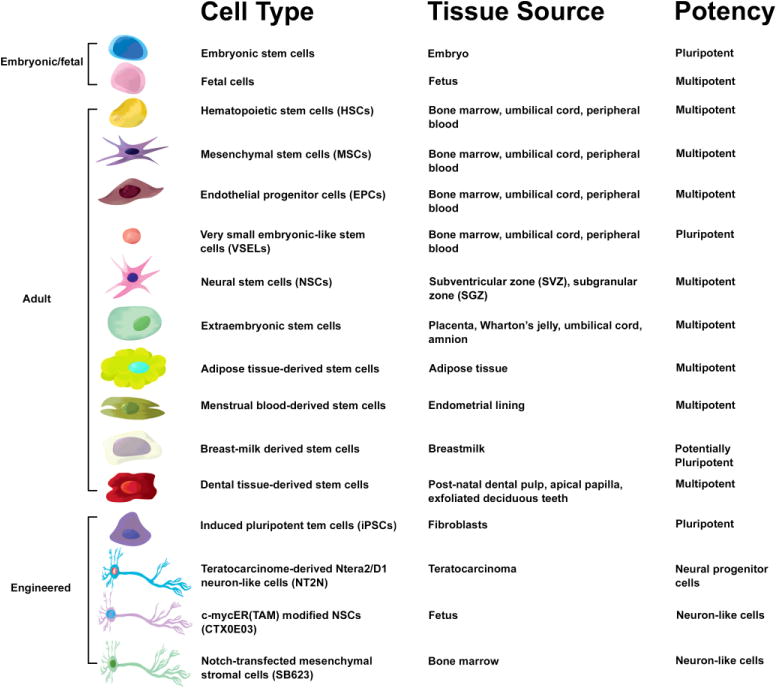
A visualization of currently available stem cell types, their source tissues, and potency. An artist’s rendering of each of the cell types discussed in this paper is presented along with information denoting its tissues sources and the potential of the cell to differentiate into mature cell lines.
4. “And the Oscar Goes To…”: Bone Marrow-Derived Mesenchymal Stem Cells Are the Optimal Model for Translational Research
In light of the advantages and disadvantages of available stem cells, bone marrow-derived mesenchymal stem cells have emerged as the leading transplantable cell type for CNS disorders, including stroke. This reputation is primarily owed to their adult tissue origin, comparative availability, proven safety profile, neuroprotective and regenerative effects, and extensive characterization in scientific literature. BM-MSCs also represent an appealing model for translational stroke research because they exhibit the capacity to differentiate into various cell lineages, including neural cells and vasculature, in vitro and in vivo. The current movement towards accepting BM-MSCs as a key cell type in the creation of clinical cell therapies echoes encouraging results from animal studies in which BM-MSC implantation encouraged functional recovery, including reduction in brain damage and improvements in motor and cognitive performance. Although the exact mechanism behind the neuroprotective and neurorestorative effects of BM-MSC transplantation remains unresolved, current evidence points to a few potential explanations, including reinnervation by direct cell replacement, growth factor secretion by BM-MSCs or their neurotrophic exosomes, and induction of endogenous brain repair processes, including neurogenesis, angiogenesis, and synaptogenesis. Nonetheless, in the clinical trials we cited here, further characterization of the mechanisms of action of stem cells are not well-defined likely due to specific study design limitations that prevent such mechanism-based analyses, i.e., under-powered trials. While early hypotheses as to the restorative mechanism of stem cell implantation concentrated on visualizing cell effects through the lens of a reductionist ligand-receptor model, we now understand that the scientific reality is more complex. A myriad of operational factors, from the regulation of signaling molecules to the stimulation of endogenous neurogenesis, may act at one time to produce a therapeutic outcome. The complexity of the stroke disease process in view of the multiple regenerative functions of stem cells explains why solitary cell therapies may not function effectively to treat ischemic stroke. Instead, adjunctive treatments, including pharmaceutical administration, the use of biomaterials, and the transplantation of additional cell types, may be essential to ensuring the optimal therapeutic profile for patients undergoing cell therapy, as they have encouraged functional recovery in similar models of inflammatory neurodegeneration (Borlongan et al., 1996; Saporta et al., 1997; Zigova et al., 1999; Borlongan, 2000; Nishino and Borlongan, 2000; Liu et al., 2014; Lozano et al., 2015; Kaelber et al., 2016, Mashkouri et al., 2016).
4.1. Not Quite the Whole Nine Yards: Investigating BM-MSC Efficacy
BM-MSCs are a particularly appealing model for stroke therapy and these cells exhibit promising results in animal models. However, significant questions still remain regarding the efficacy of BM-MSCs as a clinical tool. Animal studies have provided varied accounts as to the functional effects of BM-MSC transplantation, from trophic support to endogenous stem cell recruitment. Ultimately, laboratory efforts intend to transfer BM-MSC therapy from bench to bedside. Therefore, we will summarize recent clinical trials of BM-MSCs in human subjects, considering the functional results of these studies, their cross-comparability, and what they suggest about the future of BM-MSC-based cell therapies.
3.6.2.1. From the perspective of the clinic: Intravenous MSC administration for acute stroke and intracranial transplantation as a chronic treatment
In limited clinical trials of acute stroke, intravenous administration of autologous BM-MSCs has been tested and found to be completely biocompatible. Moreover, delayed autologous transplantation (initial infusion at 4 weeks after disease onset) of 100 million MSCs (SH-2 and SH-4 positive) in five stroke patients produced no adverse effects and appeared to improve neurological outcomes, according to the Barthel index and Rankin scale (Bang et al., 2005). However, these functional benefits effectively diminished by 12 months post-transplantation (Bang et al., 2005). A similar autologous intravenous bone marrow transplantation that delivered 7–10 million per kilogram of BM-derived mononuclear cells (MNCs), a cellular derivative of BM-MSCs, acutely (24 and 72h after stroke) resulted in more marked improvements in the Barthel index, modified Rankin scale, and National Institutes of Health Stroke Scale (NIHSS) with no adverse effects over a 6-month period in the majority of patients that received the transplant (Savitz et al., 2011). However, in response to the findings of this initial bone-marrow derived MNC stroke trial, a phase II, multicenter, parallel group, randomized trial with blinded outcome assessment of 120 patients was conducted in India (Prasad et al., 2014). Subjects did not receive immunosuppressive agents in this study. Stroke patients (n = 58) in this study who received a mean of 280.75 million MNCs at a median of 18.5 days after stroke onset showed no difference in Barthel index score, modified Rankin scale shift analysis, NIHSS score, and infarct volume as compared to non-transplanted stroke patients at 6-months post-transplantation (Prasad et al., 2014). This randomized trial suggests that intravenous transplantation of MNCs, while safe, does not appear to be an effective therapy for subacute stroke. Another stroke trial examined the therapeutic potential of a smaller subpopulation of CD34+ bone marrow MNCs (Banerjee et al., 2014). Intra-arterial delivery of 100 million autologous, immunoselected CD34+ stem/progenitor in stroke patients (n=5) presenting within 7 days of the onset of sever anterior ischemic stroke (NIHSS score of ≥8) produced improvements in modified Rankin scale and NIHSS score in addition to a decrease in lesion volume during a 6-month follow-up period (Banerjee et al 2014). Since the procedure also produced no adverse effects, this study strengthened evidence suggesting intra-arterial delivery of bone marrow-derived MNC CD34+ cells is a safe therapy (Banerjee et al., 2014).
A critical evaluation of the results of these clinical trials reveals that transplantation of BM-MSCs and their cellular derivatives, such as MNCs, is a biocompatible procedure for stroke patients. The efficacy of these procedures, however, warrants further investigation, especially in light of the small sample size and open-label designation of these studies (with the exception of the Prasad study). Moreover, a detailed review of the study methods reveals disparities across trials that would complicate cross-study comparisons. More disappointing, however, is the evident disconnect between the laboratory and clinical transplant regimes, particularly in terms of the cell identities chosen for transplantation. These clinical trials differed in the donor cells each employed and, as we have noted, the type of donor cell can significantly impact the functional outcome of cell therapy. Bang and colleagues used Src homology 2 and Src homology 4-type cells, whereas Savitz and collaborators employed a considerable consortium of antibodies (CD3, CD14, CD16, CD19, CD20, CD34, CD45, CD56, Lin 1, CD133-2) for flow cytometry to define the MNCs used in their study (Bang et al., 2005; Savitz et al., 2011). While Prasad and co-workers also employed flow cytometry to identify MNCs, they focused only on CD34 and CD45 antibodies (Prasad et al., 2014). On the other hand, Banerjee and colleagues used a magnetic cell isolation procedure to harvest only purified CD34+ cells (Banerjee et al., 2014). The apparent variability of the donor cell materials utilized in each trial unfortunately renders cross-comparisons inconclusive. In addition, each trial also differed in the timing of intervention: 4 weeks, days 1–3, 18.5 days, and within 7 days of stroke onset for the Bang, Savitz, Prasad, and Banerjee trials, respectively (Bang et al., 2005; Savitz et al., 2011; Banerjee et al., 2014; Prasad et al., 2014). Moreover, the route of delivery was inconsistent, with Bang, Savitz, and Prasad using intravenous and Banerjee using intra-arterial (Bang et al., 2005; Savitz et al., 2011; Banerjee et al., 2014; Prasad et al., 2014). However, one of the most disappointing parameters in each of these clinical trials was the functional dosage employed. Preclinical studies of many stem cells, including BM-MSCs, suggest the effective dose range for intravenous delivery is about 4 million cells in a 250 g rat or about 840 million cells for a 75 kg human being (Diamandis and Borlongan, 2015). This means that the dose in these clinical trials was significantly lower than the threshold value needed to recognize any efficacy read-out; 100 million, an average of 600 million, 100 million, and 280.75 million were used in the Bang, Savitz, Banerjee, and Prasad studies, respectively. (Bang et al., 2005; Savitz et al., 2011; Banerjee et al., 2014; Prasad et al., 2014). Savitz’s trial is an exception in that the employed dose closely approximated the recommended preclinical dose (Savitz et al., 2011). While patients in Savitz’s trial showed clinical improvement, these results should be interpreted cautiously as the study was an open-label trial (Savitz et al., 2011). For each donor type used in the described clinical trials, except for that of Savitz’s group, an assessment of the literature reveals few reported studies characterizing the safety, efficacy, and mechanism of action of these cells according to the Stem cell Therapeutics as an Emerging Paradigm for Stroke (STEPS) lab-to-clinic translational guidelines (Diamandis and Borlongan, 2015). All told, this suggests that future studies to ascertain the clinical application of stem-cell therapies will better succeed by adhering to STEPS guidelines and ensuring that laboratory science forms the basis of the clinical trial design (Borlongan, 2008, Borlongan, 2009, Diamandis and Borlongan, 2015).
Recent studies have shed new light on the cross-talk between endogenous stem cells or grafted stem cells and cells from the immune system (Kokaia et al., 2012; Morganti et al., 2015; Grotenhuis et al., 2016; Krampera et al., 2003). These studies suggest that the regenerative medicine seems heavily influenced by both cell-autonomous and non-cell autonomous mechanism, which are controlled by infiltrating circulating populations of innate (microglia, monocytes, monocyte-derived macrophages) and adaptive immune cells (B and T cells) (Kokaia et al., 2012). Certainly, there is a wide spectrum of concurrent immune responses within the infiltrating circulating population of the immune system including both pro- and anti-inflammatory phenotypes after injury, which will elicit a response in the grafted stem cells population (Morganti et al., 2015). In particular, MHC II phenotype increases collagen deposition and proliferation and gene expression of MMP1 (stimulate cells migration), PLOD2 (critical for stability of intermolecular crosslinks) and PTGS2 (modulate the inflammatory immune response) when MHCII+ macrophages were co-cultured with adipose mesenchymal stem cells (Grotenhuis et al., 2016). Adaptive immunity presents a different barrier for stem cells to be able to rescue inflammation-associated pathologies (Krampera et al., 2003). Despite host immune tolerance to stem cells, T cells or NK cells may recognize stem cells (i.e. neural stem cells) due to MHC I expression on the cell surface, leading to classical immune mediated cell cytolysis (Krampera et al., 2003). However, MSCs are able to modulate the T-cell response of naive and memory T cells even in the absence of antigen-presenting cells (APCs) or of CD4+/CD25+regulatory T cells in MSC culture (Krampera et al., 2003). Whereas the mechanisms modulating the cross-talk between immune cells and stem cells are still vague, these interactions can certainly be biphasic showing both beneficial and detrimental effects for stem cell survival and for the bystander secretion of trophic factors. Clearly additional studies are warranted to probe these stem cell-immune system interactions.
5. Conclusion
Stroke remains the third leading cause of death in the majority of developed countries, thereby posing a significant unmet clinical need with regard to effective treatment for both its acute and chronic stages (Borlongan et al., 2004). Ischemic stroke, the most prevalent class of stroke, induces acute neuroinflammation that can exacerbate the initial brain damage, but similarly achronic and systemic neuroinflammation can greatly encourage secondary cell death (Broderick et al., 1993, Borlongan et al., 2012, Dailey et al., 2013, Jin et al., 2013). Finding a treatment that ameliorates harmful inflammatory responses during these periods after the onset of stroke may present a novel therapeutic modality for stroke. Along this line of stroke therapeutics, stem cell therapy is uniquely poised to afford anti-inflammatory effects. In this review, we defined and summarized the advantages and disadvantages of clinically available cell types, including embryonic stem cells, HSCs, BM-MSCs. EPCs. VSELs, NSCs, extraembryonic stem cells, adipose tissue-derived stem cells, breastmilk-derived stem cells, menstrual blood-derived stem cells, dental tissue-derived stem cells, iPSCs, NT2N, CTX0E03, and SB623. Through a critical review of the available literature, we provide evidence that MSCs appear as an appealing stem cell candidate when contemplating treatment strategies designed to sequester neuroinflammation. The bulk of studies employing BM-MSCs as a transplantable cell source for hematologic diseases demonstrates positive outcomes without adverse effects. Moreover, BM-MSCs have multiple logistical advantages, including the ease with which allogenic and autologous samples can be harvested, their potential for diverse differentiation, their compatibility with multiple methods of administration, and their robust neurogenic, angiogenic, and restorative exosomes. The ability of BM-MSCs to induce brain neuroprotection and regeneration, as well as behavioral recovery following stroke has been documented in many in vitro and in vivo models (Lai et al., 2010; Guihong et al., 2016; Kong et al 2016). Moreover, in limited clinical trials of acute stroke, both intravenous administration of autologous BM-MSCs and delayed autologous transplantation have been found to be completely biocompatible and, in some cases, to improve neurological outcomes (Bang et al., 2005; Savitz et al., 2011; Banerjee et al., 2014; Prasad et al., 2014). Similarly, chronic stroke patients who received intracerebral transplantation of BM-MSC-related genetically modified cells tolerated such grafts with positive readouts towards efficacy (Steinberg, 2016). For these reasons, BM-MSCs appear as the most clinically relevant cell type at this time for stroke. However, as we noted above, certain cell types for which there is accumulating compelling evidence of safety and efficacy, and novel regenerative processes, beyond abrogation of neuroinflammation, may complement the therapeutic action of BM-MSCs. To this end, we encourage researchers and clinicians to consider the STEPS guidelines when designing future studies, and to ensure that laboratory science always informs the clinical application of regenerative therapies (Borlongan, 2008; Borlongan, 2009; Diamandis and Borlongan, 2015). A consortium of scientists and clinicians should strive to advance laboratory findings towards addressing unmet clinical needs for stroke in the most expeditious manner without sacrificing the scientific mandate of demonstrating the safety, efficacy, and mechanism of action of stem cell therapy.
Figure 2.
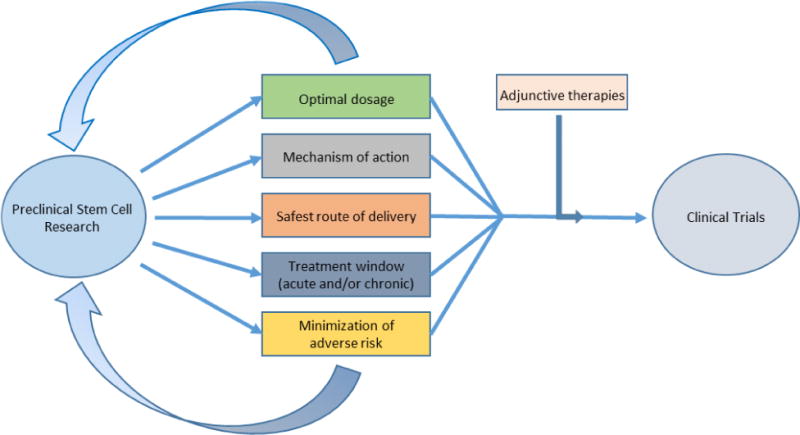
Modeling the optimal lab-to-clinic translational process. A visualization of the translational process as recommended by the STEPS guidelines, with preclinical research directing and informing clinical studies. [Adapated from Diamandis and Borlongan 2015.]
Table 1.
Significant neuroinflammatory mediators
| Inflammatory Mediator | Family | Types | Produced By | Role |
|---|---|---|---|---|
| Cytokines | Pleiotropic polypeptides (glycoproteins) | tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-20, IL-10 and transforming growth factor (TGF)-β. | Microglia Astrocytes Neurons and Endothelial cells Invading leukocytes |
Neuroinflammation (TNF-α, IL-1β, IL-6, IL-20) Neuroprotection (IL-10 and TGF-β.) |
| Chemokines | Small cytokines (classified into subgroups according to variations in cysteine residues) | monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and fractakline | Microglia Astrocytes Injured neurons |
Pro-inflammatory as chemoattractants for invading leukocytes |
| Cellular adhesion molecules (CAMs) | Cell surface proteins (often transmembrane receptors) | Immunoglobulin superfamily (IgSF), integrins, cadherins, selectins | Endothelial cells Epithelial cells Fibroblasts Leukocytes |
Pro-inflammatory by facilitating extravasation of invading leukocytes |
| Reactive oxygen species | Free oxygen radicals | superoxide anion radical (O2·−), singlet oxygen (1O2), hydroxyl radical (·OH) and perhydroxyl radical (HO2·), nitric oxide (NO) | Neuronal, endothelial* and inducible NO synthases (n-, e-, iNOS respectively), Oxidative imbalance | Ischemic cell death *Endothelial NO production can have a neuroprotective effect |
| Matrix metalloproteases | Zinc-containing endopeptidases | MMP-2 (gelatinase A) and MMP-9 (gelatinase-B) | Endothelial cells Neutrophils Macrophages |
Pro-inflammatory via degradation of BBB to facilitate invasion of peripheral leukocytes |
| Regulatory T cells | Lymphocytes | CD4+CD25+ | Dendritic or antigen-presenting cells | Immunosuppressive Mediate microglial/astrocytic activation Downregulate TNF-α and IFN-γ production Produce IL-10 (anti-inflammatory) |
Adapted from Lakhan et al., 2009 and Ceulemans et al., 2010
Table 2.
Milestone discoveries in the study of stroke-induced neuroinflammation
| Authors and Journal | Publication Date | Findings | Significance |
|---|---|---|---|
| Giulian et al., Journal of Experimental Medicine | 1986 | CNS ameboid microglia produce IL-β | First record of cerebral microglia producing cytotoxic/inflammatory compounds, suggesting they play a role in neurodegenerative pathology |
| Garcia et al, The American Journal of Pathology | 1993 | Identification of histopathological changes in brain after ischemic stroke, including time-dependent increases in necrosis and cellular damage | Suggested that neurodegeneration can affect the brain constitutively after ischemic stroke, expanded pathological perspective on the disease beyond the primary lesion |
| Clark et al, Brain Research Bulletin | 1993 | Identification of immunohistochemical changes in brain following ischemic stroke, including infiltration of neutrophils, necrosis, and activation of astroglia | Early evidence that neutrophils invade cerebral environment following stroke, suggesting peripheral immune response and activated astroglia may exacerbate insult, extra-focal tissue damage |
| Morioka et al, Journal of Comparative Neurology | 1993 | Reactive microglia found to be activated within area of ischemic injury and extra-focal areas, microglia activation persisted unilaterally after long-term survival | Reactive microglia implicated as a mediator of both extra-focal neurodegeneration and long-term neuroinflammation following ischemic stroke |
| Schroeter et al, Journal of Neuroimmunology | 1994 | Multiple classes of immune cells detected in tissues of the ischemic brain, including neutrophils, T cells, B cells, and macrophages | Significant evidence that peripheral immune cells can infiltrate cerebral environment following ischemic stroke, crossing the blood brain barrier, and possibly exacerbating insult. Overturned notion of brain as an immune-privileged organ. |
| Kim et al, J of Neuroimmunology | 1995 | Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein (MIP-1alpha) elevated in stroke brain and peaks between 24 and 48 hours after ischemic stroke | Provided evidence of macrophage-induced inflammation in the brain, suggested a possible signaling mechanism was being produced to attract pro-inflammatory macrophages |
| Jander et al, Journal of Cerebral Blood Flow & Metabolism | 1995 | MIP-alpha expression correlates with detected infiltration of peripheral macrophages after ischemic stroke | Direct evidence that macrophages from peripheral blood are responsible for producing neuroinflammation in the cerebral environment following ischemic stroke |
| Szaflarski et al, Stroke | 1995 | Cerebral ischemia stimulates local expression of inflammatory cytokines, TNF-alpha and IL-β | Suggests that neuroinflammation following ischemic stroke may be a product of both an external immune response and cytokine gene expression from endogenous brain cells |
| Gendron et al, Brain Research | 2002 | Systemic activation of T and B cell populations following ischemic stroke, absence of asymmetric suppressive effects between cerebral hemispheres, total number of spleen cells decreases after stroke | Evidence ischemic lesion produces a neuroinflammatory effect in both cortical and subcortical areas of either hemisphere, evidence that lesions produce elevated systemic inflammatory mobilization of T and B cells, early acknowledgement of spleen’s potential role in promoting neuroinflammation |
| Hill et al, Journal of Neuropathology & Experimental Neurology | 2004 | Chemokine stromal-derived factor-1 (SDF-1) expression elevated in penumbra following ischemic stroke, associated with reactive perivascular astrocytes/microglia | SDF-1 implicated as a signaling molecule in the mobilization of bone-marrow derived cells, notably inflammatory monocytes, to the brain after ischemic stroke |
| Newman et al, Stem Cells and Development | 2005 | Cytokine-induced neutrophil chemoattractant-1 (CNC-1) and IL-8 elevated in brain tissue following ischemic stroke, highest during acute phase | Discovery of first chemokine, IL-8, in ischemic brain tissue, suggests this pro-inflammatory factor may play a role in the mobilization and homing of neutrophils to the site of injury |
| Offner et al, The Journal of Immunology | 2006 | Ischemic stroke leads to splenic atrophy, reduction in number of splenocytes, resultant reduction in peripheral B cells, upregulation of CD4+FoxP3+regulatory T cells and CD11b+VLA-4-negative macrophages/monocytes | Evidence that spleen plays a role in the regulation of the immune response to ischemic stroke, influencing macrophage/regulator T cell mobilization |
| Ajmo et al, Journal of Neuroscience Research | 2008 | Splenectomy results in a significant reduction in lesion volume, numbers of activated microglia, macrophages, and neutrophils in brain tissue following ischemic stroke | Direct evidence that spleen is a major contributor to the development of secondary inflammation and resultant neurodegeneration after ischemic stroke |
| Seifert et al, Journal of Neuroimmune Pharmacology | 2012 | Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes (lymphocyte, monocytes, and neutrophils) explicitly visualized migrating to the brain after ischemic injury | Provided resolution as to the mobilization/migration behavior of splenic immune cells following ischemic stroke, demonstrated that after 96h splenocytes develop into pro-inflammatory NK cells, T cells and monocytes |
| Acosta et al, Stroke | 2015 | Intravenously injected labeled hBMSCs preferentially migrate to spleen following ischemic stroke, encourage reduction in striatal and peri-striatal infarct, activated inflammatory cells in brain tissue, and TNF-alpha expression in splenic cells, while exercising a neuroprotective effect on hippocampal neuronal cells | Suggests that hBMSCs, and generalized stem cell transplants, may provide a means to abrogate stroke induced neuroinflammation by moderating the intensity of the splenic peripheral immune response and resultant pro-inflammatory activation in brain tissue |
Highlights.
The present manuscript provides a critical analysis of clinically relevant stem cell types, by comparing their potential efficacy to sequester stroke-induced neuroinflammation and their feasibility as translational clinical cell sources.
We highlight that MSCs, with a proven track record of safety and efficacy as a transplantable cell for hematologic diseases, stand as an attractive cell type that confers superior anti-inflammatory effects in stroke both in vitro and in vivo. That stem cells can mount a robust anti-inflammatory action against stroke complements the regenerative processes of cell replacement and neurotrophic factor secretion conventionally ascribed to cell-based therapy in neurological disorders.
Acknowledgments
CVB is funded by NIH R01NS071956, NIH R01 NS090962, NIH R21NS089851, NIH R21 NS094087, and VA Merit Review I01 BX001407. Sandra A. Acosta and Cesar V. Borlongan conceptualized the manuscript. Connor Stonesifer, Sydney Corey, Shaila Ghanekar, and Zachary Diamandis provided writing and technical assistance.
Abbreviations
- AD-MSCs
adipose tissue-derived mesenchymal stem cells
- ANG-1
angiopoietin-1
- ANG-2
Angiopoietin-2
- ATP
adenosine triphosphate
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- BME
β mercaptoethanol
- BM-MNCs
bone marrow-derived mononuclear cells
- BMSCs
bone marrow-derived stem cells
- CACs
circulating angiogenic cells
- CAMs
cellular adhesion molecules
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- CNC-1
cytokine-induced neutrophil chemoattractant-1
- C1P
ceramide-1-phosphate
- CTX0E03
c-mycER(TAM) modified neural stem cells
- CXCR4
C-X-C chemokine receptor type 4
- DPSCs
dental pulp stem cells
- EPCs
endothelial progenitor cells
- EPO
erythropoietin
- rhEPO
recombinant human erythropoietin
- EPOR
erythropoietin receptor
- ERK
extracellular signal–regulated kinases
- ES
embryonic stem cells
- hESCs
human embryonic stem cells
- bFGF/FGF-2
basic fibroblast growth factor
- G-CSF
granulocyte-colony stimulating factor
- GDNF
glial cell line-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GM-CSF
granulocyte macrophage-colony-stimulating factor
- HCM
hypoxic conditioned media
- HGF
hepatocyte growth factor
- HIF-1α
hypoxia-inducible factor
- HSCs
hematopoietic stem cells
- IFN-γ
interferon-γ
- IGF-1
insulin growth factor-1
- IL-1β
interleukin 1 beta
- rhIL-3
recombinant human interleukin-3
- IL-6
interleukin 6
- IL-8
interleukin 8
- IL-10
interleukin 10
- IL-20
Interleukin 20
- iPSCs
induced pluripotent stem cells
- IRF
interferon regulatory factor
- IgSF
immunoglobulin superfamily
- MAPCs
multipotent adult progenitor cells
- MCAO
middle cerebral artery occlusion
- MCP-1
monocyte chemoattractant protein 1
- MIP-1α
macrophage inflammatory protein-1α
- MMPs
matrix metalloproteases
- MMP-2
gelatinase A
- MMP-9
gelatinase-B
- MSCs
mesenchymal stem cells
- MenSCs
Menstrual blood-derived stem cells
- Muse
multilineage-differentiating stress enduring cells
- NF
neurofilament
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NGF
nerve growth factor
- NIHSS
National Institutes of Health Stroke Scale
- NK
natural killer cell
- n-, e-iNOS
neuronal, endothelial and inducible nitric oxide synthases
- NSCs
neural stem cells
- hNSCs
human neural stem cells
- NT2N/hNT
teratocarcinoma-derived Ntera2/D1 neuron-like cells
- NT-3
neurotrophin-3
- OECs
outgrowth endothelial cells
- OGD
oxygen glucose deprivation
- PDL
poly-D-lysine
- PISCES
Pilot Investigation of Human Neural Stem Cells in Chronic Ischemic Stroke Patients
- PL
platelet lysate,
- ROS
reactive oxygen species,
- SDF-1
chemokine stromal-derived factor-1,
- SGZ
subgranular zone
- SHED
stem cells from exfoliated deciduous teeth,
- S1P
sphingosine-1-phosphate
- STEPS
Stem cell Therapeutics as an Emerging Paradigm for Stroke
- SVZ
subventricular zone,
- TGF-β
transforming growth factor beta
- TLRs
toll-like receptors
- TNF-α
tumor necrosis factor-α
- Tregs
antigen-specific regulatory T cells
- UCB-MSCs
umbilical cord blood-derived mesenchymal stem cells
- VE-cadherin
vascular endothelial cadherin
- VEGF
vascular endothelial growth factor
- VSELs
very small embryonic-like stem cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- Abdullah RH, Yaseen NY, Salih SM, Al-Juboory AA, Hassan A, Al-Shammari AM. Induction of mice adult bone marrow mesenchymal stem cells into functional motor neuron-like cells. Journal of Chemical Neuroanatomy. 2016;77:129–142. doi: 10.1016/j.jchemneu.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. The Journal of Immunology. 2014;193:1383–1391. doi: 10.4049/jimmunol.1400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46:2616–2627. doi: 10.1161/STROKEAHA.115.009854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature medicine. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Ajmo CT, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. Journal of neuroscience research. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. TLR signaling. From Innate Immunity to Immunological Memory. 2006:1–16. [Google Scholar]
- Aliaghaei A, Gardaneh M, Maghsoudi N, Salehinejad P, Gharib E. Dopaminergic Induction of Umbilical Cord Mesenchymal Stem Cells by Conditioned Medium of Choroid Plexus Epithelial Cells Reduces Apomorphine-Induced Rotation in Parkinsonian Rats. Archives of Iranian Medicine (AIM) 2016;19:561–70. [PubMed] [Google Scholar]
- Anderson EM, Mooney DJ. The combination of vascular endothelial growth factor and stromal cell-derived factor induces superior angiogenic sprouting by outgrowth endothelial cells. Journal of vascular research. 2015a;52:62–69. doi: 10.1159/000382129. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Kwee BJ, Lewin SA, Raimondo T, Mehta M, Mooney DJ. Local delivery of VEGF and SDF enhances endothelial progenitor cell recruitment and resultant recovery from ischemia. Tissue Engineering Part A. 2015b;21:1217–1227. doi: 10.1089/ten.tea.2014.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Developmental biology. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Teratocarcinomas and human embryology: pluripotent human EC cell lines. Apmis. 1998;106:158–168. doi: 10.1111/j.1699-0463.1998.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Antonucci I, Stuppia L, Kaneko Y, Yu S, Tajiri N, Bae EC, Chheda SH, Weinbren NL, Borlongan CV. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell transplantation. 2011;20:789–795. doi: 10.3727/096368910X539074. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurgical focus. 2016;40(5):E2. doi: 10.3171/2016.2.FOCUS15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Kilic Ü, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- Bai YY, Wang L, Chang D, Zhao Z, Lu CQ, Wang G, Ju S. Synergistic effects of transplanted endothelial progenitor cells and RWJ 67657 in diabetic ischemic stroke models. Stroke. 2015;46:1938–46. doi: 10.1161/STROKEAHA.114.008495. [DOI] [PubMed] [Google Scholar]
- Bai YY, Peng XG, Wang LS, Li ZH, Wang YC, Lu CQ, Ding J, Li PC, Zhao Z, Ju SH. Bone marrow endothelial progenitor cell transplantation after ischemic stroke: an investigation into its possible mechanism. CNS neuroscience & therapeutics. 2015;21:877–886. doi: 10.1111/cns.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KA, Hong M, Sadi D, Mendez I. Intrastriatal and intranigral grafting of hNT neurons in the 6-OHDA rat model of Parkinson’s disease. Experimental neurology. 2000;162:350–360. doi: 10.1006/exnr.1999.7337. [DOI] [PubMed] [Google Scholar]
- Balaji S, Han N, Moles C, Shaaban AF, Bollyky PL, Crombleholme TM, Keswani SG. Angiopoietin-1 improves endothelial progenitor cell–dependent neovascularization in diabetic wounds. Surgery. 2015;158:846–856. doi: 10.1016/j.surg.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A, Nicholls J, Williamson DA, Jensen SL, Gordon M, Habib N. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem cells translational medicine. 2014;3:1322–1330. doi: 10.5966/sctm.2013-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of neurology. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental hematology. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Webster TJ. XanoMatrix surfaces as scaffolds for mesenchymal stem cell culture and growth. International Journal of Nanomedicine. 2016;11:2655. doi: 10.2147/IJN.S101838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhartiya D, Shaikh A, Anand S, Patel H, Kapoor S, Sriraman K, Parte S, Unni S. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Human Reproduction Update. 2016;23:41–76. doi: 10.1093/humupd/dmw030. [DOI] [PubMed] [Google Scholar]
- Bhatt VR, Balasetti V, Jasem JA, Giri S, Armitage JO, Loberiza FR, Bociek RG, Bierman PJ, Maness LJ, Vose JM, Fayad P. Central nervous system complications and outcomes after allogeneic hematopoietic stem cell transplantation. Clinical Lymphoma Myeloma and Leukemia. 2015;15:606–611. doi: 10.1016/j.clml.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. The Lancet. 1999;353:29–30. doi: 10.1016/s0140-6736(99)90229-5. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VMY, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Experimental neurology. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’may be good after all! Leukemia. 2011;25:1674–1686. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke. Stroke. 2009;40:146–148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell–like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem cells and development. 2010;19:439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, Hess DC, Kondziolka D. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regenerative medicine. 2008;3:249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, E Glover L, R Sanberg P, C Hess D. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Current pharmaceutical design. 2012;18:3670–3676. doi: 10.2174/138161212802002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain research. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Stahl CE, Cameron DF, Saporta S, Freeman TB, Cahill DW, Sanberg PR. CNS immunological modulation of neural graft rejection and survival. Neurological research. 1996;18:297–304. doi: 10.1080/01616412.1996.11740425. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Fournier C, Stahl CE, Yu G, Xu L, Matsukawa N, Newman M, Yasuhara T, Hara K, Hess DC, Sanberg PR. Gene therapy, cell transplantation and stroke. Frontiers in bioscience: a journal and virtual library. 2005;11:1090–1101. doi: 10.2741/1865. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Saporta S, Poulos SG, Othberg A, Sanberg PR. Viability and survival of hNT neurons determine degree of functional recovery in grafted ischemic rats. Neuroreport. 1998;9:2837–2842. doi: 10.1097/00001756-199808240-00028. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Su TP, Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 2000;11:923–926. doi: 10.1097/00001756-200004070-00005. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. Journal of neurosurgery. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. Journal of Comparative Neurology. 2009;515:125–144. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Zhou Y, Zhou B, Lou S. Hypoxic conditioned medium from rat cerebral cortical cells enhances the proliferation and differentiation of neural stem cells mainly through PI3-K/Akt pathways. PloS one. 2014;9:e111938. doi: 10.1371/journal.pone.0111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. Journal of neuroinflammation. 2010;1(7):74. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. American Journal of Physiology-Cell Physiology. 2010;299:1562–1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. 2014;32:3075–3087. doi: 10.1002/stem.1802. [DOI] [PubMed] [Google Scholar]
- Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cellular immunology. 2012;274:46–53. doi: 10.1016/j.cellimm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Chen J, Shehadah A, Pal A, Zacharek A, Cui X, Cui Y, Roberts C, Lu M, Zeitlin A, Hariri R, Chopp M. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell transplantation. 2013;22:871–879. doi: 10.3727/096368911X637380. [DOI] [PubMed] [Google Scholar]
- Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Guo Y, Cheng W, Chen R, Liu T, Chen Z, Tan S. High glucose induces apoptosis and suppresses proliferation of adult rat neural stem cells following in vitro ischemia. BMC neuroscience. 2013;14:24. doi: 10.1186/1471-2202-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702–4717. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Liu AR, Qiu HB, Yang Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem cell research & therapy. 2015;6:44. doi: 10.1186/s13287-015-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang W, Wang JM, Duan HT, Kong JH, Wang YX, Dong M, Bi X, Song J. Differentiation of isolated human umbilical cord mesenchymal stem cells into neural stem cells. International journal of ophthalmology. 2016;9:41–7. doi: 10.18240/ijo.2016.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZZ, Jiang XD, Zhang LL, Shang JH, Du MX, Xu G, Xu RX. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neuroscience letters. 2008;445:36–41. doi: 10.1016/j.neulet.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Chi K, Fu RH, Huang YC, Chen SY, Lin SZ, Huang PC, Lin PC, Chang FK, Liu SP. Therapeutic Effect of Ligustilide-Stimulated Adipose-Derived Stem Cells in a Mouse Thromboembolic Stroke Model. Cell transplantation. 2016;25:899–912. doi: 10.3727/096368916X690539. [DOI] [PubMed] [Google Scholar]
- Chiang KH, Cheng WL, Shih CM, Lin YW, Tsao NW, Kao YT, Lin CT, Wu SC, Huang CY, Lin FY. Statins, HMG-CoA reductase inhibitors, improve neovascularization by increasing the expression density of CXCR4 in endothelial progenitor cells. PloS one. 2015;10:e0136405. doi: 10.1371/journal.pone.0136405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CH, Sinden JD, Couraud PO, Modo M. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PloS one. 2014;9:e106346. doi: 10.1371/journal.pone.0106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SY, Soker S, Jang YJ, Kwon TG, Yoo ES. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. Journal of Korean medical science. 2016;31:171–177. doi: 10.3346/jkms.2016.31.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ, Barone FC. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain research bulletin. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT. Ischemic Stroke Activates Hematopoietic Bone Marrow Stem CellsNovelty and Significance. Circulation research. 2015;116:407–417. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacu R, Arvidsson L, Andersson Å, Khademi M, Erlandsson-Harris H, Harris RA, Svensson MA, Olsson T, Brundin L. TLR activation induces TNF-α production from adult neural stem/progenitor cells. The Journal of Immunology. 2009;182:6889–6895. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS, Hartmann PE. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell and tissue research. 2007;329:129–136. doi: 10.1007/s00441-007-0390-x. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. The Journal of Immunology. 2010;185:6617–6623. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, Pabon M, Acosta S, Kaneko Y, Loveren HV, Borlongan CV. An update on translating stem cell therapy for stroke from bench to bedside. Journal of clinical medicine. 2013;2:220–241. doi: 10.3390/jcm2040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MA, Tate CC, Aizman I, McGrogan M, Case CC. Comparing the immunosuppressive potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. Journal of neuroinflammation. 2011;8:133. doi: 10.1186/1742-2094-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy. 2011;13:1205–1220. doi: 10.3109/14653249.2011.605351. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. The Journal of clinical investigation. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke. 2015;46:588–591. doi: 10.1161/STROKEAHA.114.007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem cell research & therapy. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunac A, Frelin C, Popolo-Blondeau M, Chatel M, Mahagne MH, Philip PJM. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. Journal of neurology. 2007;254:327–332. doi: 10.1007/s00415-006-0362-1. [DOI] [PubMed] [Google Scholar]
- Dunlop J, McIlvain HB, Lou Z, Franco R. The pharmacological profile of L-glutamate transport in human NT2 neurones is consistent with excitatory amino acid transporter 2. European journal of pharmacology. 1998;360:249–256. doi: 10.1016/s0014-2999(98)00675-x. [DOI] [PubMed] [Google Scholar]
- Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke. Stem cells translational medicine. 2015;4:841–851. doi: 10.5966/sctm.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Vu Q, Xie K, Yu J, Liao W, Cramer SC, Zhao W. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. Journal of Cerebral Blood Flow & Metabolism. 2013;33:1322–1334. doi: 10.1038/jcbfm.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eterno V, Zambelli A, Pavesi L, Villani L, Zanini V, Petrolo G, Manera S, Tuscano A, Amato A. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5:613–633. doi: 10.18632/oncotarget.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation research. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, Chen Y, Su H, Young WL, Yang GY. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Annals of neurology. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y, Maeda M, Moriguchi A, Sawamoto T, Kawamura A, Matsuoka N, Mutoh S, Yanagihara T. Tacrolimus, a potential neuroprotective agent, ameliorates ischemic brain damage and neurologic deficits after focal cerebral ischemia in nonhuman primates. J Cereb Blood Flow Metab. 2003;23:1183–1194. doi: 10.1097/01.WCB.0000088761.02615.EB. [DOI] [PubMed] [Google Scholar]
- Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, Chang E, Chao L, Chao J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. Journal of the American Heart Association. 2014;3:e001194. doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Williams SN, Haim ED, Tajiri N, Hernandez-Ontiveros DG, Frisina-Deyo A, Boffeli SM, Sanberg PR, Borlongan CV. Compromised blood–brain barrier competence in remote brain areas in ischemic stroke rats at the chronic stage. Journal of Comparative Neurology. 2014;522:3120–3137. doi: 10.1002/cne.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Lin R, Borlongan CV. Intravenously Transplanted Human Bone Marrow Endothelial Progenitor Cells Engraft Within Brain Capillaries, Preserve Mitochondrial Morphology, and Display Pinocytotic Activity Toward Blood-Brain Barrier Repair in Ischemic Stroke Rats. Stem Cells. 2017 doi: 10.1002/stem.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian JINYING, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. The American journal of pathology. 1993;142 [PMC free article] [PubMed] [Google Scholar]
- Gendron A, Teitelbaum J, Cossette C, Nuara S, Dumont M, Geadah D, du Souich P, Kouassi E. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain research. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation research. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grymula K, Tarnowski M, Piotrowska K, Suszynska M, Mierzejewska K, Borkowska S, Fiedorowicz K, Kucia M, Ratajczak MZ. Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment. Journal of cellular and molecular medicine. 2014;18:1797–1806. doi: 10.1111/jcmm.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhuis N, De Witte SF, van Osch GJ, Bayon Y, Lange JF, Bastiaansen-Jenniskens YM. Biomaterials Influence Macrophage-Mesenchymal Stem Cell Interaction In Vitro. Tissue Eng Part A. 2016;22:1098–107. doi: 10.1089/ten.TEA.2016.0162. [DOI] [PubMed] [Google Scholar]
- Guerin CL, Loyer X, Vilar J, Cras A, Mirault T, Gaussem P, Silvestre JS, Smadja DM. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. Thromb Haemost. 2015;113:1084–1094. doi: 10.1160/TH14-09-0748. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Fernández M, Otero-Ortega L, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Díez-Tejedor E. Adipose tissue-derived mesenchymal stem cells as a strategy to improve recovery after stroke. Expert opinion on biological therapy. 2015;15:873–881. doi: 10.1517/14712598.2015.1040386. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez M, Rodriguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Fuentes B, Diez-Tejedor E. Adipose tissue-derived stem cells in stroke treatment: from bench to bedside. Discovery medicine. 2013;16:37–43. [PubMed] [Google Scholar]
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Vallejo-Cremades MT, Sanz-Cuesta BE, Díez-Tejedor E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. Journal of translational medicine. 2015;13:46. doi: 10.1186/s12967-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Song J, Fino K, Sandhu P, Song X, Lei F, Zheng S, Ni B, Fang D, Song J. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Scientific reports. 2016;6:20588. doi: 10.1038/srep20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, Hess DC. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Progress in Neurobiology. 2008;85:318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Margulis M, Fishman PS, Lee VMY, Tang CM. Functional synapses are formed between human NTera2 (NT2N, hNT) neurons grown on astrocytes. Journal of Comparative Neurology. 1999;407:1–10. doi: 10.1002/(sici)1096-9861(19990428)407:1<1::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Trojanowski JQ, Lee VMY. Differential effects of spinal cord gray and white matter on process outgrowth from grafted human NTERA2 neurons (NT2N, hNT) Journal of Comparative Neurology. 1999;415:404–418. doi: 10.1002/(sici)1096-9861(19991220)415:3<404::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Z, O’Reilly J, Pearse Y, Stroemer P, Tang E, Sinden J, Price J, Thuret S. Human neural progenitor cell engraftment increases neurogenesis and microglial recruitment in the brain of rats with stroke. PloS one. 2012;7:e50444. doi: 10.1371/journal.pone.0050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, Trengove N, Lai CT, Filgueira L, Blancafort P, Hartmann PE. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem cells. 2012;30:2164–2174. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens AM, Sun H, Shiozawa Y, Jung Y, Wang J, Mishra A, Jiang Y, O’Neill DW, Krebsbach PH, Rodgerson DO, Taichman RS. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem cells and development. 2013;23:689–701. doi: 10.1089/scd.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell transplantation. 2013;22:1541–1552. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- Hilgendorf I, Greinix H, Halter JP, Lawitschka A, Bertz H, Wolff D. Long-Term Follow-up After Allogeneic Stem Cell Transplantation. Deutsches Ärzteblatt International. 2015;112:51–58. doi: 10.3238/arztebl.2015.0051. http://doi.org/10.3238/arztebl.2015.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EJ, Jiménez-González C, Tarczyluk M, Nagel DA, Coleman MD, Parri HR. NT2 derived neuronal and astrocytic network signalling. PloS one. 2012;7:e36098. doi: 10.1371/journal.pone.0036098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. Journal of Neuropathology & Experimental Neurology. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Holloway PM, Gavins FN. Modeling ischemic stroke in vitro: status quo and future perspectives. Stroke. 2016;47:561–569. doi: 10.1161/STROKEAHA.115.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. Journal of neuroscience research. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HH, Huang HL, Wang HC, Tsai YF, Liu TC, Chang CS, Lin SF. Acute cerebral infarct with elevated factor VIII level during the thrombocytopenic stage after hematopoietic stem cell transplant. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2014;12:171–172. [PubMed] [Google Scholar]
- Huang GJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of dental research. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wong S, Snyder EY, Hamblin MH, Lee JP. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem cell research & therapy. 2014;5:129. doi: 10.1186/scrt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Hurlbert MS, Gianani RI, Hutt C, Freed CR, Kaddis FG. Neural transplantation of hNT neurons for Huntington’s disease. Cell transplantation. 1998;8:143–151. doi: 10.1177/096368979900800106. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell–derived neural precursor cells. Journal of neuroscience research. 2008;86:1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi SI, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura SI. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–685. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nature medicine. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Engineering Part A. 2012;19:24–29. doi: 10.1089/ten.tea.2011.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Asai T, Oyama D, Agato Y, Yasuda N, Fukuta T, Shimizu K, Minamino T, Oku N. Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 2013;4:1362–70. doi: 10.1096/fj.12-221325. [DOI] [PubMed] [Google Scholar]
- Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NRS, Bagher-Ebadian H, Ali MM, Arbab AS, Janic B. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem cells translational medicine. 2013;2:703–714. doi: 10.5966/sctm.2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. Journal of Cerebral Blood Flow & Metabolism. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Jiang D, Muschhammer J, Qi Y, Kügler A, De Vries JC, Saffarzadeh M, Sindrilaru A, Beken SV, Wlaschek M, Kluth MA, Ganss C. Suppression of Neutrophil-Mediated Tissue Damage—A Novel Skill of Mesenchymal Stem Cells. Stem Cells. 2016;34:2393–2406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Ding S, Wu J, Liu X, Wu Z. Norepinephrine stimulates mobilization of endothelial progenitor cells after limb ischemia. PloS one. 2014;9:e101774. doi: 10.1371/journal.pone.0101774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. Journal of cardiovascular translational research. 2013;6:834–851. doi: 10.1007/s12265-013-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe IS, Cho GW. PDE4 Inhibition by Rolipram Promotes Neuronal Differentiation in Human Bone Marrow Mesenchymal Stem Cells. Cellular Reprogramming. 2016;18:224–229. doi: 10.1089/cell.2015.0061. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2, 3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28(11):1174–1181. doi: 10.1016/j.placenta.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kachamakova-Trojanowska N, Bukowska-Strakova K, Zukowska M, Dulak J, Jozkowicz A. The real face of endothelial progenitor cells – Circulating angiogenic cells as endothelial prognostic marker? Pharmacological Reports. 2015;4:793–802. doi: 10.1016/j.pharep.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Kaelber S, Pantcheva P, Borlongan CV. Drug-and cell-based therapies for targeting neuroinflammation in traumatic brain injury. Neural Regeneration Research. 2016;11:1575. doi: 10.4103/1673-5374.193231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaingade PM, Somasundaram I, Nikam AB, Sarang SA, Patel JS. Assessment of growth factors secreted by human breastmilk mesenchymal stem cells. Breastfeeding Medicine. 2016;11:26–31. doi: 10.1089/bfm.2015.0124. [DOI] [PubMed] [Google Scholar]
- Kaingade PM, Somasundaram I, Nikam AB, Sarang SA, Patel JS. Breastmilk-Derived Mesenchymal Stem Cells In Vitro Are Likely to Be Mediated Through Epithelial–Mesenchymal Transition. Breastfeeding Medicine. 2016;11:152–152. doi: 10.1089/bfm.2016.0023. [DOI] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. The Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Yamahara K, Soma T, Stern DM, Nakagomi T, Matsuyama T, Taguchi A. Transplantation of hematopoietic stem cells: intra-arterial versus intravenous administration impacts stroke outcomes in a murine model. Translational Research. 2016;176:69–80. doi: 10.1016/j.trsl.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Kassmer SH, Krause DS. Very small embryonic-like cells: Biology and function of these potential endogenous pluripotent stem cells in adult tissues. Molecular reproduction and development. 2013;80:677–690. doi: 10.1002/mrd.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. Journal of Cerebral Blood Flow & Metabolism. 2010;30:1487–1493. doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KMA. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. Journal of neuroimmunology. 1995;56:127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kim SU, De Vellis J. Stem cell-based cell therapy in neurological diseases: a review. Journal of neuroscience research. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci. 2012;8:1078–87. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kong D, Zhu J, Liu Q, Jiang Y, Xu L, Luo N, Zhao Z, Zhai Q, Zhang H, Zhu M, Liu X. Mesenchymal stem cells protect neurons against hypoxic-ischemic injury via inhibiting parthanatos, necroptosis, and apoptosis, but not autophagy. Cellular and molecular neurobiology. 2016:1–11. doi: 10.1007/s10571-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4+ SSEA-1+ Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood–preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kucia M, Masternak M, Liu R, Shin DM, Ratajczak J, Mierzejewska K, Spong A, Kopchick JJ, Bartke A, Ratajczak MZ. The negative effect of prolonged somatotrophic/insulin signaling on an adult bone marrow-residing population of pluripotent very small embryonic-like stem cells (VSELs) Age. 2013;35:315–330. doi: 10.1007/s11357-011-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Bhoi S, Mohanty S, Kamal VK, Rao DN, Mishra P, Galwankar S. Bone marrow hematopoietic stem cells behavior with or without growth factors in trauma hemorrhagic shock. International Journal of Critical Illness and Injury Science. 2016;6:119. doi: 10.4103/2229-5151.190654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED. Circulating endothelial progenitor cells predict coronary artery disease severity. American heart journal. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Molecular Therapy. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Journal of translational medicine. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira P, Gomes J, Pedreiro S, Pedrosa M, Martinho A, Antunes B, Ribeiro T, Santos F, Domingues R, Abecasis M, Trindade H. Human bone marrow-derived mesenchymal stromal cells differentially inhibit cytokine production by peripheral blood monocytes subpopulations and myeloid dendritic cells. Stem cells international. 2015;2015:819084. doi: 10.1155/2015/819084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Hartley RS, Trojanowski JQ. Neurobiology of human neurons (NT2N) grafted into mouse spinal cord: implications for improving therapy of spinal cord injury. Progress in brain research. 1999;128:299–307. doi: 10.1016/S0079-6123(00)28027-8. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, Kim HS. Microvesicles from brain-extract—treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Scientific Reports. 2016;6:33038. doi: 10.1038/srep33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J, Vink R. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem cells translational medicine. 2012;1:177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A stem cell legacy: Leroy Stevens 2000 [Google Scholar]
- Li G, Yu F, Lei T, Gao H, Li P, Sun Y, Huang H, Mu Q. Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies. Neural Regeneration Research. 2016;11:1015. doi: 10.4103/1673-5374.184506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang W, Wang G, Hou Y, Xu F, Liu R, Wang F, Xue J, Hu T, Luan X. Interferon-γ and tumor necrosis factor-α promote the ability of human placenta–derived mesenchymal stromal cells to express programmed death ligand-2 and induce the differentiation of CD4+ interleukin-10+ and CD8+ interleukin-10+ Treg subsets. Cytotherapy. 2015;17:1560–1571. doi: 10.1016/j.jcyt.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Li X, Zheng W, Bai H, Wang J, Wei R, Wen H, Ning H. Intravenous administration of adipose tissue-derived stem cells enhances nerve healing and promotes BDNF expression via the TrkB signaling in a rat stroke model. Neuropsychiatric disease and treatment. 2016;12:1287–93. doi: 10.2147/NDT.S104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CC, Liu HL, Chang SD, Chen SH, Lee TH. The protective effect of human umbilical cord blood CD34+ cells and estradiol against focal cerebral ischemia in female ovariectomized rat: cerebral MR imaging and immunohistochemical study. PloS one. 2016;11(1):e0147133. doi: 10.1371/journal.pone.0147133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Li Z, Ma T, Han Z, Du W, Geng J, Jia H, Zhao M, Wang J, Zhang B, Feng J. Transplantation of human placenta derived mesenchymal stem cell alleviates critical limb ischemia in diabetic nude rat. Cell Transplantation. 2016;26:45–61. doi: 10.3727/096368916X692726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Zhang H, Feng QS, Cai MB, Deng W, Qin D, Yun JP, Tsao GSW, Kang T, Esteban MA, Pei D. The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chinese journal of cancer. 2013;32:205–12. doi: 10.5732/cjc.012.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Jeong CH, Jun JA, Kim SM, Ryu CH, Hou Y, Oh W, Chang JW, Jeun SS. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem cell research & therapy. 2011;2:38. doi: 10.1186/scrt79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Liu M, Wang J, Hu X, Xu J. Study of immunomodulatory function of exosomes derived from human umbilical cord mesenchymal stem cells. Zhonghua yi xue za zhi. 2015;95:2630–2633. [PubMed] [Google Scholar]
- Liu SJ, Zou Y, Belegu V, Lv LY, Lin N, Wang TY, McDonald JW, Zhou X, Xia QJ, Wang TH. Co-grafting of neural stem cells with olfactory en sheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. Journal of neuroinflammation. 2014;11:66. doi: 10.1186/1742-2094-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wang CP, Liu M, Ji G, Guo JC. Transplantation of human embryonic neural stem cells protects rats against cerebral ischemic injury. Sheng li xue bao:[Acta physiologica Sinica] 2014;66:691–701. [PubMed] [Google Scholar]
- Liu X, Li Y, Liu Y, Luo Y, Wang D, Annex BH, Goldschmidt-Clermont PJ. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. The American journal of pathology. 2010;176:504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, Fan X, Jiang Y, Stetler RA, Liu G, Chen J. Cell based therapies for ischemic stroke: from basic science to bedside. Progress in neurobiology. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental neurology. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lulic D, Burns J, Bae EC, van Loveren H, Borlongan CV. A review of laboratory and clinical data supporting the safety and efficacy of cyclosporin A in traumatic brain injury. Neurosurgery. 2011;5:1172–85. doi: 10.1227/NEU.0b013e31820c6cdc. [DOI] [PubMed] [Google Scholar]
- Lv Y, Xu X, Zhang B, Zhou G, Li H, Du C, Han H, Wang H. Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. Journal of translational medicine. 2014;2:344. doi: 10.1186/s12967-014-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Pastor A, Ohlemeyer C, Kann O, Wiegand F, Prass K, Knapp F, Kettenmann H, Dirnagl U. Distinct physiologic properties of microglia and blood-borne cells in rat brain slices after permanent middle cerebral artery occlusion. Journal of Cerebral Blood Flow & Metabolism. 2000;20:1537–1549. doi: 10.1097/00004647-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell research. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZL, Mai XL, Sun JH, Ju SH, Yang X, Ni Y, Teng GJ. Inhibited atherosclerotic plaque formation by local administration of magnetically labeled endothelial progenitor cells (EPCs) in a rabbit model. Atherosclerosis. 2009;205:80–86. doi: 10.1016/j.atherosclerosis.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications-a systematic review of the literature. The open orthopaedics journal. 2011;5:242–8. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maienschein J. Regenerative medicine’s historical roots in regeneration, transplantation, and translation. Developmental biology. 2011;358:278–284. doi: 10.1016/j.ydbio.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Makhoul G, Jurakhan R, Jaiswal PK, Ridwan K, Li L, Selvasandran K, Duong M, Schwertani A, Cecere R. Conditioned medium of H9c2 triggers VEGF dependent angiogenesis by activation of p38/pSTAT3 pathways in placenta derived stem cells for cardiac repair. Life sciences. 2016;153:213–221. doi: 10.1016/j.lfs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Martini MM, Jeremias TDS, Kohler MC, Marostica LL, Trentin AG, Alvarez-Silva M. Human placenta-derived mesenchymal stem cells acquire neural phenotype under the appropriate niche conditions. DNA and cell biology. 2013;32:58–65. doi: 10.1089/dna.2012.1807. [DOI] [PubMed] [Google Scholar]
- Mashkouri S, Crowley MG, Liska MG, Corey S, Borlongan CV. Utilizing pharmacotherapy and mesenchymal stem cell therapy to reduce inflammation following traumatic brain injury. Neural Regeneration Research. 2016;11:1379–1384. doi: 10.4103/1673-5374.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Espinosa G, Collazo-Navarrete O, Millán-Aldaco D, Palomero-Rivero M, Guerrero-Flores G, Drucker-Colín R, Covarrubias L, Guerra-Crespo M. Mouse Embryonic Stem Cell-Derived Cells Reveal Niches that Support Neuronal Differentiation in the Adult Rat Brain. Stem Cells. 2015;33:491–502. doi: 10.1002/stem.1856. [DOI] [PubMed] [Google Scholar]
- Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, Pastore I, Marasso R, Madon E. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2003;4:158–161. doi: 10.1080/14660820310014653. [DOI] [PubMed] [Google Scholar]
- McAndrews KM, McGrail DJ, Ravikumar N, Dawson MR. Mesenchymal stem cells induce directional migration of invasive breast cancer cells through TGF-β. Scientific reports. 2015;5:16941. doi: 10.1038/srep16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin CP, Jurga M, Miller AM, Sarnowska A, Wiedner M, Boyle NT, Lynch MA, Jablonska A, Drela K, Lukomska B, Domanska-Janik K. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Archives of biochemistry and biophysics. 2013;534:88–97. doi: 10.1016/j.abb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B. Endometrial regenerative cells: a novel stem cell population. Journal of translational medicine. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minteer D, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: biology and potential applications. Mesenchymal Stem Cells-Basics and Clinical Application. 2012;I:59–71. doi: 10.1007/10_2012_146. [DOI] [PubMed] [Google Scholar]
- Miyazono M, Lee VM, Trojanowski JQ. Proliferation, cell death, and neuronal differentiation in transplanted human embryonal carcinoma (NTera2) cells depend on the graft site in nude and severe combined immunodeficient mice. Laboratory investigation; a journal of technical methods and pathology. 1995;73:273–283. [PubMed] [Google Scholar]
- Mohamad O, Drury-Stewart D, Song M, Faulkner B, Chen D, Yu SP, Wei L. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PloS one. 2013;8:e64160. doi: 10.1371/journal.pone.0064160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Nikbakht M, Mohammadi AM, Panah MZ, Ostadali MR, Nasiri H, Ghavamzadeh A. Human Platelet Lysate as a Xeno Free Alternative of Fetal Bovine Serum for the In Vitro Expansion of Human Mesenchymal Stromal Cells. International Journal of Hematology-Oncology and Stem Cell Research. 2016;10:161–71. [PMC free article] [PubMed] [Google Scholar]
- Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cellular Physiology and Biochemistry. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Riparip LK, Rosi S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PloS one. 2016;11(1):e0148001. doi: 10.1371/journal.pone.0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. Journal of Comparative Neurology. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L, Foucault-Bertaud A, Velly L, Dignat-George F. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Reviews and Reports. 2011;7:208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, Bakiasi G, Tsai LH, Aubourg P, Ransohoff RM, Jaenisch R. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nature Research. 2016;11:1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcisi R, Arikan OH, Lehmann J, ten Berge D, van Osch GJ. Differential effects of small molecule WNT agonists on the multilineage differentiation capacity of human mesenchymal stem cells. Tissue Engineering Part A. 2016;22:1264–1273. doi: 10.1089/ten.TEA.2016.0081. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VMY. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. The American journal of pathology. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnár Z, O’donnell ME, Povlishock JT, Saunders NR. Engaging neuroscience to advance translational research in brain barrier biology. Nature Reviews Neuroscience. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem cells and development. 2005;14:576–586. doi: 10.1089/scd.2005.14.576. [DOI] [PubMed] [Google Scholar]
- Nicholas JS. Ross Granville Harrison 1870–1959. The Yale journal of biology and medicine. 1960;32:407–412. [PMC free article] [PubMed] [Google Scholar]
- Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Progress in brain research. 2000;127:461–476. doi: 10.1016/s0079-6123(00)27022-2. [DOI] [PubMed] [Google Scholar]
- Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. European Journal of Neuroscience. 2004;19:2388–2398. doi: 10.1111/j.0953-816X.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. The Journal of Immunology. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell stem cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Choi C, Chang DJ, Shin DA, Lee N, Jeon I, Sung JH, Lee H, Hong KS, Ko JJ, Song J. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy. 2015;17:1090–1103. doi: 10.1016/j.jcyt.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Gen Son T, Lee JH, Roberts NJ, Mughal MR, Hutchison E, Cheng A, Arumugam TV, Lathia JD, Van Praag H. TLR2 activation inhibits embryonic neural progenitor cell proliferation. Journal of neurochemistry. 2010;114:462–474. doi: 10.1111/j.1471-4159.2010.06778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain research reviews. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Ortega L, Gutiérrez-Fernández M, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Sobrino T, Hernanz TN, Campos F, López JA, Cerdán S, Vázquez J. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem cell research & therapy. 2015;6:121. doi: 10.1186/s13287-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Yu S, Kaneko Y, Tajiri N, Bae EC, Chheda SH, Stahl CE, Yang T, Fang L, Hu K, Borlongan CV. Intravenous infusion of GDNF gene-modified human umbilical cord blood CD34+ cells protects against cerebral ischemic injury in spontaneously hypertensive rats. Brain research. 2010;1366:217–225. doi: 10.1016/j.brainres.2010.09.098. [DOI] [PubMed] [Google Scholar]
- Osman MM, Lulic D, Glover L, Stahl CE, Lau T, van Loveren H, Borlongan CV. Cyclosporine-A as a neuroprotective agent against stroke: its translation from laboratory research to clinical application. Neuropeptides. 2011;6:359–68. doi: 10.1016/j.npep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Pan HC, Chin CS, Yang DY, Ho SP, Chen CJ, Hwang SM, Chang MH, Cheng FC. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochemical research. 2009;34:1304–1316. doi: 10.1007/s11064-008-9910-7. [DOI] [PubMed] [Google Scholar]
- Park DH, Borlongan CV, Willing AE, Eve DJ, Cruz LE, Sanberg CD, Chung YG, Sanberg PR. Human umbilical cord blood cell grafts for brain ischemia. Cell transplantation. 2009;18:985–998. doi: 10.3727/096368909X471279. [DOI] [PubMed] [Google Scholar]
- Park DH, Eve DJ, Musso J, III, Klasko SK, Cruz E, Borlongan CV, Sanberg PR. Inflammation and stem cell migration to the injured brain in higher organisms. Stem cells and development. 2009;18:693–702. doi: 10.1089/scd.2009.0008. [DOI] [PubMed] [Google Scholar]
- Park HW, Moon HE, Kim HSR, Paek SL, Kim Y, Chang JW, Yang YS, Kim K, Oh W, Hwang JH, Kim JW. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through thrombospondin1, pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. Journal of neuroscience research. 2015;93:1814–1825. doi: 10.1002/jnr.23616. [DOI] [PubMed] [Google Scholar]
- Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell transplantation. 2008;17:303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- Pearson JD. Endothelial progenitor cells–an evolving story. Microvascular Research. 2010;3:162–8. doi: 10.1016/j.mvr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Peplow PV. Growth factor-and cytokine-stimulated endothelial progenitor cells in post-ischemic cerebral neovascularization. Neural regeneration research. 2014;9:1425–9. doi: 10.4103/1673-5374.139457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips MF, Muir JK, Saatman KE, Raghupathi R, Lee VMY, Trojanowski JQ, McIntosh TK. Survival and integration of transplanted postmitotic human neurons following experimental brain injury in immunocompetent rats. Journal of neurosurgery. 1999;90:116–124. doi: 10.3171/jns.1999.90.1.0116. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VM. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Experimental neurology. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM. Intravenous Autologous Bone Marrow Mononuclear Stem Cell Therapy for Ischemic Stroke. Stroke. 2014;45:3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- Puangmalai N, Somani A, Thangnipon W, Ballard C, Broadstock M. A genetically immortalized human stem cell line: a promising new tool for Alzheimer’s disease therapy. EXCLI journal. 2015;14:1135. doi: 10.17179/excli2015-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, Ratajczak MZ. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Experimental hematology. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim C, Janowska-Wieczorek A, Ratajczak J. The expanding family of bone marrow homing factors for hematopoietic stem cells: stromal derived factor 1 is not the only player in the game. The Scientific World Journal. 2012;2012:758512. doi: 10.1100/2012/758512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Liu R, Mierzejewska K, Ratajczak J, Kucia M, Zuba-Surma EK. Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation–an update and comparison to other primitive small stem cells isolated from adult tissues. Aging. 2012;4:235–246. doi: 10.18632/aging.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramirez MC, Blanquer M, Marin N, Martínez S, Moraleda JM. Mesenchymal stem cells derived from dental tissues. International endodontic journal. 2011;44:800–806. doi: 10.1111/j.1365-2591.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer research. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural regeneration research. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba F, Soleimani M, Atashi A, Mortaz E, Shahjahani M, Roshandel E, Jaseb K, Saki N. The role of the nervous system in hematopoietic stem cell mobilization. Lab Hematol. 2013;19:8–16. doi: 10.1532/LH96.12013. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Eve DJ, Willing AE, Garbuzova-Davis S, Tan J, Sanberg CD, Allickson JG, Cruz LE, Borlongan CV. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell transplantation. 2011;20:85–94. doi: 10.3727/096368910X532855. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bonemarrow stromal cells differentiate into neural cells in vitro. Experimental Neurology. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Santilli G, Lamorte G, Carlessi L, Ferrari D, Nodari LR, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PloS one. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporta S, Borlongan CV, Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose–response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- Saporta S, Cameron DF, Borlongan CV, Sanberg PR. Survival of rat and porcine Sertoli cell transplants in the rat striatum without cyclosporine-A immunosuppression. Experimental neurology. 1997;146:299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Suzuki J, Tateyama D, Oka S, Namioka T, Namioka A, Onodera R. Synergic effects of rehabilitation and intravenous infusion of mesenchymal stem cells after stroke in rats. Physical therapy. 2016;96 doi: 10.2522/ptj.20150504. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Annals of neurology. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem cells. 2008;26:1537–1546. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. Journal of neuroimmunology. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. Journal of Neuroimmune Pharmacology. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, Tritschler L, Brenot M, Guidou E, Blondeau J, Lhuillier M. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. doi: 10.1161/STROKEAHA.109.563015. [DOI] [PubMed] [Google Scholar]
- Sharma S, Bhonde R. Mesenchymal stromal cells are genetically stable under a hostile in vivo–like scenario as revealed by in vitro micronucleus test. Cytotherapy. 2015;17:1384–1395. doi: 10.1016/j.jcyt.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Shekaran A, Lam A, Sim E, Jialing L, Jian L, Wen JTP, Chan JKY, Choolani M, Reuveny S, Birch W, Oh S. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy. 2016;18:1332–1344. doi: 10.1016/j.jcyt.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Shen L, Gao Y, Qian J, Wu Y, Zhou M, Sun A, Zou Y, Ge J. The role of SDF-1α/Rac pathway in the regulation of endothelial progenitor cell polarity; homing and expression of Rac1, Rac2 during endothelial repair. Molecular and cellular biochemistry. 2012;365:1–7. doi: 10.1007/s11010-011-1083-z. [DOI] [PubMed] [Google Scholar]
- Shi X, Yan C, Liu B, Yang C, Nie X, Wang X, Zheng J, Wang Y, Zhu Y. miR-381 regulates neural stem cell proliferation and differentiation via regulating Hes1 expression. PloS one. 2015;10:e0138973. doi: 10.1371/journal.pone.0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shichinohe H, Ishihara T, Takahashi K, Tanaka Y, Miyamoto M, Yamauchi T, Saito H, Takemoto H, Houkin K, Kuroda S. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabilitation and neural repair. 2015;29:80–89. doi: 10.1177/1545968314525856. [DOI] [PubMed] [Google Scholar]
- Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ, Kucia M. Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem cells and development. 2011;21:1639–1652. doi: 10.1089/scd.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Suszynska M, Mierzejewska K, Ratajczak J, Ratajczak MZ. Very small embryonic-like stem-cell optimization of isolation protocols: an update of molecular signatures and a review of current in vivo applications. Experimental & molecular medicine. 2013;45:e56. doi: 10.1038/emm.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A, Maki T, Mandeville ET, Liang AC, Egawa N, Itoh K, Itoh N, Borlongan M, Holder JC, Chuang TT, McNeish JD. Astrocyte-derived pentraxin 3 supports blood–brain barrier integrity under acute phase of stroke. Stroke. 2016;47:1094–1100. doi: 10.1161/STROKEAHA.115.012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kim DW, Pabon M, Acosta S, Kaneko Y, Borlongan CV. Stem cells for neurovascular repair in stroke. Journal of stem cell research & therapy. 2013;4:12912. doi: 10.4172/2157-7633.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Liao L, Su X, Yu Y, Shao B, Jing H, Zhang X, Deng Z, Jin Y. Melatonin treatment improves mesenchymal stem cells therapy by preserving stemness during long-term in vitro expansion. Theranostics. 2016;6:1899–917. doi: 10.7150/thno.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor–stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, Stevanato L, Sinden JD, Modo M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem cells. 2012;30:785–796. doi: 10.1002/stem.1024. [DOI] [PubMed] [Google Scholar]
- Soffer-Tsur N, Peer D, Dvir T. ECM-based macroporous sponges release essential factors to support the growth of hematopoietic cells. Journal of Controlled Release. 2016 doi: 10.1016/j.jconrel.2016.09.021. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song M, Jue SS, Cho YA, Kim EC. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. Journal of neuroscience research. 2015;93:973–983. doi: 10.1002/jnr.23569. [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C. Clinical Outcomes of Transplanted Modified Bone Marrow–Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke. 2016;47:1817–24. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato L, Thanabalasundaram L, Vysokov N, Sinden JD. investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. PloS one. 2016;11:e0146353. doi: 10.1371/journal.pone.0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabilitation and neural repair. 2009;9:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Shu-Uin G, Kae-Siang N, Gauthaman K, Biswas A, Choolani M, Bongso A, Chui-Yee F. Human umbilical cord wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. Journal of cellular biochemistry. 2012;113:1886–1895. doi: 10.1002/jcb.24057. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Duncan K, Dailey T, Kaneko Y, Tajiri N, Borlongan CV. A possible new focus for stroke treatment–migrating stem cells. Expert opinion on biological therapy. 2015;15:949–958. doi: 10.1517/14712598.2015.1043264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26:1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- Tajiri N, Acosta S, Glover LE, Bickford PC, Simancas AJ, Yasuhara T, Date I, Solomita MA, Antonucci I, Stuppia L, Kaneko Y. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PloS one. 2012;7:e43779. doi: 10.1371/journal.pone.0043779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Borlongan CV, Kaneko Y. Cyclosporine A Treatment Abrogates Ischemia-Induced Neuronal Cell Death by Preserving Mitochondrial Integrity through Upregulation of the Parkinson’s Disease-Associated Protein DJ-1. CNS Neurosci Ther. 2016;7:602–10. doi: 10.1111/cns.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, Hernadez-Ontiveros DG, Shinozuka K, Ishikawa H, Kaneko Y, Yankee E. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Frontiers in systems neuroscience. 2014;8:116. doi: 10.3389/fnsys.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proceedings of the National Academy of Sciences. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, Xu L, Hess DC, Borlongan CV. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell transplantation. 2007;16:159–169. [PubMed] [Google Scholar]
- Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Experimental neurology. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. American Journal of Physiology-Heart and Circulatory Physiology. 2006;290:1378–1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- Thomas E, Zeps N, Cregan M, Hartmann P, Martin T. 14-3-3σ (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle. 2011;10:278–284. doi: 10.4161/cc.10.2.14470. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research. 1961;14:213–222. [PubMed] [Google Scholar]
- Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. British journal of clinical pharmacology. 2009;68:484–492. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ. Sarcoma derived from cultured mesenchymal stem cells. Stem cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Topchiy E, Panzhinskiy E, Griffin WST, Barger SW, Das M, Zawada WM. Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation. Developmental neuroscience. 2013;35:293–305. doi: 10.1159/000350502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PloS one. 2015;10:e0127302. doi: 10.1371/journal.pone.0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trialists OS. Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. The Lancet. 2004;363:352–356. doi: 10.1016/S0140-6736(04)15434-2. [DOI] [PubMed] [Google Scholar]
- Truelsen T, Krarup LH, Iversen HK, Mensah GA, Feigin VL, Sposato LA, Naghavi M. Causes of death data in the Global Burden of Disease estimates for ischemic and hemorrhagic stroke. Neuroepidemiology. 2015;45:152–160. doi: 10.1159/000441084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Yang F, Wan J, Liu Y, Zhang J, Wu B, Liu Y, Zeng S, Wang L. Light-controlled astrocytes promote human mesenchymal stem cells toward neuronal differentiation and improve the neurological deficit in stroke rats. Glia. 2014;62:106–121. doi: 10.1002/glia.22590. [DOI] [PubMed] [Google Scholar]
- Valencia J, Blanco B, Yáñez R, Vázquez M, Sánchez CH, Fernández-García M, Serrano CR, Pescador D, Blanco JF, Hernando-Rodríguez M, Sánchez-Guijo F. Comparative analysis of the immunomodulatory capacities of human bone marrow–and adipose tissue–derived mesenchymal stromal cells from the same donor. Cytotherapy. 2016;18:1297–1311. doi: 10.1016/j.jcyt.2016.07.006. [DOI] [PubMed] [Google Scholar]
- van der Strate BWA, Popa ER, Schipper M, Brouwer LA, Hendriks M, Harmsen MC, Van Luyn MJA. Circulating human CD34+ progenitor cells modulate neovascularization and inflammation in a nude mouse model. Journal of molecular and cellular cardiology. 2007;42:1086–1097. doi: 10.1016/j.yjmcc.2007.03.907. [DOI] [PubMed] [Google Scholar]
- Wang B, Pfeiffer MJ, Drexler HC, Fuellen G, Boiani M. Proteomic Analysis of Mouse Oocytes Identifies PRMT7 as a Reprogramming Factor that Replaces SOX2 in the Induction of Pluripotent Stem Cells. Journal of proteome research. 2016;15:2407–2421. doi: 10.1021/acs.jproteome.5b01083. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen Y, Yang Y, Xiao X, Chen S, Zhang C, Jacobs B, Zhao B, Bihl J, Chen Y. Endothelial progenitor cells and neural progenitor cells synergistically protect cerebral endothelial cells from Hypoxia/reoxygenation-induced injury via activating the PI3K/Akt pathway. Molecular brain. 2016;9(1):12. doi: 10.1186/s13041-016-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Duan F, Wang MX, Wang XD, Liu P, Ma LZ. Effect of stem cell-based therapy for ischemic stroke treatment: a meta-analysis. Clinical neurology and neurosurgery. 2016;146:1–11. doi: 10.1016/j.clineuro.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Wang YL, Lin SP, Nelli SR, Zhan FK, Cheng H, Lai TS, Yeh MY, Lin HC, Hung SC. Self-Assembled Peptide-Based Hydrogels as Scaffolds for Proliferation and Multi-Differentiation of Mesenchymal Stem Cells. Macromolecular Bioscience. 2016;17:4. doi: 10.1002/mabi.201600192. [DOI] [PubMed] [Google Scholar]
- Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell transplantation. 2013;22:977–991. doi: 10.3727/096368912X657251. [DOI] [PubMed] [Google Scholar]
- Wen J, Li HT, Li SH, Li X, Duan JM. Investigation of modified platelet-rich plasma (mPRP) in promoting the proliferation and differentiation of dental pulp stem cells from deciduous teeth. Brazilian Journal of Medical and Biological Research. 2016;49:e5373. doi: 10.1590/1414-431X20165373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proceedings of the National Academy of Sciences. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Stilhano RS, To VP, Tran L, Wong K, Silva EA. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P) PloS one. 2015;10:e0123437. doi: 10.1371/journal.pone.0123437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Ye M, Levy A, Rothstein J, Bergles D, Searson PC. The blood-brain barrier: an engineering perspective. Frontiers in neuroengineering. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WS K. Adult mesenchymal stem cells and cell surface characterization-a systematic review of the literature. The Open Orthopaedics Journal. 2011;5:253–60. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Yin H, Yao YY, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Human gene therapy. 2006;17:206–219. doi: 10.1089/hum.2006.17.206. [DOI] [PubMed] [Google Scholar]
- Xia CF, Smith RS, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47:752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressed multipotent mesenchymal stromal cells. Cell transplantation. 2016;26:243–257. doi: 10.3727/096368916X693031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhu H, Zhao D, Tan J. Endometrial stem cells: clinical application and pathological roles. International journal of clinical and experimental medicine. 2015;8:22039–44. [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013;44:551–554. doi: 10.1161/STROKEAHA.112.676759. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell transplantation. 2011;20:883–891. doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Saito H, Ito M, Shichinohe H, Houkin K, Kuroda S. Platelet lysate and granulocyte-colony stimulating factor serve safe and accelerated expansion of human bone marrow stromal cells for stroke therapy. Translational stroke research. 2014;5:701–710. doi: 10.1007/s12975-014-0360-z. [DOI] [PubMed] [Google Scholar]
- Yan F, Yue W, Zhang YL, Mao GC, Gao K, Zuo ZX, Zhang YJ, Lu H. Chitosan-collagen porous scaffold and bone marrow mesenchymal stem cell transplantation for ischemic stroke. Neural regeneration research. 2015;9:1421–6. doi: 10.4103/1673-5374.163466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YH, Li SH, Gao Z, Zou SF, Li HY, Tao ZY, Song J, Yang JX. Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow-derived neural stem cells via notch signaling pathway. Life sciences. 2016;166:131–138. doi: 10.1016/j.lfs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Borlongan CV, Date I. Ex vivo gene therapy: transplantation of neurotrophic factor-secreting cells for cerebral ischemia. Front Biosci. 2006;11:760–775. doi: 10.2741/1834. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM, Yu SJ, Bae E, Yu G, Xu L, McGrogan M, Bankiewicz K. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem cells and development. 2009;18:1501–1514. doi: 10.1089/scd.2009.0011. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Translational stroke research. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem cell research & therapy. 2013;4:73. doi: 10.1186/scrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Zhang HT, Cai YQ, Han YJ, Yao F, Yuan ZH, Wu BY. Anti-inflammatory effect of mesenchymal stromal cell transplantation and quercetin treatment in a rat model of experimental cerebral ischemia. Cellular and molecular neurobiology. 2016;36:1023–1034. doi: 10.1007/s10571-015-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Lu H, Kang Q, Li W, Gao M. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology. 2011;31:384–391. doi: 10.1111/j.1440-1789.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Liu X, Wei M, Nejad-Davarani SP, Wang X, Zhang ZG. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PloS one. 2014;9:e113972. doi: 10.1371/journal.pone.0113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152:389–402. doi: 10.1530/REP-16-0286. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ying G, Ren C, Jizhang Y, Brogan D, Liu Z, Li S, Ding Y, Borlongan CV, Zhang J, Ji X. Administration of human platelet-rich plasma reduces infarction volume and improves motor function in adult rats with focal ischemic stroke. Brain research. 2015;1594:267–273. doi: 10.1016/j.brainres.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Yao S, Li F, Xin L, Lai M, Bracchi-Ricard V, Xu H, Yen W, Meng W, Liu S. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells. 2012;30:510–524. doi: 10.1002/stem.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, Zhang Q, Xiang C, Hou P, Song Z, Liu Y. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell stem cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao YH, Yuan B, Chen J, Feng DH, Zhao B, Qin C, Chen YF. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS neuroscience & therapeutics. 2013;19:67–75. doi: 10.1111/cns.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Qin Z, Zhong CJ, Wang Y, Shen XY. Neuroprotective effects of bone marrow stromal cells on rat organotypic hippocampal slice culture model of cerebral ischemia. Neuroscience letters. 2003;342:93–96. doi: 10.1016/s0304-3940(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Zhou F, Gao S, Wang L, Sun C, Chen L, Yuan P, Zhao H, Yi Y, Qin Y, Dong Z, Cao L. Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model. Stem cell research & therapy. 2015;6:92. doi: 10.1186/s13287-015-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Barroso LF, Willing AE, Saporta S, McGrogan MP, Freeman TB, Sanberg PR. Dopaminergic phenotype of hNT cells in vitro. Developmental Brain Research. 2000;122:87–90. doi: 10.1016/s0165-3806(00)00055-9. [DOI] [PubMed] [Google Scholar]
- Zigova T, Willing AE, Tedesco EM, Borlongan CV, Saporta S, Snable GL, Sanberg PR. Lithium chloride induces the expression of tyrosine hydroxylase in hNT neurons. Experimental neurology. 1999;157:251–258. doi: 10.1006/exnr.1999.7054. [DOI] [PubMed] [Google Scholar]
- Zimmermann JA, Hettiaratchi MH, McDevitt TC. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem cells translational medicine. 2017;6:223–237. doi: 10.5966/sctm.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]


